Abstract
Combustion catalysts have been extensively explored to reduce the emission of hydrocarbons that are capable of triggering photochemical smog and greenhouse effect. Palladium as the most active material is widely applied in exhaust catalytic converter and combustion units, but its high capital cost stimulates the tremendous research on non-noble metal candidates. Here we fabricate highly defective cobalt oxide nanocrystals via a controllable oxidation of carbon-encapsulated cobalt nanoparticles. Strain gradients induced in the nanoconfined carbon shell result in the formation of a large number of active sites featuring a considerable catalytic activity for the combustion of a variety of hydrocarbons (methane, propane and substituted benzenes). For methane combustion, the catalyst displays a unique activity being comparable or even superior to the palladium ones.
 Palladium is an effective but expensive catalyst used in catalytic converters. Here, the authors show that defective Co3O4 nanocrystals, synthesized via oxidation of carbon-encapsulated cobalt nanoparticles, display similar or even comparable catalytic activity to palladium for hydrocarbon combustion.
Palladium is an effective but expensive catalyst used in catalytic converters. Here, the authors show that defective Co3O4 nanocrystals, synthesized via oxidation of carbon-encapsulated cobalt nanoparticles, display similar or even comparable catalytic activity to palladium for hydrocarbon combustion.
Extensive research has been performed in searching candidates for noble metal catalysts, but there is still a large demand for practically useful and acceptable catalysts for a variety of important reactions. Palladium (Pd) is the most active metal for catalytic combustion of hydrocarbons, while polyvalent metal oxides, especially cobalt oxide (Co3O4), as the promising candidates still cannot provide a satisfactory activity at the low-temperature region1,2. Undoubtedly, experimental and theoretical studies have evidenced that the geometry effect at the nanoscale can induce the unexpected properties of an active phase. Low coordinated atoms on stepped and kinked surfaces were reported to strongly bind reactants and often display an outstanding activity for a quantity of structure-sensitive reactions3,4,5. As a cheap candidate to replace noble metals for catalytic combustion, Co3O4 nanoparticles show a pronounced particle size effect on the reaction pathway of cyclohexane conversion6. Smaller nanoparticles with more edge and corner sites would lead to more strongly bound oxygen species to mediate the combustion pathway rather than oxidative dehydrogenation one. Co3O4 nanocrystals were also found to show a strong crystal plane effect for catalytic combustion of methane (CH4), that is, the activity order follows {112}>{011}>>{001} (ref. 7). Hence, the selective synthesis of Co3O4 nanocrystals containing high-index facets is a key to achieve a high reaction rate being comparable to those of noble metals.
The oxidation reaction of nanostructured metals such as cobalt (Co) and iron can generate nanoscale voids inside the nanocrystals being driven by the Kirkendall effect, which is assigned to the difference in diffusion rates between the anion and cation8,9,10. The atomic diffusion of matter is balanced by an opposite flow of vacancies that are capable to condense into pores and result in deformation or void formation. In case this process is confined into a nano-sized core, both the high surface-to-volume ratio of the particle and the absence of defects in the core remarkably enhance the rate of vacancy injection. The arc-discharging method has been applied to synthesize the carbon-encapsulated Co (Co@C) nanoparticles with a thin graphitic layer as the shell. Using the Co@C core–shell nanocapsules as the starting materials, it should be feasible to produce Co3O4 nanocrystals with a highly defective surface that may display a satisfactory activity for hydrocarbon combustion reaction.
Here we report a systematic study on the formation process and catalytic performance of highly active Co3O4 nanocrystals for the combustion of CH4 and other hydrocarbons. Co3O4 nanocrystals are produced via an in situ oxidation of Co@C core–shell nanocapsules (carbon content=6.8 wt%; ref. 11). Our studies suggest that the strain gradients induced in the nanoconfined graphitic shell are beneficial not only to generate abundant active sites for enhancing catalytic activity but also to prevent the severe aggregation of metal nanoparticles for keeping stability.
