Abstract
The loss of muscle strength and increased injury rate in aging skeletal muscle has previously been attributed to loss of muscle protein (cross-sectional area) and/or decreased neural activation. However, it is becoming clear that force transfer within and between fibers plays a significant role in this process as well. Force transfer involves a secondary matrix of proteins that align and transmit the force produced by the thick and thin filaments along muscle fibers and out to the extracellular matrix. These specialized networks of cytoskeletal proteins aid in passing force through the muscle and also serve to protect individual fibers from injury. This review discusses the cytoskeleton proteins that have been identified as playing a role in muscle force transmission, both longitudinally and laterally, and where possible highlights how disease, aging, and exercise influence the expression and function of these proteins.
Keywords: force transmission, dystrophin-glycoprotein complex, injury, aging
on average, humans lose around 45% of their muscle mass between their mid-20s and 80s (47, 70, 71). This loss in muscle mass in the absence of disease is known as sarcopenia (60). The decline in muscle mass is accompanied by, but cannot fully explain, a rapid loss in muscle strength (64). The loss in muscle strength has previously been investigated from the perspectives of loss of muscle protein mass (cross-sectional area) and decreased neural activation. A third possibility, impaired force transfer, has received the least attention in relation to aging, exercise, and disease (66, 90, 102). However, recent advances in our understanding of this process suggest that force transfer plays an important role in muscle strength and injury prevention, and this fact is the focus of this review.
Force Transfer
Over 60 years ago, Andrew Huxley and his students used electron microscopy to show that during muscle contraction the I-band (containing the thin filament) shortened whereas the A-band (containing the thick filament) remained a constant length (39). Their famous “sliding theory of muscle contraction” provided an image of a muscle shortening end to end as the A-bands drew closer together. Implied in this model is that force is transferred in a longitudinal manner as a result of sarcomere shortening. However, a deeper consideration of this model requires that there be a secondary matrix of proteins that are not visible to the electron microscope, proteins that transmit the force produced by the thick and thin filaments along the muscle fiber to the tendons. These specialized networks of cytoskeletal proteins transmit force through the muscle to the tendon and also serve to protect individual fibers from injury. An important structure in these networks is the “costamere”, which connects the sarcolemma with the contractile apparatus, as first highlighted by Pardo et al. (85). Early cell work in rat cardiomyocytes suggested that the costamere structure transmitted force to the extracellular environment (22) and that these proteins are also important in healthy skeletal muscle, playing a crucial role in muscle function and injury prevention.
The fact that skeletal muscle transmits force in both longitudinal and lateral directions was first highlighted by Street (102) in frog muscle. Longitudinal force relates to the transmission along muscle fibers from Z-line to Z-line via the myotendinous junction to the tendon. By contrast, lateral force transmission shuttles force out of each sarcomere to the overlying connective tissue and extracellular matrix and from there to the tendon (38, 120). This review discusses the cytoskeleton proteins that have been identified to play a role in muscle force transmission (longitudinal and lateral) and where possible highlights how disease, aging, and exercise influence the expression and function of these proteins.
Longitudinal Force Transmission
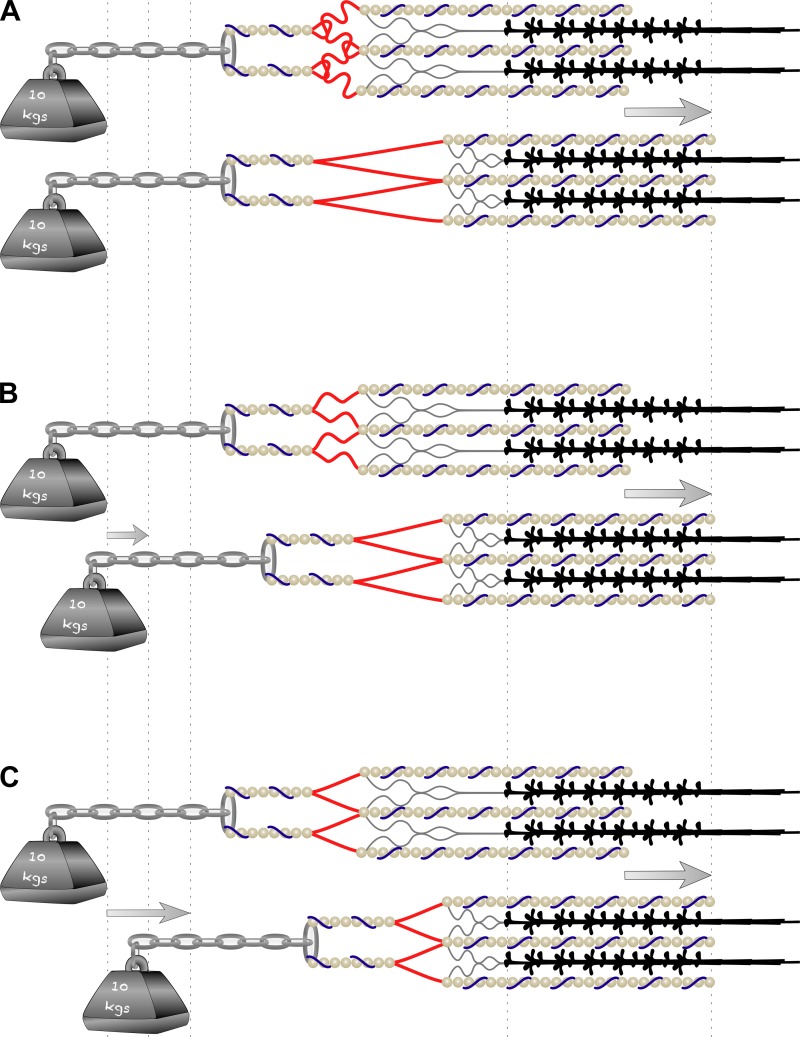
Longitudinal force transfer supports and enables the interactions between myosin and actin. This system incorporates proteins that are within the thick (titin) and thin (nebulin) filaments, the α-actinin within the Z-lines, and the proteins that anchor the thick (ankryin repeat proteins) and thin filaments (muscle LIM proteins) to the Z-line. When the longitudinal force transfer system is working properly, the force generated by actomyosin interactions within each sarcomere is transferred rapidly from sarcomere to sarcomere to the myotendinous junction to move the load. When the system is suboptimal, there is greater compliance within the system that needs to be overcome before the load can be moved. The result is that changes to proteins involved in longitudinal force transfer manifest as changes in the rate of force development and power. (Fig. 1).
Fig. 1.
Process of longitudinal force transfer in skeletal muscle contractions to overcome an external load. Dysfunctional (A) or suboptimal performance (B) of proteins involved in longitudinal force transfer (represented by the length of the Z-line in red) leads to a reduction in force and the rate of force development. In both circumstances, the elastic component of the muscle, influenced by proteins such as titin, nebulin, and α-actinin, needs to be overcome for the force produced to shift the external load. C: when the system is working properly, the force produced within a sarcomere will be transferred rapidly to the external load.
Titin.
Titin is the largest protein in the body and the third most abundant protein in mammalian skeletal muscle (45). This giant protein spans from the sarcomere's Z-line to its M-line and from this position may play a role in longitudinal force transmission. Although from a single gene, the alternative splicing of titin mRNA allows for varying sizes of different titin isoforms to be present within striated muscle (45). In human skeletal muscle, two titin isoforms have been reported (62), with no fiber type- or muscle-specific associations observed (28). In both isoforms, the I-band region of titin is comprised of the proximal and distal immunoglobulin (Ig) tandem domains, the N2A domain, and a PEVK segment. Each domain/segment appears to be influenced by sarcomere length, with the PEVK segment extended at intermediate sarcomere lengths and unfolding of the Ig domain occurring at very long sarcomere lengths, e.g., during eccentric contractions (68). Titin has been proposed as the “molecular spring”, as it is the major protein responsible for passive tension and allows for the sarcomere to be protected from overstretching (34, 36). Indeed, this protective factor is dependent on which titin isoform is expressed due to lower or higher titin-derived muscle stiffness (74). Furthermore, the stiffness of this molecular spring may be actively regulated through titin-actin interactions as well as the structural arrangement of the spring elements within the I-band region (103). Evidence also suggests that Ca2+-induced changes in titin can increase titin and sarcomere stiffness (44, 103). However, debate remains surrounding the regulation of non-cross-bridge force production (91).
