Abstract
The onset of diabetic nephropathy (DN) is associated with both systemic and renal changes. Fibroblast growth factor (FGF)-21 prevents diabetic complications mainly by improving systemic metabolism. In addition, low-dose radiation (LDR) protects mice from DN directly by preventing renal oxidative stress and inflammation. In the present study, we tried to define whether the combination of FGF21 and LDR could further prevent DN by blocking its systemic and renal pathogeneses. To this end, type 2 diabetes was induced by feeding a high-fat diet for 12 wk followed by a single dose injection of streptozotocin. Diabetic mice were exposed to 50 mGy LDR every other day for 4 wk with and without 1.5 mg/kg FGF21 daily for 8 wk. The changes in systemic parameters, including blood glucose levels, lipid profiles, and insulin resistance, as well as renal pathology, were examined. Diabetic mice exhibited renal dysfunction and pathological abnormalities, all of which were prevented significantly by LDR and/or FGF21; the best effects were observed in the group that received the combination treatment. Our studies revealed that the additive renal protection conferred by the combined treatment against diabetes-induced renal fibrosis, inflammation, and oxidative damage was associated with the systemic improvement of hyperglycemia, hyperlipidemia, and insulin resistance. These results suggest that the combination treatment with LDR and FGF21 prevented DN more efficiently than did either treatment alone. The mechanism behind these protective effects could be attributed to the suppression of both systemic and renal pathways.
Keywords: low-dose radiation, fibroblast growth factor-21, insulin resistance, inflammation, oxidative stress
diabetic nephropathy (DN) is a severe complication of diabetes and the leading cause of end-stage renal disease (38). DN initiates with the thickening of the glomerular basement membrane, which is followed by mild and moderate mesangial expansion, capillary collapse in the renal tubule, epithelial cell degeneration, and a gradual increase in proteinuria, and finally leads to renal fibrosis and kidney failure (43, 56). Hyperglycemia, hyperlipidemia, and subsequent insulin resistance are the initial and systemic pathogeneses of DN, which is also associated with renal pathogeneses, such as oxidative stress, inflammation, and fibrosis. There are currently multiple therapies to treat patients with DN, including reducing blood sugar levels, lowering lipid levels, enhancing insulin sensitivity, and reducing oxidative stress, inflammation, or fibrosis (1, 19, 50). However, most of these treatments just slow rather than arrest the progression toward DN, since blocking any single pathway is not sufficient to prevent the development of DN (8, 19). Therefore, identifying a treatment that inhibits both the systemic and renal pathogeneses will prevent the development of DN efficiently.
Fibroblast growth factor (FGF)-21 is a novel member of the FGF family that functions as an endocrine hormone rather than regulating cellular proliferation and differentiation (3, 29). FGF21 acts on multiple tissues to regulate carbohydrate and lipid metabolism. Large amounts of evidence have demonstrated that FGF21 also induces beneficial effects in diabetes and its related complications by lowering blood glucose levels, stimulating lipid β-oxidation, and enhancing insulin sensitivity (25, 26). In addition, FGF21 protects the heart against oxidative stress-induced damage in two different mice models [isoproterenol-induced cardiac hypertrophy (40) and the LPS-induced proinflammatory pathway (7) by inducing the expression of antioxidant proteins, including uncoupling proteins and SOD-2 (41)]. FGF21 also protects the liver from d-gal-induced oxidative stress by activating Nrf2 and subsequent antioxidant genes (54). Moreover, Kim et al. (27) demonstrated that FGF21 protects the kidneys in a mouse model of type 2 diabetes by improving insulin sensitivity. Our previous study (56) also demonstrated the beneficial effect of FGF21 on type 1 diabetes-induced renal damage.
Low-dose radiation (LDR) generally refers to radiation at a dose less than 100 mGy for low linear energy transfer (LET) (32), which induces beneficial effects, including stimulating DNA/RNA repair, increasing the cellular antioxidant capacity, prolonging life span, and activating immune functions (13, 14). These cellular hormetic effects of LDR protect cells in vitro or tissues in vivo against gene mutations, DNA damage, and the chromosomal aberrations caused by subsequent large doses of radiation or toxic chemicals via an adaptive response (9, 10, 12). Increasing evidence has suggested that LDR reduces the incidence of diabetes and also exerts beneficial effects on diabetic complications such as DN (35, 45). In addition, our previous studies (43) showed that exposure to LDR greatly prevented type 2 diabetes-induced renal injury and also inhibited renal oxidative stress, inflammation, and fibrosis without altering blood glucose levels. We (51, 57) further demonstrated that LDR could increase Akt phosphorylation and the expression of Nrf2 and its downstream antioxidants significantly.
Taken together, the evidence above suggests that both FGF21 and LDR induce beneficial effects on DN. FGF21 might mainly suppress the systemic pathogenesis, whereas LDR might protect the kidneys from diabetes mainly via anti-inflammatory, antioxidative, and antifibrotic mechanisms (51, 56, 57). Therefore, the aim of the present study was to investigate whether the combination treatment with LDR and FGF21 could exert additive effects on the prevention of DN via both systemic and renal mechanisms.
MATERIALS AND METHODS
Ethics statement.
Eight-week-old male C57BL/6J mice (weighting 18–22 g) were purchased from the Experimental Animal Center of Beijing University of Medical Science (Beijing, China) and allowed to acclimate for 2 wk. All mice were housed in the Experimental Animal Center of Jilin University at 22°C with 12:12-h light-dark cycles and free access to rodent chow and tap water. The animal production license is No. SCXK 2007-0003. All animal procedures were approved by the University Animal Care and Use Committee (permit number 2007-0011), which is certified by the Chinese Association of Accreditation of Laboratory Animal Care.
Animals and type 2 diabetes model.
The high-fat diet (HFD)/streptozotocin (STZ) protocol was applied to establish the model of type 2 diabetes (34, 43, 49). Mice were fed a HFD (40% of calories from fat; Shanghai SLAC Laboratory Animal, Shanghai, China) for 12 wk to induce metabolic syndrome, characterized by obesity, abnormal glucose tolerance, and insulin resistance. Age-matched nondiabetic mice were fed a standard diet (SD, 10% of calories from fat; Shanghai SLAC Laboratory Animal). The obese mice were divided randomly into two groups: one group was given a single injection of 50 mg/kg body wt STZ ip to induce hyperglycemia and the onset of type 2 diabetes, whereas the other group was given an ip injection of an equivalent volume of citrate buffer (Con). Mice were considered diabetic when their blood glucose exceeded 12 mmol/l. Subsequently, the diabetic mice were divided into four subgroups: diabetes mellitus (DM) plus LDR treatment (DM/LDR), DM plus FGF21 treatment (DM/FGF21), and DM plus the combination of LDR and FGF21 treatment (DM/Com).
Whole body LDR and FGF21 treatment.
A 180-kVp X-ray generator (Model XSZ-Z20/20, China) was used to deliver radiation at a dose rate of 12.5 mGy/min (120 kv, 13 mA). Diabetic mice in the DM/LDR and DM/Com groups received whole body irradiation every 2 days at a dose of 50 mGy/day for 4 wk, based on our previous observations (56). Mice in the DM/FGF21 or DM/Com groups were given intraperitoneal (ip) injections of 1.5 mg/kg FGF21 daily for 8 wk. Therefore, the DM/Com group received both LDR and FGF21 treatment for 4 wk followed by an additional 4 wk of FGF21 treatment alone.
