Abstract
Urea transporter (UT)-A1 in the kidney inner medulla plays a critical role in the urinary concentrating mechanism and thereby in the regulation of water balance. The 14-3-3 proteins are a family of seven isoforms. They are multifunctional regulatory proteins that mainly bind to phosphorylated serine/threonine residues in target proteins. In the present study, we found that all seven 14-3-3 isoforms were detected in the kidney inner medulla. However, only the 14-3-3 γ-isoform was specifically and highly associated with UT-A1, as demonstrated by a glutathione-S-transferase-14-3-3 pulldown assay. The cAMP/adenylyl cyclase stimulator forskolin significantly enhanced their binding. Coinjection of 14-3-3γ cRNA into oocytes resulted in a decrease of UT-A1 function. In addition, 14-3-3γ increased UT-A1 ubiquitination and protein degradation. 14-3-3γ can interact with both UT-A1 and mouse double minute 2, the E3 ubiquitin ligase for UT-A1. Thus, activation of cAMP/PKA increases 14-3-3γ interactions with UT-A1 and stimulates mouse double minute 2-mediated UT-A1 ubiquitination and degradation, thereby forming a novel regulatory mechanism of urea transport activity.
Keywords: protein kinase A, accessory protein, protein turnover, urea transporter
urea plays a critical role in the urinary concentrating mechanism and, therefore, in the regulation of water balance. The major mechanism for delivering urea to the inner medullary (IM) interstitium is urea reabsorption from the terminal IM collecting duct (IMCD), which is mediated by urea transporter (UT)-A1 (19, 27). A UT-A1/A3 knockout mouse has impaired urea clearance and urinary concentrating ability (7).
UT-A1 activity is mainly regulated by vasopressin in vivo. The addition of vasopressin to the bath of a perfused rat terminal IMCD results in binding to V2 receptors, stimulation of adenylyl cyclase III and VI, generation of cAMP, and enhanced facilitated urea transport (33, 35). UT-A1 has several putative phosphorylation sites within the intracellular domains, including four consensus cAMP-dependent PKA phosphorylation sites (14). Phosphorylation of UT-A1 is an important mechanism by which vasopressin rapidly increases urea permeability in vivo (35). The activation of UT-A1 phosphorylation by PKA coincides with increased transepithelial urea permeability in perfused IMCDs (33, 35) and in UT-A1-expressing cultured Madin-Darby canine kidney (MDCK) cells (8). UT-A1 phosphorylation by PKA is mainly at Ser84 in the NH2-terminus (11) and two sites, Ser486 and Ser499, within the large intracellular loop (2). Ser486 and Ser499 are important for vasopressin-regulated accumulation of UT-A1 in the cell membrane and UT activity.
The 14-3-3 proteins are a family of highly conserved eukaryotic proteins that regulate many cellular processes by binding to phosphorylated serine/threonine residues in diverse target proteins (9). In mammals, there are seven 14-3-3 isoforms (β, ε, γ, η, σ, τ, and ζ), which are encoded by separate genes. 14-3-3 proteins are widely expressed in almost all tissues. The ubiquitous nature of 14-3-3 proteins reflects their fundamental roles in eukaryotic biology (9). The presence of seven 14-3-3 isoforms suggests the existence of isoform-specific interactions with different targets. Interaction of 14-3-3 with its various target proteins has multiple functional consequences (3, 9, 10, 22, 26). Studies on extracts of proliferating HeLa cells using 14-3-3 affinity chromatography have identified >200 human phosphoproteins that could bind to 14-3-3 (22), suggesting that 14-3-3 proteins may be central to integrating the regulation of biosynthetic metabolism, cell proliferation, survival, and other processes in human cells.
A number of membrane proteins, such as the CFTR (16), Na+/Ca2+ exchanger (NCX) (24), human ether-a-go-go-related gene (HERG) K+ channel (13), Ca2+-ATPase pump (25), Na+/H+ exchanger isoform 1 (NHE1) (15), and α2-adrenergic receptor (23), directly interact with 14-3-3 proteins. Their interactions play important roles in regulating membrane protein cell surface expression, stability, and activity. The goal of the present study was to investigate the possible role of 14-3-3 proteins in kidney UT-A1 regulation. We found that UT-A1 binds to the 14-3-3 γ-isoform in a phosphorylation-dependent manner. However, this binding negatively regulates UT-A1 transport activity. We further found that 14-3-3γ binding increases UT-A1 protein ubiquitination and degradation, thereby downregulating UT-A1 function.
MATERIALS AND METHODS
Animal and tissue preparation.
All animal protocols were approved by the Institutional Animal Care and Use Committee of Emory University. Sprague-Dawley rats (Charles River Laboratories) weighing 200–250 g were used for the evaluation of 14-3-3 expression in the kidney. The kidneys were removed, and the cortex, outer medulla (OM), and IM were dissected. Tissues used for RNA were snap frozen in liquid nitrogen, and those for Western blot analysis were lysed immediately in RIPA buffer (4).
RT-PCR.
Total RNA was extracted from tissues with TRIzol reagent (Invitrogen) and reverse transcribed to single-stranded cDNA with a first-strand cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions. PCR was performed using an Advantage 2 PCR kit (Clontech). The primer sequences for each 14-3-3 isoform are shown in Table 1.
Table 1.
PCR primer sequences
| Primer sequences |
||||
|---|---|---|---|---|
| Gene | Accession No. | Sense | Antisense | Product size, bp |
| 14-3-3τ | D17614 | 5′-GAGTCTGAGCTGAGGTCCATCT-3′ | 5′-AAAAGGTTTTCTTGAGGGGAAG-3′ | 524 |
| 14-3-3σ | XM_233856 | 5′-CTGAACTTTTCCGTCTTCCACTAT-3′ | 5′-CTACTCCGTTTAAGTCCTCTCTGC-3′ | 300 |
| 14-3-3γ | S55305 | 5′-CAAGCCGCTCCTCTCTTTTCC-3′ | 5′-TAAAGACTGCAGTAGTAGCAT-3′ | 879 |
| 14-3-3η | BC081825 | 5′-GAGCGAGCGAGCAGGCGGTGCG-3′ | 5′-GCAACCATCAGTCCAGCAAATGCC-3′ | 985 |
| 14-3-3ε | BC063163 | 5′-AGTCGGAGACGCTATCCGCTT-3′ | 5′-AGCCTCTATGCAGTCCTGTTA-3′ | 1,112 |
| 14-3-3β | D17446 | 5′-AGTGAGCTGGTACAGAAAG-3′ | 5′-CTGCAAGGCTTAGGCTGTG-3′ | 852 |
| 14-3-3ζ | BC070941 | 5′-GCAGTTACTGAGAGACAACTTGACA-3′ | 5′-GCAAACAGCAGGTAACTTTACAAAT-3′ | 692 |
| GAPDH | NM_017008 | 5′-GACAAGATGGTGAAGGTCGG-3′ | 5′-CATGGACTGTGGTCATGAGC-3′ | 538 |
Plasmid construction.