Results
Fabrication and characterization of active Co3O4 nanocrystals
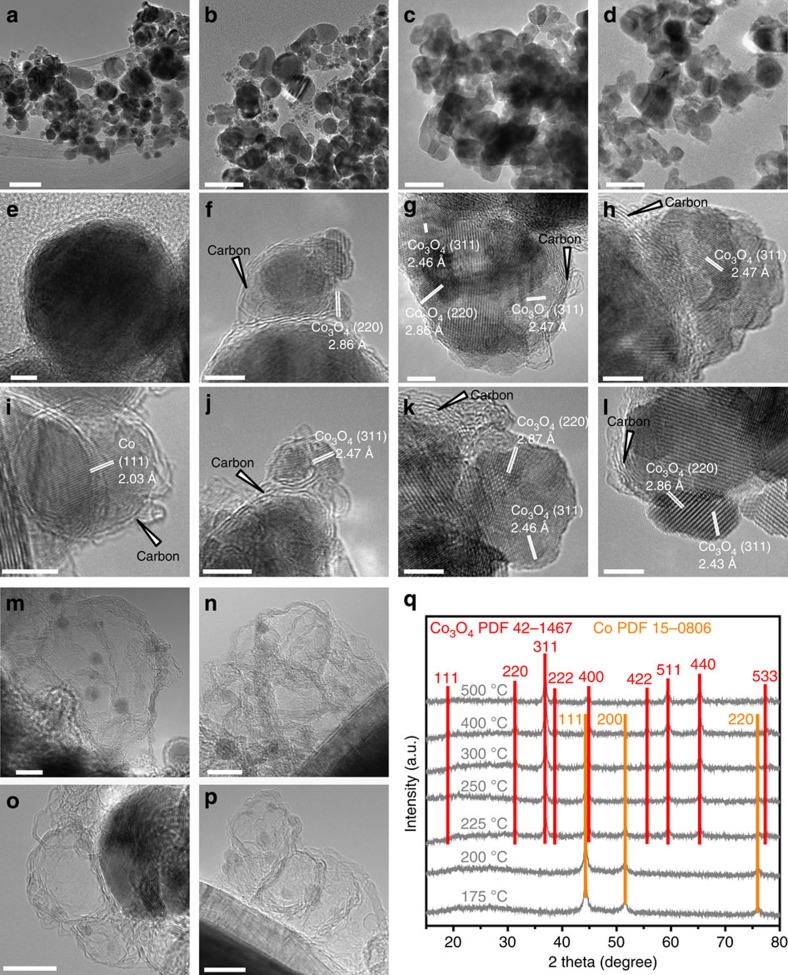
To follow the structural change of the Co@C nanocapsules in a flow of diluted oxygen, we used transmission electron microscope (TEM) to detail the morphological properties of the sample after the oxidation at various temperatures. As shown in Fig. 1a–l, the Co@C nanocapsules present as mostly regular spheres with a diameter ranging from 8 to 50 nm. A typical core–shell structure comprises several atomic layers of graphitic carbon as a shell and metallic Co as a core featuring with d002 spacing of 2.03 Å. After the oxidation at 200 °C, the carbon shell of nanocapsules with a diameter of ∼5 nm was cracked due to the burning by O2, while big nanocapsules with highly graphitic shells still kept unchanged. This difference can be related with the chemical reactivity of the carbon atoms on the surface of small nanocapsules with a high surface-to-volume ratio12. One metallic core was simultaneously oxidized and disintegrated into several Co3O4 nanocrystals with lattice distances of 2.44 and 2.86 Å, being assigned to {311} and {220} planes, respectively. These oxide particles spilled from the rupture while the carbon shells were severely deformed (Fig. 1m–p), indicating that a large strain force was produced during the oxidative destruction of nanocapsules. As the treatment temperature was elevated, the Co3O4 nanocrystals started to aggregate but still kept an irregular shape, while the oxidation of Co nanoparticles without carbon shells is apt to produce oxide particles with a regular shape (Supplementary Fig. 1).