Glucose and insulin tolerance tests.
To evaluate glucose tolerance, mice were given an injection of d-glucose (1.5 g/kg ip) after an overnight fast (12 h) with free access to water. Venous blood was collected 30 min before the injection of glucose (time 0), as well as 15, 30, 60, and 120 min after injection from the tail of each mouse, and glucose levels were measured using a OneTouch SureStep complete blood glucose monitor (LifeScan, Milpitas, CA). To evaluate insulin tolerance, a single dose of 0.5 U/kg Novolin R regular insulin (Novo Nordisk, Bagsvaerd, Denmark) was administered ip after a 4-h fast with free access to water, and the blood glucose levels were measured as described above.
Measuring renal function.
Mice were placed in metabolic cages individually with free access to tap water on the day before euthanasia to collect 24-h urine samples. Total urinary protein (UP), urinary microalbumin (mAlb), and urinary creatinine (U-Cre) contents were measured as parameters of renal function using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems (Itasea, MN) according to the manufacturer's instructions.
Measuring serum and renal lipid profiles.
Mice were euthanized after being anesthetized, and blood was collected by cardiac puncture and centrifuged at 2,000 g for 20 min at 4°C to prepare serum. Serum triglycerides (TG), total cholesterol (CHO), high-density lipoprotein (HDL)-cholesterol, and low-density lipoprotein (LDL)-cholesterol levels were determined using an Olympus Au800 automatic biochemical analyzer (Olympus, Japan).
To measure TG and free fatty acid (FFA) contents in the kidney, ∼80 mg of kidney tissue from each mouse was homogenized in 250 μl of buffer containing 150 mM NaCl and 10 mM Tris (pH 7.5), and then extracted using 200 μl of methanol and 400 μl of chloroform, as described by Bligh and Dyer (6). The rest of the procedure was same as that described above, using an Olympus Au800 automatic biochemical analyzer.
Total protein extraction and Western blotting.
Renal tissues were homogenized in lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA), and the supernatants were collected by centrifugation at 12,000 g and 4°C. After determination of the total protein concentration, equal amounts of each sample were run on 10% SDS-polyacrylamide gel electrophoresis and then transferred to nitrocellulose membranes. After blocking with nonfat milk for 1 h at room temperature, the membranes were incubated overnight at 4°C with the following primary antibodies: nuclear factor E2-related factor 2 (Nrf-2, 1:1,000), superoxide dismutase-1 (SOD-1, 1:2,000), NAD(P)H:quinone oxidoreductase-1 (NQO-1, 1:1,000), heme oxygenase-1 (HO-1, 1:2,000), 3-nitrotyrosine (3-NT, 1:1,000), intercellular adhesion molecule 1 (ICAM-1, 1:2,000), plasminogen activator inhibitor 1 (PAI-1, 1:2,000), connective tissue growth factor (CTGF, 1:2,000), tumor necrosis factor-α (TNF-α, 1:1,000), and β-actin (1:2,000). All antibodies were purchased from Abcam (Cambridge, MA), except for β-actin (Cell Signaling Technology, Danvers, MA). After three washes in Tris-buffered saline containing 0.05% Tween 20, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Antigen-antibody complexes were then visualized using an enhanced chemiluminescence kit (Amersham, Piscataway, NJ), and the intensity of the protein bands was quantified using Quantity One software (v. 4.6.2; Bio-Rad, Hercules, CA).
Histological examination.
Kidney tissues were fixed in 10% formalin at room temperature for 48 h. After dehydration, the tissue blocks were embedded in paraffin and cut into 4-mm-thick sections. They were then stained with hematoxylin and eosin (H&E) for general morphological examination, periodic acid-Schiff (PAS) for glomerulosclerosis evaluation, and Masson's Trichrome staining for interstitial expansion assessment, as described previously (24, 44, 56). Five different fields were selected randomly from each slice, each containing at least 50 glomeruli. The degree of glomerulosclerosis was divided into five grades from 0 to 4 according to the number of sclerotic lesions in each glomerulus (grade 0, normal glomerulus; grade 1, sclerotic area of 1–25%; grade 2, sclerotic area of 26–50%; grade 3, sclerotic area of 51–75%; grade 4, sclerotic area of 76–100%). The glomerulosclerosis index (GSI) was calculated using following formula:
GSI = [(N1 × 1) + (N2 × 2) + (N3 × 3) + (N4 × 4)]/Ntotal
where N represents the number of the corresponding degree of glomerulosclerosis and Ntotal is the total number of glomeruli. Similarly, the extent of interstitial expansion was quantified by calculating the tubular interstitial collagen-positive area (blue) of Masson's Trichrome staining using Image-Pro Plus 6.0 software (Media Cybernetics, Silver Spring, MD). Twenty consecutive glomeruli were examined for each section, and the mean percentage of collagen-positive lesions was calculated for each mouse.
Assaying lipid oxidation.
A thiobarbituric acid (TBA) assay was used to measure relative malondialdehyde (MDA) production as an index of lipid peroxidation, as described previously (11). Briefly, tissue proteins were collected by centrifuging at 12,000 g at 4°C for 15 min, and the protein concentrations were measured using Bradford assays. Then, 50 μl of sample was mixed with 20 μl of 8.1% sodium dodecyl sulfate (SDS), 150 μl of 20% acetic acid, and 210 μl of 0.0571% TBA and was incubated at 90°C for 70 min. Samples were centrifuged at 4,000 rpm for 15 min at 4°C, harvested, and transferred to 96-well plates, and the optical density was read at 540 nm.
Statistical analysis.
Data were collected from nine mice in each group, and the results are presented as means ± SE. Statistical analyses were performed using one-way or two-way ANOVA, followed by post hoc multiple comparisons using Bonferroni's tests. All analyses were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Statistical significance was defined as P < 0.05.
RESULTS
Type 2 diabetic mouse model.
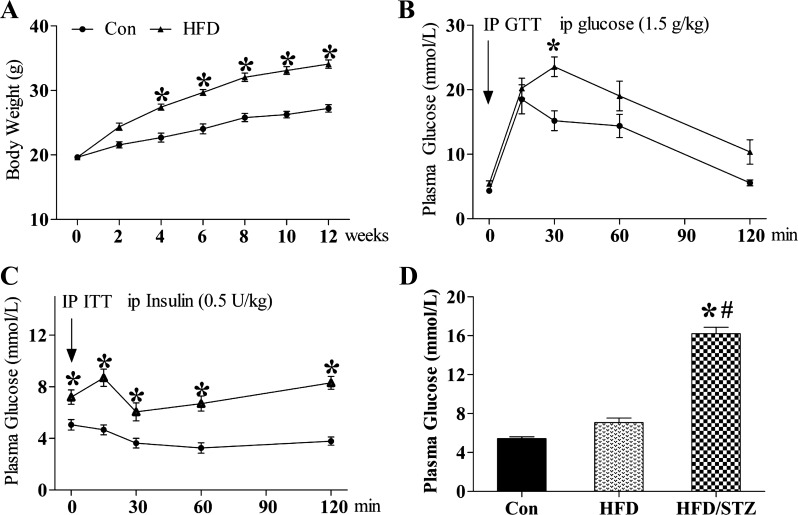
Clinically, the major features of type 2 diabetes are insulin resistance together with obesity, hyperlipidemia, and hyperglycemia. Therefore, in the current study mice were fed a HFD to induce obesity, hyperlipidemia, and insulin resistance and were then treated using a single injection of STZ to induce hyperglycemia and establish a model of type 2 diabetes. As shown in Fig. 1, there was a significant increase in their body weight starting 4 wk after HFD feeding compared with SD feeding (Con), and the difference between the two groups increased gradually in a time-dependent manner (Fig. 1A). In the glucose tolerance test (GTT), the blood glucose level in the HFD-fed mice after glucose infusion was greater than that in the SD-fed mice at 30 min, suggesting that the HFD-fed mice were glucose intolerant (Fig. 1B). In addition, the blood glucose level in the HFD-fed mice remained higher than that in the SD-fed mice until the final measurement, although there were no statistically significant differences between the two groups (Fig. 1B). Furthermore, blood glucose levels during the insulin tolerance test (ITT) were significant higher in HFD-fed mice than in SD-fed mice at all time points (Fig. 1C). The results above suggested that the mice fed a HFD for 12 wk had already developed glucose intolerance and insulin resistance (Fig. 1, B and C). A single injection of STZ induced a significant increase in blood glucose compared with both HFD-fed alone and SD-fed (Con) groups. But no significant difference was observed between SD- and HFD-fed groups (Fig. 1D).