All seven 14-3-3 isoforms in pGEX vector were generated in H. Fu's laboratory as previously described (5). Rat UT-A1 cDNA was cloned into the Xenopus expression vector pGH19 (pGH19-UT-A1) (6). 14-3-3γ and 14-3-3σ cDNAs from pGEX vector were subcloned into pGH19. NH2-terminal FLAG (DYKDDDDK)-tagged 14-3-3γ and 14-3-3σ were generated by PCR and cloned into pcDNA3 vector. pcDNA3-hemagglutinin-MDM2 was kindly provided by Dr. Hua Lu (12).
Cell culture, transfection, biotinylation, and immunoprecipitation.
UT-A1-MDCK cells (8) and human embryonic kidney (HEK)-293 cells were maintained in DMEM supplemented with 10% FCS at 37°C in 5% CO2. HEK-293 cells were grown in six-well plates to 80% confluency and transfected with the indicated plasmids using Lipofectamine (Invitrogen) for 48 h. Before cell harvest, some cells were treated with 6 μM of the proteasome inhibitor MG-132 for 6 h and/or 10 μM forskolin (FSK; Sigma) for 5, 15, and 30 min. Cell surface biotinylation was performed as previously described (6). Cells were lysed in RIPA buffer. Equal amounts of postnuclear supernatants were used for glutathoine-S-transferase (GST) pulldown, Western blot analysis, and immunoprecipitation with the relevant antibodies.
GST pulldown assay.
pGEX-4T2-14-3-3 constructs were transformed in Esherichia coli BL21 cells (Stratagene), and GST-14-3-3 fusion proteins were prepared as previously described (5). UT-A1-MDCK cells were grown in six-well plates to confluency and treated with 10 μM FSK for the indicated times. Cells were scraped into RIPA buffer, disrupted with a Polytron homogenizer, and centrifuged at 10,000 rpm for 10 min. Equal amounts (500 μg) of cleared total lysates prepared from UT-A1 MDCK cells were first preincubated with GST beads alone for 2 h at 4°C with constant rotation. After centrifugation, the supernatant was collected and processed for GST pulldown assay by incubation with GST or GST-14-3-3 at 4°C overnight. After being washed, bound proteins were eluted in 50 μl Laemmli buffer by boiling for 5 min and used for immunoblot analysis with UT-A1 or pan-14-3-3 antibody.
Lambda phosphatase treatment.
For dephosphorylation treatment, UT-A1 MDCK cells were lysed in Nonidet P-40 buffer [50 mM Tris·HCl (pH 8.0), 150mM NaCl, and 1% Nonidet P-40] (34). Cell lysates (500 μg/100 μl) were incubated with or without 2 μl lambda phosphatase (P0753, New England Biolabs) in the presence of 1 mM MnCl2 at 30°C for 30 min. After incubation, samples were used for GST-14-3-3 pulldown. 14-3-3-bound UT-A1 was analyzed by Western blot analysis with a UT-A1 antibody.
Western blot analysis.
Tissues were lysed in RIPA buffer (4). The protein concentration was determined using BCA reagent (Pierce). Fifty micrograms of the lysates were used for immunoblot analysis. Membranes were routinely processed by blotting with 2.5% milk dissolved in 0.1% PBST and incubated overnight with primary antibody and then for 1 h with horseradish peroxidase-conjugated secondary antibody. Immunoreacting proteins were detected using an enhanced chemiluminescence kit (Amersham). The list of 14-3-3-specific isoform antibodies is shown in Table 2. FLAG monoclonal antibody was purchased from Sigma (F1804). Ubiquitin antibody (P4D1) was from Santa Cruz Biotechnology (sc-8017). Rabbit polyclonal UT-A1 antibody was as previously described (28). Secondary horseradish peroxidase-conjugated goat anti-rabbit IgG and secondary FITC-conjugated goat anti-rabbit IgG were purchased from Amersham. ImageJ software (National Institutes of Health) was used to quantify band density.
Table 2.
Antibodies specific to 14-3-3 isoforms for Western blot analysis
| 14-3-3 Isoform | Company | Catalog | Raised in |
|---|---|---|---|
| 14-3-3τ | Santa Cruz Biotechnology | sc-732 | Rabbit |
| 14-3-3σ | Upstate | 05-632 | Mouse |
| 14-3-3γ | IBL | 18647 | Rabbit |
| 14-3-3η | IBL | 18645 | Rabbit |
| 14-3-3ε | Santa Cruz Biotechnology | sc-1020 | Rabbit |
| 14-3-3β | GTX | 12341 | Mouse |
| 14-3-3ζ | Santa Cruz Biotechnology | sc-1019 | Rabbit |
| pan-14-3-3 | Santa Cruz Biotechnology | sc-629 | Rabbit |
Immunohistochemistry.
Kidney paraffin sections were dewaxed in xylene, hydrated, and then antigen retrieved in 0.01 M citrate buffer (pH6.0). Sections were incubated with 1% BSA and PBS for 20 min followed by primary antibody (rabbit anti-UT-A1 antibody at 1:200 or rabbit anti-14-3-3γ antibody at 1:100) at 4°C overnight and FITC-conjugated secondary goat anti-rabbit antibody (Sigma) at room temperature for 1 h. After being washed in PBS, slides were mounted with Vectashield (Vector Laboratories) and examined under a fluorescence microscope.
cRNA preparation, oocyte microinjection, and urea uptake measurement.
To measure UT-A1 transport activity, a Xenopus oocyte system was used. Capped cRNAs were synthesized with T7 polymerase using the mMESSAGE mMACHINE T7 Ultra Kit (Ambion). Five nanograms of UT-A1 and 14-3-3γ cRNAs were microinjected into stage IV–V oocytes. Three days later, UT activity was assessed by 14C-labeled urea flux, and protein expression was detected by Western blot analysis as previously described (6).
