Abstract
In polycystic kidney disease (PKD), the rate of cyst formation and disease progression is highly variable. The lack of predictability in disease progression may be due to additional environmental factors or pathophysiological processes called “third hits.” Diabetes is a growing epidemic, and recent studies suggest that PKD patients may be at an increased risk for this disease. We sought to determine if hyperglycemia enhances the initiation and rate of cystogenesis. Tamoxifen was administered to adult Ift88 conditional floxed allele mice to induce cilia loss in the presence of Cre. Subsequent administration of streptozotocin resulted in equivalent hyperglycemia in cilia+ and cilia− mice. Hyperglycemia with loss of cilia increased the rate of cyst formation and cell proliferation. Structural and functional alterations in the kidney, including focal glomerular foot process effacement, interstitial inflammation, formation of primitive renal tubules, polyuria, and increased proteinuria, were also observed in hyperglycemic cilia− mice. Gene array analysis indicated enhanced Wnt and epithelial-to-mesenchymal transition signaling in the kidney of hyperglycemic cilia− mice. These data show that hyperglycemia, in the absence of cilia, results in renal structural and functional damage and accelerates cystogenesis, suggesting that diabetes is a risk factor in the progression of PKD.
Keywords: polycystic kidney disease, diabetes, streptozotocin, Wnt, epithelial-to-mesenchymal transition
polycystic kidney disease (PKD) is a relatively common inherited genetic disorder that ultimately leads to a high rate of renal failure. In human autosomal-dominant or autosomal-recessive PKD, one copy of the gene pkd1, pkd2, or pkhd1 is mutated at conception. It is thought that, over time, a second somatic mutation, referred to as the “second hit,” occurs in a small number of renal epithelial cells and results in the development of renal cysts (25, 39). Recently, the concept of a “third hit” has emerged; the third-hit hypothesis suggests that additional elements, such as environmental factors or pathophysiological conditions, can contribute to the rate of cyst initiation and progression (12, 21, 37). This hypothesis helps explain the wide variations in disease progression, especially within affected families.
It is well established that PKD is a ciliopathic disorder. Nearly all the protein products of the genes involved in human cyst formation are located within the cilium-centrosome complex (36). In addition, these proteins are necessary for proper cilia function. Germline deletion of cystogenes or cilia [through inhibition of intraflagellar transport (IFT)] is usually embryonic-lethal. Establishment of the conditional floxed allele mouse, in which the cystoproteins or IFT proteins can be deleted at any time, was crucial to the advent of the third-hit theory. While deletion of the cystoproteins/IFT proteins before postnatal day 12 results in severe developmental abnormalities and widespread cysts, deletion of these proteins in the adult mouse results in slow-onset cystogenesis, with no significant cystic burden detected for ≥6 mo (9, 21, 27, 29).
This delay in cystogenesis provides a model that can be used to study additional factors and conditions that alter the initiation and rate of cystogenesis. This model has been used to identify ischemia-reperfusion injury, nephrotoxic kidney injury, and hypertrophic signaling due to a reduction in renal mass (unilateral nephrectomy) as third-hit events that promote cystogenesis (2, 3, 12, 27, 37). The common finding in these models is that the perturbation, in the absence of functional cilia or cystoproteins, leads to accelerated cystogenesis. Other findings include increased cell proliferation, inflammation, and renal damage, as well as alterations in cell signaling pathways, including mammalian target of rapamycin and Wnt. Additionally, reduction of renal mass following loss of cilia resulted in an elevation of functional and structural hypertrophy. These findings suggest that the absence of cilia or cystoproteins alters cell signaling pathways that are involved in renal repair and hypertrophic signaling. Another common pathophysiological condition that leads to renal damage and hypertrophic signaling is hyperglycemia; however, there is little evidence that elevated extracellular glucose levels influence the rate of cystogenesis in PKD.
Recently, Reed et al. (31) compared the clinical characteristics of autosomal-dominant PKD (ADPKD) patients with type 2 diabetes with those of matched ADPKD controls. The primary finding was that renal volumes in ADPKD patients with type 2 diabetes were double those in ADPKD controls, suggesting that diabetes might lead to an increase in cystic burden. The epidemic levels of diabetes and the complex altered cell signaling events that occur in this disease are compelling reasons to determine if there is an interaction between hyperglycemia and PKD on the rate of cystogenesis as well as on the pathogenic response to hyperglycemia.
METHODS
Animals.
