Abstract
Angiotensin-converting enzyme 2 (ACE2) knockout is associated with reduced fetal weight at late gestation; however, whether uteroplacental vascular and/or hemodynamic disturbances underlie this growth-restricted phenotype is unknown. Uterine artery reactivity and flow velocities, umbilical flow velocities, trophoblast invasion, and placental hypoxia were determined in ACE2 knockout (KO) and C57Bl/6 wild-type (WT) mice at day 14 of gestation. Although systolic blood pressure was higher in pregnant ACE2 KO vs. WT mice (102.3 ± 5.1 vs. 85.1 ± 1.9 mmHg, n = 5–6), the magnitude of difference was similar to that observed in nonpregnant ACE2 KO vs. WT mice. Maternal urinary protein excretion, serum creatinine, and kidney or heart weights were not different in ACE2 KO vs. WT. Fetal weight and pup-to-placental weight ratio were lower in ACE2 KO vs. WT mice. A higher sensitivity to Ang II [pD2 8.64 ± 0.04 vs. 8.5 ± 0.03 (−log EC50)] and greater maximal contraction to phenylephrine (169.0 ± 9.0 vs. 139.0 ± 7.0% KMAX), were associated with lower immunostaining for Ang II receptor 2 and fibrinoid content of the uterine artery in ACE2 KO mice. Uterine artery flow velocities and trophoblast invasion were similar between study groups. In contrast, umbilical artery peak systolic velocities (60.2 ± 4.5 vs. 75.1 ± 4.5 mm/s) and the resistance index measured using VEVO 2100 ultrasound were lower in the ACE2 KO vs. WT mice. Immunostaining for pimonidazole, a marker of hypoxia, and hypoxia-inducible factor-2α were higher in the trophospongium and placental labyrinth of the ACE2 KO vs. WT. In summary, placental hypoxia and uterine artery dysfunction develop before major growth of the fetus occurs and may explain the fetal growth restricted phenotype.
Keywords: hypoxia, vascular reactivity, angiotensin II, vasoconstriction, fetal growth restriction
intrauterine growth restriction (IUGR) is a leading cause of perinatal morbidity and mortality in humans (31). Maternal health factors such as chronic hypertension, preeclampsia, diabetes mellitus, chronic renal disease, poor nutrition, and smoking (57) and fetal genetic abnormalities increase the risk of IUGR. The uteroplacental vasculature undergoes tremendous adaptations during pregnancy, including vasculo/angiogenesis and remodeling of spiral arteries (44). These changes allow the expansion of blood volume in the uteroplacental unit and sufficient blood flow from the placenta to the fetus (44). Fetal metabolic demands progressively increase in the second half of pregnancy (37). Normal fetal growth is vastly dependent on adequate placental development, whereas increased vascular resistance and hypoxia are associated with reduced fetal weight (10a). However, the molecular basis of uteroplacental dysfunction in IUGR is not well understood.
The components of the renin-angiotensin system (RAS) are important mediators of uteroplacental vasculature. Angiotensin-converting enzyme 2 (ACE2) is a key enzyme of the RAS that degrades angiotensin II (Ang II) and forms Ang-(1–7). ACE2 plays a key regulatory role in the control of arterial pressure and vascular function (24) by balancing the vasoconstrictor Ang II/Ang II receptor 1 (AT1R) and vasodilator Ang-(1–7)/MAS (AT1–7R) arms of the renin-angiotensin system. ACE2 expression in human placenta was characterized by Valdes et al. (56) in the invading and intravascular trophoblasts, decidual cells, and umbilical cord, suggesting a paracrine influence of this enzyme in the uteroplacental unit. Moreover, human umbilical vein endothelial cells (HUVECs) isolated from pregnancies with small for gestational age (SGA) newborns had significantly lower levels of ACE2 mRNA compared with pregnancies associated with average for gestational age (AGA) newborns (48), suggesting that lower ACE2 levels in HUVECs are associated with IUGR.
Several studies have investigated ACE2 expression in the uteroplacental unit of pregnant animals. ACE2 levels were higher in the uterus of normotensive pregnant rats compared with normotensive nonpregnant rats (39). ACE2 levels were lower in hypertensive pregnant rats that had reduced uterine perfusion pressure (RUPP) surgery compared with normotensive pregnant rats (39). RUPP surgery induces the reduction in uterine perfusion with subsequent placental ischemia by placing clips around the descending aorta and around both main uterine arteries (1, 39). It has been also shown that placentas generate threefold more ACE2 mRNA than gravid uterus or the kidneys, suggesting that the placenta is a significant contributor to the generation of ACE2 levels (systemic or uteroplacental) seen during rat pregnancy (27). In agreement with the hypothesis that ACE2 deficiency is associated with IUGR, a recent study from our group demonstrated that ACE2 knockout (KO) mice developed maternal and fetal growth restriction at late pregnancy (3). These changes were accompanied by increased levels of placental Ang II and decreased circulating Ang-(1–7), a result of ACE2 knockout and reduced degradation of Ang II (3). Mean blood pressure was elevated in virgin ACE2 KO mice and did not change with pregnancy (3). Since cardiac output was similarly increased in ACE2 KO and C57Bl/6 mice (3), the absence of blood pressure response in ACE2 KO mice during pregnancy implies a higher peripheral resistance. These findings suggest that the dysregulated RAS has a pathogenic role in the development of IUGR (3). Moreover, a recent study by Ibrahim et al. (21) showed that ACE2 activator, xanthenone, decreased blood pressure in hypertensive pregnant rats, suggesting that ACE2 activation is protective against hypertension during pregnancy.
In male animals, ACE2 knockout increases endothelial dysfunction and the expression of inflammatory mediators in the aorta (55). ACE2 knockout also exacerbates Ang II-mediated aortic remodeling in mice due to increased oxidative stress and vascular smooth muscle cell apoptosis (46). On the other hand, ACE2 overexpression in hypertensive rats improves endothelial function (52, 53), suggesting a protective effect of ACE2 in vasculature.
Disturbances in the uteroplacental circulation have been implicated in the development of IUGR; however, the role of ACE2 in the regulation of the uteroplacental vasculature is unknown. The use of a pregnant ACE2 KO mouse model allowed us to test the hypothesis that ACE2 knockout induces uteroplacental dysfunction as early as day 14 of gestation before major growth of the fetus occurs (10a).
MATERIALS AND METHODS
Animals.
The study was approved by the Institutional Animal Care and Use Committee of the Wake Forest School of Medicine (WFSM). ACE2 KO mice developed on a C57Bl/6 background were received from Susan B. Gurley MD and Thomas Coffman MD of Duke University; the colony was established at the Hypertension and Vascular Research Center at WFSM. As previously described (3), C57Bl/6 mice, purchased from Harlan Laboratories, were used in control experiments. Standard rodent chow (Lab Diet 5P00 - Prolab RMH 3000; PMI Nutrition International, Brentwood, MO) and water were available ad libitum throughout the experimental protocols.
Timeline for the experiments.
Homozygous female ACE2 KO mice between 12 and 18 wk of age were mated with male ACE2 KO mice. Day 0 of pregnancy was established by the presence of a vaginal plug or sperm in the vaginal smear. Mice received ultrasound scans at day 13 of gestation. In the evening of the same day, animals were placed in metabolic cages for 24-h urine collection (MMC 100; Hatteras Instruments, Cary, NC). Blood pressures were taken in the morning of the following day, and the animals were euthanized in the afternoon of day 14 of gestation (0.7 term) by decapitation. In addition, on the day of euthanasia, some animals were injected with hypoxyprobe-1 to assess placental hypoxia and were euthanized 1 h later according to the manufacturer's instructions (25, 44). The main uterine artery was dissected and immediately placed in oxygenated ice-cold Krebs buffer for assessment of vascular reactivity. A 5-mm segment of the uterine artery and one uteroplacental unit per mouse were fixed in 10% formalin for 24 h followed by 70% ethanol. Maternal tibia length was determined in each animal. To account for differences in maternal body weights between animals within each study group, maternal and fetal characteristics were standardized to maternal tibia length (8, 65). All of the animals had blood pressure and ultrasound scans recorded. Additional animals were added for vascular experiments.