Statistical analysis.
Urea flux data are expressed as means ± SD. Statistical analysis was performed by one-way ANOVA followed by Tukey honestly significant difference tests.
RESULTS
Multiple 14-3-3 isofoms were detected in the kidney IM.
We first examined the expression pattern of seven 14-3-3 isoforms in the kidney. Total RNA was extracted from the rat kidney IM. RT-PCR was performed with specific primers for each specific 14-3-3 isoform. As shown in Fig. 1A, all seven isoforms were detected in the IM. The same results were seen in brain tissues.
Fig. 1.
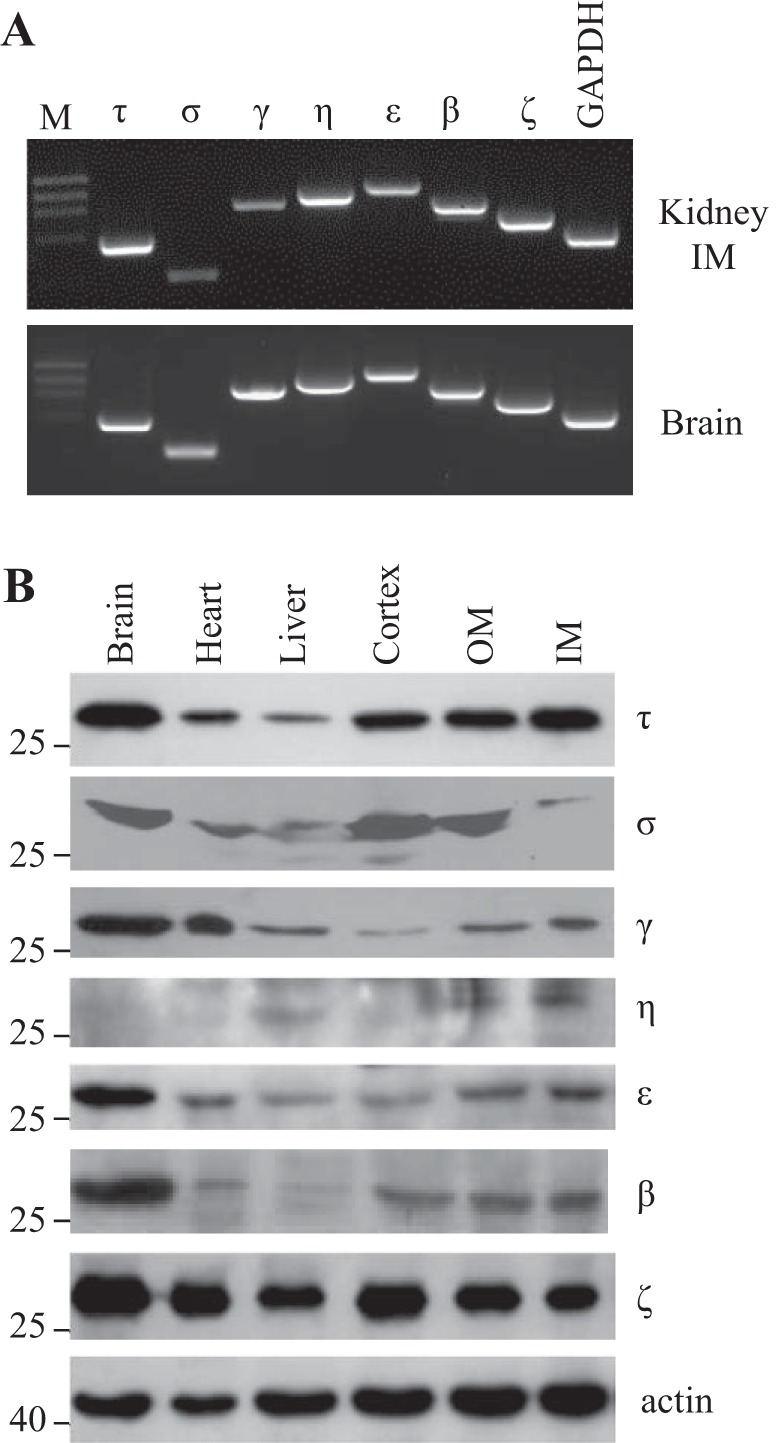
14-3-3 expression in the kidney. A: RT-PCR analysis of 14-3-3 isoforms in the kidney inner medulla (IM). M, DNA marker. B: Western blot analysis of 14-3-3 isoform expression in the brain, heart, liver, and kidney [cortex, outer medulla (OM), and IM].
To examine 14-3-3 protein expression in the kidney, subfractions of the kidney cortex, OM, and IM were isolated and used for immunoblot analysis with isoform-specific antibodies to 14-3-3. 14-3-3ζ and 14-3-3τ are highly and widely expressed in the kidney as well as in the brain, heart, and liver. 14-3-3γ, 14-3-3η, and 14-3-3ε are found in the cortex, OM, and IM but are mainly expressed in the IM. 14-3-3β is equally expressed in the kidney cortex, OM, and IM (Fig. 1B).
UT-A1 specifically bound to 14-3-3γ.
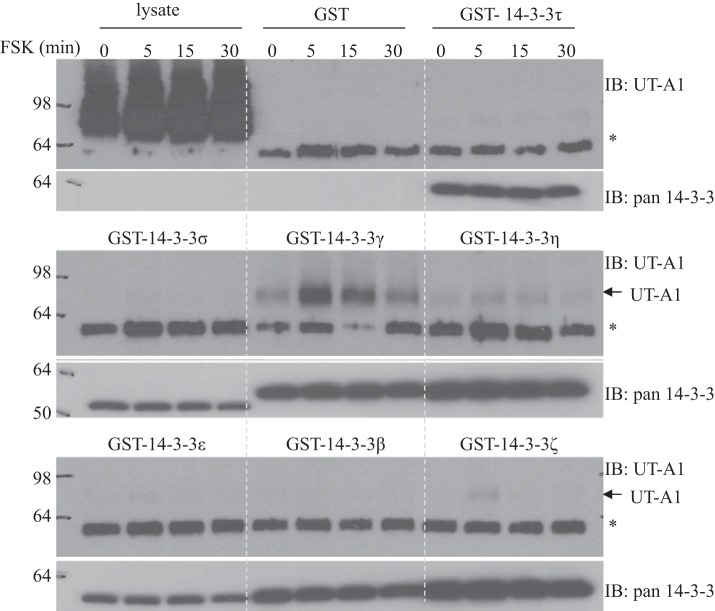
To investigate the possibility that UT-A1 interacts with 14-3-3, we performed pulldown experiments using all seven GST-14-3-3 fusion proteins. UT-A1 MDCK cells were treated with 10 μM FSK for 0, 5, 15, and 30 min. Cells were lysed with RIPA buffer. The clear supernatants were used for GST-14-3-3 pulldown assay. As shown in Fig. 2, UT-A1 specifically bound to the 14-3-3 γ-isoform. The binding of UT-A1 to 14-3-3γ was significantly increased in cells stimulated by FSK. A minor amount of UT-A1 was also pulled down by 14-3-3η and 14-3-3ζ.