From a skeletal muscle performance perspective, a decrease in titin has been observed during disuse and following high-intensity eccentric resistance exercise (104, 106). Twenty-four hours after 10–13 sets of 10 knee extensions at a workload of 120% concentric force, Trappe et al. (104) observed a 30% and a 15% reduction in titin and another important sarcomeric protein, nebulin, respectively. The authors suggested that this loss in titin was due to either direct damage or postinjury degradation. It is important to note that they did not differentiate titin into its different isoforms (titin-1 and titin-2); thus, the isoform-specific changes in human skeletal muscle following exercise remain to be determined. A more recent study, by Udaka et al. (106), highlighted the alterations in skeletal muscle through reduced titin expression. The authors performed 6 wk of hindlimb immobilization on Wistar rats and subsequently extracted the whole soleus muscle posttreatment. They observed an ∼45% loss in titin following disuse. The reduction in titin was associated with significant decreases in thick filament length, along with abnormalities in sarcomeric structure and altered interfilament lattice spacing.
Another factor highlighted from the Udaka study (106), was a reduction in calcium ion (Ca2+) sensitivity as a consequence of sarcomere structure abnormalities following titin loss. Recently, Mateja et al. (59) utilized a homozygous mutant rat strain that expresses longer titin isoforms (3.75 MDa) (33) compared with wild-type (3.44-3.30 MDa) to investigate the role of titin in myofilament length-dependent activation. The authors observed much lower passive forces (assessed by force probe displacement during a single myofibril [Ca2+] activation-relaxation cycle) in the mutant (N2BA-G titin isoform) rats compared with the wild-type, which was accompanied by a decrease in maximal force and Ca2+ sensitivity at short and long sarcomere lengths. It would therefore appear that titin does not affect muscle contraction by directly altering the sarcomeric structure. Other mechanisms suggested within the cardiac literature have ranged from altering the Ca2+ affinity of troponin, interfilament spacing, myosin structure, and thin filament activation (23, 27). The last possibility may reflect the fact that titin, within the thick filament, could affect nebulin, within the thin filament, and alter muscle contraction and subsequent longitudinal force transfer (see below). Overall, the role of titin as a molecular spring, protecting the sarcomere from overstretching and contributing towards the passive tension in the muscle, are pivotal to longitudinal force transfer and the rate of force development.
Nebulin.
Nebulin (700–900 kDa) is another giant sarcomeric protein that spans the length of the thin filament. Nebulin anchors at the Z-line via its COOH terminus, whereas the NH2-terminal region stretches toward the pointed end of the thin filament (112). Like titin, nebulin plays a role in muscle contraction, calcium homeostasis, and cross-bridge cycling kinetics (16, 46, 77, 80). The importance of nebulin on these contractile processes within skeletal muscle is commonly observed with nemaline myopathy (NM, a nondystrophic congential myopathy) (87). This disease occurs due to pathologically low levels of nebulin. Fifty percent of NM cases are due to a genetic mutation in the nebulin gene, with the most important clinical feature of the condition being muscle weakness (83).
The analysis of skeletal muscle from both NM patients and nebulin knockout (NE-KO) animal models has enhanced our understanding of the role of nebulin in muscle contraction and thus force transfer (16, 77, 80, 118). One component of muscle weakness observed in the NM patients is the dysregulation of thin filament length (81, 83). In the NM patients, Ottenheijm et al. (83) observed a left shift in the length-tension relationship due to shorter thin filaments compared with controls. The shift in the length-tension curve was accompanied by a decrease in maximal force (∼65%) in NM patients compared with controls, indicating that both length-dependent and maximal force were decreased in the absence of nebulin. This is most likely due to fewer cross-bridge interactions due to the shorter thin filaments. These findings have been confirmed in the NE-KO model (2, 32, 116), highlighting the importance of nebulin in thin filament length and muscle force production.
Along with the decrease in thin filament length in the absence of nebulin, changes in cross-bridge cycling and calcium sensitivity are also common observations in nebulin-deficient muscles (16, 77, 80). Chandra et al. (16) utilized the NE-KO model to investigate the calcium-dependent regulation of thin filament activation via the troponin and tropomyosin complex, as nebulin is able to associate with this complex (58, 78). The authors observed a reduction in calcium sensitivity and cooperativity of activation, highlighted by reductions in the force-pCa relationship (relative force produced when stimulated with calcium) between wild-type and NE-KO skinned muscle fibers. Interestingly, the authors postulated that nebulin increases the number of force-generating cross-bridges, which contribute to thin filament activation within skeletal muscle. Overall, the dysregulation in thin filament length highlights the role of nebulin in muscle force production, where changes in the thin filament length affect thin-thick filament interactions, impacting on cross-bridge kinetics and thus decreasing the longitudinal force-generating capacity at a given sarcomere length within skeletal muscle (82). However, whether nebulin plays a role in the rate of force development, aging or exercise has yet to be determined.
α-Actinin.
The Z-line in mammalian skeletal muscle is largely composed of α-actinin-2 (ACTN2) and/or α-actinin-3 (ACTN3), which link adjacent actin thin filaments (101). These proteins are essential for maintaining the spatial relationship between myofilaments and stabilizing the myofilament lattice (7). ACTN2 is expressed in all human muscle fiber types, whereas ACTN3 expression is restricted to fast glycolytic type II muscle fibers (109), those responsible for rapid force production (26). The predominant role of ACTN3 in force transfer is highlighted at the genetic level. In healthy individuals of European decent, ∼18% are deficient in ACNT3 due to a common nonsense polymorphism in the ACTN3 gene, R577X (76). The early identification of this polymorphism has led to intense investigation as to the role of ACTN3 deficiency in muscle damage, strength/power, and human performance (15, 19, 56, 100, 110, 119).
In XX genotype individuals (i.e., ACTN3 deficient), muscle force is reduced, and these individuals experience increased muscle damage compared with RR genotype counterparts (19, 67, 109–111). The impact of ACTN3 on muscle function is further observed in elite athletic performance, where endurance athletes have a higher frequency of ACTN3 deficiency than power/sprint individuals (94, 119). One possible explanation for this observation is that ACTN3 is stiffer and therefore transmits force faster than ACTN2. What is certain is that single muscle fibers from the ACTN3 knockout mouse display an ∼40% greater force deficit postexercise following eccentric contractions compared with wild-type animals (99). These observations highlight an important role for α-actinin in the force transmission properties of the Z-line. Furthermore, α-actinin can affect both rate of force development and injury, suggesting that it plays a role in both longitudinal and lateral force transmission (see below). Subsequent studies are needed to address the influence of aging on the α-actinin proteins in relation to force transmission, especially as the ACTN3 genotype has been observed to impact on muscle force and strength in humans and animals toward later life (41, 98, 111). It is important to note that, although the loss of ACTN3 has been observed in muscular dystrophies (107), within healthy populations this has no impact on muscle force and power due to compensation from ACTN2 and desmin (75, 99).
Ankryin repeat proteins.