Fig. 1.
Establishing a type 2 diabetic mouse (DM) model using the HFD/STZ (high-fat diet/streptozotocin) model. C57BL/6J mice were fed a HFD (40% of calories from fat) for 12 wk to induce obesity. Body weight (A), glucose tolerance (B), and insulin sensitivity (C) were examined. Three days after injection of STZ, blood glucose was measured (D). Mice were regarded as diabetic once hyperglycemia was observed (>12 mmol/l). Data are presented as means ± SE; n = 9 per group. GTT, glucose tolerance test; ITT, insulin tolerance test. *P < 0.05 vs. HFD group; #P < 0.05 vs. DM group.
The combination of LDR and FGF21 improved insulin sensitivity in type 2 diabetic mice significantly compared with treatment of either alone.
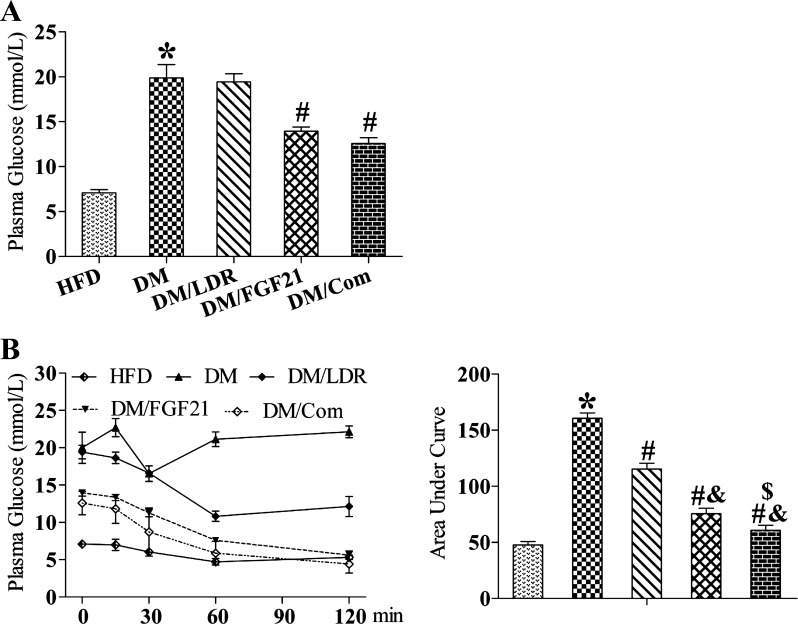
Diabetic mice were fed a HFD in the presence of FGF21 alone, LDR alone, or the combination of both treatments. At the end of experiment, FGF21 and the combination treatment, not LDR alone, decreased blood glucose levels significantly compared with the DM group (Fig. 2A). Although the blood glucose levels in the combination group were decreased slightly compared with FGF21 treatment alone, the difference was not statistically significant (Fig. 2A). In addition, both LDR and FGF21 significantly ameliorated diabetes-induced insulin resistance (Fig. 2B); however, the combination treatment improved insulin sensitivity additively compared with either single treatment alone (Fig. 2B).
Fig. 2.
Effects of combination treatment strategy on hyperglycemia and insulin resistance. Blood glucose levels (A) and insulin tolerance (B) were examined in each group. LDR, low-dose radiation; FGF, fibroblast growth factor. Data are presented as means ± SE; n = 9 per group. *P < 0.05 vs. HFD group; #P < 0.05 vs. DM group; &P < 0.05 vs. LDR group; $P < 0.05 vs. FGF21 group.
Effects of the different treatments on renal dysfunction in diabetic mice.
Diabetes increased the levels of UP and mAlb significantly, indicating the induction of renal dysfunction (Table 1). However, FGF21 treatment prevented diabetes-induced renal dysfunction significantly, as shown by reduced UP and mAlb and increased U-Cre levels. Exposure to LDR also reduced UP and mAlb significantly but did not affect U-Cre levels. Renal dysfunction, which is reflected by increased UP and mAlb levels, was diminished further in mice that received the combination treatment compared with either treatment alone. Both FGF21 alone and the combination treatment, but not LDR alone, increased U-Cre excretion significantly compared with the DM group (Table 1).
Table 1.
Renal function of mice fed a HFD with or without additional treatments
| Parameters | HFD | DM | DM/LDR | DM/FGF21 | DM/Com |
|---|---|---|---|---|---|
| Urine protein, μg/day | 120.9 ± 10.5 | 985.4 ± 25.9* | 750.5 ± 30.2# | 664.2 ± 34.0# | 480.2 ± 30.6#&$ |
| Urine mAlb, μg/day | 17.8 ± 4.7 | 305.2 ± 24.7* | 184.8 ± 21.2# | 138.8 ± 23.1# | 102.5 ± 20.3#&$ |
| Urine creatinine | 33.8 ± 2.0 | 31.2 ± 1.0 | 35.5 ± 1.2 | 37.6 ± 1.5# | 38.3 ± 1.4# |
Data are presented as means ± SE; n = 9/group.
mAlb, microalbumin; HFD, high-fat diet; DM, diabetes mellitus; LDR, low-dose radiation; FGF, fibroblast growth factor; Com, combination LDR + FGF21.
P < 0.05 vs. HFD group;
P < 0.05 vs. DM group;
P < 0.05 vs. LDR group;
P < 0.05 vs. FGF21 group.
Effects of the different treatments on diabetes-induced renal hypertrophy and pathological changes.