Blood pressure recordings.
Systolic blood pressures (SBP) were recorded in trained conscious mice under restraint using an automated tail cuff system (SC-100, Hatteras Instruments) as described (28). We have experience with tail cuff blood pressure recordings, and our previous studies showed consistent and reproducible results (28, 62, 63). Metal holders specifically designed to restrain animals with minimal stress during blood pressure recordings were provided by Hatteras Instruments. Occluding (8 mm) and sensor (8 mm) cuffs were used for the measurements of SBP. Mice were acclimated to the system and to the warming-box exposure for 7 days before the actual recordings were performed. On the day of the experiment, mice were placed in warming boxes for 15 min prior to blood pressure recordings. SBPs were measured 10 times in each mouse; the total time for blood pressure recording was no more than 20 min. The data for each animal were averaged and reported as the mean ± SE for each experimental group.
Ultrasound of uterine and umbilical arteries.
Mice were anesthetized with 1.5% isoflurane and placed on a heated platform for ultrasound imaging. Heart rate was determined using the ECG electrodes connected to the platform. All hair on the abdomen was removed by application of a shaving gel. Images were obtained using an MS550S transducer and Vevo 2100 ultrasound system (Visual Sonics, Toronto, ON, Canada) (10a). The angle between the ultrasound probe and the direction of the flow was kept at less than 50 degrees (33 degrees on average) during the recordings of the velocity waveforms. The velocities of the main uterine arteries were recorded below the bladder and at the level where the main uterine artery branches from the internal iliac artery. Umbilical arteries were visualized in the longitudinal plane of the fetoplacental unit using a two-dimensional mode. Color Doppler and pulsed wave (PW) Doppler modes were applied to establish a typical uterine or umbilical artery wave and to record peak systolic (Vmax) and minimum diastolic (Vmin) velocities. The resistance index was determined as (Vmax − Vmin)/Vmax (10a). The results were obtained in the arteries from two or three fetoplacental units in each animal and averaged.
Vascular reactivity.
Uterine artery segments (maximum length 2 mm) were mounted between an isometric force transducer (Kistler Morce DSC 6, Seattle, WA) and a displacement device on a myograph (Multi Myograph, model 620M; Danish Myo Technologies, Aarhus, Denmark) using two stainless steel wires (diameter 25 μm), as previously described (29, 50). All isolated uterine arteries were first tested for stress-stretch responses during the normalization procedure, followed by the maximum responses to potassium chloride, phenylephrine, and acetylcholine. Then the contractile responses of uterine artery segments to Ang II were examined, Ang II response was also tested after preincubation with Ang-(1–7).
Experimental protocol.
The myograph organ bath (5 ml) was filled with Krebs buffer maintained at 37°C and aerated with 95% O2-5% CO2. The vessels were washed and incubated for 30 min before the normalization procedure was performed. Each arterial segment was stretched in a stepwise manner. The internal circumference and corresponding wall tension at each stretch were calculated and plotted to produce a resting wall tension-internal circumference curve for that artery, using the DMT Normalization Module (ADInstruments). Arterial segments were normalized to 0.9·L100, where L100 is the internal circumference of the vessels at a transmural pressure of 100 mmHg (38). Optimal diameters (OD) were calculated as OD = 0.9·L100/π. After obtaining the OD, a 30-min equilibration period preceded the addition of test substances. Stress-stretch responses were obtained during the normalization procedure and were plotted and the data were adjusted to the exponential equation Y = Y0*exp(k*X), where k is the rate constant. Values of k were analyzed for each artery and then compared between groups. Our previous studies showed that the contractile response to KCl in the uterine artery attained maximal values at 75 mM (49). Therefore, after equilibration, uterine arteries were exposed to 75 mM KCl for 5 min and then washed with Krebs buffer. The procedure was repeated three times, and the last measurement was considered the maximal response to K+ (KMAX). After washing and resting, uterine artery segments were exposed to a cumulative concentration-response curve of phenylephrine (PE) by exposing arteries to six (10−10 to 10−5 M) increasing concentrations in one-log steps, with each subsequent dose being introduced only after a steady response had been reached. The responses to PE were expressed as the percentage of maximal contraction induced by KCl. Arteries were then washed again, and vasodilation to acetylcholine was tested in the uterine arteries preconstricted with a submaximal dose of PE (10−6 to 10−5 M). After attaining an equivalent level of contraction, a concentration response curve to acetylcholine (10−10 to 10−4 M) was calculated. Uterine artery segments were washed and, under resting tension, were exposed to a cumulative concentration-response curve of Ang II by exposing arteries to 10−10 to 10−7 M increasing concentrations in one-fourth log steps, with each subsequent dose being introduced after a steady response had been reached (every 2 min). The response to Ang II was expressed as the percent (%) of maximal contraction induced by KCl. Ang II response was also tested after preincubation with Ang-(1–7) (106 M) alone or in the presence of MAS (AT1–7R) antagonist (105 M) in some vessels.
Immunohistochemistry.
After fixation in formalin and ethanol, one uterine artery segment and one placental section were embedded in separate paraffin blocks, and cut into 5-μm sections. Immunostaining was performed using the avidin biotin complex (ABC) method as described (62). Staining for all proteins required antigen retrieval treatment with sodium citrate buffer (pH 6.0) at 90–95°C for 30 min. Uterine arteries were incubated with the following primary antibodies: rabbit polyclonal affinity purified endothelial nitric oxide synthase, anti-eNOS (dilution: 1:100; BD Biosciences, San Diego, CA; cat. no. 610297), anti-AT1R (dilution: 1:400; Santa Cruz, cat. no. sc-1173), anti-AT2R (dilution: 1:400; Abcam, cat. no. ab19134), anti-MAS/Ang-(1–7)R (dilution: 1:400, Alomone Labs, cat. no. AAR-013), monoclonal anti-actin α-smooth muscle (2.5 μg/ml; Sigma-Aldrich, St. Louis, MO; cat. no. A5228), monoclonal anti-skeletal myosin (dilution: 1:800; Sigma-Aldrich; cat. no. M4276), and secondary biotinylated goat anti-rabbit or anti-mouse antibody (dilution: 1:400; Vector Laboratories, Burlingame, CA). Uteroplacental unit sections were stained with anti-cytokeratin KRT7 rabbit polyclonal antibody (dilution: 1:200; Proteintech Group; Chicago, IL; cat. no. 15539-1-AP). Mouse on mouse (M.O.M.) protocol was used for actin and myosin staining (Vector Laboratories, Burlingame, CA). One placenta and one uterine artery per stain were examined under a light microscope and photographed at ×50 or ×200 magnifications with a QImaging Retiga 1300R CCD digital camera and SimplePCI (version 6) software: 6 fields per uterine artery section and 12 sections per one placenta (4 sections per each: labyrinth, junctional zone, and mesometrium). Images were analyzed using Adobe Photoshop 7.0 by the investigator blinded to the experimental groups. Staining for eNOS, actin, or myosin was normalized to the intensity of the background and to the maximal value of the red, green, and blue intensity (RGB) component and reported as relative intensity units (41, 62). The following stains were performed to assess morphological features of uterine arteries: periodic acid Schiff stain (PAS, Sigma-Aldrich; cat. no. 395B-1KT) to detect fibrinoid content, Verhoeff's stain (American MasterTech; cat. no. KTVEL) to detect elastic fibers, and Picrosirius red stain (Sigma-Aldrich; assay components cat. no. 197378-100G; P6744-16A; 36-554-8) to assess connective tissue components (collagen and muscle cells). The area of cells stained by PAS and Verhoeff's stain was normalized to the total area of vessel. The Picrosirius red intensity was expressed as the media-to-adventitia area ratio. PAS intensity in the media of uterine artery was normalized to the intensity of the background and to the maximal value of 255 of the RGB component and reported as relative intensity units (41, 62).