The muscle ankryin repeat proteins (MARPs) are a family of proteins located within the I-band of the sarcomere (42). MARPs are expressed in both cardiac and skeletal muscle, and they bind to the N2A region of titin as well as to the nebulin anchoring protein myopalladin (42). The MARP family consists of muscle (Ankrd2/ARPP), cardiac (Ankrd1/CARP), and diabetes-associated (Ankrd23/DARP) ankryin repeat proteins (42). In terms of muscle function, ARPP and CARP have received more attention. DARP has been observed in both heart and skeletal muscle only following upregulation in type 2 diabetes and insulin-resistant animals (40). ARPP and CARP have been implicated in muscular dystrophies (72, 73), the response to acute resistance exercise (17), and overload-induced hypertrophy (14) and show altered expression following denervation (105).
In terms of muscle force transmission, Barash et al. (3) utilized single, double, and triple knockout mouse models for the MARP family to investigate the structural/functional role for this family of proteins in response to loading. For the triple-knockout model (ARPP, CARP, and DARP) there were no significant reductions in muscle fiber size or fiber type; however, there was a significant increase in resting sarcomere length in the EDL muscle, which was accompanied by longer isoforms of titin. This observation provides insight into the contribution the MARP family may have toward the passive mechanical behavior of muscle. Furthermore, eccentric contractions resulted in a greater reduction in torque (i.e., more muscle injury) in the triple-knockout mice compared with wild-type mice. However, there was no functional impairment at later points in the recovery period. Furthermore, in the single- and double-knockout models there were no significant reductions in muscle torque compared with wild-type mice. Thus, it appears that the MARP family of proteins have a high level of redundancy. The structural and functional integrity of muscle is compromised only when all MARP proteins are absent, and this increased level of injury is especially obvious when the muscle is placed under an eccentric load.
Muscle LIM protein.
The muscle LIM protein (MLP) is a Z-line protein that binds to structural proteins such as nebulin-related protein (N-RAP), titin, and α-actinin (1, 88). The position of MLP within the sarcomere suggests that it may play a role in longitudinal force transmission or as a muscle stress sensor. Indeed, in response to a physical stimulus, MLP can translocate between the cytoplasm and nucleus (25). This property presents the possibility that MLP is not only involved in structural adaptations within striated muscle but can additionally function as some form of mechanically activated transcription factor (1, 43).
To test the mechanical role of MLP, Schneider et al. (96) electrically stimulated the tibialis anterior (TA) muscle and contralateral soleus (SOL) muscle of male Wistar rats for a period of 24 h/day at low frequency and collected muscles after 12 h and 4 and 8 days of stimulation. The authors observed a baseline difference in MLP expression between SOL and TA muscles, with expression being higher in the SOL. Low-frequency stimulation resulted in an upregulation of MLP in the TA muscles after 4 and 8 days. This finding was accompanied by an increase in the expression of type I fibers, so it was difficult to determine whether the increase in loading or the transition from fast-to-slow phenotype was the cause of the increase in MLP. A follow-up study by Willmann et al. (115), using a 6-wk synergist ablation model, also showed an increase in MLP. However, once again there was a fast-to-slow phenotype shift that was accompanied by increased MLP expression with loading. Thus, whether it was a direct effect of the loading remained unclear.
To address the role of MLP following loading in the absence of a shift in muscle fiber phenotype, a number of groups have studied the early response of MLP to resistance exercise and contraction-induced injury (4, 5, 17). Six hours after a single bout of resistance exercise (10 sets of 6 repetitions with electrical stimulation) in female Wistar rats, MLP mRNA increased 4.3-fold (17). Moreover, Barash et al. (5) observed significant (8- to 11-fold) increases in both MLP and MARP gene expression 12–24 h following eccentric contraction-induced injury in mice. However, how these rapid increases in MLP mRNA are reflected in changes in MLP protein within the sarcomere and how this contributes to muscle adaptation/function remains poorly understood.
The Lieber laboratory (4) generated an MLP knockout mouse to better determine its structural and functional role in muscle. Muscle fibers isolated from the knockout mice showed a decrease in resting sarcomere length, highlighting that MLP may have an effect on muscle structure through its association with titin (45). Furthermore, following an eccentric loading protocol, the authors observed a delay in the recovery of force in the MLP knockout mice. There was no difference in the mRNA response of CARP and ARPP in an effort to compensate for the loss of MLP, even though the proteins show a similar location and putative function. Overall, these data suggest that MLP may play a role in longitudinal muscle force transmission, which is important for normal muscle maintenance and the capacity for skeletal muscle to respond to physical stimuli.
Lateral Force Transmission
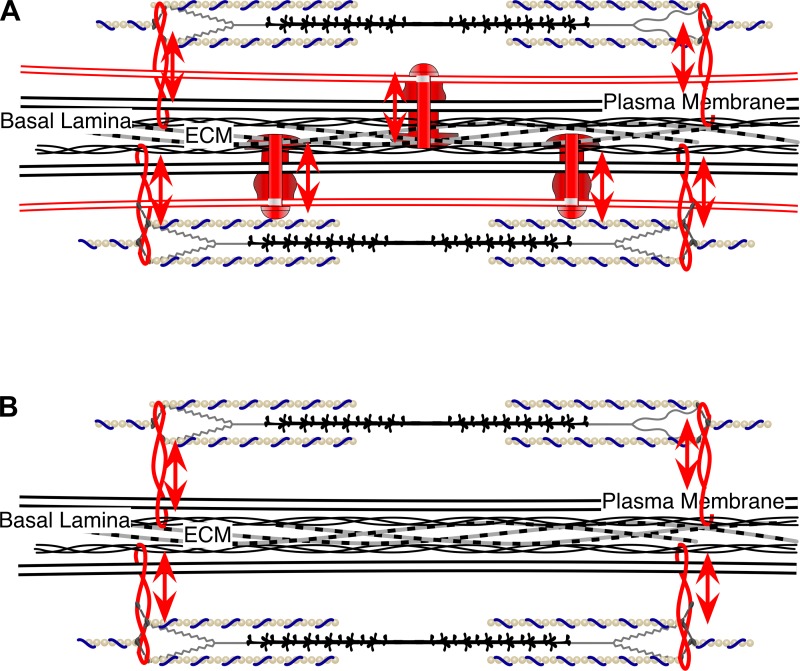
An early review by Bloch and Gonzalez-Serratos (9) highlighted the concept that force transmission does not occur only in a longitudinal direction. The alternative path has been called lateral force transmission since the force produced in each sarcomere would be passed laterally from the Z-line to the sarcolemma and externally to the extracellular matrix. The transmission of force laterally through the sarcolemma occurs via costameres (10). Costameres are comprised of large membrane-cystoskeletal complexes (dystrophin-associated glycoprotein complex, integrins, etc.), which appear to couple the intracellular matrix (actin and demin filaments) to the extracellular matrix like the rivets used to fasten two adjacent materials (see Fig. 2). Much like rivets, the lateral force transmission system is best suited to withstand shear loads that can result in damage to the sarcolemma. For instance, both costameres and microtubule structures provide strong mechanical links between the sarcolemma and the contractile apparatus (89, 95). This is exemplified by the functional role of dystrophin (discussed in more detail below), which has been classed as a “cytolinker” and forms strong connections between the sarcolemma and actin filaments (89, 95). Studies that have attempted to measure lateral force transmission have found that more than 80% of force is transferred via this lateral pathway (90, 102). Furthermore, the proteins involved and their role in protecting the sarcolemma make lateral force transmission extremely important in injury prevention (Fig. 2).
Fig. 2.
Role of lateral force transmission in membrane stability and integrity. A: dystrophin-glycoprotein complex (DGC) and integrins act like rivets and ties that couple the intracellular matrix to the extracellular matrix (ECM) and prevent shear stress from damaging the sarcolemma. B: loss of these complexes in muscular dystrophies weakens the connection between the intracellular and extracellular matrices, rendering individual fibers more susceptible to damage from shear. Therefore, loss in lateral force transmission appears to lead toward sarcolemma damage and membrane disruption.
Dystrophin-associated glycoprotein complex.