Maintenance of nonmotile cilia is critically dependent on a functioning IFT system. Thus, knockout of a critical IFT component leads to loss of cilia within several days. Development of Ift88 floxed allele mice has been previously reported (9, 14). Briefly, the Ift88flox/flox mouse has LoxP sites flanking exons 4–6 of Ift88. Ift88 conditional knockout mice were generated by cross-breeding Ift88flox/flox female mice with male mice that express the tamoxifen-inducible systemic Cre with actin promoter (CAGGCre-ERTM) (13). Genotyping was performed as described elsewhere (14), and mice were randomly assigned to groups. The male-to-female ratio was comparable across groups. For induction of Cre, tamoxifen was administered once a day for 5 consecutive days when male and female mice were ∼8 wk of age. Tamoxifen (Sigma, St. Louis, MO) was dissolved in corn oil (Sigma; 10 mg/ml) and administered intraperitoneally (0.5 ml) to Cre+ and Cre− mice. At 1 wk following tamoxifen injection, mice were rendered diabetic using the low-dose streptozotocin (STZ) induction protocol described by the Animal Models of Diabetic Complications Consortium (1). Briefly, STZ was administered intraperitoneally (50 mg/kg in 0.1 mmol/l sodium citrate buffer, pH 4.5) once a day for 5 consecutive days. Mice were fasted for 4 h prior to injection. Blood glucose levels from fasted mice were measured periodically beginning 1 wk after STZ injection using the OneTouch Ultra 2 glucometer (LifeScan, Milpitas, CA). Mice with a blood glucose level ≥200 mg/dl were considered diabetic. At 6 wk or 3 mo post-STZ, mice were euthanized by isoflurane overdose followed by aortic transection. Tissues were removed and preserved in 10% buffered formalin or flash-frozen. All mice were maintained in accordance with regulations of the Medical University of South Carolina Animal Care and Use Committee, which approved the study.
Western blot analysis.
Kidney tissue was homogenized and proteins were extracted using RIPA buffer with protease and phosphatase inhibitors (Thermo Scientific, Waltham, MA). Samples (40–50 μg of protein) were reduced with tris(2-carboxyethyl)phosphine (Thermo Scientific), and proteins were separated by SDS-PAGE on TGX gels (Bio-Rad, Hercules, CA). Proteins were transferred to nitrocellulose and immunoblotted with primary antibodies to Wnt10a (Abcam, Cambridge, MA) and GAPDH (Millipore, Billerica, MA). All membranes were blocked in 5% BSA. Immunoblots were visualized using enhanced chemiluminescence (ECL Plus, GE Healthcare, Piscataway, NJ) on film.
Immunohistochemistry.
Sections (5 μm) were cut from paraffin-embedded kidneys and stained using an immunoperoxidase method (Vectastain Elite ABC System, Vector Labs, Burlingame, CA) for Wnt10a or β-catenin (Abcam) according to protocol. Sections were stained with ImmPACT diaminobenzidine (Vector Labs) and counterstained with hematoxylin. Sections incubated without primary antibody were used as a negative control. Slides were imaged using a Leica DMI 4000B microscope (Leica Microsystems, Bannockburn, IL).
Quantitative PCR.
Kidney tissue was homogenized, and RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. RNA concentration was determined using a NanoDrop 2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). RNA (1 μg) was converted to cDNA using the RT2 First-Strand cDNA kit (Qiagen) and stored at −20°C until use. For pathway analysis, three plates per group of the Mouse WNT Signaling Pathway PCR Array (PAMM-043A, Qiagen) and Mouse EMT PCR Array (PAMM-090A, Qiagen) were used according to the manufacturer's protocol. Briefly, cDNA was mixed with the RT2 SYBR Green quantitative PCR master mix, and 25 μl were added to each well containing different primers. The plates were sealed and spun for 1 min at 1,000 g before initiation of the quantitative PCR program with the following cycling conditions: 10 min at 95°C, 40 cycles of 15 s at 95°C, and 1 min at 60°C. A dissociation curve was constructed by ramping the temperature from 60°C to 95°C. Comparative threshold (CT) values were normalized against reference genes on the array. Values were considered significant at P ≤ 0.05 and a fold-change of ≥2.
Glomerular filtration rate.
Mice were anesthetized with isoflurane and placed on a heated table to maintain body temperature at 37°C. The right jugular vein was cannulated for infusion of replacement fluid (1% BSA in 0.9% NaCl) at 0.2 ml/h. The left femoral artery was cannulated, and blood pressure was measured with a Digi-Med blood pressure analyzer system (Micro Med, Louisville, KY). The urinary bladder was cannulated to allow collection of urine. Animals were given a 0.2-ml bolus of [14C]inulin (9 μCi in 1% BSA in 0.9% NaCl) followed by an infusion at 0.2 ml/h. After 60 min of equilibration, inulin clearance was measured over two 30- to 40-min periods.
Arterial blood (∼80 μl) was collected at the midpoint of each urine collection. Urine volume was measured gravimetrically. Aliquots of urine and blood were counted in a Beckman LS6500 liquid scintillation counter, and the data were used to calculate inulin clearance.
Noninvasive blood pressure measurements.
Diastolic blood pressure, systolic blood pressure, and heart rate were directly determined by tail-cuff flow occlusion using the CODA system (Kent Scientific, Torrington, CT) in restrained, but nonanesthetized, mice.
Histological analysis.
For light microscopy, 5-μm sections were cut from paraffin-embedded kidneys and stained with hematoxylin-eosin, Masson's trichrome, periodic acid-Schiff (PAS), or Jones' methenamine silver. Sections were scored by an independent observer blinded to groups. Glomeruli, tubules, vessels, and degree of inflammation were scored on a scale of 0–3. At least 20 blinded morphometric measurements were obtained per mouse. For bromodeoxyuridine (BrdU) staining, mice were injected intraperitoneally with BrdU 2 h before euthanization. BrdU staining was performed using a BrdU staining kit (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. A single observer blinded to groups counted BrdU-positive cells. BrdU-positive cells in 10 fields of view (×40 magnification) were counted per slide (n = 3 per group).