Figure 1. Morphological properties of oxidized Co@C nanocapsules.
TEM and HRTEM images of (a,e,i) the pristine and samples that were oxidized at (b,f,j) 200, (c,g,k) 225 and (d,h,l) 250 °C, as well as (m–p) the residual carbon shells in the oxidized samples at 225 °C after washing by hydrochloric acid. Scale bars, 50 (a–d), 5 (e–l) and 10 nm (m–p). (q) XRD spectra of oxidized samples.
The phase transformation of Co@C nanocapsules in the oxidative atmosphere can be related with the carbon shell as the template. It can be clearly seen in the TEM images (Fig. 1a–l) that defective carbon shell encapsulated or supported Co3O4 nanoparticles as the transition state. We note that our case obviously differs from the oxidation of exposed metallic Co nanoparticles undergoing successive diffusion of atoms and unrestrained release of lattice stress13. Rapid exposure of the metallic core to an oxygen-rich atmosphere will first cause the complete oxidation into Co3O4, being confirmed by the absence of other Co oxides in powder X-ray diffraction (XRD) patterns (Fig. 1q). Meanwhile, the abrupt transformation from the high-density metallic Co (8.79 g cm−3) to low-density spinel Co3O4 (6.06 g cm−3) would cause ∼45% of volume increase14. Since the oxidation temperature is far below the melting points of Co and its oxides15,16, the rapid expansion of the confined core is expected to produce an extensive strain force, inducing the rupture or fragmentation of oxides (Fig. 1a–l). The high-resolution TEM (HRTEM) images of the seriously distorted carbon shells in Fig. 1m–p are particularly important to demonstrate the instant release of deformation force from the core. Quite rough shape and irregular morphology of the extruded Co3O4 nanoparticles indicate the existence of some high-index facets that are capable to provide a superior activity for the combustion reaction7, which can be supported by the diffraction of many high-index crystal planes in the XRD pattern of the used catalyst (Supplementary Fig. 2).
Catalytic activity and kinetic measurement
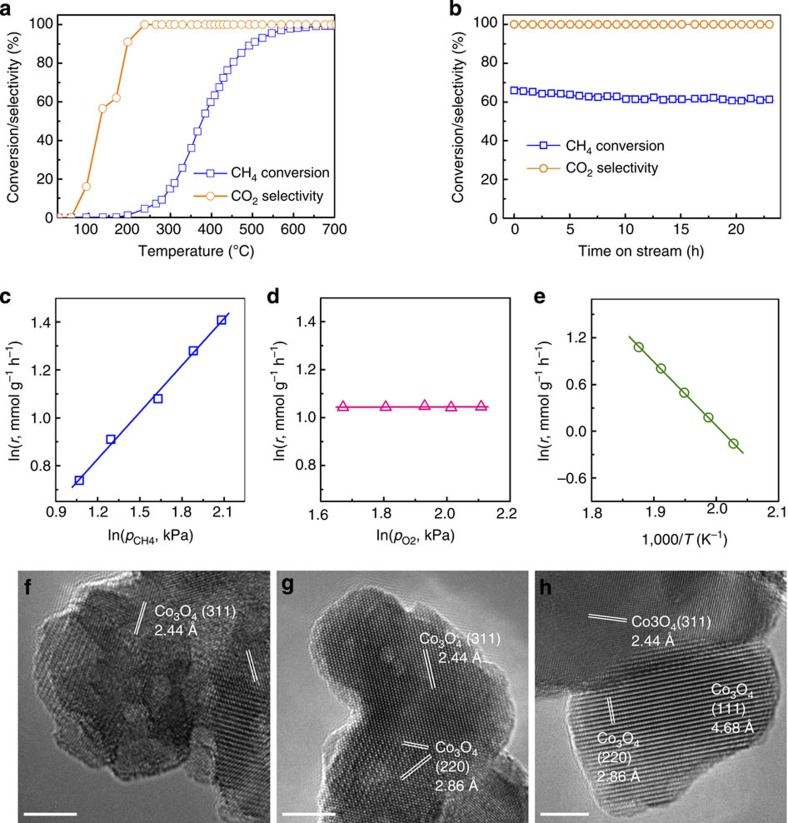
Catalytic combustion of CH4 (CH4+2O2→CO2+2H2O) was used as a probe reaction to evaluate the catalytic properties. The reaction was conducted at 25–700 °C in an oxygen-rich environment with an O2 to CH4 ratio of 2.5. For each test, the product mixture with CO2, CO and residual CH4 gives an almost close carbon balance of 100±3%. In a blank experiment without catalyst, the conversion of CH4 was negligible even at the temperature as high as 700 °C. Temperature dependency of CH4 conversion in the combustion reaction over the catalyst is presented in Fig. 2a, showing that the light-off temperature in the conversion curve was ∼220 °C with 100% selectivity to CO2. The temperature at a conversion of 50% (T50), an important indicator to the activity of a combustion catalyst, is 376 °C, reporting a considerable reaction rate at T50, that is, 26.8 mmol g−1 h−1. Such an activity at a low temperature is superior to