Abstract
Background: CD154 and its soluble counterpart (sCD154) are proteins of the tumor necrosis factor (TNF) family and exhibit proinflamatory and procoagulant properties. Higher circulating sCD154 levels have been found in ischemic stroke patients than in controls. However, the association between circulating sCD154 levels and mortality in ischemic stroke patients has not been reported, and was the focus of this study. Methods: This was a multicenter, observational and prospective study carried out in six Spanish Intensive Care Units. We measured serum sCD154 from 50 patients with severe malignant middle cerebral artery infarction (MMCAI), defined as Glasgow Coma Scale (GCS) lower than 9, at the moment of the severe MMCAI diagnosis and from 50 healthy controls. The end-point of the study was 30-day mortality. Results: We found higher serum sCD154 levels in patients with severe MMCAI than in healthy controls (p < 0.001). We found higher serum sCD154 levels (p < 0.001) in non-surviving (n = 26) than in surviving MMCAI patients (n = 24). Multiple binomial logistic regression analysis showed that serum sCD154 levels >1.41 ng/mmL were associated with 30-day mortality (OR = 10.25; 95% CI = 2.34–44.95; p = 0.002). Conclusions: The new more important finding of our study was that serum sCD154 levels in MMCAI patients were associated with mortality.
Keywords: sCD154, cerebral infarction, patients, mortality
1. Introduction
Ischemic stroke is an important cause of disability, mortality and resources consumption [1]. CD154 is a protein of the tumor necrosis factor (TNF) family, and is expressed by platelets, B cells, monocytic cells, natural killer cells, mast cells, and basophils. CD40 is also expressed on T cells, and predominantly on T cells that promote inflammation. Th40 cells are highly associated with autoimmune diseases including type 1 diabetes and multiple sclerosis, and produces TNF and interleukin (IL)-6 [2]. CD154 and its soluble counterpart (sCD154) are proteins that exhibit proinflammatory and procoagulant properties when binding to their cell surface receptor CD154 [3]. CD154 is a member of the TNF receptor family that is expressed on the surface of many cells, such as endothelial cells, smooth muscle cells, B cells, monocytes, and microglia.
Higher CD154 platelet expression in ischemic stroke patients than in controls [4,5], higher circulating sCD154 levels in ischemic stroke patients than in controls [6,7,8,9,10,11,12,13], and higher CD154 platelet expression in ischemic stroke patients with poor functional outcome [14,15] have been reported. However the association between circulating sCD154 levels and mortality in ischemic stroke patients has not been reported, and that was the objective of this study.
2. Methods
2.1. Design and Subjects
This is a multicenter, observational, prospective study carried out in six Intensive Care Units of Spain. The study was approved by the Institutional Review Board of the six participant hospitals: Hospital Universitario de Canarias (La Laguna, Santa Cruz de Tenerife, Spain), Hospital Universitario Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain), Hospital General de La Palma (La Palma, Spain), Hospital Clínico Universitario de Valencia (Valencia, Spain), Hospital Insular (Las Palmas de Gran Canaria, Spain), and Hospital Universitario Dr. Negrín (Las Palmas de Gran Canaria, Spain). Written informed consent from the patients or from their legal guardians was obtained.
We included 50 patients with severe malignant middle cerebral artery infarction (MMCAI) and 50 healthy volunteer control subjects. Severity of MMCAI was classified according to Glasgow Coma Scale (GCS) [16], and we included patients with GCS ≤8. Exclusion criteria were: age less than 18 years, pregnancy, inflammatory or malignant disease.
2.2. Variables Recorded
The following variables were recorded for each patient: sex, fibrinolityc therapy, decompressive craniectomy, age, temperature, sodium, glycemia, leukocytes, pressure of arterial oxygen (PaO2), PaO2/pressure of arterial oxygen/fraction inspired oxygen (FI02) ratio, bilirubin, creatinine, hemoglobin, GCS, lactic acid, platelets, international normalized ratio (INR), activated partial thromboplastin time (aPTT), fibrinogen, Acute Physiology and Chronic Health Evaluation II (APACHE II) score [17]. The end-point of the study was 30-days mortality.