Transmission electron microscopy.
Kidney tissue was fixed in 2.5% each formaldehyde and glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4 (Electron Microscopy Sciences, Hatfield, PA), overnight at room temperature. Samples were rinsed several times with 0.1 M sodium cacodylate buffer, pH 7.4, and then postfixed for 1 h with 1% osmium tetroxide in cacodylate buffer. Tissue was rinsed and dehydrated through a series of graded ethanol and 100% propylene oxide, infiltrated with a 1:1 mixture of 100% propylene oxide and embedding resin (Embed 812, Electron Microscopy Sciences), and embedded and polymerized in 100% resin. Thick (1-μm) sections were stained with toluidine blue, and thin (70- to 90-nm) sections were mounted on copper grids and stained with uranyl acetate and lead citrate. Thin sections were examined with an electron microscope (Tecnai Spirit, FEI, Hillsboro, OR) operated at 120 kV. Digital images were obtained with a CCD camera (Advanced Microscopy Techniques).
Urinalysis.
Mice were individually housed in metabolic cages with free access to food and water. After 24–48 h of acclimation to metabolic cages, a 24-h urine collection period was initiated. Urine was collected on ice and centrifuged at 1,000 g for 10 min at 4°C. Urine volume was determined and osmolality was measured using a vapor pressure osmometer (Vapro 5520, Wescor, Logan, UT). Creatinine and albumin were measured using the QuantiChrom assay kit (BioAssay Systems, Hayward, CA), and urinary glucose, protein, creatinine, ketone, specific gravity, pH, blood, nitrite, and leukocytes were measured with a Clinitek Status+ Analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY). Multistix PRO 10LS dipsticks (Siemens Healthcare Diagnostics) were used for urinalysis. For absolute protein measurements, ratios of absorbance at 260 nm to absorbance at 280 nm (A260/A280) were obtained using a spectrophotometer (Nanodrop 2000, Thermo Fisher Scientific, Wilmington, DE). Absolute glucose was determined with the OneTouch Ultra 2 glucometer (LifeScan) and verified with the CLINITEK Status+ Analyzer range values. The OneTouch Ultra 2 glucometer has a maximum readout of 600 mg/dl; therefore, any values >600 mg/dl were reported as 600 mg/dl.
Statistical analysis.
Values are means ± SE; statistical significance of differences in mean values was assessed by Student's t-test or ANOVA with Tukey's post hoc test, as appropriate. Differences between means were considered significant at P < 0.05.
RESULTS
Renal function and systemic hemodynamics.
To determine if hyperglycemia augments cystogenesis and renal damage, we used the Ift88 conditional floxed murine model in which deletion of cilia after postnatal day 12 does not lead to significant cyst formation for ≥6 mo. Mice are designated cilia+ for Cre− mice and cilia− for Cre+ mice, because tamoxifen in Cre+ mice resulted in >80% deletion of cilia (14). All mice received tamoxifen, and hyperglycemia was induced by injection of STZ, a model of type 1 diabetes. Mice with a fasting blood glucose ≥200 mg/dl were considered diabetic. Fourteen percent of the cilia+ mice and none of the cilia− mice failed to become hyperglycemic. Where appropriate, the results obtained in the current study are compared with normoglycemic two-kidney cilia+ and cilia− mice studied at 3 wk and 3 mo (3). As shown in Table 1, there were no differences between diabetic cilia+ and cilia− mice in terms of body weight, kidney weight, heart rate, or systolic and diastolic blood pressures at 6 wk or 3 mo. Additionally, there were no significant differences in these parameters between male and female mice (data not shown). Male cilia− mice had significantly higher blood glucose levels than female cilia− mice (P < 0.05, data not shown), but, overall, the presence of cilia had no significant effect on blood glucose levels of hyperglycemic mice (Table 1). Cilia− mice were polyuric compared with cilia+ mice; however, glomerular filtration rate (GFR) and arterial blood pressures were similar between the two groups (Table 2). Both groups exhibited proteinuria. The level of protein excretion was much greater in the cilia− than cilia+ mice, but the albumin-to-creatinine ratio was similar between the two groups (Table 2).
Table 1.