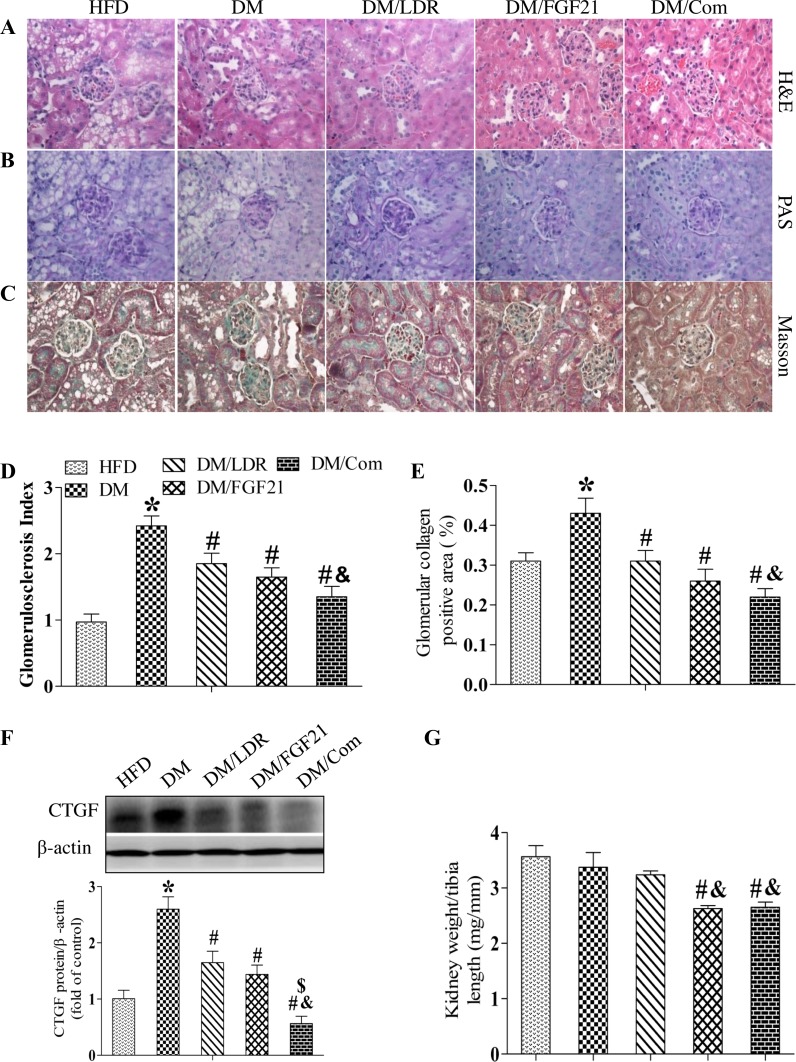
Generally, renal dysfunction reflects pathological changes in the kidney. Consistent with the functional findings (Table 1), mesangial cell proliferation, capillary wall thickness, capillary collapse, tubular dilation, and epithelial cell degeneration were all obvious in diabetic kidneys after H&E staining (Fig. 3A). These pathological changes were suppressed significantly by LDR or FGF21 treatment alone (Fig. 3A), and to a greater extent by the combination treatment.
Fig. 3.
Effects of combination treatment strategy on diabetes-induced histopathological changes in the kidneys of mice. Representative images of hematoxylin and eosin (A, H&E) staining, periodic acid-Schiff (B, PAS) staining, and Masson's Trichrome staining (C, Masson) to detect renal pathological changes, glomerulosclerosis, and collagen deposition, respectively. Magnification ×400. Glomerulosclerosis and collagen accumulation were examined in PAS- or Masson's-stained slices, respectively, and quantified using Image-Pro Plus 6.0 software (D and E). Renal connective tissue growth factor (CTGF) expression was measured using Western blotting (F). Renal hypertrophy was examined by assessing the change in the kidney weight-to-tibia length ratio (G). Data are presented as means ± SE; n = 9 per group. *P < 0.05 vs. HFD group; #P < 0.05 vs. DM group; &P < 0.05 vs. LDR group.
Next, the kidney tissues were stained with PAS to detect glomerulosclerosis. As shown in Fig. 3, B and D, the GSI was increased significantly in diabetic mice, which was prevented significantly by treatment with either LDR or FGF21 alone and to a greater extent by the combination treatment.
Masson's Trichrome staining revealed significant collagen accumulation in the kidneys of diabetic mice, indicating that interstitial fibrosis had developed (Fig. 3, C and E). Treatment with either LDR or FGF21 alone prevented these pathological changes significantly, and the preventive effects were additive in the combination treatment group. To confirm these observations, we assessed CTGF expression in the kidneys as a classical molecular marker of fibrosis using western blotting. Consistent with the results of Masson's staining, renal CTGF expression was increased significantly in the DM group which was remarkably suppressed by treatment with wither LDR and FGF21 alone. Furthermore a additive suppression was observed in the combination treatment group (Fig. 3F).
Diabetes-induced kidney injury is always associated with renal hypertrophy, which is characterized by an increased kidney weight to tibia length ratio. The ratio decreased slightly after exposure to LDR compared with DM and HFD groups. In contrast, treatment with FGF21 alone or in combination with LDR decreased the ratio significantly compared with the DM group (Fig. 3G).
Effects of the different treatments on systemic and renal dyslipidemia in type 2 diabetic mice.
In addition to hyperglycemia, dyslipidemia is a crucial systemic pathogenesis of DN. As shown in Table 2, hyperlipidemia was evident in the DM groups, as shown by increased serum TG levels. Consistent with the serum profiles, kidney tissue H&E staining revealed an increased number of fat vacuoles in the kidney (Fig. 3A); the renal TG and FFA levels were also increased significantly in the diabetic mice (Table 2). These results suggest that renal lipid accumulation was increased in the DM group. However, treatment with FGF21 normalized all of the above abnormal profiles remarkably. Similar effects were observed in the DM/LDR mice, except that there was no change in CHO levels compared with the DM group. Importantly, the combination treatment normalized serum and renal TG, serum LDL, and renal FFA levels (Table 2) and significantly reduced the number of renal fat vacuoles (Fig. 3A).
Table 2.
Serum and renal lipid metabolic parameters in mice fed a HFD with or without additional treatments
| Parameters | HFD | DM | DM/LDR | DM/FGF21 | DM/Com |
|---|---|---|---|---|---|
| Serum TG, mmol/l | 1.39 ± 0.17 | 6.58 ± 0.70* | 4.28 ± 0.72# | 3.60 ± 0.65# | 3.06 ± 0.48#&$ |
| Serum CHO, mmol/l | 4.41 ± 0.47 | 4.70 ± 0.38 | 4.58 ± 0.34 | 3.83 ± 0.68# | 3.65 ± 0.37# |
| Serum HDL, mmol/l | 1.95 ± 0.25 | 1.65 ± 0.24 | 2.35 ± 0.28# | 2.42 ± 0.17# | 2.47 ± 0.21# |
| Serum LDL, mmol/l | 0.45 ± 0.07 | 0.66 ± 0.16 | 0.53 ± 0.07 | 0.50 ± 0.08# | 0.35 ± 0.07#&$ |
| Renal TG, mg/g tissue | 6.17 ± 1.20 | 9.82 ± 1.42* | 6.20 ± 1.13# | 4.45 ± 1.20# | 3.52 ± 1.50#&$ |
| Renal FFA, mg/g tissue | 9.58 ± 1.72 | 16.25 ± 1.64* | 10.46 ± 1.55# | 8.20 ± 1.69# | 7.15 ± 1.82#&$ |
Data are presented as means ± SE; n = 9/group.
TG, triglycerides; CHO, cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; FFA, free fatty acids.
P < 0.05 vs. HFD group;
P < 0.05 vs. DM group.
P < 0.05 vs. LDR group;
P < 0.05 vs. FGF21 group.
Effects of the different treatments on diabetes-induced renal oxidative stress and inflammation.