2.3. Blood Sample Collection
We recollected blood samples from 50 patients with severe MMCAI at the moment of the diagnosis to measure serum sCD154 levels, serum TNF-alpha levels, and plasma tissue factor (TF) levels. In addition, there were recollected blood samples from 50 healthy controls to measure serum sCD154 levels. To avoid the possible dispersion of serum level results, all the samples were processed at the same time and in the same laboratory, at the end of the recruitment process.
2.4. Laboratory Determinations
Venous blood samples were collected in serum separator tubes (SST) for determination of serum sCD154 and TNF-alpha levels, and in citrate tubes to determine plasma TF levels. Blood samples were centrifuged within 30 min at 1000× g for 15 min. The serum and plasma were removed and frozen at −80 °C until measurement. The determination of serum sCD154 and TNF-alpha levels, and plasma TF levels were centralized in the Laboratory Department of the Hospital Universitario de Canarias (La Laguna, Santa Cruz de Tenerife, Spain).
Serum sCD154 levels were assayed by specific ELISA (Bender MedSystems GmbH, Vienna, Austria). The intra-assay and inter-assay coefficients of variation (CV) were 4% (n = 8) and 6.8% (n = 8) respectively; and detection limits for the assays was 0.06 ng/mL.
Serum TNF-alpha levels were measured by a solid-phase, chemiluminiscents immunometrics assays kit (Immulite®, Siemens Healthcare Diagnostics Products, Llanberis, United Kingdom). The intra-assay and inter-assay CV were <3.6% (n = 20) and <6.5% (n = 20) respectively; and detection limits for the assays was 1.7 pg/mL.
Plasma TF levels were assayed by specific ELISA (Imubind® Tissue Factor ELISA, American Diagnostica, Inc., Stanford, CT, USA). The intra-assay and inter-assay CV were <7.2% (n = 20) and <8% (n = 20) respectively; and detection limits for the assays was 10 pg/mL.
2.5. Statistical Methods
Continuous variables are reported as medians and interquartile ranges. Categorical variables are reported as frequencies and percentages. Comparisons of continuous variables between groups were carried out using Wilcoxon-Mann-Whitney test. Comparisons between groups on categorical variables were carried out with chi-square test.
Multiple binomial logistic regression analysis was applied to determine the independent contribution of serum sCD154 levels on 30-day mortality, controlling for GCS and age. Odds Ratio and 95% confidence intervals were calculated as measurement of the clinical impact of the predictor variables.
Receiver operating characteristic (ROC) analysis was carried out to determine the goodness-of-fit of the of serum sCD154 levels to predict 30-day mortality. Kaplan-Meier analysis of survival at 30 days and comparisons by log-rank test were carried out using serum sCD154 levels lower/higher than 1.41 ng/mL as the independent variable and survival at 30 days as the dependent variable. The association between continuous variables was carried out using Spearman’s rank correlation coefficient. A p value of less than 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and NCSS 2000 (Kaysville, UT, USA) and LogXact 4.1 (Cytel Co., Cambridge, MA, USA).
3. Results
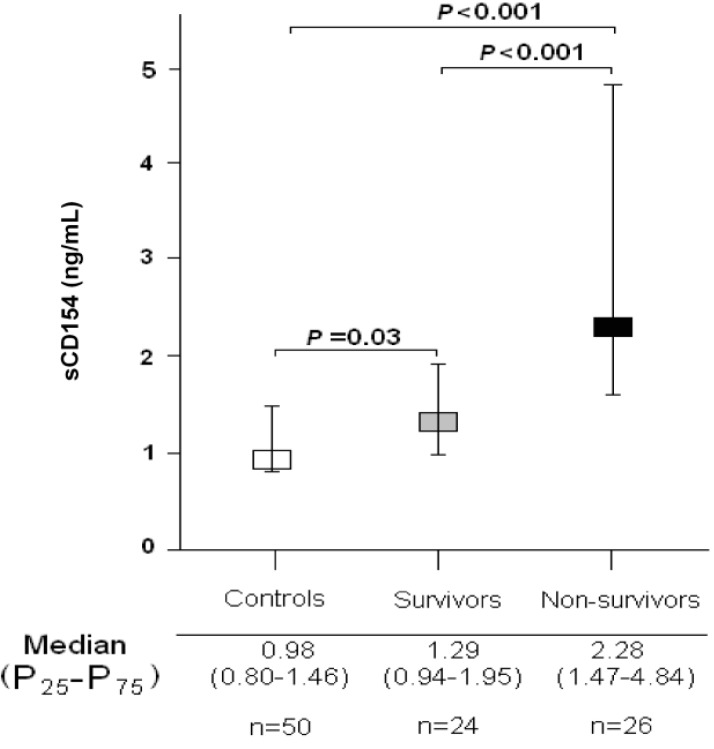
Table 1 shows the comparisons of age, sex and serum sCD154 levels between patients with severe MMCAI patients (n = 50) and healthy controls (n = 50). There were no significant differences between patients with severe MMCAI and healthy controls groups on age and sex. However, we found higher serum sCD154 levels in patients with severe MMCAI patients than in healthy controls (p < 0.001); in addition, serum sCD154 levels were higher in surviving (p = 0.03) and non-surviving patients with severe MMCAI patients (p < 0.001) compared to healthy controls (Figure 1).