the transition metal oxides but close to or even higher than the supported Pd catalysts (Supplementary Fig. 3). The time on stream experiment reveals a satisfactory durability at 420 °C. Although a slight decrease in conversion was observed over the initial several hours, the activity had stabilized and remained at above 60% for ∼20 h. During the whole period, the selectivity to CO2 did not change and kept at almost 100% (Fig. 2b). The catalyst is also capable to efficiently combust propane, aromatics (benzene, toluene, xylenes, ethylbenzene and styrene) and carbon monoxide17 at relatively low temperatures (Supplementary Fig. 4).
Figure 2. Reaction data.
(a) Light-off curves of CH4 combustion against the increasing temperature and (b) catalytic stability at 420 °C under conditions: 0.1 g catalyst, 6.7% CH4, O2/CH4=2.5, helium balance, space velocity 18 l gcat−1 h−1. Dependencies of reaction rate (r, mmol g−1 h−1) on partial pressures of (c) CH4 (pCH4) and (d) O2 (pO2) at 260 °C, under conditions: 5 mg catalyst, 1.6–5.0 kPa CH4, 5.3–8.4 kPa O2, helium balance, space velocity 800 l gcat−1 h−1. (e) Arrhenius-type plot for CH4 combustion under conditions: 5 mg catalyst, 3.0 kPa CH4, 7.6 kPa O2, helium balance, 220–260 °C, space velocity 800 l gcat−1 h−1. T, Kelvin temperature. HRTEM images of the used catalysts after the reaction at temperatures of (f) 225, (g) 250 and (h) 400 °C. Scale bars, 5 nm (f–h).
To elucidate the reaction mechanism of this process, we conducted the kinetic experiments in a differential reactor to rule out both mass and heat transport effects. Reaction rate approached the same value at a gaseous hourly space velocity of reactants being as high as 18,000 ml g−1 h−1. The reaction orders of CH4 and O2 were determined from the logarithm plot of reaction rates versus partial pressure of each reactant, while the apparent activation energy of CH4 combustion was derived from the Arrhenius plot (Fig. 2c–e). The reaction orders of CH4 and O2 were 0.652 and 0.003, respectively, both of which are quite close to those on noble metal catalysts such as Pd, rhodium and so on. (Supplementary Table 1). The tiny reaction order of O2 indicates that the reaction rate is independent on the partial pressure of O2, and the catalyst surface is saturated by oxygen species without competition between CH4 and O2 molecules18. The apparent activation energy of 68±1 kJ mol−1 is even lower than some reported values of activation energies for platinum (67–138 kJ mol−1; ref. 19) and Pd catalysts (70–90 kJ mol−1; refs 19, 20). This value is also very close to those of some Co3O4 catalysts21,22, suggesting an identical structure of the active centres. We therefore attribute the reason for the high catalytic activity to the richness in active sites on the surface of our catalyst. HRTEM images of the used catalysts after reaction at various temperatures were depicted in Fig. 2f–h, indicating that the catalyst surface is highly defective to present a great deal of steps and kinks.