The dystrophin-associated glycoprotein (DAG) complex is fundamental to the process of lateral force transmission. The complex is comprised of dystrophin, dystroglycan (α- and β-), and sarcoglycan (α-, β-, γ-, and δ-) proteins. The importance of the DAG complex in muscle has been highlighted by studies on muscular dystrophy. The predominant pathology in most muscular dystrophies is an inability of the muscle to produce functional copies of all of the dystrophin-associated complex proteins (10, 117). This family of muscle diseases is characterized by a progressive loss of muscle mass and muscle force, caused by muscle injury and degeneration, which ultimately impacts overall locomotion and respiration (54, 69). The role of dystrophin in force transmission (specifically lateral transmission) is centered on the connection between the sarcomere and the extracellular matrix, which aids in both sarcolemma integrity (55) and intracellular Ca2+ homeostasis (21). Indeed, in mdx mice, Garcia-Pelagio et al. (30) observed, by applying suction pressures to isolated extensor digitorum longus (EDL) muscles, a reduction in muscle stiffness and increased sarcolemma deformation and compromised links between costameres and the connections to nearby myofibrils. Overall, the loss in dystrophin is accompanied by significant reductions in muscle force capacity and renders muscle fibers more susceptible to contraction-induced injury, especially following eccentric contractions (51).
Ramaswamy et al. (90) elegantly demonstrated that in the absence of dystrophin lateral force transmission was impaired. The measurement of lateral force transmission was achieved through the development of a “yoke” apparatus that was sewn to the epimysium of muscles midway between the tendons. Using a force transducer attached either to the tendon or to the yoke, Ramaswamy et al. observed that in young healthy wild-type mice and rats there was little or no difference in force measured at the yoke or at the tendon. Therefore, in otherwise healthy muscle, at least 80% of force produced in the muscle was transmitted laterally to the tendon. The authors then observed a reduction in lateral force transmission in muscles associated with the disruption of the dystrophin-glycoprotein complex (DGC), either in very old rats or in mdx mice that lacked dystrophin. It was suggested that the decrease in lateral force transmission resulting from disruptions in the DGC complex leads to sarcomere instability and subsequent contraction-induced injury. The observed loss of dystrophin in very old rats, alongside reduced lateral transmission in mdx mice, highlights a potential age-related susceptibility to muscle injury. The authors observed no differences between young and very old rats for α- and β-dystroglycan proteins, and so it would appear that the loss of dystrophin alone is sufficient to decrease lateral force transmission.
In contrast to the effects of aging on dystrophin levels reported by Ramaswamy et al., Rice et al. (92) observed increases in dystrophin, β-dystroglycan, and α-sarcoglycan with aging in the EDL of 30 and 36 mo old rats. However, in the soleus muscle, these protein levels decreased with aging. This suggests that the effects of aging on dystrophin levels may be muscle and activity dependent. Rice et al. did not perform muscle force measurements; so how these alterations in cytoskeleton proteins with aging impact force transmission between muscle types requires further investigation. The observations by Rice et al. highlight the possibility that phenotypically different muscles may show alterations in cytoskeleton proteins with aging, and this may underlie the susceptibility of certain muscles to contraction-induced injury.
Alongside dystrophin, the sarcoglycan subcomplex has been implicated as a sensor for mechanical loading and force transmission. Interestingly, α-sarcoglycan-null mice exhibit larger muscle mass than age-matched wild-type mice, yet the absolute force production was maintained near control levels at all ages (20). However, when eccentric contractions were performed in mice lacking γ-sarcoglycan, which results in the additional loss of β- and δ-sarcoglycan (35), these (20-wk-old) mice exhibited a significantly greater drop in force (−30%) than wild-type mice (6). Overall, the involvement of the DGC complex toward age-related changes in muscle force transmission remains to be fully elucidated. However, it is clear that loss of the DGC makes muscles prone to injury.
As with titin, a single bout of eccentric contractions, which reduced contractile function (maximal tetanic tension) and sarcolemmal integrity, is associated with a loss of dystrophin [Lovering and De Deyne (51)]. Lovering and De Deyne observed a significant association between a disruption in sarcolemmal integrity (identified by Evans blue dye assay) and loss of dystrophin labeling. It was not clear from the study, however, whether the decreased dystrophin was a cause or a consequence of the disruption of sarcolemmal integrity. Even so, the loss of dystrophin was not associated with changes in the remainder of the subsarcolemmal complex (α- or β-dystroglycan). Additionally, there was no change in desmin or α-actinin levels, supporting the importance of dystrophin in both sarcolemmal structure and force transmission. Interestingly, the observations by Lovering and De Deyne, were from TA muscle, which, like the EDL muscle (55, 65), is highly susceptible to contraction-induced injury (24). Thus, in skeletal muscle where dystrophin expression is low, muscles are more susceptible to contraction-induced injury due to the inability to laterally transmit the forces generated and prevent sarcolemmal shearing.
Integrins.
In skeletal muscle, the DGC complex works together with integrins to transmit force laterally. The integrins are transmembrane heterodimers of noncovalently bound α- and β-subunits for which 18 and 8 subunits (respectively) have been characterized (61, 108). The predominant integrin in adult skeletal muscle is α7β1, with the α7-subunit responsible for binding to laminin within the basal lamina and the β1-subunit involved with linking to actin through various subsarcolemmal proteins such as α-actinin, desmin, and paxillin [for a detailed review see Mayer (61)]. The β1-integrins are expressed in mammalian muscle at the myotendinous junction, within costameres at the sarcolemma and at the neuromuscular junction. In skeletal muscle, integrins function as cell surface adhesion receptors that can mediate cell-cell and cell-matrix interactions. This has led to the investigation of these complexes in mechanotransduction and force transmission during muscle contraction.
Two early studies by Boppart and colleagues (11, 12) utilized animal models that either overexpressed or knocked out α7-integrin to address its role in skeletal muscle damage. The mice with α7-integrin overexpression undertook 30 min of downhill running (20° decline) and the gastrocnemius and soleus muscles were then assessed for muscle damage and intracellular signaling. Boppart et al. (11) observed a reduction in the number of muscle fibers damaged 24 h postexercise (as assessed from the number of Evans blue dye-positive fibers) in the α7-integrin-overexpressing mice compared with wild-type. Interestingly, the reduced muscle damage was accompanied by attenuation of both MAP kinase signaling immediately postexercise and Akt-mTOR-p70S6K phosphorylation 1 and 3 h postexercise. The authors postulated that the alteration in signaling might be due to the increased connection between laminin and the underlying myofibrillar proteins leading to enhanced “stiffness” and a subsequent decrease in mechanosensitivity. On the other hand, when α7-integrin knockout mice were exposed to single or repeated bouts of exercise (12), they demonstrated significantly higher levels of muscle damage than wild-type mice. Interestingly, the level of injury was exacerbated in the myotendinous region of the muscle after a subsequent exercise bout. Furthermore, in wild-type mice, the downhill running protocol led to increased expression and localization of α7A and α7B at the myotendinous junction. Along with these findings in transgenic mice, the integrin complexes are also altered during muscle contraction, hypertrophy, and contraction-induced injury in wild-type mice (12, 49, 53, 121). These observations provide evidence toward the role of α7β1-integrins in lateral force transmission and attenuating the development of contraction-induced injury.
Another instance where α7β1-integrin appears to contribute to force transmission and maintenance of muscle integrity is when considered in relation to Duchenne muscular dystrophy. With the loss of dystrophin in muscle, there is increased expression of α7β1-integrins (37, 113). To determine the importance of α7β1-integrins, Welser et al. (114) generated α7-integrin knockout mice. These mice showed normal sarcolemmal integrity, possibly due to the upregulation of dystrophin, but muscle strength was significantly lower, highlighting the importance of integrins in lateral force transmission. In support of α7β1-integrin and dystrophin working together to transmit force laterally, mice lacking both dystrophin and α7-integrin develop a more severe muscular dystrophy then either individual knockout strains (93). In fact, α7-integrin/dystrophin double-knockout mice die within 4 wk of birth due to extensive loss of membrane integrity, muscle degeneration, and necrosis. Therefore, it would appear that the DGC complex and integrins combine to maintain lateral force transmission and the structural and functional integrity of skeletal muscle (93).