Trophoblast invasion.
Decidual and mesometrial areas of the placenta of ACE2 KO and C57Bl/6 mice were examined for the depth of the trophoblast invasion and for the presence of trophoblast cells in the arterial walls (47). Trophoblast cells were identified by positive staining for cytokeratin KRT7, as described above. The distribution of extravillous (EVC) and arterial trophoblasts (ATB) and the extent of their invasion into mesometrium were analyzed using semiquantitative analysis as described by Pijnenborg et al. (47) as follows: 1 = high numbers evenly spread; 2 = high numbers focally spread; 3 = low numbers evenly spread; 4 = low numbers focally spread.
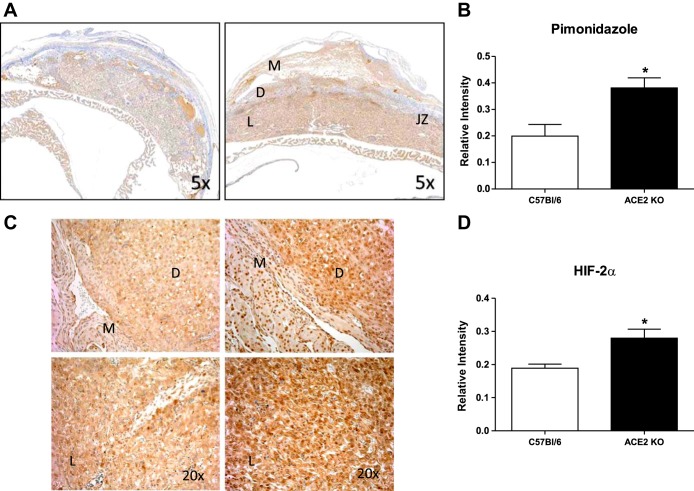
Assessments of placental hypoxia.
1) Hypoxyprobe-1 was injected intraperitoneally in the ACE2 KO and C57Bl/6 mice at day 14 of gestation (60 mg/kg maternal body wt). One hour later, the mother was euthanized, and placentas were fixed in 10% formalin for 24 h followed by 70% ethanol. After the fixation, placentas were embedded in paraffin, cut into 5-μm sections, and immunostained for hypoxyprobe-1 following the manufacturer's protocol (25, 44). Hypoxic areas were identified in sections of the placenta using a Hypoxyprobe-1TM kit (Chemicon, Temecula, CA; cat. no. HP1-100kit) that contains pimonidazole hydrocholoride and a primary antibody for the detection of pimonidazole. Pimonidazole binds to thiol-containing proteins in hypoxic cells; its level in the cells has been correlated with oxygen electrode measurements (51). 2) HIF-2α is a transcription factor that induces the expression of hypoxia-regulated genes. Levels of HIF-2α are increased under hypoxic conditions (11, 19). In addition, HIF-2α protein, but not HIF-1α, was induced in cytotrophoblast cells by hypoxia (18). Immunostaining for HIF-2α (1:100 antibody dilution, Abcam, San Francisco, CA; cat. no. ab199) was performed on paraffin-embedded sections using the ABC-DAB protocol described above. The relative intensities of pimonidazole and HIF-2α staining were analyzed in the whole placenta section as described above.
Statistical analysis.
Comparisons between ACE2 KO and C57Bl/6 groups were evaluated using unpaired t-tests. Data obtained from the concentration-dependent responses of each arterial segment in each group were fitted to a logistic curve to determine maximal response and sensitivity. The curve had the form Y = bottom + (top - bottom)/(1 +10(LogEC50 − X) × Hill Slope)) where X represents the logarithm of the drug concentration and Y the response. Values of sensitivity were expressed as pD2 (pD2 = -Log EC50), with EC50 values obtained from the curve fittings. GraphPad Prism IV (San Diego, CA) plotting and statistical software were used. All measurements were expressed as means ± SE. The criterion for statistical significance was P < 0.05.
RESULTS
Physiological indexes.
SBP was higher in pregnant ACE2 KO than in WT C57Bl/6 mice (Table 1) as previously observed at late gestation in the ACE2 KO pregnant mouse (3). The magnitude of difference in SBP in pregnant mice was similar to the difference observed in nonpregnant mice (ACE2 KO: 106.8 ± 3.2 vs. C57Bl/6: 94.2 ± 2.2 mmHg, n = 4). Moreover, maternal urinary protein excretion, serum creatinine, and kidney and heart weights were similar between groups (Table 1). Maternal weight was significantly lower in ACE2 KO mice, and this difference was sustained when total fetal weight was subtracted from the maternal weight. Maternal weight was independent of maternal food intake (Table 1). Fetal weight was lower in the ACE2 KO mice than in WT mice. There were no differences in placental weights; however, pup-to-placental weight ratio, an indirect measure of placental insufficiency, was significantly lower in the ACE2 KO vs. WT mice (Table 1).
Table 1.
Physiological characteristics of ACE2 KO and C57Bl/6 mice at day 14 of gestation
| Characteristics | C57Bl/6 (n = 5) | ACE2 KO (n = 6) |
|---|---|---|
| Mother's BW(g)/tibia length (cm) | 17.90 ± 0.20 | 16.42 ± 0.37* |
| (Mother's BW–total pup BW), g/tibia length (cm) | 16.63 ± 0.30 | 15.30 ± 0.38* |
| Pup BW (g)/tibia length (cm) | 0.15 ± 0.01 | 0.11 ± 0.01* |
| Pup BW, unadjusted (g) | 0.21 ± 0.01 | 0.17 ± 0.01* |
| Pup number | 8.70 ± 0.35 | 8.0 ± 0.28 |
| Placental wt (g)/tibia length (cm) | 0.05 ± 0.002 | 0.05 ± 0.001 |
| Placental wt, unadjusted (g) | 0.07 ± 0.003 | 0.08 ± 0.003* |
| Pup BW/placental weight ratio (per tibia length) | 3.15 ± 0.28 | 2.44 ± 0.11* |
| Pup BW/placental weight ratio (unadjusted) | 2.91 ± 0.18 | 2.34 ± 0.13* |
| Mean kidney wt (g)/tibia length (cm) | 0.15 ± 0.004 | 0.14 ± 0.003 |
| Heart wt (g)/tibia length (cm) | 0.11 ± 0.005 | 0.12 ± 0.004 |
| Systolic blood pressure (mmHg) | 85.10 ± 1.90 | 102.30 ± 5.10* |
| Maternal heart rate (beats/min) | 361.20 ± 28.9 | 349.20 ± 30.20 |
| Proteinuria (mg protein/g of mother's BW) | 1.02 ± 0.09 | 1.03 ± 0.42 |
| Maternal serum creatinine (mg/dl) | 0.29 ± 0.02 | 0.34 ± 0.03 |
| Maternal Food Intake (g) | 3.40 ± 0.24 | 4.0 ± 0.21 |
Data are means ± SE.
ACE2 KO, angiotensin-converting enzyme 2 knockout mice; BW, body weight.
P < 0.05 vs. C57Bl/6 mice.
Uterine artery reactivity and immunohistochemistry.