Fig. 2.
Glutathione-S-transferase (GST)-14-3-3 pulldown assay. UT-A1 Madin-Darby canine kidney cells pretreated with 10 μM forskolin (FSK) for 0, 5, 15, and 30 min. Cell lysates were prepared for GST-14-3-3 precipitation and detected by immunoblot analysis (IB) with urea transporter (UT)-A1 antibody. The same membranes were stripped and reprobed with pan-14-3-3 antibody. *Unknown nonspecific bands.
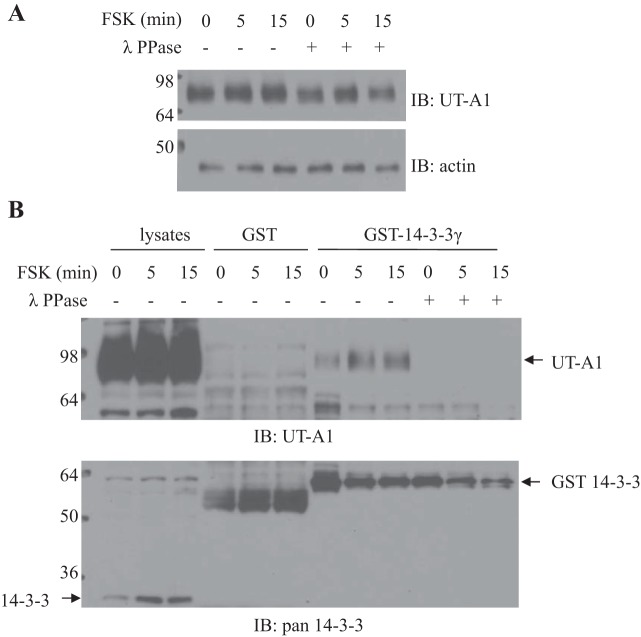
UT-A1 association with 14-3-3γ was phosphorylation dependent.
To more specifically determine whether UT-A1 binds to 14-3-3γ in a phosphorylation-dependent manner, cell lysates were pretreated with lambda protein phosphatase 1 and then applied for a GST-14-3-3 pulldown experiment. Phosphatase incubation caused UT-A1 bands to migrate fast (Fig. 3A), indicating that UT-A1 is modulated by posttranslational phosphorylation. As shown in Fig. 3B, phosphatase treatment impaired UT-A1 association with 14-3-3.
Fig. 3.
Dephosphorylation of UT-A1 by protein phosphatase 1. UT-A1 MDCK cells treated with 10 μM FSK for 0, 5, and 15 min were lysed with 1% Nonidet P-40 buffer. Cell lysates were incubated with or without lambda protein phosphatase (λ PPase) at 30°C for 30 min. Next, cell lysates were used for Western blot analysis for UT-A1 expression (4–20% SDS-PAGE gradient gel; A) or applied for GST-14-3-3 pulldown assay (10% SDS-PAGE gel; B).
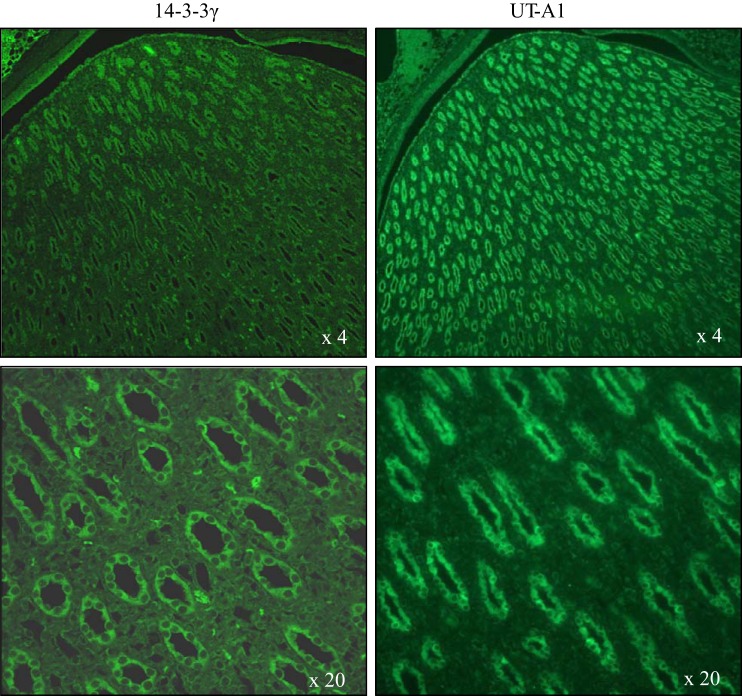
Both UT-A1 and 14-3-3γ were expressed in kidney IMCD epithelial cells.
To explore the physiological significance of 14-3-3γ regulating UT-A1 activity in vivo, we used immunohistochemistry to examine whether they colocalize in IMCD cells. Since the antibodies to UT-A1 and 14-3-3γ are both rabbit in origin, we were unable to do double immunostaining but only single immunostaining for each antibody separately. UT-A1 is mainly expressed in terminal IMCD epithelial cells. As shown in Fig. 4, both UT-A1 and 14-3-3γ were detected in kidney IMCD epithelial cells, suggesting the possibility of their working together.
Fig. 4.
Immunofluorescence microscopy. Rat kidney paraffin sections were incubated with rabbit anti-UT-A1 antibody or rabbit anti-14-3-3γ antibody followed by FITC-conjugated secondary goat anti-rabbit antibody. The immunostaining was examined by fluorescence microscopy.