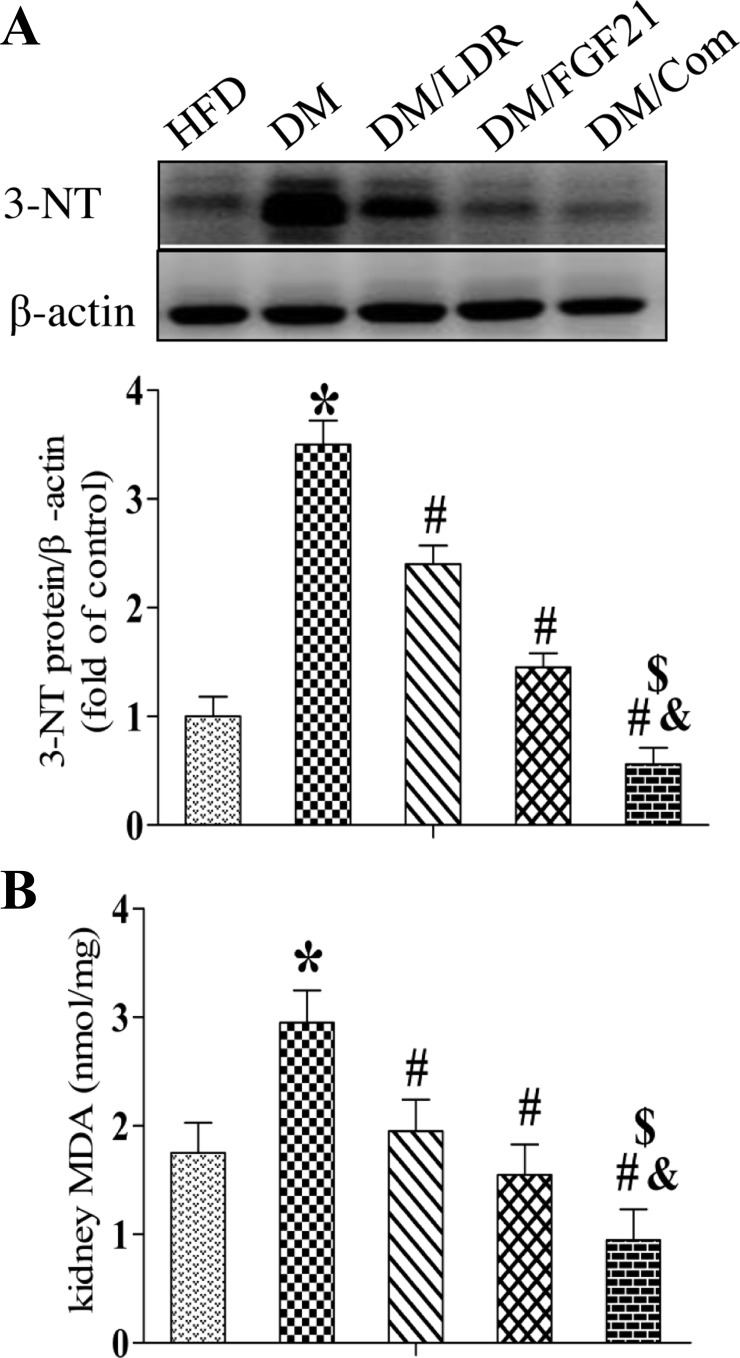
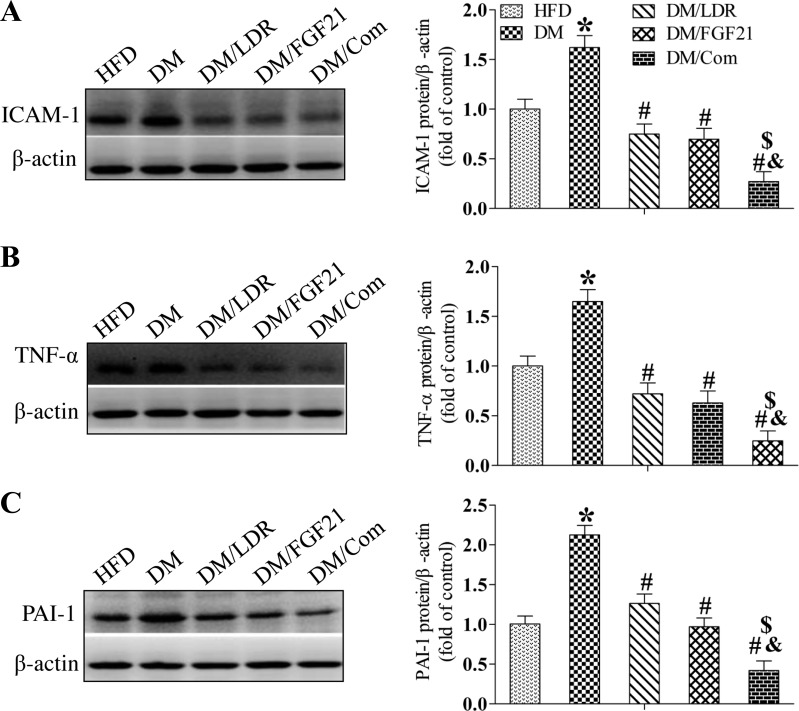
As markers of nitrosative or oxidative damage, respectively, renal 3-NT (Fig. 4A) and MDA (Fig. 4B) levels were increased significantly in the DM group but not in the DM/LDR, DM/FGF21, and DM/Com groups (Fig. 4, A and B). To confirm these observations, Western blotting was used to examine the levels of classic inflammatory factors in the kidney, including ICAM-1 (Fig. 5A), TNF-α (Fig. 5B), and PAI-1 (Fig. 5C). Consistent with the above results, diabetes increased the expression of these inflammatory factors in the kidney significantly. However, the increase was prevented by treatment with LDR or FGF21 alone and to an even greater extent by the combination treatment.
Fig. 4.
Effects of combination treatment strategy on diabetes-induced oxidative stress in kidneys. Expression of oxidative damage markers 3-nitrotyrosine (3-NT; A) and renal malondialdehyde (MDA; B) were measured using Western blotting and ELISA, respectively. Data are presented as means ± SE; n = 9 per group. *P < 0.05 vs. HFD group; #P < 0.05 vs. DM group; &P < 0.05 vs. LDR group; $P < 0.05 vs. FGF21 group.
Fig. 5.
Effects of combination treatment strategy on renal inflammation in type 2 diabetic mice. Renal tissues were collected from the different groups at the indicated times, and intercellular adhesion molecule 1 (ICAM-1), tumor necrosis factor-α (TNF-α), and plasminogen activator inhibitor 1 (PAI-1) expression was measured using Western blotting (A–C). Data are presented as means ± SE; n = 9 per group. *P < 0.05 vs. HFD group; #P < 0.05 vs. DM group; &P < 0.05 vs. LDR group; $P < 0.05 vs. FGF21 group.
Effects of the different treatments on the renal expression of antioxidants in diabetic mice.