Table 1.
Characteristics of healthy controls and patients with severe MMCAI.
| Healthy Controls (n = 50) | Patients (n = 50) | p-Value | |
|---|---|---|---|
| Gender female—n (%) | 15 (30.0%) | 17 (34.0%) | 0.83 |
| Age—median years (p 25–75) | 59 (50–68) | 60 (51–69) | 0.47 |
| Serum sCD154—median ng/mL (p 25–75) | 0.98 (0.80–1.46) | 1.79 (1.22–3.56) | <0.001 |
Figure 1.
Serum sCD154 levels in severe malignant middle cerebral artery infarction patients and healthy controls.
Table 2 shows the comparisons between non-surviving (n = 26) and surviving (n = 24) MMCAI patients. There were no significant differences between non-surviving and surviving patients in temperature, sodium, PaO2, PaO2/FI02 ratio, lymphocytes, leukocytes, lactic acid, INR, hemoglobin, glycemia, GCS score, sex, fibrinogen, creatinine, bilirubin, aPTT, APACHE-II score and age. We found that non-surviving showed compared to surviving patients lower platelet count, and higher circulating levels of TNF-alpha, TF, and sCD154 (p < 0.001). In addition, non-surviving patients had a lower ICU stay duration than surviving (4 (2–9) vs. 23 (16–40) days; p < 0.001).
Table 2.
Clinical and biochemical characteristics of MMCAI patients according to 30-day survival.
| Survivors (n = 24) | Non-Survivors (n = 26) | p Value | |
|---|---|---|---|
| TNF-alpha (pg/mL)—median (p 25–75) | 9.25 (9.02–10.63) | 12.95 (10.03–15.08) | 0.01 |
| TF (pg/mL)—median (p 25–75) | 156 (127–196) | 279 (181–400) | 0.02 |
| Temperature (°C)—median (p 25–75) | 36.5 (35.7–37.0) | 37.0 (35.7–37.8) | 0.26 |
| Sodium (mEq/L)—median (p 25–75) | 140 (138–145) | 140 (137–146) | 0.91 |
| sCD154 (ng/mL)—median (p 25–75) | 1.29 (0.94–1.95) | 2.28 (1.47–4.84) | <0.001 |
| Platelets—median × 103/mm3 (p 25–75) | 227 (183–308) | 152 (123–190) | 0.003 |
| PaO2 (mmHg)—median (p 25–75) | 110 (101–194) | 104 (85–139) | 0.10 |
| PaO2/FI02 ratio—median (p 25–75) | 246 (192–327) | 248 (175–320) | 0.41 |
| Lymphocytes—median × 103/mm3 (p 25–75) | 1.5 (0.9–1.8) | 1.1 (0.5–2.1) | 0.30 |
| Leukocytes—median × 103/mm3 (p 25–75) | 12.8 (9.8–16.9) | 14.4 (11.9–21.9) | 0.49 |