14-3-3γ expression inhibited UT-A1 urea transport activity.
To evaluate the functional consequence of 14-3-3 on UT-A1 urea transport activity, UT-A1 and 14-3-3γ cRNAs were synthesized and coinjected into Xenopus oocytes. 14-3-3σ, which was not bound to UT-A1 (Fig. 2), was used as a negative control. Three days later, UT-A1 urea transport activity was determined by 14C-labeled urea uptake. Figure 5A shows that coinjection of 14-3-3γ reduced UT-A1 urea transport activity. The decreased urea transport activity corresponded to decreased UT-A1 protein expression (Fig. 5B). Coexpression of 14-3-3σ also showed a tendency toward decreasing UT-A1 expression and activity.
Fig. 5.
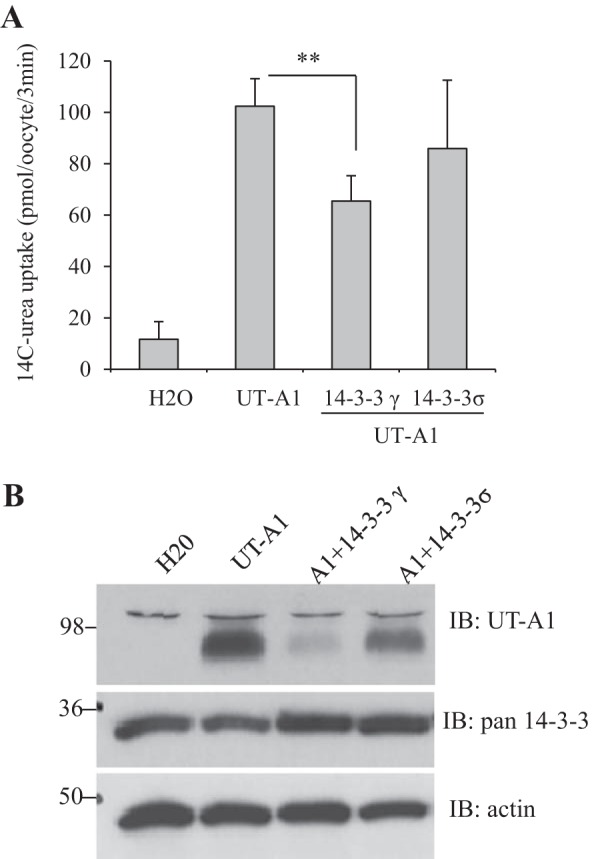
Effect of 14-3-3γ coexpression on UT-A1 urea transport activity. Oocytes were coinjected with cRNAs encoding UT-A1 (2 ng/cell) alone or with 14-3-3γ or 14-3-3σ (5 ng/cell) for 3 days. A: urea transport activity was measured by 14C-labeled urea flux (n = 5∼6 oocytes/3 min). **P < 0.01. B: 10 oocytes from each group were lysed in RIPA buffer. Protein expression was examined by Western blot analysis with UT-A1, pan-14-3-3, and actin antibodies.
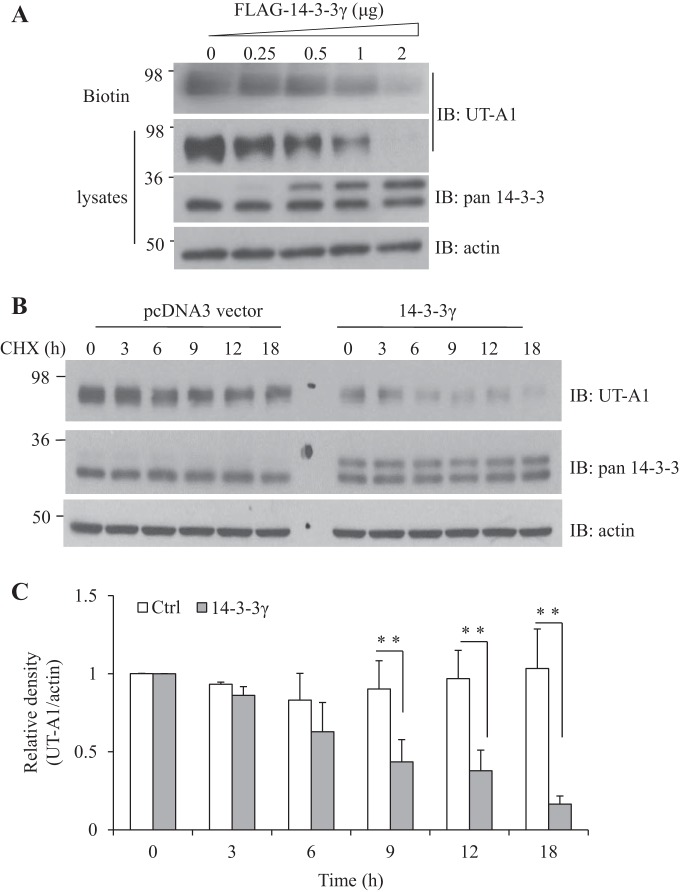
14-3-3γ increased UT-A1 degradation.
To further explore the effect of 14-3-3γ on UT-A1 protein expression, UT-A1 HEK-293 cells were transfected with different amounts of pcDNA3-FLAG-14-3-3γ for 48 h. 14-3-3 protein expression was examined by pan-14-3-3 antibody. The upper bands in Fig. 6A indicate transfected FLAG 14-3-3γ; the lower bands in Fig. 6A indicated endogenous 14-3-3. 14-3-3γ dose dependently decreased total and cell surface UT-A1 protein expression (Fig. 6A). To determine if 14-3-3γ promotes UT-A1 degradation, UT-A1 HEK-293 cells were transfected with FLAG-14-3-3γ for 48 h. Cells were then treated with 100 μg/ml cycloheximide to block protein synthesis, and UT-A1 protein degradation was chased after cycloheximide treatment by Western blot analysis of total cell lysates. As shown in Fig. 6, B and C, 14-3-3γ significantly increased UT-A1 degradation.
Fig. 6.