Structural stability of Co3O4 catalyst
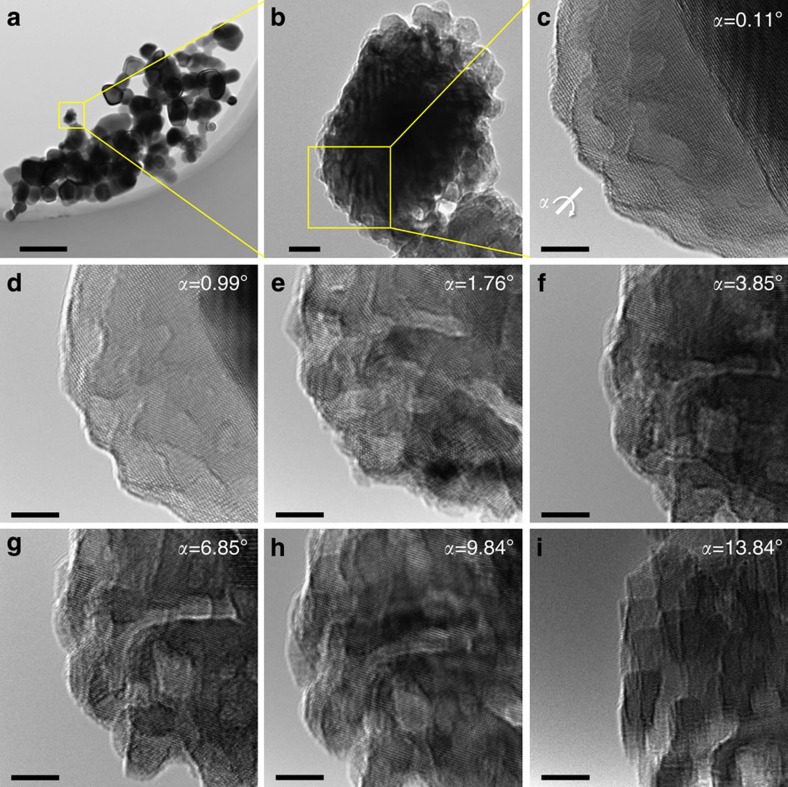
It is worth mentioning that the obtained Co3O4 nanoparticles displayed an unexpected thermal stability against severe sintering at a high temperature. We detailed the microstructure of the catalyst after the reaction at 700 °C (Fig. 3) by tilting the sample holder inside the TEM chamber. The images clearly reveal the existence of concavities or cracks on the surface and an interconnecting characteristic at a specific angle. The diameter of pores or channels is estimated to be 2–4 nm, being higher than mean free path of reactants and products to allow the efficient mass transport. The nanoscale and devious channels are expected to possess a large number of surface atomic steps on the interior wall that often acted as the catalytically active sites in molecular dissociation and consequential reaction. Although the phase transition at the elevated temperatures led to particle aggregation, the defective carbon shell still plays a pivotal role to prevent serious sintering at the initial stage. The temperature-programmed oxidation test shows that the carbon shells totally disappeared at ∼550 °C (Supplementary Fig. 5). As the metallic Co atoms reacted with O2, the oxidation of metallic subsurface at the defective sites was much faster than that in the regions capsulated by well-defined graphitic layers due to the diffusion of oxygen. The atomic diffusion of residual metallic Co into the oxygen-rich phase being driven by the Kirkendall effect finally resulted in a number of disordered voids or channels inside the enlarged particles9. Note that the residual carbon shells can also block the approach of reactant molecules to active sites, which would certainly reduce the overall catalytic activity to some extent.