Characteristics of Ift88 mice with hyperglycemia
| Blood Pressure, mmHg |
|||||||
|---|---|---|---|---|---|---|---|
| Group | Body Wt, g | Kidney Wt, g | Kidney Wt/Body Wt | Plasma Glucose | Systolic | Diastolic | Heart Rate, beats/min |
| 6 wk | |||||||
| Cilia+ | 25.3 ± 1.6 (9) | 0.43 ± 0.03 (9) | 0.017 ± 0.001 (9) | 383.1 ± 49.1 (9) | 127.3 ± 9.3 (3) | 99.3 ± 9.0 (3) | 734.1 ± 18.2 (3) |
| Cilia− | 26.7 ± 3.0 (6) | 0.46 ± 0.06 (6) | 0.018 ± 0.002 (6) | 459.5 ± 60.4 (6) | 137.5 ± 14.0 (5) | 111.5 ± 14.0 (5) | 697.3 ± 23.0 (5) |
| 3 mo | |||||||
| Cilia+ | 23.1 ± 1.5 (5) | 0.45 ± 0.03 (5) | 0.020 ± 0.002 (5) | 331.8 ± 22.1 (5) | 111.3 ± 11.7 (3) | 75.3 ± 7.0 (3) | 720.4 ± 16.4 (3) |
| Cilia− | 26.6 ± 1.9 (9) | 0.46 ± 0.04 (9) | 0.018 ± 0.002 (9) | 386.9 ± 38.6 (9) | 122.2 ± 12.4 (5) | 92.6 ± 13.7 (5) | 673.5 ± 12.9 (5) |
Values are means ± SE of number of animals shown in parentheses.
Table 2.
Urinalysis and renal function data
| Group | Volume, ml/24 h | Glucose, mg/dl | Protein, mg/day | Albumin/Creatinine | GFR, μl·min−1•g kidney wt−1 |
|---|---|---|---|---|---|
| 6 wk | |||||
| Cilia+ | 1.3 ± 0.5 (4) | 275 ± 111 (4) | 96 ± 31 (4) | 897 ± 34 (5) | |
| Cilia− | 12.6 ± 1.6* (3) | 600 ± 1* (3) | 203 ± 75 (3) | 833 ± 45 (5) | |
| 3 mo | |||||
| Cilia+ | 1.0 ± 0.2 (4) | 116 ± 26 (4) | 70 ± 18 (4) | 1.35 ± 0.18 (4) | |
| Cilia− | 11.4 ± 4.2* (6) | 417 ± 85* (6) | 212 ± 50* (6) | 2.78 ± 0.76 (6) | |
Values are means ± SE of number of animals shown in parentheses. Urine volume, urine glucose, urine protein, and albumin-to-creatinine ratio were measured in 24-h urine collected in metabolic cages. Glomerular filtration rate (GFR) was measured in a separate group of anesthetized mice.
P < 0.05 vs. cilia+.
Renal histology and pathology.
Renal tissue was stained with hematoxylin-eosin, Masson's trichrome, PAS, and Jones' silver at 6 wk and 3 mo post-STZ (Fig. 1). Cilia+ sections at 6 wk and 3 mo appeared normal, with the absence of cystic structures and no inflammation or glomerular abnormalities. These results are similar to our previous findings for the normoglycemic cilia+ group at 3 wk and 3 mo (3) and are also consistent with reports that diabetic mice are relatively resistant to development of diabetic nephropathy (4, 5). Cilia− sections exhibited cysts in up to 15% of the renal parenchyma. As a direct comparison at 3 mo, the cystic burden in the hyperglycemic cilia− mice was greater than our previous finding of only sporadic cysts in normoglycemic cilia− mice. There was no evidence of overt fibrosis, as there were minimal differences in Masson's trichrome staining between the cilia+ and cilia− hyperglycemic mice. However, there was evidence for eosinophilic proteinaceous material in tubules. Jones' silver staining identified areas of increased glomerular mesangial matrix, thickened Bowman's capsule, and widened vascular walls immediately adjacent to the glomerulus. Although not significant, females trended toward worsened pathology.
Fig. 1.
Hyperglycemia induced renal cysts in cilia− mice. A and B: hematoxylin-eosin (H&E), trichrome, periodic acid-Schiff (PAS), and Jones' silver (Silver) staining of renal sections from cilia+ and cilia− mice at 6 wk (A) and 3 mo (B) after streptozotocin (STZ) injection. Magnification ×40.
Other abnormalities were present in renal sections from cilia− mice (Fig. 2). Focal interstitial inflammation at 3 mo was primarily associated with cystic regions and was more prominent in female mice (Fig. 2A). The most striking abnormality was the presence of immature or primitive renal tubules (Fig. 2B). Additionally, glycogenated nuclei were observed in cilia+ and cilia− sections (Fig. 2C), although correlation analysis indicated that the degree of glycogenated nuclei was directly related to plasma glucose level (r = 0.52, P = 0.01). A correlation analysis was used to determine if the severity of pathological changes was related to plasma glucose, but glucose did not correlate to pathology scores for cilia+ (r = 0.49, P = 0.10) or cilia− (r = −0.36, P = 0.22) mice. There were no differences in hypertrophic measurements of glomerular diameter or proximal or distal tubular cell heights between STZ-treated mice at 3 mo, although we did observe cellular hypertrophy (12–15%) of glomeruli and tubular epithelial cells (data not shown) compared with the nondiabetic controls reported in our previous work (3).
Fig. 2.