Desmin.
Desmin is an intermediate filament in striated muscle located at the Z-line of the sarcomere. Desmin is thought to play an essential role in the structural and mechanical integrity of the contractile apparatus by forming a three-dimensional connection between the contractile apparatus, the subsarcolemma cystoskeleton, and the nucleus (29, 84, 86). The loss of desmin in humans results in a myopathy. Similarly, in mice lacking desmin, there are alterations in muscle structure (i.e., irregular myofiber organization and Z-line streaming), which are accompanied by reduced strength and increased muscle fatigue (48, 52). Much like other components of the lateral force transmission system, desmin-null mice are more susceptible to an absolute load-induced muscle injury (48). More recent work has suggested another consequence of the altered lateral force transmission in the desmin knockout mice: altered nuclear deformation during loading. Palmisano et al. (84) used confocal imaging of single muscle fibers with and without desmin to directly measure changes in muscle structure. In the absence of desmin, as fibers were forcibly lengthened the Z-lines became irregular and the overlying nuclei showed no deformation. In contrast, nuclei in wild-type muscle fibers increased their length/width ratio to match the increased sarcomere length induced by the stretch. Using a mathematical model, the authors suggested that the role for desmin in force transmission was dependent on its localization within the fiber. When desmin was localized within the fiber or distributed across the fiber, there was no effect on force transfer. However, if desmin was localized in the subsarcolemmal region, small changes in desmin led to large improvements in force transfer. This study highlights the role of desmin in force transfer and its fundamental role in mechanical sensing within skeletal muscle.
Synemin.
Similar to desmin in its role, synemin is an intermediate filament protein (α-isoform, 210 kDa; β-isoform, 180 kDa) which has been suggested to provide potential links between the costameres and the contractile apparatus at the Z-disks (8, 31). In a study by Garcia-Pelagio et al. (31), this protein appears to act as a mechanical sensor, with synemin knockout mice displaying a reduction in force production (assessed by electrical stimulation) and mean fiber diameter. Additionally, the mice displayed an increase in necrotic fiber abundance and susceptibility to muscle injury following lengthening contractions. It is important to note that the loss in synemin did not result in alterations in the organization of the contractile apparatus or costameres. Overall, these observations highlight an inability of the muscle to respond to mechanical loads with the loss of synemin. However, there is limited evidence for the role of synemin toward force transmission in aging and disease (myofibrillar myopathies) (79). These recent studies on desmin (84) and synemin (31) point toward the importance of intermediate filament proteins in mechanical sensing and force transfer in skeletal muscle (Fig. 3).
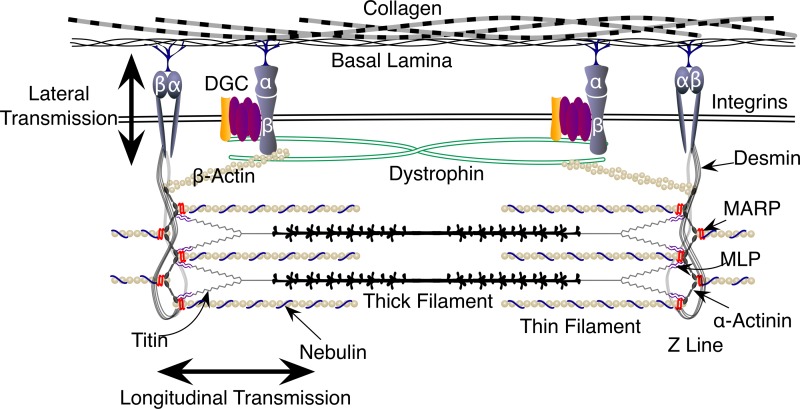
Fig. 3.
Schematic diagram of the various cytoskeleton proteins involved in lateral and longitudinal force transfer. The dystrophin-associated glycoprotein and integrin complexes are key components in lateral force transmission, enabling skeletal muscle to accommodate shear loads and protect the muscle from contraction-induced injury. Titin, nebulin, the MARP (muscle ankryin repeat proteins) family, and muscle LIM protein (MLP) are involved in longitudinal force transfer and are important in the rate of force development in the muscle. Desmin and α-actinin link the two modes of force transmission and therefore play a key role in both processes.
Future Directions
Our understanding of the role of various cytoskeleton proteins in both longitudinal and lateral force transmission has been generated by studying disease and exercise and by using animal (knockout) models and clinical human studies. Recent evidence has brought attention to the important role of force-transmitting proteins in aging and alterations in muscle force production and injury (90, 92). The limited evidence surrounding aging and force transmission is especially pertinent given two factors. First, with advancing age there is a rapid decline in muscle strength and mass (63, 64). Second, the chronic prevalence of muscle injury can lead to uncontrolled tissue damage, repair, and eventually the substitution of myofibers with nonfunctional fibrotic tissues, as observed in severe muscular dystrophies (57, 97). This last factor may play a critical role in age-related muscle loss. To further enhance our knowledge, studies will need to investigate the roles of the various proteins highlighted (e.g., dystrophin, desmin, nebulin) in relation to aging, but also develop experimental techniques similar to those developed by Ramaswamy and colleagues (90) (e.g., yoke apparatus/exercise models), to investigate the impact of lateral force transmission on the aging process.
Summary
The ability of skeletal muscle to produce and transfer force longitudinally relates to the hexagonal structure of the thick and thin filaments within the sarcomere and the stiffness of these structures along their length. Central to longitudinal force transmission are the titin and nebulin molecules that form the backbone of the thick and thin filaments respectively and their connections with α-actinin within the Z-line. The stiffer and stronger these proteins are, the more force a muscle can produce and the faster that force can be developed. Therefore, a key measurement for determining a longitudinal force-transmitting protein is the rate of force development. The functional integrity between the sarcomere and extracellular matrix is also important in force transfer (laterally), with various cytoskeleton proteins and laminin receptors playing a role. The proteins involved in lateral force transmission (dystrophin, α7β1-integrins, and desmin) are essential to the health of individual muscle fibers. Without these proteins, muscle is more susceptible to injury, particularly at the sarcolemma; therefore, proteins mutated in muscular dystrophies and lost during aging are often involved in lateral force transmission. Over the past decade, our understanding of the roles of cytoskeleton proteins has improved, and we have begun to identify how these proteins might be involved in longitudinal vs. lateral force transmission. Force transmission is fundamental to muscle function, maintaining sarcolemmal integrity, and protection from contraction-induced muscle injury. There is, however, limited research on how force transmission proteins respond to exercise and how much they contribute to the loss of strength and increased susceptibility to contraction-induced muscle injury during aging (13, 18, 50). A better understanding of the components within muscle that transfer force will aid in reducing muscle injury and maintaining muscle strength, resulting in better quality of life for the elderly.
GRANTS
This work was supported by a project grant from the National Institute on Aging of the National Institutes of Health under award number R01 AG-045375.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.C.H., M.A.W., and K.B. prepared figures; D.C.H., M.A.W., and K.B. drafted manuscript; D.C.H., M.A.W., and K.B. edited and revised manuscript; D.C.H., M.A.W., and K.B. approved final version of manuscript.