Figure 3. Morphology of catalysts after reaction at 700 °C.
TEM images during tilting the sample holder from 0.11° to 13.84°. Scale bars, 200 (a), 10 (b) and 5 nm (c–i).
Discussion
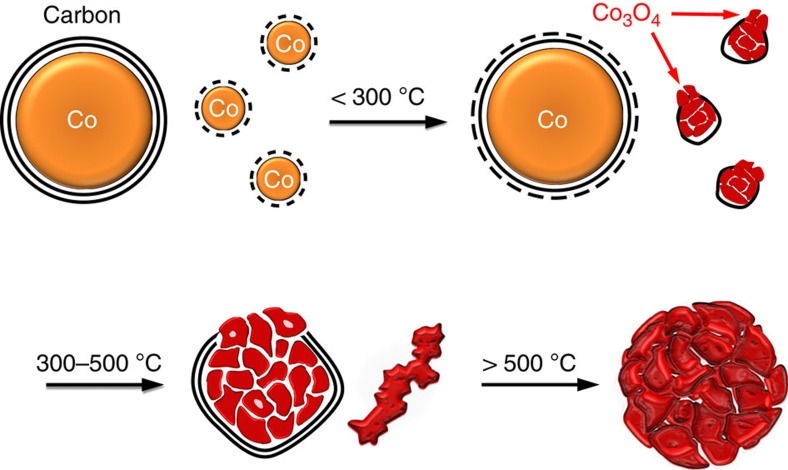
We have demonstrated here the highly active and durable Co3O4 nanocrystals deriving from Co@C nanocapsules, which efficiently catalysed the combustion of various hydrocarbons especially CH4. The formation process of Co3O4 nanocrystals is illustrated in Fig. 4. The attacks of oxygen result in the partial combustion of carbon shells and consequently the oxidation of subjacent metallic Co atoms, raising Co oxides islands due to the inward diffusion of oxygen. Meanwhile, the immediate volumetric expansion inside the residual carbon shells gives rise to a large strain force to induce the disintegration of bulk oxides. Finally, at elevated temperatures, the oxides nanocrystals with an enlarged particle size and nanoscale interconnecting pores were formed after the complete burning of carbon shells. The defective surface comprising mostly high-index facets endows a considerable activity for combustion of alkanes, and the deviously interconnecting channels allow an efficient mass transfer of reactants. Provided more efforts were made to optimize the structural parameters of nanocapsules, this strategy can be used to synthesize a variety of oxides catalysts for applications in more catalysis processes.
Figure 4. Scheme for the transformation process of the Co3O4 catalyst.
The attenuation of carbon shells in oxygen-containing reaction condition allows the inward diffusion of oxygen and the oxidation of subjacent metallic Co atoms gradually. The volumetric expansion due to phase transformation gives rise to a large strain force to induce the disintegration of bulk oxides. The Co3O4 nanocrystals were finally formed with nanoscale interconnecting pores at elevated temperatures.
Methods
Preparation of Co@C
The Co@C nanocapsules were prepared by evaporating pure Co ingot with the modified arc-discharge technique. The Co ingot with purity 99.99% was used as anode target while a graphite needle with a diameter of 8 mm was served as cathode. As the vacuum of chamber reached 5 × 10−3 Pa, the argon and hydrogen with purity of 99.99% were introduced into the chamber to be served as the source of plasma, which lead chamber pressure increase of 0.2 and 0.02 MPa, respectively. Subsequently, 20 ml pure ethanol served as the carbon source was introduced into the chamber. During the arc-discharging process, the Co atoms were evaporated out from the Co ingot to form the Co nanoparticles, and then encapsulated by the carbon atoms decomposed from the ethanol to finally form the Co@C nanocapsules.