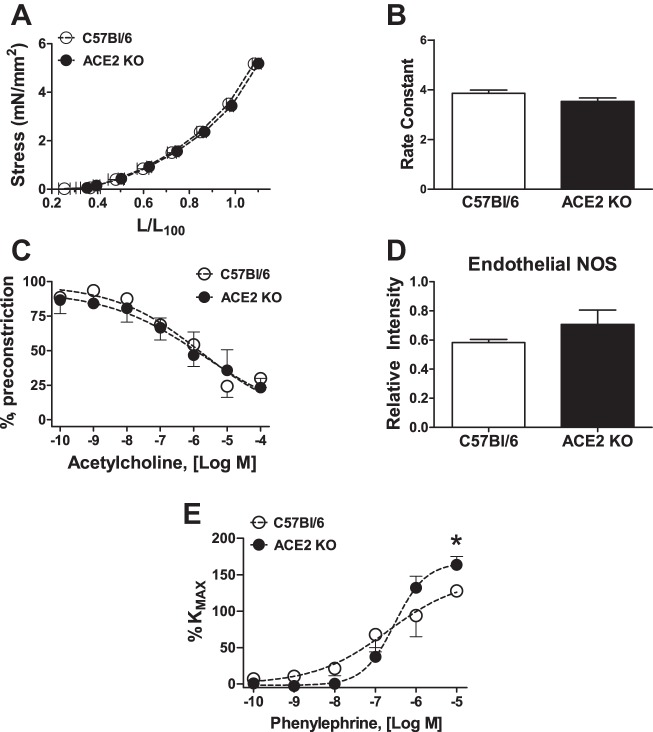
ODs of uterine artery segments were not different between groups (236.0 ± 12.0 vs. 240.0 ± 7.0 μm, n = 4 in each group). Resting tension also was similar in both groups (1.75 ± 0.09 vs. 1.77 ± 0.09 mN/mm2). Similarly, the stress-stretch response and the rate constant were not statistically different between studied groups (Fig. 1, A and B). No difference in vasodilation in response to acetylcholine was observed in C57Bl/6 vs. ACE2 KO mice (Fig. 1C and Table 2). In addition, the intensity of the uterine artery eNOS immunostaining was similar between C57Bl/6 and ACE2 KO mice (Fig. 1D).
Fig. 1.
Uterine artery responses in angiotensin-converting enzyme 2 knockout (ACE2 KO) and C57Bl/6 mice at day 14 of gestation. Stress-stretch response (A) and its rate constant (B) (n = 4 in each group); vasodilatory response to acetylcholine (C) and endothelial NOS immunostaining (D) (n = 4 in each group); contraction to phenylephrine (E) (n = 5–7). Data are means ± SE. *P < 0.05 vs. C57Bl/6.
Table 2.
Vascular reactivity in C57Bl/6 and ACE2 KO mice at day 14 of gestation
| Characteristics | C57Bl/6 | ACE2 KO |
|---|---|---|
| KMAX | 1.13 ± 0.12 | 1.90 ± 0.15 * |
| Phe MAX (%KMAX) | 139.0 ± 7.0 | 169.0 ± 9.0 * |
| pD2 (−log EC50) | 6.76 ± 0.47 | 6.48 ± 0.13 |
| ACh MAX | 73 ± 7 | 76 ± 7 |
| pD2 (−log EC50) | 6.43 ± 0.4 | 6.19 ± 0.57 |
| Ang IIMAX (%KMAX) | 91.0 ± 5.0 | 93.0 ± 13.0 |
| pD2 (−log EC50) | 8.5 ± 0.03 | 8.64 ± 0.04 * |
Data are means ± SE.
KMAX, maximal response to potassium chloride (K+); PheMAX, maximal response to phenylephrine; ACh, acetylcholine; pD2, negative logarithm of the molar concentration producing the half-maximum response (EC50).
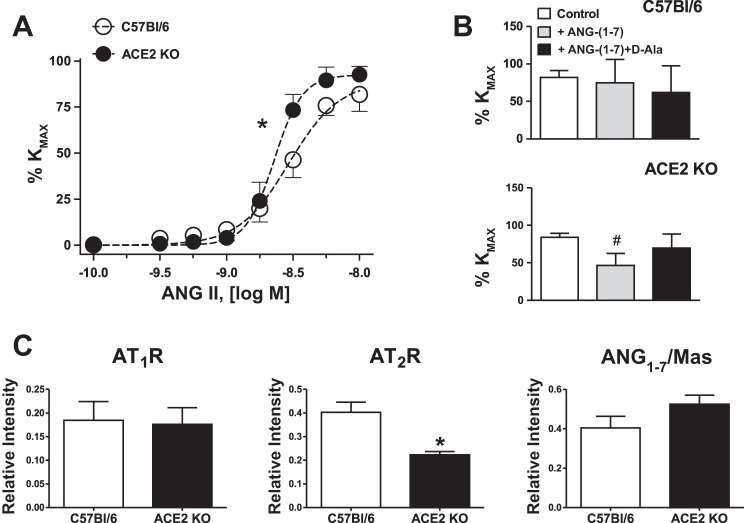
In contrast to the acetylcholine response, active tension, assessed as a response to 75 mM KCl, was significantly greater in uterine arteries of ACE2 KO (Table 2). In addition, as shown in Fig. 1E and Table 2, uterine arteries from ACE2 KO mice displayed greater maximal response to phenylephrine, with no difference in sensitivity. Since the sensitivity to Ang II decreases during pregnancy, we compared the reactivity of uterine arteries to Ang II. Uterine arteries from ACE2 KO mice were more sensitive to Ang II compared with C57Bl/6 mice (n = 5–6; Fig. 2A and Table 2). Preincubation with Ang-(1–7) at the concentration of 1 μM for 30 min decreased maximal contraction to Ang II in ACE2 KO but not in WT mice (Fig. 2B). A preincubation with Ang-(1–7) antagonist, [d-Ala7]-Ang-(1–7) increased the Ang II contraction in the uterine artery of ACE2 KO but not in WT mice (Fig. 2B). There were no differences in the expression of AT1R or AT1–7/MAS receptors in the uterine arteries of ACE2 KO vs. C57BL/6 mice (Fig. 2C). However, the expression of AT2R determined by the immunostaining was lower in the uterine arteries of ACE2 KO vs. C57BL/6 mice (Fig. 2C).
Fig. 2.
Uterine arteries responses to Ang II (A and B) and semiquantitative analysis of angiotensin receptor (ATR) expression (C) in uterine arteries of ACE2 KO and C57Bl/6 mice at day 14 of gestation. A: concentration-dependent response to Ang II. B: maximal response to Ang II (10−8 M): Ang II alone, after preincubation with Ang-(1–7) (10−6M) or a combination of Ang-(1–7) with AT1–7/MAS receptor antagonist [d-Ala7]-Ang-(1–7) (10−5 M) for 20 min. Data are means ± SE. *P < 0.05 vs. C57Bl/6; #P < 0.05 vs. ACE2 KO control (Ang II contraction); n = 5–7 for vascular responses; n = 4 in each group for immunostaining analysis.
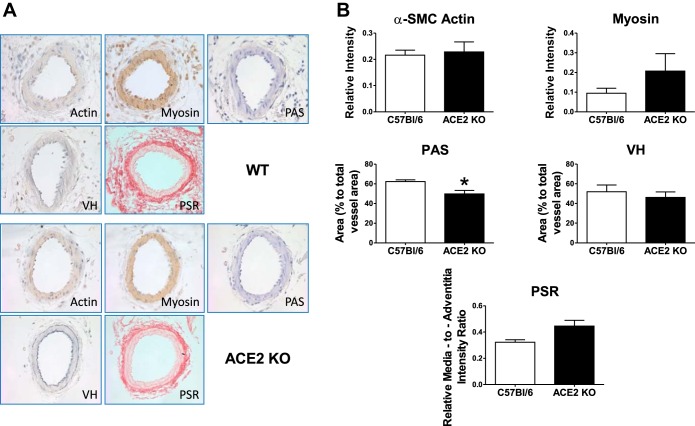
Greater responses to phenylephrine and Ang II in ACE2 KO mice were associated with lower fibrinoid content as assessed by PAS staining (n = 4 in each group) and a tendency for a higher collagen expression in the media of uterine artery as assessed by Picrosirius red staining (P = 0.05, n = 4–5; Fig. 3B). There were no differences observed in the intensity of actin or myosin between ACE2 KO and C57Bl/6 mice (n = 3–4). The area of Verhoeff staining indicative of elastin distribution in the uterine arteries was also similar in the two strains (Fig. 3B; n = 3–4).
Fig. 3.