ACKNOWLEDGMENTS
Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Arber S, Halder G, Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell 79: 221–231, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Bang ML, Li X, Littlefield R, Bremner S, Thor A, Knowlton KU, Lieber RL, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J Cell Biol 173: 905–916, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barash IA, Bang ML, Mathew L, Greaser ML, Chen J, Lieber RL. Structural and regulatory roles of muscle ankyrin repeat protein family in skeletal muscle. Am J Physiol Cell Physiol 293: C218–C227, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barash IA, Mathew L, Lahey M, Greaser ML, Lieber RL. Muscle LIM protein plays both structural and functional roles in skeletal muscle. Am J Physiol Cell Physiol 289: C1312–C1320, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol 286: C355–C364, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Barton ER. Impact of sarcoglycan complex on mechanical signal transduction in murine skeletal muscle. Am J Physiol Cell Physiol 290: C411–C419, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Beggs AH, Byers TJ, Knoll J, Boyce FM, Bruns G, Kunkel LM. Cloning and characterization of two human skeletal muscle alpha-actinin genes located on chromosomes 1 and 11. J Biol Chem 267: 9281–9288, 1992. [PubMed] [Google Scholar]
- 8.Bellin RM, Huiatt TW, Critchley DR, Robson RM. Synemin may function to directly link muscle cell intermediate filaments to both myofibrillar Z-lines and costameres. J Biol Chem 276: 32330–32337, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Bloch RJ, Gonzalez-Serratos H. Lateral force transmission across costameres in skeletal muscle. Exerc Sport Sci Rev 31: 73–78, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Bloch RJ, Reed P, O'Neill A, Strong J, Williams M, Porter N, Gonzalez-Serratos H. Costameres mediate force transduction in healthy skeletal muscle and are altered in muscular dystrophies. J Muscle Res Cell Motil 25: 590–592, 2004. [PubMed] [Google Scholar]
- 11.Boppart MD, Burkin DJ, Kaufman SJ. α7β1-Integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol Cell Physiol 290: C1660–C1665, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Boppart MD, Volker SE, Alexander N, Burkin DJ, Kaufman SJ. Exercise promotes alpha7 integrin gene transcription and protection of skeletal muscle. Am J Physiol Regul Integr Comp Physiol 295: R1623–R1630, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol Cell Physiol 258: C436–C442, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Carson JA, Nettleton D, Reecy JM. Differential gene expression in the rat soleus muscle during early work overload-induced hypertrophy. FASEB J 16: 207–209, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Chan S, Seto JT, MacArthur DG, Yang N, North K, Head S. A gene for speed: contractile properties of isolated whole EDL muscle from an α-actinin-3 knockout mouse. Am J Physiol Cell Physiol 295: C897–C904, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Chandra M, Mamidi R, Ford S, Hidalgo C, Witt C, Ottenheijm C, Labeit S, Granzier H. Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. J Biol Chem 284: 30889–30896, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YW, Nader GA, Baar KR, Fedele MJ, Hoffman EP, Esser KA. Response of rat muscle to acute resistance exercise defined by transcriptional and translational profiling. J Physiol 545: 27–41, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi SJ, Lim JY, Nibaldi EG, Phillips EM, Frontera WR, Fielding RA, Widrick JJ. Eccentric contraction-induced injury to type I, IIa, and IIa/IIx muscle fibers of elderly adults. Age 34: 215–226, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarkson PM, Devaney JM, Gordish-Dressman H, Thompson PD, Hubal MJ, Urso M, Price TB, Angelopoulos TJ, Gordon PM, Moyna NM. ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. J Appl Physiol 99: 154–163, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Consolino CM, Duclos F, Lee J, Williamson RA, Campbell KP, Brooks SV. Muscles of mice deficient in α-sarcoglycan maintain large masses and near control force values throughout the life span. Physiol Genomics 22: 244–256, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Constantin B, Sebille S, Cognard C. New insights in the regulation of calcium transfers by muscle dystrophin-based cytoskeleton: implications in DMD. J Muscle Res Cell Motil 27: 375–386, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Danowski BA, Imanaka-Yoshida K, Sanger JM, Sanger JW. Costameres are sites of force transmission to the substratum in adult rat cardiomyocytes. J Cell Biol 118: 1411–1420, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol 48: 851–858, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil 22: 467–475, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Ecarnot-Laubriet A, De Luca K, Vandroux D, Moisant M, Bernard C, Assem M, Rochette L, Teyssier JR. Downregulation and nuclear relocation of MLP during the progression of right ventricular hypertrophy induced by chronic pressure overload. J Mol Cell Cardiol 32: 2385–2395, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Egan B, Zierath Juleen R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Farman GP, Allen EJ, Schoenfelt KQ, Backx PH, de Tombe PP. The role of thin filament cooperativity in cardiac length-dependent calcium activation. Biophys J 3, 99: 2978–2986, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fry AC, Staron RS, James CBL, Hikida RS, Hagerman FC. Differential titin isoform expression in human skeletal muscle. Acta Physiol Scand 161: 473–479, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, disease. Annu Rev Biochem 63: 345–382, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Pelagio KP, Bloch RJ, Ortega A, Gonzalez-Serratos H. Biomechanics of the sarcolemma and costameres in single skeletal muscle fibers from normal and dystrophin-null mice. J Muscle Res Cell Motil 31: 323–336, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Pelagio KP, Muriel J, Desmond PF, Lovering RM, Lund L, Bond M, Bloch RJ. Myopathic changes in murine skeletal muscle lacking synemin. Am J Physiol Cell Physiol 308: C448–C462, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gokhin DS, Bang ML, Zhang J, Chen J, Lieber RL. Reduced thin filament length in nebulin-knockout skeletal muscle alters isometric contractile properties. Am J Physiol Cell Physiol 296: C1123–C1132, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greaser ML, Krzesinski PR, Warren CM, Kirkpatrick B, Campbell KS, Moss RL. Developmental changes in rat cardiac titin/connectin: transitions in normal animals and in mutants with a delayed pattern of isoform transition. J Muscle Res Cell Motil 26: 325–332, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Greaser ML, Pleitner JM. Titin isoform size is not correlated with thin filament length in rat skeletal muscle. Front Physiol 5: 35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hack AA, Ly CT, Jiang F, Clendenin CJ, Sigrist KS, Wollmann RL, McNally EM. γ-Sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J Cell Biol 142: 1279–1287, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herzog W, Duvall M, Leonard TR. Molecular mechanisms of muscle force regulation: a role for titin? Exerc Sport Sci Rev 40: 50–57, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Hodges B, Hayashi Y, Nonaka I, Wang W, Arahata K, Kaufman S. Altered expression of the alpha7beta1 integrin in human and murine muscular dystrophies. J Cell Sci 110: 2873–2881, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech 32: 329–345, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Huxley AF, Niedergerke R. Structural changes in muscle during contraction. Nature 173: 971–973, 1954. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda K, Emoto N, Matsuo M, Yokoyama M. Molecular identification and characterization of a novel nuclear protein whose expression is up-regulated in insulin-resistant animals. J Biol Chem 278: 3514–3520, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Judson RN, Wackerhage H, Hughes A, Mavroeidi A, Barr RJ, Macdonald HM, Ratkevicius A, Reid DM, Hocking LJ. The functional ACTN3 577X variant increases the risk of falling in older females: results from two large independent cohort studies. J Gerontol Series A Biol Sci Med Sci 66: 130–135, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Kojic S, Radojkovic D, Faulkner G. Muscle ankyrin repeat proteins: their role in striated muscle function in health and disease. Crit Rev Clin Lab Sci 48: 269–294, 2011. [DOI] [PubMed] [Google Scholar]