Characterization
The morphology and microstructure characterization was performed by a HRTEM (Techni F20) with a double tilt holder operating at emission voltages of 200 kV. The phase composition of the samples were analysed by powder XRD with Cu-Kα (λ=0.154,056 nm). The phase transition process of Co@C in oxygen-containing condition was simulated by the temperature-programmed oxidation analysis in air on a thermogravimetric analyser (Netzsch 449F3) at a heating rate of 10 °C min−1, which the outlet attached to an AVI Omnistar 200 mass spectrometer to monitor the emergent gases. The adsorption–desorption isotherms of nitrogen were performed on a Micromeritics ASAP 2020 instrument. The CO chemisorption was carried out on a Micromeritics AutoChem II 2920, and the procedure was the same as that described in the reference23. The results were shown in Supplementary Fig. 6a,b.
Catalytic evaluation
The catalytic combustion reaction was carried out in an immobilized bed quartz reactor (8 mm i.d.) in a tri-heating system at the atmospheric pressure. Co@C (100 mg) or carbon-free Co powder diluted with quartz sands was fixed between two layers of quartz wool to maintain a 10 mm height of the bed (Supplementary Fig. 6c). The feed gases consisted of CH4 (hydrocarbons or CO), O2 and He were controlled individually by three mass flow controllers (Brooks, USA) to vary the total flows and partial pressures of the reactants. The product components leaving the reactor were monitored by an on-line gas chromatograph (Agilent 7890A, USA) with flame ionization detector and thermal conductivity detector. Blank experiments showed that reaction rates were negligible without catalyst.
Additional information
How to cite this article: Wang, H. et al. In situ oxidation of carbon-encapsulated cobalt nanocapsules creates highly active cobalt oxide catalysts for hydrocarbon combustion. Nat. Commun. 6:7181 doi: 10.1038/ncomms8181 (2015).
Supplementary Material
Supplementary Figures 1-6, Supplementary Table 1 and Supplementary References
Acknowledgments
We gratefully acknowledged the discussion with Dr Di Wang (Karlsruhe Institute of Technology) and the financial support from National Natural Science Foundation of China (51422212, 51202262, 51271178, 51331006 and 21403261), and the Laboratory of Science and Technology on Surface Physics and Chemistry (ZDXKFZ201304).
Footnotes
Author contributions J.Z. and S.M. proposed and supervised the overall project. H.W. and S.M. conducted the Co@C preparation and the tilting TEM characterization. C.C. carried out all the oxidation treatments and catalytic experiments, as well as the manuscript preparation. J.Z. performed HRTEM characterizations and structural analysis, as well as revised the manuscript. T.Y., H.G., Y.Z. and L.P. helped the catalysts preparation and characterization. Z.Z. and D.S.S. contributed to scientific discussion.