Effect of 14-3-3γ on UT-A1 stability. A: 14-3-3γ coexpression reduces UT-A1 abundance. UT-A1 human embryonic kidney (HEK)-293 cells were transfected with different amounts of FLAG-14-3-3γ for 48 h. Total and cell membrane (biotinylated) UT-A1 was detected by Western blot analysis with UT-A1 antibody. The same membrane was stripped and reprobed for pan-14-3-3 and actin. B: 14-3-3γ increased UT-A1 protein degradation. UT-A1 HEK-293 cells were transfected with 14-3-3γ or pcDNA3 vector. After 48 h, cells were treated with 100 μg/ml cycloheximide (CHX), and total UT-A1 protein was chased for the indicated times. C: densitometry analysis of UT-A1 protein abundance of Fig. 6B from three separate experiments. The relative density of UT-A1 was normalized to the actin, and time 0 was set as 1. n = 3. **P < 0.01.
14-3-3γ increased UT-A1 ubiquitination.
To investigate whether the increase in UT-A1 degradation by 14-3-3γ was linked to an increase in protein ubiquitination, HEK-293 cells were transfected with UT-A1 and 14-3-3γ for 48 h. Before cell collection, cells were pretreated with 10 μM MG-132 for 6 h. Ubiquitinated UT-A1 was detected from UT-A1-immunoprecipitated samples using ubiquitin antibody. As shown in Fig. 7, the total abundance of UT-A1 was decreased, but ubiquitinated UT-A1 was increased after 14-3-3γ transfection, suggesting that 14-3-3γ promotes UT-A1 ubiquitination.
Fig. 7.
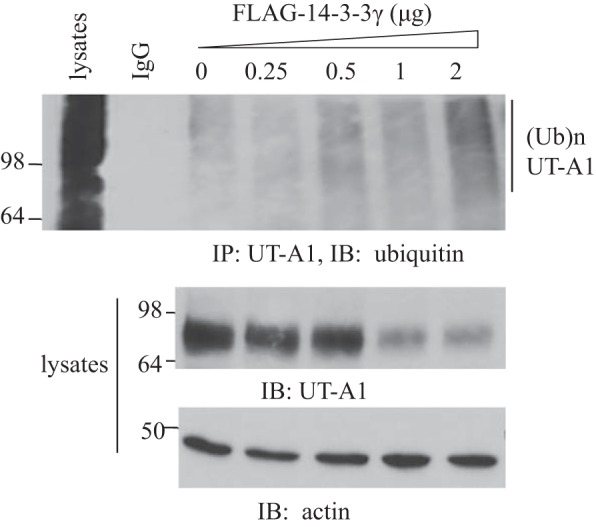
14-3-3γ regulation of UT-A1 ubiquitination. UT-A1 HEK-293 cells were transfected with different amounts of 14-3-3γ for 48 h. Total cell lysates were immunoprecipitated with UT-A1 antibody and blotted with ubiquitin antibody.
14-3-3γ promoted mouse double minute 2 binding to UT-A1.
Our previous study (4) showed that mouse double minute 2 (MDM2) mediates UT-A1 ubiquitination and that overexpression of MDM2 promotes UT-A1 degradation. We then asked whether the enhancement of UT-A1 ubiquitination by 14-3-3γ could be due to the recruitment of the E3 ubiquitin ligase MDM2. We performed an immunoprecipitation experiment with HEK-293 cells cotransfected with UT-A1, hemagglutinin-MDM2, and FLAG-14-3-3γ. Interestingly, the coimmunoprecipitation assay showed that both MDM2 and UT-A1 were immunoprecipitated by FLAG-14-3-3γ (Fig. 8), indicating that 14-3-3γ acts as an important recruiter of MDM2, which then mediates UT-A1 ubiquitination and degradation.
Fig. 8.
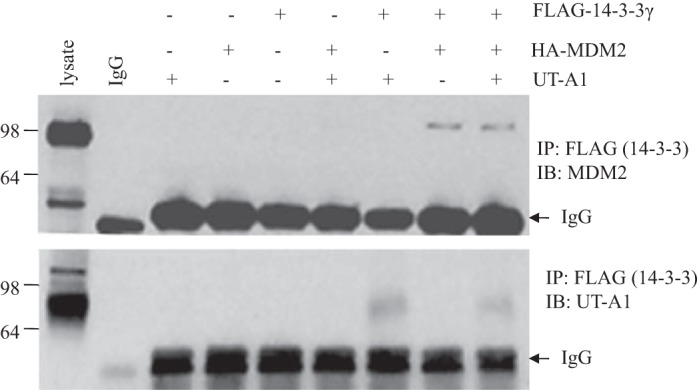
14-3-3γ interacts with both mouse double minute 2 (MDM2) and UT-A1. HEK-293 cells were transfected with different combinations of UT-A1, FLAG-14-3-3γ, or MDM2. Total cell lysates were immunoprecipitated with FLAG antibody followed by immunoblot analysis for MDM2 and UT-A1. HA, hemagglutinin.
DISCUSSION
In the present study, we identified 14-3-3γ as a novel UT-A1 partner. The properties of 14-3-3, which recognizes phosphorylated target proteins, suggest that it plays a potentially important role in the regulation of UT-A1 urea transport activity in the kidney through a phosphorylation signal cascade.
Unlike the ε-isoform of 14-3-3, which is the major species expressed in the heart (14), our data reveal that there is no single isoform specifically expressed in the kidney IM. All seven 14-3-3 isoforms were detected in the kidney IM. Interestingly, 14-3-3σ was not listed in a transcription gene profile study of isolated IMCD cells by Knepper's group (32) and our RNA-Seq analysis of gene expression from the IM (unpublished observations), but, in the present study, we found 14-3-3σ expression in the kidney IM at low levels (Fig. 1). This was verified by a DNA sequence with the RT-PCR product. However, our GST pulldown experiment showed that 14-3-3γ is the specific isoform that binds to UT-A1 in the kidney IM; that is, although multiple 14-3-3 isoforms are expressed in IMCD cells, UT-A1 specifically associates with 14-3-3γ. The multiple other 14-3-3 isoforms that are expressed in the IM may contribute to other aspects of IM physiology.
Different proteins associate with different 14-3-3 isoforms. NHE1 associates with 14-3-3β (15). HERG K+ channels bind to 14-3-3ε (13). Some membrane proteins can interact with multiple 14-3-3 isoforms. For example, NCX2 interacts with 14-3-3 β-, ζ-, θ-, and ε-isoforms (24). At least three isoforms of 14-3-3 (η, τ, and ζ) have been reported to interact with the cardiac voltage-gated Na+ channel Nav1.5 (1). Unlike NCX2 and Nav1.5, which interact with multiple 14-3-3 isoforms, our data show that UT-A1 more specifically binds to 14-3-3γ.