Odd pathological manifestations in hyperglycemic cilia− mice. A–C: hematoxylin-eosin-stained sections from mice at 3 mo post-STZ show interstitial inflammatory infiltrates (A), primitive renal tubules (arrows; B), and glycogenated nuclei (arrows; C). Magnification ×40. D and E: slides were reviewed by a blinded investigator, and pathology (glomeruli, tubules, vessels, and degree of inflammation) was scored on a scale of 0–3. D: cilia− mice at 3 mo post-STZ showed significantly worsened pathology than cilia+ STZ-treated mice. E: linear regression analysis revealed no significant correlation between plasma glucose and renal pathology score in cilia+ and cilia− mice at 6 wk or 3 mo post-STZ.
As a direct comparison with our previous work (3), normoglycemic cilia− mice presented with minimal cyst formation at 3 mo. There was little evidence of inflammation or pathological changes in the renal parenchyma, and silver-stained sections from these mice were normal. After induction of hyperglycemia by administration of STZ (current study), cystic burden increased along with tissue inflammation and pathological changes, as noted above.
Detailed cellular morphology was assessed using electron microscopy. In hyperglycemic cilia+ kidneys, there was no evidence of altered renal epithelial cell morphology (not shown) and glomerular architecture was normal (Fig. 3A). In hyperglycemic cilia− kidneys, there was evidence of renal epithelial cell stress and necrosis (Fig. 3, B and D) and fusion of glomerular podocyte foot processes (Fig. 3C).
Fig. 3.
Focal foot process effacement in cilia− mice. A–D: electron micrographs of kidney sections from mice 6 wk post-STZ. A: cilia+ hyperglycemic mouse kidney showing normal podocyte foot processes and basement membrane. B–D: a distal tubular or collecting duct cell exhibiting epithelial cell stress (B), fusion of podocyte food processes (C), and renal tubular epithelia cell death and necrosis (D) in hyperglycemic cilia− mice.
Enhanced cell proliferation.
Cell proliferation is considered one of the classical features of PKD. BrdU was used to assess renal cell proliferation in response to hyperglycemia at 3 mo post-STZ (Fig. 4). BrdU staining was greatly enhanced in cilia− mice compared with cilia+ mice, with most of the BrdU-positive cells found in cystic regions, and was most noticeable in distal tubule/collecting duct segments.
Fig. 4.
Renal tubular epithelial cell proliferation is enhanced in cystic epithelium of STZ-treated cilia− mice. A: bromodeoxyuridine (BrdU)-stained kidney sections from cilia+ and cilia− mice at 3 mo post-STZ. Magnification ×40. B: number of BrdU-positive tubular epithelial cells per image field (n = 3 per group). *P < 0.05.
Wnt and epithelial-to-mesenchymal transition signaling.
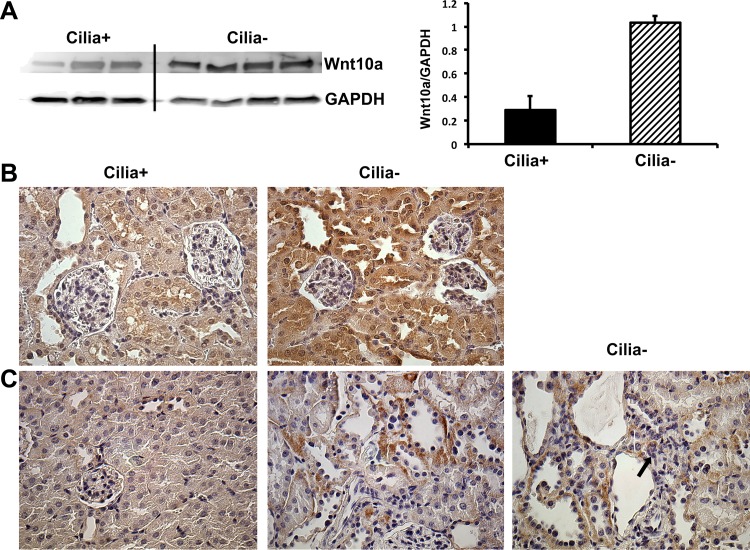
Two of the pathways that might contribute to cyst growth and the pathological changes that occur with hyperglycemia in cilia− mice are Wnt signaling and activation of the epithelial-to-mesenchymal transition (EMT), as both of these pathways have been implicated in the progression of PKD and diabetic kidney disease (15, 19, 38, 41). Pathway-specific gene array analysis was used to compare the activation of these pathways between cilia+ and cilia− hyperglycemic mice. As shown in Figs. 5 and 6, there is clear evidence for enhanced activation of Wnt and EMT signaling in hyperglycemic cilia− mice compared to cilia+ mice. For instance, there were increases in Snai1 and Snai2 (Fig. 5), both of which repress E-cadherin expression. There was also an increase in canonical Wnt signaling (Sfrp2, Sfrp4, Sox17, Frat1, Fzd3, Lef1, Pygo1, Wnt10a, Wnt16, and Wnt7b) and noncanonical calcium ion-dependent signaling (Wnt10a, Wnt16, and Wnt7b; Fig. 6). Western blot analysis confirmed enhanced Wnt signaling at the protein level, as Wnt10a was increased in the hyperglycemic cilia− mouse kidney (Fig. 7A), where it localizes to the distal tubule/collecting duct cells (Fig. 7B). Additionally, staining for β-catenin, which is involved in canonical Wnt signaling and EMT, identified increased expression of β-catenin in cystic regions as well as within primitive renal tubules (Fig. 7C).