References
- Ciuparu D., Lyubovsky M. R., Altman E., Pfefferle L. D. & Datye A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev. 44, 593–649 (2002). [Google Scholar]
- Cargnello M. et al. Exceptional activity for methane combustion over modular Pd@CeO2 subunits on functionalized Al2O3. Science 337, 713–717 (2012). [DOI] [PubMed] [Google Scholar]
- Norskov J. K. & Abild-Pedersen F. Catalysis: bond control in surface reactions. Nature 461, 1223–1225 (2009). [DOI] [PubMed] [Google Scholar]
- Lee I., Delbecq F., Morales R., Albiter M. A. & Zaera F. Tuning selectivity in catalysis by controlling particle shape. Nat. Mater. 8, 132–138 (2009). [DOI] [PubMed] [Google Scholar]
- Van Santen R. A. Complementary structure sensitive and insensitive catalytic relationships. Acc. Chem. Res. 42, 57–66 (2009). [DOI] [PubMed] [Google Scholar]
- Tyo E. C. et al. Oxidative dehydrogenation of cyclohexane on cobalt oxide (Co3O4) nanoparticles: The effect of particle size on activity and selectivity. ACS Catal 2, 2409–2423 (2012). [Google Scholar]
- Hu L. H., Peng Q. & Li Y. D. Selective synthesis of Co3O4 nanocrystal with different shape and crystal plane effect on catalytic property for methane combustion. J. Am. Chem. Soc. 130, 16136–16137 (2008). [DOI] [PubMed] [Google Scholar]
- Smigelskas A. D. & Kirkendall E. O. Zinc diffusion in alpha-brass. Trans. Am. Inst. Min. Metal. Eng. 171, 130–142 (1947). [Google Scholar]
- Yin Y. D. et al. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 304, 711–714 (2004). [DOI] [PubMed] [Google Scholar]
- Zhou J. S. et al. Carbon-encapsulated metal oxide hollow nanoparticles and metal oxide hollow nanoparticles: a general synthesis strategy and its application to lithium-ion batteries. Chem. Mater. 21, 2935–2940 (2009). [Google Scholar]
- Wang H. et al. Optimal electromagnetic-wave absorption by enhanced dipole polarization in Ni/C nanocapsules. Appl. Phys. Lett. 101, 083116–1 (2012). [Google Scholar]
- Müller J.-O., Frank B., Jentoft R. E., Schlögl R. & Su D. S. The oxidation of soot particulate in the presence of NO2. Catal. Today 191, 106–111 (2012). [Google Scholar]
- Ha D. H., Moreau L. M., Honrao S., Hennig R. G. & Robinson R. D. The oxidation of cobalt nanoparticles into Kirkendall-hollowed CoO and Co3O4: The diffusion mechanisms and atomic structural transformations. J. Phys. Chem. C 117, 14303–14312 (2013). [Google Scholar]
- Nam K. M. et al. Syntheses and characterization of wurtzite CoO, rocksalt CoO, and spinel Co3O4 nanocrystals: Their interconyersion and tuning of phase and morphology. Chem. Mater. 22, 4446–4454 (2010). [Google Scholar]
- Sun J. & Simon S. L. The melting behavior of aluminum nanoparticles. Thermochim. Acta 463, 32–40 (2007). [Google Scholar]
- Jian Z. Y., Kuribayashi K. & Jie W. Q. Solid-liquid interface energy of metals at melting point and undercooled state. Mater. Trans. 43, 721–726 (2002). [Google Scholar]
- Xie X. et al. Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458, 746–749 (2009). [DOI] [PubMed] [Google Scholar]
- Yao Y. F. Y. Oxidation of alkanes over noble-metal catalysts. Ind. Eng. Chem. Prod. Res. Dev. 19, 293–298 (1980). [Google Scholar]
- Beck I. E. et al. Platinum nanoparticles on Al2O3: Correlation between the particle size and activity in total methane oxidation. J. Catal. 268, 60–67 (2009). [Google Scholar]
- Ribeiro F. H., Chow M. & Dallabetta R. A. Kinetics of the complete oxidation of methane over supported palladium catalysts. J. Catal. 146, 537–544 (1994). [Google Scholar]
- Teng F. et al. High combustion activity of CH4 and catalluminescence properties of CO oxidation over porous Co3O4 nanorods. Appl. Catal. B 110, 133–140 (2011). [Google Scholar]
- Kirchnerova J., Alifanti M. & Delmon B. Evidence of phase cooperation in the LaCoO3-CeO2-Co3O4 catalytic system in relation to activity in methane combustion. Appl. Catal. A 231, 65–80 (2002). [Google Scholar]
- Zhu Z. Z. et al. Highly active and stable Co3O4/ZSM-5 catalyst for propane oxidation: effect of the preparation method. ACS Catal. 3, 1154–1164 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-6, Supplementary Table 1 and Supplementary References