Images (A) and semiquantitative analysis (B) of morphological markers of arterial stiffness (PSR), elasticity (VH), fibrinoid content (PAS), and structural proteins (actin, myosin) of uterine arteries in ACE2 KO and C57Bl/6 mice. PAS, periodic acid Schiff stain; PSR, Picrosirius red stain; VH, Verhoeff's stain. Data are means ± SE. *P < 0.05 vs. C57Bl/6. Magnification ×40.
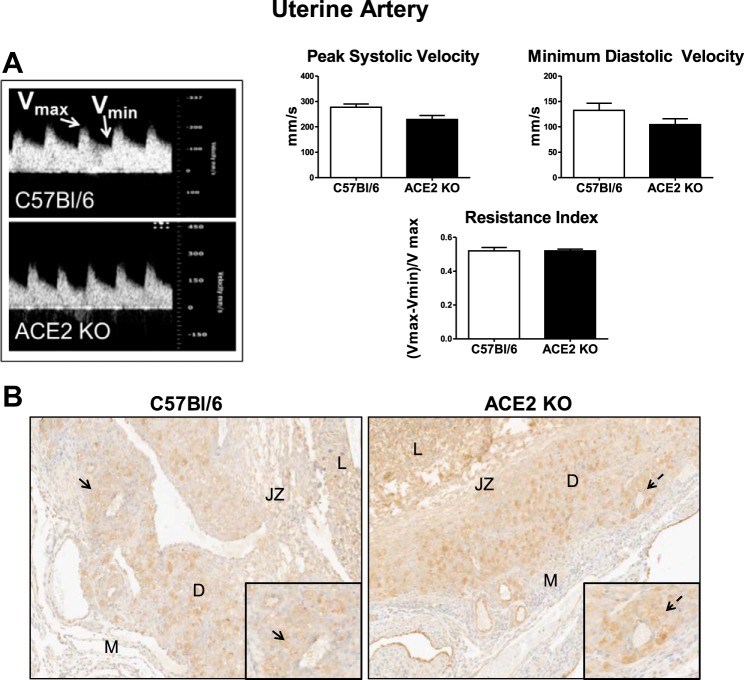
Uterine artery ultrasound data.
There were no differences in the uterine artery peak systolic or minimum diastolic velocities or resistance index of ACE2 KO compared with C57Bl/6 mice (n = 4 in each group; Fig. 4A).
Fig. 4.
Analysis of uterine artery hemodynamics and trophoblast invasion in ACE2 KO and C57Bl/6 mice at day 14 of gestation. A: pulsed wave Doppler image and representative section of peak systolic (Vmax) and minimum diastolic (Vmin) velocities and analysis of the data in C57Bl/6 and ACE2 KO mice. Data are means ± SE; n = 4–6. B: distribution of cytokeratin-positive cells in the decidua (D) and mesometrium (M) of C57Bl/6 and ACE2 KO mice at ×5 and ×20 (inset) magnification; n = 3–4. L, labyrinth; JZ, junctional zone. Arrows indicate decidual arteries that are shown on the ×20 inset image.
Trophoblast invasion.
The extent of extravillous (EVTB) and arterial trophoblast (ATB) invasion in the decidua and mesometrium was compared between ACE2 KO and C57Bl/6 mice. No differences in EVTB or ATB distribution were found between ACE2 KO and C57Bl/6 mice (3.7 ± 0.1 vs. 3.4 ± 0.4 number of EVTB and ATB combined). In both strains, the EVTBs evenly invaded into the decidua, reaching the upper limit of the decidua (Fig. 4B). Both strains showed variable signs of ATB invasion into the decidual arteries, going from no invasion in some arteries to complete invasion (Fig. 4B, inset). There were low numbers of ATBs and EVTBs in arterial walls within the mesometrium in both strains.
Placental hypoxia.
Cross-sections of the placenta were immunostained for the hypoxia marker pimonidazole and HIF-2α. As shown in Fig. 5, A and B, pimonidazole staining was higher in the trophospongium and the labyrinth of the ACE2 KO vs. C57Bl/6 placentas (1.9-fold, n = 4–5). In addition, HIF-2α immunostaining (Fig. 5, C and D) was also greater in decidual and labyrinth areas of the ACE2 KO mice compared with C57Bl/6 (n = 4–5).
Fig. 5.
Expression of hypoxia markers in the placenta of C57Bl/6 and ACE2 KO mice. A and B: pimonidazole staining and analysis. C and D: hypoxia-inducible factor-2α (HIF-2α) staining and analysis. Data are means ± SE; n = 4–5. *P < 0.05 vs. C57Bl/6. Magnification: A, ×5; C, ×20.
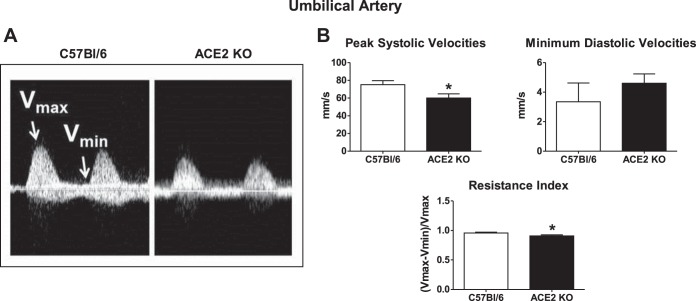
Umbilical artery ultrasound data.
Peak systolic velocities of umbilical arteries were significantly lower in the ACE2 KO mice (Fig. 6; n = 8–9). Minimum diastolic velocities of the umbilical arteries were similar in ACE2 KO and C57Bl/6 mice (Fig. 6; n = 8–9). The resistance index of umbilical arteries in ACE2 KO was lower than that of C57Bl/6 mice (0.90 ± 0.01 vs. 0.95 ± 0.01, n = 8–9; Fig. 6).
Fig. 6.
Analysis of umbilical artery velocities and the resistance index of C57Bl/6 and ACE2 KO mice at midgestation. A: representative wave forms of Vmax and Vmin points of umbilical artery velocity measurement. Arrows indicate Vmax and Vmin of the umbilical artery velocity wave. B: average measurements of Vmax and Vmin and the calculated resistance index. Data are means ± SE; n = 8–9. *P < 0.05 vs. C57Bl/6.
DISCUSSION
We recently reported that ACE2 knockout is associated with reduced fetal weight at late pregnancy (3); however, the mechanisms underlying this growth-restricted phenotype are not known. In the present study, we found that the reduced fetal weight in ACE2 KO mice at day 14 of gestation is associated with uteroplacental dysfunction evidenced by greater uterine artery reactivity to vasoconstrictors, abnormal uterine artery remodeling, and placental hypoxia.
Most fetal growth in mice occurs during the last trimester of pregnancy (30). Intrauterine growth restriction is characterized by an abnormal vascular resistance and compromised blood flow (22). During normal pregnancy, blood flow progressively increases in the uteroplacental circulation, including the uterine artery and its branches and the umbilical artery (10a). The main uterine arteries provide ∼80% of total uteroplacental flow (44). As a result of proper remodeling, uteroplacental vessels become more resistant to vasoactive substances compared with nonremodeled vessels (44). In addition, local mediators play a prominent role in the regulation of uteroplacental vascular reactivity (44). The relaxation of uterine arteries during normal pregnancy is mediated by nitric oxide- and endothelial-derived hyperpolarizing factors and prostacyclin (10, 42). Contrary to the responses of uterine artery isolated from pregnant eNOS knockout IUGR mice or small myometrial arteries isolated from human IUGR pregnancies (26, 61), we found similar relaxation responses to acetylcholine in ACE2 KO and WT mice, suggesting that ACE2 may not interfere with endothelial-dependent relaxation at day 14 of gestation in mice. Moreover, global gene knockout may result in a series of compensatory events that may mask the effects of ACE2 knockout in pregnancy. For example the activation of neprilysin or other enzymes that can generate Ang-(1–7) (13) may be upregulated in the placenta of ACE2 KO mice. The levels of placental Ang-(1–7) were similar in ACE2 KO and WT mice at late gestation (3). Since Ang-(1–7) increases the release of nitric oxide, it may contribute to the acetylcholine-induced relaxation of uteroplacental vessels.