Fig. 5.
Genes involved in epithelial-to-mesenchymal transition (EMT) were upregulated in the 6-wk cilia− hyperglycemic mouse kidney. EMT array analysis identified several genes upregulated at 6 wk post-STZ, while only 1 gene was significantly downregulated at 3 mo post-STZ. Differences >2-fold and P < 0.05 were considered significant.
Fig. 6.
Genes in the Wnt pathway were significantly upregulated in the hyperglycemic cilia− mouse kidneys at 6 wk and 3 mo post-STZ. Differences >2-fold and P < 0.05 were considered significant.
Fig. 7.
Wnt10a and β-catenin are enhanced in the kidney of cilia− mice at 3 mo post-STZ. A: Western blot for Wnt10a and GAPDH. B and C: Wnt10a (B) and β-catenin (C) stained kidney sections from cilia+ and cilia− mice at 3 mo post-STZ. Staining was enhanced in distal tubule/collecting duct, and β-catenin staining colocalized with primitive renal tubules (arrow in C).
DISCUSSION
Primary cilia on renal tubular epithelial cells sense fluid flow and help maintain cell structure and function. Deletion, malformation, or loss of function of the cilium results in renal cyst formation, as seen in PKD. While embryonic deletion of cilia or cystoproteins is lethal or causes massive cyst formation, deletion of cilia or cystoproteins in the adult mouse does not lead to significant cyst formation for months (9, 21, 27, 29). This delay in cystogenesis has provided a model that can be used to study factors or conditions that can modify the initiation and rate of cystogenesis. This is important, since there is a lack of understanding regarding factors that influence the rate of cystogenesis.
Recently, we reported that nephrectomy-induced renal hypertrophy dramatically increased the initiation and rate of cystic disease in the absence of cilia (3). Additionally, we found that, in the absence of cilia, reduced renal mass led to a significant increase in structural and functional hypertrophy. These findings suggest that growth factors, activated in response to reduced renal mass, stimulate cystogenesis and that cilia help control the degree of hypertrophy in response to growth factor stimulation. Hyperglycemia is another condition that results in hypertrophic signaling leading to structural and functional hypertrophy (7, 35). A unique feature of diabetes-related hypertrophy is that the signaling pathways are initially mediated by signaling pathways resulting in increased cell proliferation (17). Shortly after proliferation, however, the environment switches to promote cellular senescence and hypertrophy (17, 33). It has been suggested that functional hypertrophy (increased GFR) contributes to the development of diabetic nephropathy (23).
In developed countries, diabetes mellitus is reaching epidemic proportions. In the United States alone, >11% of adults are diabetic, while 35% of adults are classified as prediabetic, and these numbers are only expected to increase (6). There are no studies in experimental models of PKD examining the effects of hyperglycemia on cystic development. However, the importance of examining PKD and hyperglycemia/diabetes has been highlighted by studies with ADPKD patients. ADPKD patients, like the general population, are at risk of developing diabetes due to increased body mass indexes (34). In addition, ADPKD patients have higher fasting blood glucose levels and a trend toward higher hemoglobin A1c levels than healthy controls (28). A recent study found that total renal volume was approximately doubled in ADPKD patients with type 2 diabetes compared with matched patients with ADPKD alone (31). This is significant, since kidney volume was found to be a predictor of both cystic volume and the rate of decline in renal function (11). Consequently, there are compelling reasons to study the effects of hyperglycemia in a mouse model of PKD.
In the current study we used the Ift88 conditional mouse model made hyperglycemic with STZ. The rate of cyst formation and progression was enhanced by STZ-induced hyperglycemia. This conclusion is based on our recent study in which we did not find significant cyst formation in nondiabetic, two-kidney cilia− mice at 3 mo (3). By 6 wk post-STZ, cysts had already developed in the cilia− mice, while no cysts were present in the hyperglycemic cilia+ mice. STZ was administered in the “low-dose” protocol, which has been shown to minimize its potential harmful effects. Although we cannot rule out a direct effect of STZ on cystic development, cysts were evaluated 6 wk and 3 mo post-STZ, which would support a role for hyperglycemia in the growth of cysts. Accordingly, cell proliferation was also increased in the STZ-treated cilia− mice, and this increased proliferation was primarily associated with cyst-lining epithelial cells. Cell proliferation has been considered to be one of the hallmarks of PKD, in that it is necessary for cyst development and expansion (20, 30). However, this idea has been challenged with the suggestion that proliferation is a secondary effect (29). Although hyperglycemia can result in an early increase in cell proliferation, the switch from proliferation to hypertrophy normally occurs within 1 wk post-STZ (17). Additionally, the association of proliferative cells with the cystic epithelium suggests that the increased proliferation is related to cystogenesis and PKD and not necessarily driven primarily by hyperglycemia. There was inflammation associated with cystic regions, and inflammatory cells have been shown to promote cyst growth by stimulating cell proliferation (18). It is unclear if the increased cell proliferation is due to paracrine proliferative signals released by inflammatory cells, although the lack of inflammatory cells in all cystic regions suggests that there are other mechanisms that induce proliferation and cystogenesis. Body weight, blood pressure, kidney weight, and GFR did not differ between cilia+ and cilia− mice. There was a substantial increase in urine output and some evidence of proteinuria in cilia− mice compared with cilia+ mice. Although we do not have a clear explanation for the substantial differences between groups, the global Ift88 knockout model exhibits hyperphagia (9), and in PKD, in general, there is a urine-concentrating defect that may include defects at the collecting duct in the kidney and the osmoreceptors/vasopressin mechanism in the brain (16, 24).