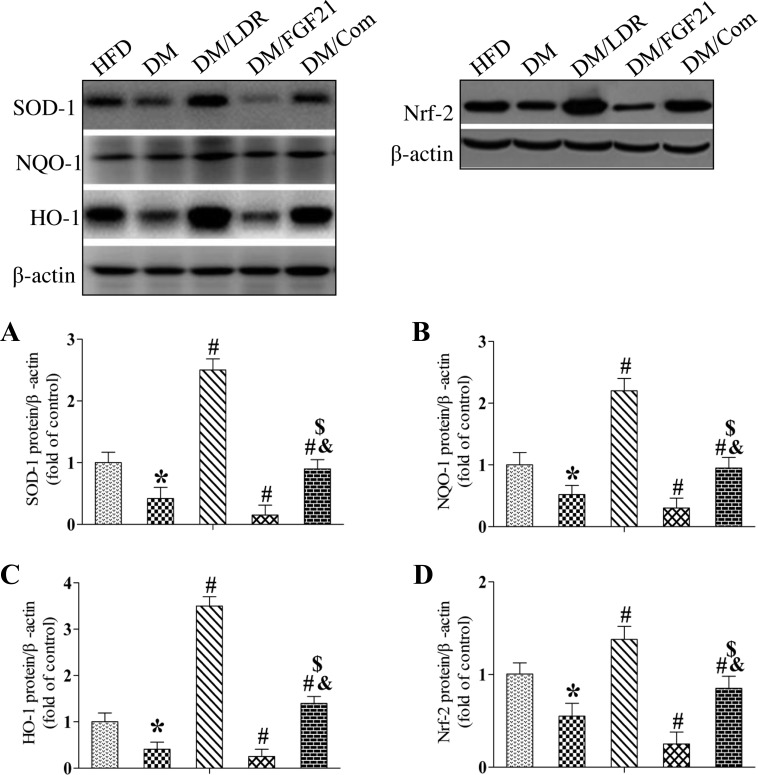
Next, we measured the renal expression of multiple antioxidants in treated and untreated diabetic mice. Western blotting revealed that the expression of SOD-1 (Fig. 6A), NQO-1 (Fig. 6B), and HO-1 (Fig. 6C) was decreased significantly in the kidneys of DM mice and DM/FGF21 mice. Interestingly, exposure to LDR reversed, but FGF21 treatment further enhanced the diabetes-induced suppression of these antioxidants significantly, and the expression of these antioxidants was much higher in the DM/LDR group than in the DM group (Fig. 6, A–C). The expression of the antioxidants was similar in DM/Com and control mice. These results suggest that LDR, but not FGF-21, increased renal antioxidant levels significantly under diabetic conditions.
Fig. 6.
Effects of combination treatment strategy on expression of renal nuclear factor E2-related factor 2 (Nrf-2) and its downstream targets in type 2 diabetic mice. Renal tissues were collected from the different groups at indicated times, and superoxide dismutase-1 (SOD-1), NAD(P)H:quinone oxidoreductase-1 (NQO-1), heme oxygenase-1 (HO-1), and Nrf-2 expression was measured using Western blotting (A–D). Data are presented as means ± SE; n = 9 per group. *P < 0.05 vs. HFD group; #P < 0.05 vs. DM group; &P < 0.05 vs. LDR group; $P < 0.05 vs. FGF21 group.
Expression of the above-mentioned antioxidants is regulated positively by the transcription factor Nrf2.
We demonstrated previously that Nrf2 is upregulated in diabetic mice exposed to LDR (51, 57). Therefore, we next determined whether the altered antioxidant expression in the DM/LDR and DM/Com groups was due to altered renal Nrf2 levels (Fig. 6D). As expected, the pattern of Nrf2 expression was similar to the antioxidant expression in the different groups, suggesting that diabetes downregulated and LDR upregulated renal antioxidant levels mainly by altering Nrf2 expression.
DISCUSSION
The kidney is the main target organ of diabetes, which leads to the development of DN. DN is characterized by renal dysfunction and pathological changes such as renal hypertrophy, fibrosis, and glomerulosclerosis (18, 21, 33). The pathogenesis of DN is highly complex and is the consequence of both systemic and renal pathogeneses. Therefore, we hypothesized that improving the systemic pathogenesis of diabetes while protecting the kidney from diabetes-induced damage might additively prevent the development of DN.
FGF21 is a member of the FGF family, which exerts systemic therapeutic effects to improve hyperglycemia, dyslipidemia, and insulin resistance (2, 16). Serum FGF21 levels were increased significantly in humans with both acute and chronic renal dysfunction during early- to end-stage kidney disease, which might be an adaptive response to diabetes to confer protective effects (30). FGF21 suppresses diabetes-induced inflammation, oxidative stress, and fibrosis in multiple organs, which could be attributed to its effects on maintaining glucose and lipid homeostasis and improving insulin resistance (22, 27, 56). Similarly, renal protective effects of FGF21 were also observed in our recent study performed in an acute lipotoxic model of type 1 diabetes (56).
High doses of ionizing radiation (HDR) induce a variety of harmful effects, including acute death and late carcinogenesis. Although LDR was also considered dangerous according to the linear no-threshold (LNT) hypothesis (32), increasing evidence has suggested that LET radiation at dose levels <100 mGy could induce beneficial effects, including extending life span, enhancing immunity, and improving DNA repair (17, 23, 36). Epidemiological surveys have analyzed individuals exposed to <100 mGy radiation. Generally, there was 1) no increase or even a reduced risk of the incidence of solid cancer, 2) no increase in the incidence of leukemia, and 3) no increase in cardiovascular diseases (5, 15, 28, 31, 37). A study supported by the Radiation Effects Research Foundation showed that the dose-response relationship supported the LNT model in principle in the dose range 0–150 mGy; however, the dose-response relationship <100 mGy tends to fluctuate, which limits statistical significance in the increase in the incidence of cancer at lower doses (42). Our recent studies demonstrated that LDR also induces beneficial effects on diabetes and its associated complications (43, 56, 57). The available data suggest that preexposure to LDR reduces the incidence of alloxan-induced diabetes and also delays the onset of hyperglycemia in diabetes-prone nonobese diabetic mice (46, 48). This protection is associated with the prevention of oxidative damage (51–53, 57). Our previous study revealed that, in addition to antioxidative effects, LDR also prevents inflammation to protect against DN (43, 51, 55, 56). Patients with DN have poor renal function; however, most drugs used to treat DN are excreted through the kidney, which further increases the renal working load in diabetic patients. Therefore, the use of LDR as a noninvasive approach has been investigated to prevent chronic renal diseases (4, 47). In the present study, we combined LDR and FGF21 as a therapeutic strategy for the first time to explore whether simultaneously blocking the systemic and renal pathogeneses could further prevent the development of DN.
We developed a human-like type 2 diabetic model using a HFD/STZ protocol. After the model of type 2 diabetes was established successfully, the mice were exposed to LDR (50 mGy/day, every other day for 4 wk) with and without FGF21 (1.5 mg/kg, daily for 8 wk) based on doses optimized in our previous studies (43).
In the early stage of DN, some abnormalities are frequently associated with enlarged kidneys. We evaluated renal hypertrophy by calculating the kidney weight-to-tibia length ratio. The HFD- and diabetes-induced increase in this ratio was suppressed slightly by LDR and significantly by FGF21 alone and the combination treatment. In addition to renal hypertrophy, morphological analysis revealed obvious glomerular basement membrane thickening, mesangial cell proliferation, mesangial matrix expansion, glomerulosclerosis, and fibrosis in the diabetic kidneys. Diabetes also caused severe renal dysfunction, as characterized by increased UP levels and mAlb excretion. These pathological and functional diabetic changes were reduced by each single-treatment strategy and suppressed further by the combination treatment. Taken together, these findings suggest that the combination of LDR and FGF21 exerts additive renal protective effects in a model of diabetes.