Here, we demonstrate that ACE2 KO mice have a higher response of the uterine artery to potassium chloride than WT mice, suggesting increased hypertrophy or hyperplasia of vascular smooth muscle cells (43). However, immunostaining for contractile proteins such as α-smooth muscle cell actin showed no difference and myosin staining only tended to increase. It is possible that our study was underpowered to detect significant differences in contractile proteins between groups. Optimal diameters and the stress-stretch responses are directly related to the elastic properties of the arterial tissue; no difference in these parameters suggests similar elastic properties of uterine arteries in ACE2 KO and WT mice. Similarly, equivalent values of resting tension between studied groups would suggest that the vascular hypertrophic and hyperplastic processes are unaffected by ACE2 at least in midpregnancy at the level of the uterine artery. The arterial response to potassium chloride is dependent on the influx of extracellular calcium and the activity of voltage-gated calcium channels (23). It is possible that higher expression of these channels or increased calcium signaling may augment the response to potassium chloride in the uterine artery of ACE2 KO mice. We demonstrate that uterine arteries of ACE2 KO mice were more responsive to phenylephrine than WT controls. As an α1-adrenergic agonist, phenylephrine can constrict blood vessels through the opening of voltage-gated calcium channels and through G protein-coupled receptor signaling (9, 34, 66). The even higher KMAX of phenylephrine exceeding that of high potassium suggests that the latter pathway may be accentuated in the uterine artery of ACE2 KO mice. Increased contraction of the main uterine artery to phenylephrine late in pregnancy was also seen in eNOS knockout mice, which develop fetal growth restriction at late pregnancy (26). Furthermore, compared with normal pregnant women, higher contraction to arginine vasopressin was reported in small myometrial arteries of pregnant women who had fetal growth restriction, suggesting that greater uterine artery contraction is associated with reduced fetal growth (61). Reduced sensitivity of pressor response to Ang II is a characteristic feature of normotensive pregnancy (2, 15, 16). The concentration-dependent vasoconstriction to Ang II was greater in the uterine arteries from pregnant ACE2 KO mice vs. WT mice. Upregulation of AT1R or increased infiltration of neutrophils in the uterine artery has been suggested to play a role in the increased sensitivity to Ang II during pregnancy (2, 5, 15, 16, 36). It is well documented that the response of uterine artery to Ang II is opposed by the AT2R possibly via mechanisms involving nitric oxide production (6, 32, 33). The activation of AT2R also reduces blood pressure and induces vasodilation of local vascular beds in normotensive C57Bl/6 at midgestation and in hypertensive AT1a receptor knockout mice (6, 50). Since the expression of AT2R was reduced in the uterine arteries of ACE2 KO mice, it is possible that vasodilation mediated by the AT2R is attenuated in this vascular bed and the protection conferred by the AT2R may be lost. Of interest, total knockout of AT2R in mice induces late-pregnancy hypertension and a significant T cell phenotypic switch toward a proinflammatory T-helper 1 phenotype, emphasizing the role of AT2R in blood pressure regulation during pregnancy (35). Similar to other models associated with fetal growth restriction such as a transgenic rat model (49) or testosterone propionate-treated rats (7), increased sensitivity to Ang II and higher maximal contraction to phenylephrine in the ACE2 KO mouse suggest an overall increased sensitivity of uterine artery to vasoconstrictors (2, 16). Although the exact mechanisms of this phenomenon in ACE2 KO mice is unknown, incomplete remodeling in the uterine artery of ACE2 KO mice may contribute to higher contractility to vasoconstrictors. These findings emphasize the role of ACE2 knockout in uterine artery dysfunction.
Our data also show Ang-(1–7) induced attenuation of Ang II constriction in the uterine artery of ACE2 KO mice. This effect was achieved, at least in part, via Ang-(1–7) acting on its own receptor, because the Ang-(1–7) antagonist increased the Ang-(1–7) attenuation of the vasoconstriction to Ang II. Tallant et al. (54) demonstrated that Ang-(1–7) can bind to AT1R in vitro when used at higher micromolar concentrations. Therefore, the possibility of Ang-(1–7) binding to AT1R and thereby limiting AT1R bioavailability for Ang II is feasible. In addition, Ang-(1–7) can activate AT2R so the lower density of AT2R might limit actions of Ang (1–7) in vivo (58). Neves (40) reported that Ang-(1–7) induces vasodilation of mesentery artery in normotensive pregnant rats, suggesting a regulatory role for this peptide in regional blood flow adaptations in pregnancy. Moreover, we (64) recently reported that Ang-(1–7) increases skin blood flow in normotensive pregnant women, suggesting a protective role of Ang-(1–7) in skin microcirculation. Nevertheless, a beneficial role of Ang-(1–7) in the regulation of uterine vasculature during hypertensive pregnancy, particularly in one associated with the activation of the RAS, needs further investigation.
Increased uterine blood flow during normal pregnancy is likely due to remodeling of the uteroplacental vasculature. The predictive and diagnostic value of reduced uterine artery flow in pregnancies with IUGR is controversial. Absent or reversed diastolic flow has been shown in such pregnancies (17), but not consistently (45). Abnormal uterine artery resistance can be influenced by incomplete systemic and local adaptations to pregnancy, including endothelial dysfunction and stiffness of maternal vasculature and placental pathology alterations (12). In our study, we found no differences in uterine artery velocities or resistance in pregnant ACE2 KO vs. WT mice at day 14 of gestation, suggesting that modest fetal growth restriction in ACE2 KO mouse is not accompanied by alterations in uterine artery hemodynamics despite morphological changes. Nor did we find differences in trophoblast invasion between strains. In contrast to human pregnancy, trophoblast invasion in mice is limited to the decidual compartment (14) and continues into late stages of gestation. Variable degrees of arterial trophoblast invasion into the decidual arteries may be more consistent with day 14 of gestation rather than a difference due to the ACE2 knockout. Differences in trophoblast invasion or uterine artery flow between ACE2 KO and WT mice may develop as pregnancy progresses and the fetus grows. In addition, an increased sensitivity of uterine artery to vasoconstrictors may be an early event that does not reflect alterations in uterine artery flow at this stage of gestation. Moreover, sustained uterine flow may prevent further decreases in fetal weight in ACE2 KO mice.
Umbilical artery ultrasound can be used to identify placental insufficiency complications and fetal growth restriction. Clinically, alterations in umbilical flow velocity are more reliable in detecting early-onset fetal growth restriction before 34 wk of gestation than later in gestation (30). During normal pregnancy, progressive increase in flow velocity of umbilical artery from mid to late gestation is essential for the expansion of the fetoplacental vasculature in the labyrinth as an adaptation to growing fetus (10a). Reduced umbilical artery flow velocity in ACE2 knockout may suggest an incomplete adaptation of labyrinth. Our finding of a lower umbilical arterial resistance in association with increased hypoxia was unexpected. However, it is known that abnormalities in the umbilical artery resistance are strongly related to IUGR (30). The reduction in resistance index was associated with a reduction in peak systolic velocity, an unusual pattern that may reflect impaired placentation or suboptimal fetal vascular development that could lead to hypoxia. Moreover, abnormal placentation and placental vascular disease in addition to hypoxia of the uteroplacental unit have been associated with the development of fetal growth restriction (20). Increased hypoxia of the placenta results in the presence of less vascular labyrinth with abnormalities in the fetoplacental capillary bed such as the reduced number of vascular segments, vascular volume, and the total length of arterial tree (20). Moreover, hypoxia increases maximal responses to potassium chloride and the thromboxane A2 mimetic U46619 in human isolated chorionic plate arteries (59, 60), suggesting that reduced oxygen tension may augment vascular reactivity of placental vessels. Attenuated umbilical flow velocity, frequently associated with reduced fetal weight, may be a compensatory adaptation in response to placental hypoxia observed in ACE2 KO mice.