Protein phosphorylation is the primary mechanism that controls 14-3-3 binding. A previous study (22) has shown that >200 phosphoproteins associate with 14-3-3. Phosphorylation is a key regulatory mechanism for UT-A1 urea transport activation and activity in response to vasopressin in vivo (2, 33, 35). In the present study, we found that the interaction of 14-3-3γ and UT-A1 was dramatically enhanced when UT-A1 MDCK cells were treated with the cAMP/adenylyl cyclase stimulator FSK. This suggests that 14-3-3 modulates UT-A1 function particularly when it is phosphorylated. This was further confirmed by lambda protein phosphatase treatment (Fig. 3), which showed that dephosphorylation reduces UT-A1 association with 14-3-3γ.
Association with 14-3-3 isoforms often activates and stabilizes the target proteins, although there are a few cases where binding inactivates the protein, reflecting the diversity of 14-3-3 functions. 14-3-3ε accelerates and enhances HERG K+ channel activation; the interaction stabilizes the lifetime of the PKA-phosphorylated state of the channel by shielding the phosphates from cellular phosphatases (13). 14-3-3 binding to phosphorylated CFTR augments its biogenesis by reducing retrograde retrieval of CFTR to the endoplasmic reticulum, and cAMP/PKA stimulation results in more CFTR protein (16). 14-3-3β increases apical membrane epithelial Na+ channel density and enhances Na+ absorption by binding to phosphorylated Nedd4-2 to block its interaction with epithelial Na+ channels (17, 21). In the present study, we found that 14-3-3γ negatively regulated UT-A1 urea transport activity. An inhibitory effect of 14-3-3ε was also observed on NCX (24) and Ca2+-ATPase (25). However, the mechanism underlying the negative regulation by 14-3-3 is unknown. We found that overexpression of 14-3-3γ resulted in a reduction of UT-A1 protein expression, consistent with the decrease in urea transport activity. This suggests that 14-3-3γ regulation of UT-A1 function might be occurring by altering protein degradation. This speculation was verified when cotransfection of 14-3-3γ in HEK-293 cells reduced total and cell membrane UT-A1 protein abundance (Fig. 6A) and increased UT-A1 degradation (Fig. 6B).
Vasopressin regulates urea permeability in the IMCD through increases in UT-A1 phosphorylation and apical plasma membrane accumulation (2, 33). We recently reported that FSK, the adenylyl cyclase stimulator often used for in vitro experiments to stimulate UT-A1 urea transport activity, can also promote UT-A1 ubiquitination and protein degradation, revealing the other side of UT-A1 phosphorylation by vasopressin or FSK (29, 30). This observation has led to the hypothesis that phosphorylation could be required for UT-A1 ubiquitination. In fact, the ubiquitination of a number of important proteins is triggered by phosphorylation (18, 20, 31). This may have important physiological significance. When the cell is stimulated by vasopressin in vivo, UT-A1 is phosphorylated and turns to an active form. However, UT-A1 phosphorylation also triggers the degradation system. This could be the general mechanism that allows the cell to return to an unstimulated, basal state after responding to stimulation by vasopressin. The findings of our present study strengthen the hypothesis of phosphorylation-ubiquitination-degradation-downregulation. Figure 9 shows the important role of 14-3-3γ in the regulation of UT-A1 function after UT-A1 is activated in response to vasopressin. Once UT-A1 is activated (phosphorylated), it incurs 14-3-3γ binding, and 14-3-3γ protein recruits MDM2 and stimulates UT-A1 ubiquitination and degradation. This process forms the feedback to attenuate the response and ultimately return cells back to the basal state after vasopressin stimulation.
Fig. 9.
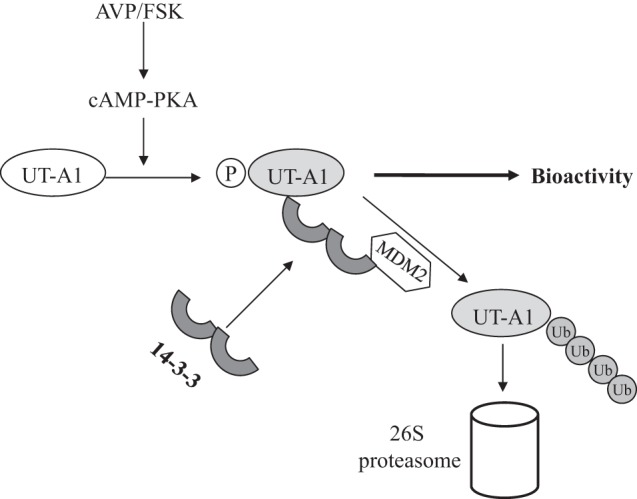
Model of UT-A1 activation, ubiquitination, and degradation. UT-A1 is activated by arginine vasopressin (AVP) in vivo (or FSK in vitro) to an active form for urea transport activity. Once UT-A1 is phosphorylated, it causes a number of phosphorylation-dependent protein bindings, including that of 14-3-3 protein. 14-3-3 subsequently recruites the ubiquitination E3 ligase MDM2 and, therefore, promotes UT-A1 ubiquitination and degradation, attenuates the cell's response to vasopressin stimulation, and eventually brings cells back to their basal condition.
GRANTS
G. Chen was supported by National Institutes of Health (NIH) Grant R01-DK-087838. J. M. Sands was supported by NIH grants R01-DK-41707 and R01-DK-89828. H. Fu was supported by NIH Grant P01-CA-116676. H. Cai was supported by Department of Veteran Affairs MERIT Award 5I01BX000994.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: X.F., Z.L., Y.D., J.D.K., and G.C. performed experiments; X.F., Z.L., H.F., J.M.S., and G.C. analyzed data; X.F., H.F., H.C., J.M.S., and G.C. interpreted results of experiments; X.F., Z.L., and G.C. prepared figures; H.F. and G.C. conception and design of research; H.F., J.D.K., H.C., J.M.S., and G.C. edited and revised manuscript; G.C. drafted manuscript; G.C. approved final version of manuscript.