Although reduced renal mass and hyperglycemia have been shown to lead to hypertrophic signaling (7, 10, 35), there were some surprising differences between these two models in the absence of cilia (3). Cystic disease was not as prominent in the hyperglycemic mice at 3 mo as in the nephrectomized mice. However, noncystic renal parenchyma was worse in the hyperglycemic mice, as this region contained primitive tubules and signs of tubular stress, which were clearly demonstrated in electron-microscopic images. The tubular vacuolated nuclei and podocyte foot process effacement could be related to the relative respective increase in urinary excretion of glucose and proteins in mice with ciliary loss and hyperglycemia. The functional hypertrophy response was also different in these two models. GFR was initially elevated in nephrectomized cilia− mice, while GFR did not differ between cilia+ and cilia− STZ-treated mice. The differences in hypertrophic responses between these two models are not known but could be related to the differences in the strength of hypertrophic signaling or in the activation or inhibition of cell signaling pathways.
The most surprising finding in this study was the presence of immature/primitive renal tubules. This was particularly interesting, given the recent suggestion that cilia are required to manage tissue regeneration in response to renal injury (40). Primitive tubules are normally seen in the developing kidney or in pediatric diseases such as Wilms' tumors. There is evidence for the activation of β-catenin/Wnt signaling in Wilms' tumors (8), and it has been suggested that β-catenin/Wnt signaling is activated in PKD as well (22, 32), although this is debatable, as it does not appear to be true in all models (26). In our studies we found increased EMT and Wnt signaling in hyperglycemic cilia− kidneys. In particular, Wnt10a, which has been implicated in diabetic nephropathy, was elevated in distal tubular/collecting duct areas, which are prone to become cystic. Thus it is possible that, in the presence of hyperglycemia, persistent β-catenin/Wnt signaling due to the lack of cilia is responsible for renal cyst formation, initiation of cellular damage, induction of inflammation, and formation of primitive tubules. Indeed, we found colocalization of β-catenin expression with primitive tubule formation.
In conclusion, we found that, with the loss of cilia, hyperglycemia accelerates cyst formation, increases inflammation, upregulates Wnt and EMT signaling, induces renal tubular and glomerular damage, and triggers the formation of primitive renal tubules. Interestingly, it would appear that cilia help “protect” the kidney from the detrimental effects of hyperglycemia. This work, along with recent human studies in ADPKD patients with diabetes (31), would suggest that the presence of both diseases may significantly accelerate disease progression and renal failure.
GRANTS
This work was supported by Veterans Affairs Health Administration Merit Awards 1I01BX002007 (P. D. Bell) and 5101CX000415-02 and 5101BX000487-04 (C. F. Baicu and M. R. Zile), National Institutes of Health Grants DK-32032 (P. D. Bell), P30 DK-074038 (Core C, P. D. Bell; B. K. Yoder, Principal Investigator), and T32 DI-007752 (P. D. Bell, Principal Investigator), a Tuberous Sclerosis Alliance Fellowship (B. J. Siroky), a Tuberous Sclerosis Alliance Courage Award (J. J. Bissler), and funds from Dialysis Clinic, Inc.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.S. and P.D.B. developed the concept and designed the research; K.M.S., W.R.F., C.F.B., S.L.S., M.Y.A., T.S., J.A.F., M.A.B., and P.D.B. performed the experiments; K.M.S., H.Y., S.L.S., T.S., J.A.F., G.P.S., B.J.S., J.J.B., and P.D.B. analyzed the data; K.M.S., J.A.F., B.J.S., J.J.B., and P.D.B. interpreted the results of the experiments; K.M.S. and S.L.S. prepared the figures; K.M.S. and P.D.B. drafted the manuscript; K.M.S., S.L.S., and P.D.B. edited and revised the manuscript; K.M.S. and P.D.B. approved the final version of the manuscript.