Next, we assessed whether the combination treatment could be attributed to preventing both systemic and renal pathogenic changes. First, we examined blood glucose levels and serum lipid profiles as global indicators of diabetes. Our data revealed that FGF21, but not LDR, treatment lowered blood glucose levels significantly. The combination treatment exhibited a similar lowering effect on blood glucose to FGF21 treatment alone, suggesting that the hypoglycemic activity of the combination treatment could be attributed completely to FGF21. Hyperglycemia is always correlated positively with insulin resistance in patients with type 2 diabetes. As expected, FGF21 treatment improved insulin sensitivity significantly, which was enhanced further by the combination treatment. However, LDR also improved insulin sensitivity, implying that there must be other mechanisms by which LDR contributes to the improved insulin sensitivity. Strong evidence suggests that dyslipidemia is a key cause of insulin resistance. Excessive levels of fatty acids and their derivatives function as signaling molecules that activate protein kinases (20). These kinases can then impair insulin signaling, finally leading to insulin resistance (39, 43). Since our animal model was characterized by obesity and insulin resistance (43), we next examined whether the different therapeutic strategies exerted beneficial effects to correct the abnormal lipid profiles in diabetic mice. Consistent with our hypothesis, obvious dyslipidemia was observed in diabetic mice, as characterized by increased serum TG levels. TG and FFA levels were also upregulated in diabetic kidneys. Treatment with either LDR or FGF21 alone corrected the diabetes-induced abnormal lipid profiles greatly, which was enhanced further by the combination treatment. Therefore, it is likely that the additive effects of LDR and FGF21 on insulin sensitivity were due mainly to correction of the abnormal lipid profile.
Next, we assessed whether the combination strategy also afforded local renal protection at the cellular and molecular levels, in addition to the abovementioned systemic effects. Large bodies of evidence have demonstrated that oxidative stress, inflammation, and subsequent fibrosis are the main pathogeneses of diabetes in the kidney (1, 19, 50). In the current study, we confirmed that diabetes increased the levels of 3-NT and MDA significantly as markers of nitrosative and oxidative damage and decreased the expression of multiple antioxidants and their upstream transcription factor Nrf2. Moreover, inflammatory cell infiltration and increased levels of multiple inflammatory factors such as ICAM-1, TNF-α, and PAI-1 were also observed in diabetic kidneys. Fibrosis, which reflects late-stage pathological damage, was also observed in the kidneys of diabetic mice, as determined using renal CTGF expression and Masson's Trichrome staining. Consistent with our hypothesis, all these pathological changes were suppressed significantly by either LDR or FGF21 treatment, and the combination treatment further protected the kidneys from injury. Therefore, the combination of LDR and FGF21 could confer protection against renal damage.
In summary, the present study has shown that exposing mice to the combination of LDR and FGF21 exerted additive effects to further protect against type 2 diabetes-induced renal damage. These effects were exerted by blocking hyperglycemia, dyslipidemia, renal oxidative stress, inflammation, and fibrosis. In addition, FGF21 regulates carbohydrate and lipid metabolism without inducing hypoglycemia and cell growth. Meanwhile, since whole body exposure to LDR is easy to perform, is noninvasive, and does not increase the kidney workload, this combination strategy might provide a novel therapeutic approach for diabetic patients in the near future.
GRANTS
This study was supported in part by grants from the National Science Foundation of China as a Young Scientist Award (81000294, to C. Zhang), the National Science Foundation of China (81370917, to C. Zhang; 81471045, to X. Lin), the Research Development Fund of Wenzhou Medical University (QTJ13005, to C. Zhang), the National Institutes of Health (1R01 DK091338-01A1, to L. Cai), and the Natural Science Foundation of Zhejiang Province (Y14H070033, to H. Yang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S., X. Li, and C.Z. conception and design of research; L.C., L.Y., F.Z., P.C., x.L., L.H., Y.T., H.Y., C.Z., and L.C. performed experiments; M.S., X. Lu, and C.Z. analyzed data; M.S., S.J., Y.T., and C.Z. prepared figures; M.S., C.Z., and L.C. edited and revised manuscript; M.S., L.Y., F.Z., X. Lu, X. Li, P.C., x.L., L.H., S.J., Y.T., H.Y., C.Z., and L.C. approved final version of manuscript; C.Z. and L.C. interpreted results of experiments; C.Z. and L.C. drafted manuscript.
REFERENCES
- 1.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, Ukpds G. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Alisi A, Panera N, Nobili V. Commentary: FGF21 holds promises for treating obesity-related insulin resistance and hepatosteatosis. Endocrinology 155: 343–346, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Antonellis PJ, Kharitonenkov A, Adams AC. Physiology and Endocrinology Symposium: FGF21: insights into mechanism of action from preclinical studies. J Anim Sci 92: 407–413, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Aunapuu M, Pechter U, Gerskevits E, Marjamagi MM, Suuroja S, Arend A, Kolts I, Kuhnel W, Ots M. Low-dose radiation modifies the progression of chronic renal failure. Ann Anat 186: 277–282, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Averbeck D. Does scientific evidence support a change from the LNT model for low-dose radiation risk extrapolation? Health Phys 97: 493–504, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 7.Bocharov AV, Baranova IN, Vishnyakova TG, Remaley AT, Csako G, Thomas F, Patterson AP, Eggerman TL. Targeting of scavenger receptor class B type I by synthetic amphipathic α-helical-containing peptides blocks lipopolysaccharide (LPS) uptake and LPS-induced pro-inflammatory cytokine responses in THP-1 monocyte cells. J Biol Chem 279: 36072–36082, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, Investigators RS. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cai L. Research of the adaptive response induced by low-dose radiation: where have we been and where should we go? Hum Exp Toxicol 18: 419–425, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Cai L, Jiang J, Wang B, Yao H, Wang X. Induction of an adaptive response to dominant lethality and to chromosome damage of mouse germ cells by low dose radiation. Mutat Res 303: 157–161, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes 54: 1829–1837, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Cai L, Wang P. Induction of a cytogenetic adaptive response in germ cells of irradiated mice with very low-dose rate of chronic gamma-irradiation and its biological influence on radiation-induced DNA or chromosomal damage and cell killing in their male offspring. Mutagenesis 10: 95–100, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese EJ. Hormesis: changing view of the dose-response, a personal account of the history and current status. Mutat Res 511: 181–189, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Calabrese EJ, Baldwin LA. Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol 43: 175–197, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Cuttler JM, Pollycove M. Nuclear energy and health: and the benefits of low-dose radiation hormesis. Dose Response 7: 52–89, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emanuelli B, Vienberg SG, Smyth G, Cheng C, Stanford KI, Arumugam M, Michael MD, Adams AC, Kharitonenkov A, Kahn CR. Interplay between FGF21 and insulin action in the liver regulates metabolism. J Clin Invest 124: 515–527, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farooque A, Mathur R, Verma A, Kaul V, Bhatt AN, Adhikari JS, Afrin F, Singh S, Dwarakanath BS. Low-dose radiation therapy of cancer: role of immune enhancement. Expert Rev Anticancer Ther 11: 791–802, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Franceschini N, Shara NM, Wang H, Voruganti VS, Laston S, Haack K, Lee ET, Best LG, Maccluer JW, Cochran BJ, Dyer TD, Howard BV, Cole SA, North KE, Umans JG. The association of genetic variants of type 2 diabetes with kidney function. Kidney Int 82: 220–225, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa M, Gohda T, Tanimoto M, Tomino Y. Pathogenesis and novel treatment from the mouse model of type 2 diabetic nephropathy. Sci World J 2013: 928197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harjai KJ. Potential new cardiovascular risk factors: left ventricular hypertrophy, homocysteine, lipoprotein(a), triglycerides, oxidative stress, and fibrinogen. Ann Intern Med 131: 376–386, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Heerspink HJ, de Zeeuw D. The kidney in type 2 diabetes therapy. Rev Diabet Stud 8: 392–402, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iglesias P, Selgas R, Romero S, Diez JJ. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur J Endocrinol 167: 301–309, 2012. [DOI] [PubMed] [Google Scholar]