REFERENCES
- 1.Allouis M, Le Bouffant F, Wilders R, Péroz D, Schott JJ, Noireaud J, Le Marec H, Mérot J, Escande D, Baró I. 14-3-3 is a regulator of the cardiac voltage-gated sodium channel Nav1.5. Circ Res 98: 1538–1546, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Blount MA, Mistry AC, Fröhlich O, Price SR, Chen G, Sands JM, Klein JD. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am J Physiol Renal Physiol 295: F295–F299, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridges D, Moorhead GB. 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE 2004: 242, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Huang H, Fröhlich O, Yang Y, Klein JD, Price SR, Sands JM. MDM2 E3 ubiquitin ligase mediates UT-A1 urea transporter ubiquitination and degradation. Am J Physiol Renal Physiol 295: F1528–F1534, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y, Masters SC, Khuri FR, Fu H. Monitoring 14-3-3 protein interactions with a homogeneous fluorescence polarization assay. J Biomol Screen 11: 269–276, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Feng X, Huang H, Yang Y, Fröhlich O, Klein JD, Sands JM, Chen G. Caveolin-1 directly interacts with UT-A1 urea transporter: the role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane. Am J Physiol Renal Physiol 296: F1514–F1520, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Urea transport in MDCK cells that are stably transfected with UT-A1. Am J Physiol Cell Physiol 286: C1264–C1270, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol 40: 617–647, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Hausser A, Link G, Hoene M, Russo C, Selchow O, Pfizenmaier K. Phospho-specific binding of 14-3-3 proteins to phosphatidylinositol 4-kinase IIIβ protects from dephosphorylation and stabilizes lipid kinase activity. J Cell Sci 119: 3613–3621, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Hwang S, Gunaratne R, Rinschen MM, Yu MJ, Pisitkun T, Hoffert JD, Fenton RA, Knepper MA, Chou CL. Vasopressin increases phosphorylation of Ser84 and Ser486 in Slc14a2 collecting duct urea transporters. Am J Physiol Renal Physiol 299: F559–F567, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Y, Zeng SX, Lee H, Lu H. MDM2 mediates p300/CREB-binding protein-associated factor ubiquitination and degradation. J Biol Chem 279: 20035–20043, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Kagan A, Melman YF, Krumerman A, McDonald TV. 14-3-3 amplifies and prolongs adrenergic stimulation of HERG K+ channel activity. EMBO J 21: 1889–1898, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karakashian A, Timmer RT, Klein JD, Gunn RB, Sands JM, Bagnasco SM. Cloning and characterization of two new isoforms of the rat kidney urea transporter: UT-A3 and UT-A4. J Am Soc Nephrol 10: 230–237, 1999. [DOI] [PubMed] [Google Scholar]
- 15.Lehoux S, Abe J, Florian JA, Berk BC. 14-3-3 binding to Na+/H+ exchanger isoform-1 is associated with serum-dependent activation of Na+/H+ exchange. J Biol Chem 276: 15794–15800, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Liang X, Da Paula AC, Bozóky Z, Zhang H, Bertrand CA, Peters KW, Forman-Kay JD, Frizzell RA. Phosphorylation-dependent 14-3-3 protein interactions regulate CFTR biogenesis. Mol Biol Cell 23: 996–1009, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang X, Peters KW, Butterworth MB, Frizzell RA. 14-3-3 isoforms are induced by aldosterone and participate in its regulation of epithelial sodium channels. J Biol Chem 281: 16323–16332, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev 13: 1181–1189, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci USA 93: 5495–5500, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orian A, Gonen H, Bercovich B, Fajerman I, Eytan E, Israel A, Mercurio F, Iwai K, Schwartz AL, Ciechanover A. SCFβ(-TrCP) ubiquitin ligase-mediated processing of NF-κB p105 requires phosphorylation of its C-terminus by IκB kinase. EMBO J 19: 2580–2591, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through βPix/14-3-3/Nedd4-2. J Am Soc Nephrol 21: 833–843, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozuelo Rubio M, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N, Mackintosh C. 14-3-3 affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem J 379: 395–408, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prezeau L, Richman JG, Edwards SW, Limbird LE. The ζ isoform of 14-3-3 proteins interacts with the third intracellular loop of different α2-adrenergic receptor subtypes. J Biol Chem 274: 13462–13469, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Pulina MV, Rizzuto R, Brini M, Carafoli E. Inhibitory interaction of the plasma membrane Na+/Ca2+ exchangers with the 14-3-3 proteins. J Biol Chem 281: 19645–19654, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Rimessi A, Coletto L, Pinton P, Rizzuto R, Brini M, Carafoli E. Inhibitory interaction of the 14-3-3ε protein with isoform 4 of the plasma membrane Ca2+-ATPase pump. J Biol Chem 280: 37195–37203, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Riou P, Kjær S, Garg R, Purkiss A, George R, Cain RJ, Bineva G, Reymond N, McColl B, Thompson AJ, O'Reilly N, McDonald NQ, Parker PJ, Ridley AJ. 14-3-3 proteins interact with a hybrid prenyl-phosphorylation motif to inhibit G proteins. Cell 153: 640–653, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sands JM. Renal urea transporters. Curr Opin Nephrol Hypertens 13: 525–532, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Su H, Liu B, Fröhlich O, Ma H, Sands JM, Chen G. Small GTPase Rab14 downregulates UT-A1 urea transport activity through enhanced clathrin-dependent endocytosis. FASEB J 27: 4100–4107, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su H, Carter CB, Laur O, Sands JM, Chen G. Forskolin stimulation promotes urea transporter UT-A1 ubiquitination, endocytosis, and degradation in MDCK cells. Am J Physiol Renal Physiol 303: F1325–F1332, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su H, Chen M, Sands JM, Chen G. Activation of the cAMP/PKA pathway induces UT-A1 urea transporter monoubiquitination and targets it for lysosomal degradation. Am J Physiol Renal Physiol 305: F1775–F1782, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol 9: 661–664, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wall SM, Han JS, Chou CL, Knepper MA. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am J Physiol Renal Fluid Electrolyte Physiol 262: F989–F998, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Xiao L, Chen Y, Ji M, Volle DJ, Lewis RE, Tsai MY, Dong J. KIBRA protein phosphorylation is regulated by mitotic kinase aurora and protein phosphatase 1. J Biol Chem 286: 36304–36315, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002. [DOI] [PubMed] [Google Scholar]