| Lactic acid (mmol/L)—median (p 25–75) | 1.25 (0.93–1.68) | 1.50 (1.01–3.15) | 0.08 |
| INR—median (p 25–75) | 1.07 (1.01–1.20) | 1.20 (1.07–1.48) | 0.16 |
| Hemoglobin (g/dL)—median (p 25–75) | 12.0 (11.3–13.8) | 12.0 (11.0–15.1) | 0.92 |
| Glycemia (g/dL)—median (p 25–75) | 133 (105–170) | 135 (110–154) | 0.92 |
| GCS score—median (p 25–75) | 7 (6–8) | 6 (4–8) | 0.10 |
| Gender female—n (%) | 8 (33.3) | 9 (34.6) | 0.99 |
| Fibrinogen (mg/dL)—median (p 25–75) | 440 (335–494) | 409 (322–598) | 0.71 |
| Decompressive craniectomy—n (%) | 7 (29.2) | 5 (19.2) | 0.51 |
| Creatinine (mg/dL)—median (p 25–75) | 0.80 (0.60–1.10) | 1.01 (0.85–1.45) | 0.052 |
| Bilirubin (mg/dL)—median (p 25–75) | 0.50 (0.38–0.90) | 0.53 (0.30–1.20) | 0.76 |
| aPTT (seconds)—median (p 25–75) | 28 (25–29) | 26 (25–33) | 0.96 |
| APACHE-II score—median (p 25–75) | 20 (16–25) | 22 (19–29) | 0.14 |
| Age (years)—median (p 25–75) | 47 (32–67) | 66 (45–76) | 0.14 |
p 25–75 = percentile 25th–75th; PaO2 = pressure of arterial oxygen/fraction inspired oxygen; FIO2 = pressure of arterial oxygen/fraction inspired oxygen; TNF = tumor necrosis factor; TF = tissue factor; INR = international normalized ratio; GCS = Glasgow Coma Scale; aPTT = activated partial thromboplastin time; APACHE II = Acute Physiology and Chronic Health Evaluation.
We found in multiple binomial logistic regression analysis that serum sCD154 levels >1.41 ng/mmL were associated with 30-day mortality (OR = 10.25; 95% CI = 2.34–44.95; p = 0.002) controlling for GCS and age (Table 3).
Table 3.
Multiple binomial logistic regression analysis to predict 30-day mortality.
| Variable | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Serum sCD154 > 1.41 ng/mmL | 10.25 | 2.34–44.95 | 0.002 |
| GCS score | 0.72 | 0.49–1.06 | 0.09 |
| Age (years) | 1.05 | 0.99–1.11 | 0.08 |
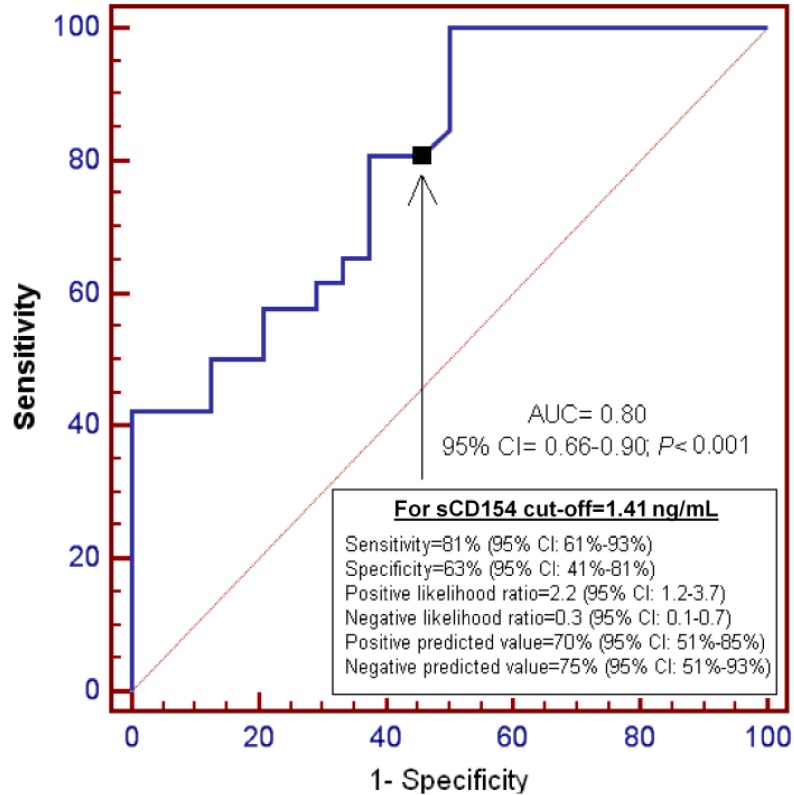
Figure 2 shows ROC analysis of serum sCD154 levels to predict 30-day mortality. We found that the area under the curve (AUC) was 0.80 (95% CI = 0.66–0.90; p < 0.001). Diagnostic goodness-of-fit for serum CD154 levels of 1.41 ng/mL were the following: sensitivity = 81% (95% CI = 61%–93%), specificity = 63% (95% CI = 41%–81%).