As shown in our study, the ACE2 KO model allows the investigation of the role of ACE2 in hemodynamic and vascular alterations during IUGR pregnancy. Since this model exhibits no major organ damage, it can be particularly useful for the investigation of the hemodynamic and placentation abnormalities that occur during pregnancy, including the early stages of pregnancy that may be critical to the development of IUGR. As stated above, one of the limitations of this model is global ACE2 gene knockout that may result in a series of compensatory events that may mask the contribution of ACE2 deletion to pregnancy and IUGR. Future studies will focus on the direct targeting of ACE2 in the placenta to assess the contribution of placental ACE2 to hypoxia as well as on distinguishing the role of the fetal vs. maternal ACE2 in IUGR.
In summary, IUGR is a leading cause of perinatal morbidity and mortality in humans; however, the molecular mechanisms of this pregnancy-induced complication are not well understood. Using mice with total ACE2 knockout allowed us to investigate the contribution of ACE2 to the development of IUGR. Our study revealed that uterine artery dysfunction in ACE2 KO mice is associated with placental hypoxia and greater uterine artery reactivity to vasoconstrictors, suggesting a protective role of ACE2 in the uteroplacental unit. Increased contractility of uterine artery in ACE2 KO mice may represent an early event that as pregnancy progresses may translate into hemodynamic changes in the uterine artery. Placental hypoxia and uterine artery dysfunction develop before the major growth of the fetus occurs and may explain the growth-restricted phenotype. Our findings also emphasize the similarities of the ACE2 KO model of fetal growth restriction to human pregnancy associated with fetal growth restriction.
GRANTS
This work was supported by an American Heart Association Scientist Development Grant (No. 13SDG17390009 to Liliya M. Yamaleyeva), NICHD R21-HL-110072 (to K. B. Brosnihan and L. M. Yamaleyeva), NHLBI P01-HL-51952 (to K. B. Brosnihan), and the Department of Defense Brazilian-US training grant P116M100027. We gratefully acknowledge grant support in part provided by Unifi, Greensboro, NC, and the Farley-Hudson Foundation, Jacksonville, NC.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The authors have no relationships to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: L.M.Y. and K.B.B. conception and design of research; L.M.Y., V.M.P., S.H.L., L.Y., C.M., M.d., P.L.B., and S.B.G. performed experiments; L.M.Y., V.M.P., S.H.L., and L.Y. analyzed data; L.M.Y., V.M.P., S.H.L., J.V., C.M., and K.B.B. interpreted results of experiments; L.M.Y. and V.M.P. prepared figures; L.M.Y. and K.B.B. drafted manuscript; L.M.Y., V.M.P., S.H.L., J.V., and K.B.B. edited and revised manuscript; L.M.Y., V.M.P., J.V., C.M., S.B.G., and K.B.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Karen Klein MA for manuscript editing.
REFERENCES
- 1.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, Granger JP. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37: 1191–1195, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin N, Rymer J, Todd SD, Thom M, Ritter JM. Sensitivity to angiotensin II of forearm resistance vessels in pregnancy. Br J Clin Pharmacol 32: 523–525, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharadwaj MS, Strawn WB, Groban L, Yamaleyeva LM, Chappell MC, Horta C, Atkins K, Firmes L, Gurley SB, Brosnihan KB. Angiotensin-converting enzyme 2 deficiency is associated with impaired gestational weight gain and fetal growth restriction. Hypertension 58: 852–858, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrell JH, Lumbers ER. Angiotensin receptor subtypes in the uterine artery during ovine pregnancy. Eur J Pharmacol 330: 257–267, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Chen K, Merrill DC, Rose JC. The importance of angiotensin II subtype receptors for blood pressure control during mouse pregnancy. Reprod Sci 14: 694–704, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chinnathambi V, Blesson CS, Vincent KL, Saade GR, Hankins GD, Yallampalli C, Sathishkumar K. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension 64: 405–414, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung E, Yeung F, Leinwand LA. Calcineurin activity is required for cardiac remodelling in pregnancy. Cardiovasc Res 100: 402–410, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen-Tannoudji J, Mhaouty S, Elwardy-Merezak J, Lecrivain JL, Robin MT, Legrand C, Maltier JP. Regulation of myometrial Gi2, Gi3, and Gq expression during pregnancy. Effects of progesterone and estradiol Biol Reprod 53: 55–64, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Cooke CL, Davidge ST. Endothelial-dependent vasodilation is reduced in mesenteric arteries from superoxide dismutase knockout mice. Cardiovasc Res 60: 635–642, 2003. [DOI] [PubMed] [Google Scholar]
- 10a.Croy BA, Yamada AT, DeMayo FJ, Adamson SL, eds. The Guide to Investigation of Mouse Pregnancy (1st Ed.). Elsevier, 2014. [Google Scholar]
- 11.Elvert G, Kappel A, Heidenreich R, Englmeier U, Lanz S, Acker T, Rauter M, Plate K, Sieweke M, Breier G, Flamme I. Cooperative interaction of hypoxia-inducible factor-2alpha (HIF-2alpha) and Ets-1 in the transcriptional activation of vascular endothelial growth factor receptor-2 (Flk-1). J Biol Chem 278: 7520–7530, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Everett TR, Lees CC. Beyond the placental bed: placental and systemic determinants of the uterine artery Doppler waveform. Placenta 33: 893–901, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Ferrario CM, Trask AJ, Jessup JA. Advances in the biochemical and functional roles of angiotensin converting enzyme 2 and angiotensin-(1–7) in the regulation of cardiovascular function. Am J Physiol Heart Circ Physiol 289: H2281–H2290, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca BM, Correia-da-Silva G, Teixeira NA. The rat as an animal model for fetoplacental development: a reappraisal of the post-implantation period. Reprod Biol 12: 97–118, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52: 2682–2689, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gant NF, Whalley PJ, Everett RB, Worley RJ, MacDonald PC. Control of vascular reactivity in pregnancy. Am J Kid Dis IX: 303–307, 1987. [DOI] [PubMed] [Google Scholar]
- 17.Gebb J, Dar P. Colour Doppler ultrasound of spiral artery blood flow in the prediction of pre-eclampsia and intrauterine growth restriction. Best Pract Res Clin Obstet Gynaecol 25: 355–366, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Genbacev O, Krtolica A, Kaelin W, Fisher SJ. Human cytotrophoblast expression of the von Hippel-Lindau protein is downregulated during uterine invasion in situ and upregulated by hypoxia in vitro. Dev Biol 233: 526–536, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23: 9361–9374, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr 2010: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim HS, Froemming GR, Omar E, Singh HJ. ACE2 activation by xanthenone prevents leptin-induced increases in blood pressure and proteinuria during pregnancy in Sprague-Dawley rats. Reprod Toxicol 49C: 155–161, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol 283: R29–R45, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kostrzewska A, Laudanski T, Batra S. Effect of calcium and calmodulin antagonists on contractile responses of the human uterine artery. Br J Pharmacol 94: 1037–1042, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulandavelu S, Whiteley KJ, Bainbridge SA, Qu D, Adamson SL. Endothelial NO synthase augments fetoplacental blood flow, placental vascularization, and fetal growth in mice. Hypertension 61: 259–266, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Kusinski LC, Stanley JL, Dilworth MR, Hirt CJ, Andersson IJ, Renshall LJ, Baker BC, Baker PN, Sibley CP, Wareing M, Glazier JD. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am J Physiol Regul Integr Comp Physiol 303: R86–R93, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Levy A, Yagil Y, Bursztyn M, Barkalifa R, Scharf S, Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol 295: R1953–R1961, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Lindsey SH, da Silva AS, Silva MS, Chappell MC. Reduced vasorelaxation to estradiol and G-1 in aged female and adult male rats is associated with GPR30 downregulation. Am J Physiol Endocrinol Metab 305: E113–E118, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2. Lewis rat. Hypertension 58: 665–671, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer C, Joseph KS. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet Gynecol 41: 136–145, 2013. [DOI] [PubMed] [Google Scholar]