- 23.James S, Enger S, Makinodan T. DNA strand breaks and DNA repair response in lymphocytes after chronic in vivo exposure to very low doses of ionizing radiation in mice. Mutat Res Fund Mol M 249: 255–263, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Ji H, Pesce C, Zheng W, Kim J, Zhang Y, Menini S, Haywood JR, Sandberg K. Sex differences in renal injury and nitric oxide production in renal wrap hypertension. Am J Physiol Heart Circ Physiol 288: H43–H47, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Kharitonenkov A, Adams AC. Inventing new medicines: The FGF21 story. Mol Metab 3: 221–229, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest 115: 1627–1635, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HW, Lee JE, Cha JJ, Hyun YY, Kim JE, Lee MH, Song HK, Nam DH, Han JY, Han SY, Han KH, Kang YS, Cha DR. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 154: 3366–3376, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Koana T, Tsujimura H. A U-shaped dose-response relationship between x radiation and sex-linked recessive lethal mutation in male germ cells of Drosophila. Radiat Res 174: 46–51, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Li H, Zhang J, Jia W. Fibroblast growth factor 21: a novel metabolic regulator from pharmacology to physiology. Front Med 7: 25–30, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Lin Z, Zhou Z, Liu Y, Gong Q, Yan X, Xiao J, Wang X, Lin S, Feng W, Li X. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One 6: e18398, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot 29: A43–A59, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Gong P, Bernstein LR, Bi Y, Gong S, Cai L. Apoptotic cell death induced by low-dose radiation in male germ cells: hormesis and adaptation. Crit Rev Toxicol 37: 587–605, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Meguro S, Tomita M, Kabeya Y, Katsuki T, Oikawa Y, Shimada A, Kawai T, Itoh H, Atsumi Y. Factors associated with the decline of kidney function differ among eGFR strata in subjects with type 2 diabetes mellitus. Int J Endocrinol 2012: 687867, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 55: 1695–1704, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Nomura T, Li XH, Ogata H, Sakai K, Kondo T, Takano Y, Magae J. Suppressive effects of continuous low-dose-rate gamma irradiation on diabetic nephropathy in type II diabetes mellitus model mice. Radiat Res 176: 356–365, 2011. [DOI] [PubMed] [Google Scholar]
- 36.Nomura T, Sakai K, Ogata H, Magae J. Prolongation of life span in the accelerated aging klotho mouse model, by low-dose-rate continuous gamma irradiation. Radiat Res 179: 717–724, 2013. [DOI] [PubMed] [Google Scholar]
- 37.Ogura K, Magae J, Kawakami Y, Koana T. Reduction in mutation frequency by very low-dose gamma irradiation of Drosophila melanogaster germ cells. Radiat Res 171: 1–8, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. New Engl J Med 345: 870–878, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med 119: S10–S16, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Planavila A, Iglesias R, Giralt M, Villarroya F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc Res 90: 276–284, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Planavila A, Redondo-Angulo I, Ribas F, Garrabou G, Casademont J, Giralt M, Villarroya F. Fibroblast growth factor 21 protects the heart from oxidative stress. Cardiovasc Res cvu263, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 168: 1–64, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Shao M, Lu X, Cong W, Xing X, Tan Y, Li Y, Li X, Jin L, Wang X, Dong J, Jin S, Zhang C, Cai L. Multiple low-dose radiation prevents type 2 diabetes-induced renal damage through attenuation of dyslipidemia and insulin resistance and subsequent renal inflammation and oxidative stress. PLoS One 9: e92574, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song Y, Li C, Cai L. Fluvastatin prevents nephropathy likely through suppression of connective tissue growth factor-mediated extracellular matrix accumulation. Exp Mol Pathol 76: 66–75, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi M, Kojima S, Yamaoka K, Niki E. Prevention of type I diabetes by low-dose gamma irradiation in NOD mice. Radiat Res 154: 680–685, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Takehara Y, Yamaoka K, Hiraki Y, Yoshioka T, Utsumi K. Protection against alloxan diabetes by low-dose 60Co gamma irradiation before alloxan administration. Physiol Chem Phys Med NMR 27: 149–159, 1995. [PubMed] [Google Scholar]
- 47.van Kleef EM, Zurcher C, Oussoren YG, Te Poele JA, van der Valk MA, Niemer-Tucker MM, van der Hage MH, Broerse JJ, Robbins ME, Johnston DA, Stewart FA. Long-term effects of total-body irradiation on the kidney of Rhesus monkeys. Int J Radiat Biol 76: 641–648, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Wang GJ, Li XK, Sakai K, Lu C. Low-dose radiation and its clinical implications: diabetes. Hum Exp Toxicol 27: 135–142, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Watts LM, Manchem VP, Leedom TA, Rivard AL, McKay RA, Bao D, Neroladakis T, Monia BP, Bodenmiller DM, Cao JX, Zhang HY, Cox AL, Jacobs SJ, Michael MD, Sloop KW, Bhanot S. Reduction of hepatic and adipose tissue glucocorticoid receptor expression with antisense oligonucleotides improves hyperglycemia and hyperlipidemia in diabetic rodents without causing systemic glucocorticoid antagonism. Diabetes 54: 1846–1853, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 290: 2159–2167, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing X, Zhang C, Shao M, Tong Q, Zhang G, Li C, Cheng J, Jin S, Ma J, Wang G, Li X, Cai L. Low-dose radiation activates Akt and Nrf2 in the kidney of diabetic mice: a potential mechanism to prevent diabetic nephropathy. Oxid Med Cell Longev 2012: 291087, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamaoka K, Kojima S, Nomura T. Changes of SOD-like substances in mouse organs after low-dose X-ray irradiation. Physiol Chem Phys Med NMR 31: 23–28, 1999. [PubMed] [Google Scholar]
- 53.Yamaoka K, Mori S, Nomura T, Taguchi T, Ito T, Hanamoto K, Kojima S. Elevation of antioxidant potency in mice brain by low-dose X-ray irradiation and its effect on Fe-NTA-induced brain damage. Physiol Chem Phys Med NMR 34: 119–132, 2002. [PubMed] [Google Scholar]
- 54.Yu Y, Bai F, Liu Y, Yang Y, Yuan Q, Zou D, Qu S, Tian G, Song L, Zhang T. Fibroblast growth factor (FGF21) protects mouse liver against d-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol Cell Biochem: 1–13, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, Jin S, Guo W, Li C, Li X, Rane MJ, Wang G, Cai L. Attenuation of diabetes-induced cardiac inflammation and pathological remodeling by low-dose radiation. Radiat Res 175: 307–321, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Zhang C, Shao M, Yang H, Chen L, Yu L, Cong W, Tian H, Zhang F, Cheng P, Jin L, Tan Y, Li X, Cai L, Lu X. Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal inflammation. PLoS One 8: e82275, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang C, Xing X, Zhang F, Shao M, Jin S, Yang H, Wang G, Cui J, Cai L, Li W, Lu X. Low-dose radiation induces renal SOD1 expression and activity in type 1 diabetic mice. Int J Radiat Biol 90: 224–230, 2014. [DOI] [PubMed] [Google Scholar]