- 43.Kong Y, Flick MJ, Kudla AJ, Konieczny SF. Muscle LIM protein promotes myogenesis by enhancing the activity of MyoD. Mol Cell Biol 17: 4750–4760, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labeit D, Watanabe K, Witt C, Fujita H, Wu Y, Lahmers S, Funck T, Labeit S, Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc Natl Acad Sci USA 100: 13716–13721, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270: 293–296, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Labeit S, Ottenheijm CA, Granzier H. Nebulin, a major player in muscle health and disease. FASEB J 25: 822–829, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Resp Env Exerc Physiol 46: 451–456, 1979. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, Mericskay M, Agbulut O, Butler-Browne G, Carlsson L, Thornell LE, Babinet C, Paulin D. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J Cell Biol 139: 129–144, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Milner DJ, Boppart MD, Ross RS, Kaufman SJ. beta1D chain increases alpha7beta1 integrin and laminin and protects against sarcolemmal damage in mdx mice. Hum Mol Genet 21: 1592–1603, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lovering RM, Brooks SV. Eccentric exercise in aging and diseased skeletal muscle: good or bad? J Appl Physiol 116: 1439–1445, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lovering RM, De Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol 286: C230–C238, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lovering RM, O'Neill A, Muriel JM, Prosser BL, Strong J, Bloch RJ. Physiology, structure, and susceptibility to injury of skeletal muscle in mice lacking keratin 19-based and desmin-based intermediate filaments. Am J Physiol Cell Physiol 300: C803–C813, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lueders TN, Zou K, Huntsman HD, Meador B, Mahmassani Z, Abel M, Valero MC, Huey KA, Boppart MD. The α7β1-integrin accelerates fiber hypertrophy and myogenesis following a single bout of eccentric exercise. Am J Physiol Cell Physiol 301: C938–C946, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 535: 591–600, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA. Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice. Am J Physiol Cell Physiol 279: C1290–C1294, 2000. [DOI] [PubMed] [Google Scholar]
- 56.MacArthur DG, Seto JT, Chan S, Quinlan KG, Raftery JM, Turner N, Nicholson MD, Kee AJ, Hardeman EC, Gunning PW. An Actn3 knockout mouse provides mechanistic insights into the association between α-actinin-3 deficiency and human athletic performance. Hum Mol Genet 17: 1076–1086, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Mann CJ, Perdiguero E, Kharraz Y, Aguilar S, Pessina P, Serrano AL, Muñoz-Cánoves P. Aberrant repair and fibrosis development in skeletal muscle. Skelet Muscle 1: 21–21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marttila M, Hanif M, Lemola E, Nowak KJ, Laitila J, Grönholm M, Wallgren-Pettersson C, Pelin K. Nebulin interactions with actin and tropomyosin are altered by disease-causing mutations. Skelet Muscle 4: 15, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mateja RD, Greaser ML, de Tombe PP. Impact of titin isoform on length dependent activation and cross-bridge cycling kinetics in rat skeletal muscle. Biochim Biophys Acta 1833: 804–811, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matthews GD, Huang CL, Sun L, Zaidi M. Translational musculoskeletal science: is sarcopenia the next clinical target after osteoporosis? Ann NY Acad Sci 1237: 95–105, 2011. [DOI] [PubMed] [Google Scholar]
- 61.Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem 278: 14587–14590, 2003. [DOI] [PubMed] [Google Scholar]
- 62.McGuigan MR, Sharman MJ, Newton RU, Davie AJ, Murphy AJ, McBride JM. Effect of explosive resistance training on titin and myosin heavy chain isoforms in trained subjects. J Strength Cond Res 17: 645–651, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol Series A Biol Sci Med Sci 57: B359–B365, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 3: 260, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moens P, Baatsen P, Maréchal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil 14: 446–451, 1993. [DOI] [PubMed] [Google Scholar]
- 66.Monti RJ, Roy RR, Hodgson JA, Reggie Edgerton V. Transmission of forces within mammalian skeletal muscles. J Biomech 32: 371–380, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Moran CN, Yang N, Bailey ME, Tsiokanos A, Jamurtas A, MacArthur DG, North K, Pitsiladis YP, Wilson RH. Association analysis of the ACTN3 R577X polymorphism and complex quantitative body composition and performance phenotypes in adolescent Greeks. Eur J Hum Genet 15: 88–93, 2007. [DOI] [PubMed] [Google Scholar]
- 68.Morgan D. New insights into the behavior of muscle during active lengthening. Biophys J 57: 209, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moser H. Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum Genet 66: 17–40, 1984. [DOI] [PubMed] [Google Scholar]
- 70.Murray MP, Duthie EH Jr, Gambert SR, Sepic SB, Mollinger LA. Age-related differences in knee muscle strength in normal women. J Gerontol 40: 275–280, 1985. [DOI] [PubMed] [Google Scholar]
- 71.Murray MP, Gardner GM, Mollinger LA, Sepic SB. Strength of isometric and isokinetic contractions: knee muscles of men aged 20 to 86. Phys Ther 60: 412–419, 1980. [DOI] [PubMed] [Google Scholar]
- 72.Nakada C, Tsukamoto Y, Oka A, Nonaka I, Sato K, Mori S, Ito H, Moriyama M. Altered expression of ARPP protein in skeletal muscles of patients with muscular dystrophy, congenital myopathy and spinal muscular atrophy. Pathobiology 71: 43–51, 2004. [DOI] [PubMed] [Google Scholar]
- 73.Nakada C, Tsukamoto Y, Oka A, Nonaka I, Takeda S, Sato K, Mori S, Ito H, Moriyama M. Cardiac-restricted ankyrin-repeated protein is differentially induced in duchenne and congenital muscular dystrophy. Lab Invest 83: 711–719, 2003. [DOI] [PubMed] [Google Scholar]
- 74.Neagoe C, Opitz CA, Makarenko I, Linke WA. Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J Muscle Res Cell Motil 24: 175–189, 2003. [DOI] [PubMed] [Google Scholar]
- 75.Norman B, Esbjornsson M, Rundqvist H, Osterlund T, von Walden F, Tesch PA. Strength, power, fiber types, and mRNA expression in trained men and women with different ACTN3 R577X genotypes. J Appl Physiol 106: 959–965, 2009. [DOI] [PubMed] [Google Scholar]
- 76.North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, Beggs AH. A common nonsense mutation results in α-actinin-3 deficiency in the general population. Nat Genet 21: 353–354, 1999. [DOI] [PubMed] [Google Scholar]
- 77.Ochala J, Lehtokari VL, Iwamoto H, Li M, Feng HZ, Jin JP, Yagi N, Wallgren-Pettersson C, Pénisson-Besnier I, Larsson L. Disrupted myosin cross-bridge cycling kinetics triggers muscle weakness in nebulin-related myopathy. FASEB J 25: 1903–1913, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogut O, Hossain MM, Jin JP. Interactions between nebulin-like motifs and thin filament regulatory proteins. J Biol Chem 278: 3089–3097, 2003. [DOI] [PubMed] [Google Scholar]
- 79.Olive M, Goldfarb L, Dagvadorj A, Sambuughin N, Paulin D, Li Z, Goudeau B, Vicart P, Ferrer I. Expression of the intermediate filament protein synemin in myofibrillar myopathies and other muscle diseases. Acta Neuropathol (Berl) 106: 1–7, 2003. [DOI] [PubMed] [Google Scholar]
- 80.Ottenheijm CA, Fong C, Vangheluwe P, Wuytack F, Babu GJ, Periasamy M, Witt CC, Labeit S, Granzier H. Sarcoplasmic reticulum calcium uptake and speed of relaxation are depressed in nebulin-free skeletal muscle. FASEB J 22: 2912–2919, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ottenheijm CA, Granzier H. Lifting the nebula: novel insights into skeletal muscle contractility. Physiology 25: 304–310, 2010. [DOI] [PubMed] [Google Scholar]