REFERENCES
- 1.Animal Models of Diabetic Complications Consortium. Low-Dose Streptozotocin Induction Protocol (Mouse). AMDCC, 2003. [Google Scholar]
- 2.Bastos AP, Piontek K, Silva AM, Martini D, Menezes LF, Fonseca JM, Fonseca II, Germino GG, Onuchic LF. Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J Am Soc Nephrol 20: 2389–2402, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell PD, Fitzgibbon W, Sas K, Stenbit AE, Amria M, Houston A, Reichert R, Gilley S, Siegal GP, Bissler J, Bilgen M, Chou PC, Guay-Woodford L, Yoder B, Haycraft CJ, Siroky B. Loss of primary cilia upregulates renal hypertrophic signaling and promotes cystogenesis. J Am Soc Nephrol 22: 839–848, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breyer MD, Bottinger E, Brosius FC 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K, Animal Models of Diabetic Complications Consortium. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Brosius FC 3rd, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T, Animal Models of Diabetic Complications Consortium. Mouse models of diabetic nephropathy. J Am Soc Nephrol 20: 2503–2512, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. [Google Scholar]
- 7.Christiansen JS, Gammelgaard J, Frandsen M, Parving HH. Increased kidney size, glomerular filtration rate and renal plasma flow in short-term insulin-dependent diabetics. Diabetologia 20: 451–456, 1981. [DOI] [PubMed] [Google Scholar]
- 8.Clark PE, Polosukhina D, Love H, Correa H, Coffin C, Perlman EJ, de Caestecker M, Moses HL, Zent R. β-Catenin and K-RAS synergize to form primitive renal epithelial tumors with features of epithelial Wilms' tumors. Am J Pathol 179: 3045–3055, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol 17: 1586–1594, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine LG, Norman J. Cellular events in renal hypertrophy. Annu Rev Physiol 51: 19–32, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP, Consortium of Radiologic Imaging Studies of Polycystic Kidney Disease Investigators. Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Happe H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol 244: 305–318, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, Yoder BK. Intraflagellar transport is essential for endochondral bone formation. Development 134: 307–316, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Hills CE, Squires PE. The role of TGF-β and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev 22: 131–139, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Ho TA, Godefroid N, Gruzon D, Haymann JP, Marechal C, Wang X, Serra A, Pirson Y, Devuyst O. Autosomal dominant polycystic kidney disease is associated with central and nephrogenic defects in osmoregulation. Kidney Int 82: 1121–1129, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Huang HC, Preisig PA. G1 kinases and transforming growth factor-β signaling are associated with a growth pattern switch in diabetes-induced renal growth. Kidney Int 58: 162–172, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends Mol Med 16: 349–360, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanoix J, D'Agati V, Szabolcs M, Trudel M. Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene 13: 1153–1160, 1996. [PubMed] [Google Scholar]
- 21.Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJ. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet 16: 3188–3196, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100: 5286–5291, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Maldonado M, Yium JJ, Eknoyan G, Suki WN. Adult polycystic kidney disease: studies of the defect in urine concentration. Kidney Int 2: 107–113, 1972. [DOI] [PubMed] [Google Scholar]
- 25.Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol 17: 1015–1025, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Ocbina PJ, Tuson M, Anderson KV. Primary cilia are not required for normal canonical Wnt signaling in the mouse embryo. PLos One 4: e6839, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietrzak-Nowacka M, Safranow K, Byra E, Binczak-Kuleta A, Ciechanowicz A, Ciechanowski K. Metabolic syndrome components in patients with autosomal-dominant polycystic kidney disease. Kidney Blood Press Res 32: 405–410, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramasubbu K, Gretz N, Bachmann S. Increased epithelial cell proliferation and abnormal extracellular matrix in rat polycystic kidney disease. J Am Soc Nephrol 9: 937–945, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Reed B, Helal I, McFann K, Wang W, Yan XD, Schrier RW. The impact of type II diabetes mellitus in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 27: 2862–2865, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the β-catenin gene. Oncogene 20: 5972–5981, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Satriano J, Mansoury H, Deng A, Sharma K, Vallon V, Blantz RC, Thomson SC. Transition of kidney tubule cells to a senescent phenotype in early experimental diabetes. Am J Physiol Cell Physiol 299: C374–C380, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrier RW, McFann KK, Johnson AM. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int 63: 678–685, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Seyer-Hansen K, Hansen J, Gundersen HJ. Renal hypertrophy in experimental diabetes. A morphometric study. Diabetologia 18: 501–505, 1980. [DOI] [PubMed] [Google Scholar]
- 36.Siroky BJ, Guay-Woodford LM. Renal cystic disease: the role of the primary cilium/centrosome complex in pathogenesis. Adv Chronic Kidney Dis 13: 131–137, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Togawa H, Nakanishi K, Mukaiyama H, Hama T, Shima Y, Sako M, Miyajima M, Nozu K, Nishii K, Nagao S, Takahashi H, Iijima K, Yoshikawa N. Epithelial-to-mesenchymal transition in cyst lining epithelial cells in an orthologous PCK rat model of autosomal-recessive polycystic kidney disease. Am J Physiol Renal Physiol 300: F511–F520, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Watnick T, Germino GG. Molecular basis of autosomal dominant polycystic kidney disease. Semin Nephrol 19: 327–343, 1999. [PubMed] [Google Scholar]
- 40.Weimbs T. Third-hit signaling in renal cyst formation. J Am Soc Nephrol 22: 793–795, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou T, He X, Cheng R, Zhang B, Zhang RR, Chen Y, Takahashi Y, Murray AR, Lee K, Gao G, Ma JX. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia 55: 255–266, 2012. [DOI] [PubMed] [Google Scholar]