- 31.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med 312: 82–90, 1985. [DOI] [PubMed] [Google Scholar]
- 32.McMullen JR, Gibson KJ, Lumbers ER, Burrell JH. Selective down-regulation of AT2 receptors in uterine arteries from pregnant ewes given 24-h intravenous infusions of angiotensin II. Regul Pept 99: 119–129, 2001. [DOI] [PubMed] [Google Scholar]
- 33.McMullen JR, Gibson KJ, Lumbers ER, Burrell JH, Wu J. Interactions between AT1 and AT2 receptors in uterine arteries from pregnant ewes. Eur J Pharmacol 378: 195–202, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Mhaouty-Kodja S, Houdeau E, Legrand C. Regulation of myometrial phospholipase C system and uterine contraction by beta-adrenergic receptors in midpregnant rat. Biol Reprod 70: 570–576, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Mirabito KM, Hilliard LM, Wei Z, Tikellis C, Widdop RE, Vinh A, Denton KM. Role of inflammation and the angiotensin type 2 receptor in the regulation of arterial pressure during pregnancy in mice. Hypertension 2014. [DOI] [PubMed] [Google Scholar]
- 36.Mishra N, Nugent WH, Mahavadi S, Walsh SW. Mechanisms of enhanced vascular reactivity in preeclampsia. Hypertension 58: 867–873, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mu J, Adamson SL. Developmental changes in hemodynamics of uterine artery, utero- and umbilicoplacental, and vitelline circulations in mouse throughout gestation. Am J Physiol Heart Circ Physiol 291: H1421–H1428, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 41: 19–26, 1977. [DOI] [PubMed] [Google Scholar]
- 39.Neves LAA, Stovall K, Joyner J, Valdes G, Gallagher PE, Ferrario CM, Merrill DC, Brosnihan KB. ACE2 and Ang-(1–7) in the uterus during early and late gestation. Am J Physiol Regul Integr Comp Physiol 294: R151–R161, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Neves LAA, Williams AF, Averill DB, Ferrario CM, Walkup MP, Brosnihan KB. Pregnancy enhances the angiotensin (Ang)-(1–7) vasodilator response in mesenteric arteries and increases the renal concentration and urinary excretion of Ang-(1–7). Endocrinology 14: 3338–3343, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen DH, Zhou T, Shu J, Mao JH. Quantifying chromogen intensity in immunohistochemis-try via reciprocal intensity. Cancer InCytes 2(1), e. 2013. [Google Scholar]
- 42.Ni Y, Meyer M, Osol G. Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. Am J Obstet Gynecol 176: 856–864, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Osol G, Cipolla M. Pregnancy-induced changes in the three-dimensional mechanical properties of pressurized rat uteroplacental (radial) arteries. Am J Obstet Gynecol 168: 268–274, 1993. [DOI] [PubMed] [Google Scholar]
- 44.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 24: 58–71, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pastore MB, Jobe SO, Ramadoss J, Magness RR. Estrogen receptor-alpha and estrogen receptor-beta in the uterine vascular endothelium during pregnancy: functional implications for regulating uterine blood flow. Semin Reprod Med 30: 46–61, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel VB, Zhong JC, Fan D, Basu R, Morton JS, Parajuli N, McMurtry MS, Davidge ST, Kassiri Z, Oudit GY. Angiotensin-converting enzyme 2 is a critical determinant of angiotensin II-induced loss of vascular smooth muscle cells and adverse vascular remodeling. Hypertension 64: 157–164, 2014. [DOI] [PubMed] [Google Scholar]
- 47.Pijnenborg R, Bland JM, Robertson WB, Brosens I. Uteroplacental arterial changes related to interstitial trophoblast migration in early human pregnancy. Placenta 4: 397–413, 1983. [DOI] [PubMed] [Google Scholar]
- 48.Pisaneschi S, Strigini FA, Sanchez AM, Begliuomini S, Casarosa E, Ripoli A, Ghirri P, Boldrini A, Fink B, Genazzani AR, Coceani F, Simoncini T. Compensatory feto-placental upregulation of the nitric oxide system during fetal growth restriction. PLoS One 7: e45294, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulgar VM, Yamaleyeva LM, Varagic J, McGee CM, Bader M, Dechend R, Howlett AC, Brosnihan KB. Increased angiotensin II contraction of the uterine artery at early gestation in a transgenic model of hypertensive pregnancy is reduced by inhibition of endocannabinoid hydrolysis. Hypertension 64: 619–625, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pulgar VM, Yamashiro H, Rose JC, Moore LG. Role of the AT2 receptor in modulating the angiotensin II contractile response of the uterine artery at mid-gestation. J Renin Angiotensin Aldosterone Syst 12: 176–183, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raleigh JA, Chou SC, Arteel GE, Horsman MR. Comparisons among pimonidazole binding, oxygen electrode measurements, and radiation response in C3H mouse tumors. Radiat Res 151: 580–589, 1999. [PubMed] [Google Scholar]
- 52.Rentzsch B, Todiras M, Iliescu R, Popova E, Campos LA, Oliveira ML, Baltatu OC, Santos RA, Bader M. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension 52: 967–973, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res 92: 401–408, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol 289: H1560–H1566, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Thomas MC, Pickering RJ, Tsorotes D, Koitka A, Sheehy K, Bernardi S, Toffoli B, Nguyen-Huu TP, Head GA, Fu Y, Chin-Dusting J, Cooper ME, Tikellis C. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ Res 107: 888–897, 2010. [DOI] [PubMed] [Google Scholar]
- 56.Valdes G, Neves LA, Anton L, Corthorn J, Chacon C, Germain AM, Merrill DC, Ferrario CM, Sarao R, Penninger J, Brosnihan KB. Distribution of angiotensin-(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 27: 200–207, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Vandenbosche RC, Kirchner JT. Intrauterine growth retardation. Am Fam Physician 58: 1384, 1998. [PubMed] [Google Scholar]
- 58.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension 45: 960–966, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Wareing M, Crocker IP, Warren AY, Taggart MJ, Baker PN. Characterization of small arteries isolated from the human placental chorionic plate. Placenta 23: 400–409, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Wareing M, Greenwood SL, Baker PN. Reactivity of human placental chorionic plate vessels is modified by level of oxygenation: differences between arteries and veins. Placenta 27: 42–48, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Wareing M, Myers JE, O'Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. J Clin Endocrinol Metab 90: 2550–2555, 2005. [DOI] [PubMed] [Google Scholar]
- 62.Yamaleyeva LM, Gilliam-Davis S, Almeida I, Brosnihan KB, Lindsey SH, Chappell MC. Differential regulation of circulating and renal ACE2 and ACE in hypertensive mRen2. Lewis rats with early-onset diabetes. Am J Physiol Renal Physiol 302: F1374–F1384, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaleyeva LM, Lindsey SH, Varagic J, Zhang LL, Gallagher PE, Chen AF, Chappell MC. Amelioration of renal injury and oxidative stress by the nNOS inhibitor L-VNIO in the salt-sensitive mRen2. Lewis congenic rat. J Cardiovasc Pharmacol 59: 529–538, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaleyeva LM, Merrill DC, Ebert TJ, Smith TL, Mertz HL, Brosnihan KB. Hemodynamic responses to angiotensin-(1–7) in women in their third trimester of pregnancy. Hypertens Pregnancy 1–14, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol Heart Circ Physiol 243: H941–H947, 1982. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H, Xiao D, Longo LD, Zhang L. Regulation of alpha1-adrenoceptor-mediated contractions of uterine arteries by PKC: effect of pregnancy. Am J Physiol Heart Circ Physiol 291: H2282–H2289, 2006. [DOI] [PubMed] [Google Scholar]