- 82.Ottenheijm CA, Granzier H, Labeit S. The sarcomeric protein nebulin: another multifunctional giant in charge of muscle strength optimization. Front Physiol 3: 37, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ottenheijm CA, Witt CC, Stienen GJ, Labeit S, Beggs AH, Granzier H. Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum Mol Genet 18: 2359–2369, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palmisano MG, Bremner SN, Hornberger TA, Meyer GA, Domenighetti AA, Shah SB, Kiss B, Kellermayer M, Ryan AF, Lieber RL. Skeletal muscle intermediate filaments form a stress-transmitting and stress-signaling network. J Cell Sci 128: 219–224, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pardo JV, Siliciano J, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA 80: 1008–1012, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res 301: 1–7, 2004. [DOI] [PubMed] [Google Scholar]
- 87.Pelin K, Hilpelä P, Donner K, Sewry C, Akkari PA, Wilton SD, Wattanasirichaigoon D, Bang ML, Centner T, Hanefeld F. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA 96: 2305–2310, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pomiès P, Louis HA, Beckerle MC. CRP1, a LIM domain protein implicated in muscle differentiation, interacts with α-actinin. J Cell Biol 139: 157–168, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prins KW, Humston JL, Mehta A, Tate V, Ralston E, Ervasti JM. Dystrophin is a microtubule-associated protein. J Cell Biol 186: 363–369, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramaswamy KS, Palmer ML, van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J Physiol 589: 1195–1208, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rassier DE, Leite FS, Nocella M, Cornachione AS, Colombini B, Bagni MA. Non-crossbridge forces in activated striated muscles: a titin dependent mechanism of regulation? J Muscle Res Cell Motil 36: 37–45, 2015. [DOI] [PubMed] [Google Scholar]
- 92.Rice KM, Preston DL, Neff D, Norton M, Blough ER. Age-related dystrophin-glycoprotein complex structure and function in the rat extensor digitorum longus and soleus muscle. J Gerontol Series A Biol Sci Med Sci 61: 1119–1129, 2006. [DOI] [PubMed] [Google Scholar]
- 93.Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and α7 integrin. J Cell Sci 119: 2185–2195, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Roth SM, Walsh S, Liu D, Metter EJ, Ferrucci L, Hurley BF. The ACTN3 R577X nonsense allele is under-represented in elite-level strength athletes. Eur J Hum Genet 16: 391–394, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rybakova IN, Patel JR, Ervasti JM. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J Cell Biol 150: 1209–1214, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schneider AG, Sultan KR, Pette D. Muscle LIM protein: expressed in slow muscle and induced in fast muscle by enhanced contractile activity. Am J Physiol Cell Physiol 276: C900–C906, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Serrano AL, Muñoz-Cánoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316: 3050–3058, 2010. [DOI] [PubMed] [Google Scholar]
- 98.Seto JT, Chan S, Turner N, MacArthur DG, Raftery JM, Berman YD, Quinlan KG, Cooney GJ, Head S, Yang N. The effect of α-actinin-3 deficiency on muscle aging. Exp Gerontol 46: 292–302, 2011. [DOI] [PubMed] [Google Scholar]
- 99.Seto JT, Lek M, Quinlan KG, Houweling PJ, Zheng XF, Garton F, MacArthur DG, Raftery JM, Garvey SM, Hauser MA, Yang N, Head SI, North KN. Deficiency of alpha-actinin-3 is associated with increased susceptibility to contraction-induced damage and skeletal muscle remodeling. Hum Mol Genet 20: 2914–2927, 2011. [DOI] [PubMed] [Google Scholar]
- 100.Seto JT, Quinlan KG, Lek M, Zheng XF, Garton F, MacArthur DG, Hogarth MW, Houweling PJ, Gregorevic P, Turner N, Cooney GJ, Yang N, North KN. ACTN3 genotype influences muscle performance through the regulation of calcineurin signaling. J Clin Invest 123: 4255–4263, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sjöblom B, Salmazo A, Djinović-Carugo K. α-Actinin structure and regulation. Cell Mol Life Sci 65: 2688–2701, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol 114: 346–364, 1983. [DOI] [PubMed] [Google Scholar]
- 103.Tatsumi R, Maeda K, Hattori A, Takahashi K. Calcium binding to an elastic portion of connectin/titin filaments. J Muscle Res Cell Motil 22: 149–162, 2001. [DOI] [PubMed] [Google Scholar]
- 104.Trappe TA, Carrithers JA, White F, Lambert CP, Evans WJ, Dennis RA. Titin and nebulin content in human skeletal muscle following eccentric resistance exercise. Muscle Nerve 25: 289–292, 2002. [DOI] [PubMed] [Google Scholar]
- 105.Tsukamoto Y, Senda T, Nakano T, Nakada C, Hida T, Ishiguro N, Kondo G, Baba T, Sato K, Osaki M. Arpp, a new homolog of carp, is preferentially expressed in type 1 skeletal muscle fibers and is markedly induced by denervation. Lab Invest 82: 645–655, 2002. [DOI] [PubMed] [Google Scholar]
- 106.Udaka J, Ohmori S, Terui T, Ohtsuki I, Ishiwata Si Kurihara S, Fukuda N. Disuse-induced preferential loss of the giant protein titin depresses muscle performance via abnormal sarcomeric organization. J Gen Physiol 131: 33–41, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vainzof M, Costa CS, Marie SK, Moreira ES, Reed U, Passos-Bueno MR, Beggs AH, Zatz M. Deficiency of alpha-actinin-3 (ACTN3) occurs in different forms of muscular dystrophy. Neuropediatrics 28: 223–228, 1997. [DOI] [PubMed] [Google Scholar]
- 108.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res 305: 285–298, 2001. [DOI] [PubMed] [Google Scholar]
- 109.Vincent B, De Bock K, Ramaekers M, Van den Eede E, Van Leemputte M, Hespel P, Thomis MA. ACTN3 (R577X) genotype is associated with fiber type distribution. Physiol Genomics 32: 58–63, 2007. [DOI] [PubMed] [Google Scholar]
- 110.Vincent B, Windelinckx A, Nielens H, Ramaekers M, Van Leemputte M, Hespel P, Thomis MA. Protective role of α-actinin-3 in the response to an acute eccentric exercise bout. J Appl Physiol 109: 564–573, 2010. [DOI] [PubMed] [Google Scholar]
- 111.Walsh S, Liu D, Metter EJ, Ferrucci L, Roth SM. ACTN3 genotype is associated with muscle phenotypes in women across the adult age span. J Appl Physiol 105: 1486–1491, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang K, Wright J. Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J Cell Biol 107: 2199–2212, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weir AP, Burton EA, Harrod G, Davies KE. A-and B-utrophin have different expression patterns and are differentially up-regulated in mdx muscle. J Biol Chem 277: 45285–45290, 2002. [DOI] [PubMed] [Google Scholar]
- 114.Welser JV, Rooney JE, Cohen NC, Gurpur PB, Singer CA, Evans RA, Haines BA, Burkin DJ. Myotendinous junction defects and reduced force transmission in mice that lack α7 integrin and utrophin. Am J Pathol 175: 1545–1554, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Willmann R, Kusch J, Sultan KR, Schneider AG, Pette D. Muscle LIM protein is upregulated in fast skeletal muscle during transition toward slower phenotypes. Am J Physiol Cell Physiol 280: C273–C279, 2001. [DOI] [PubMed] [Google Scholar]
- 116.Witt CC, Burkart C, Labeit D, McNabb M, Wu Y, Granzier H, Labeit S. Nebulin regulates thin filament length, contractility, and Z-disk structure in vivo. EMBO J 25: 3843–3855, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Worton R. Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science 270: 755–756, 1995. [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto DL, Vitiello C, Zhang J, Gokhin DS, Castaldi A, Coulis G, Piaser F, Filomena MC, Eggenhuizen PJ, Kunderfranco P. The nebulin SH3 domain is dispensable for normal skeletal muscle structure but is required for effective active load bearing in mouse. J Cell Sci 126: 5477–5489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, North K. ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet 73: 627–631, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang C, Gao Y. Effects of aging on the lateral transmission of force in rat skeletal muscle. J Biomech 47: 944–948, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, Boppart MD. The alpha(7)beta(1)-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol 111: 1134–1141, 2011. [DOI] [PubMed] [Google Scholar]