Figure 2.
Receiver operation characteristic analysis using serum sCD154 levels as predictor of mortality at 30 days.
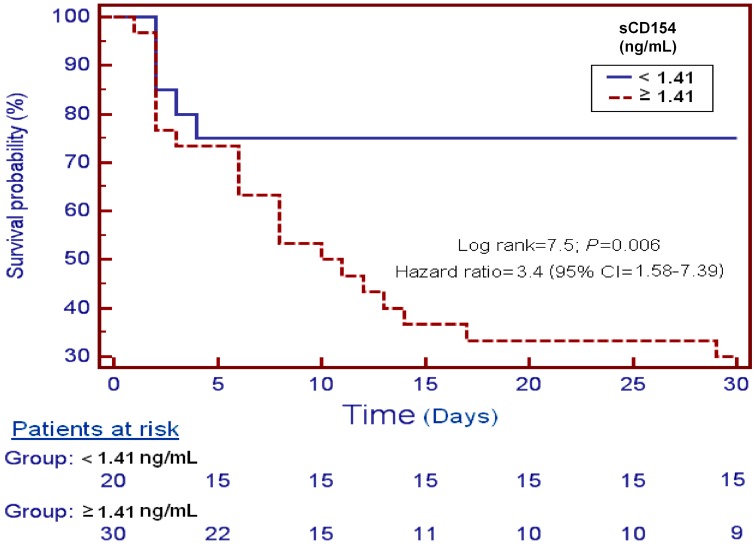
Figure 3 shows survival analysis of patients with serum sCD154 levels higher and lower of 1.41 ng/mmL, and survival at 30 days as the dependent variable. We found that patients with serum sCD154 levels higher than 1.41 ng/mmL showed higher mortality at 30 days than patients with lower levels (Hazard ratio = 3.4; 95% CI = 1.58–7.39; p = 0.006).
Figure 3.
Survival curves at 30 days using serum sCD154 levels higher or lower than 1.41 ng/mL.
We found an association of circulating levels of sCD154 with GCS (rho = −0.21; p = 0.04), TF (rho = 0.52; p < 0.001) and TNF-alpha (rho = 0.54; p < 0.001).
4. Discussion
The novel findings of our study were the following: (a) non-surviving severe MMCAI patients had higher serum sCD154 levels than surviving patients; (b) there is an association between circulating levels of sCD154, GCS, TF and TNF-alpha in patients with severe MMCAI; (c) serum sCD154 levels could be used as prognostic biomarker in patients with severe MMCAI.
We found higher serum sCD154 in MMCAI patients than in controls, and previously, higher CD154 platelet expression in ischemic stroke patients than in controls were reported [4,5], as well as higher circulating sCD154 levels in ischemic stroke patients than in controls [6,7,8,9,10,11,12,13].
Previously higher CD154 platelet expression in ischemic stroke patients with poor functional outcome [14,15] was reported. However, in our study we found for the first time, an association between circulating levels of sCD154 and stroke severity assessed by GCS, and higher serum sCD154 levels in non-surviving severe MMCAI patients than in surviving patients, an association between serum sCD154 levels and mortality in logistic regression analysis, and that serum sCD154 levels could predict mortality according to the ROC analysis. The findings are in consonance with the results of previous studies showing higher sCD154 in ischemic stroke patients with poor functional outcome [14,15] and with the findings of other studies reporting an association between serum sCD154 levels and mortality in patient with acute coronary syndrome [18], sepsis [19,20] and brain trauma injury [21].
Circulating sCD154 levels in MMCAI patients could play a physiological role by its proinflammatory [22,23] and procoagulant [24,25,26,27,28,29] actions. CD154 is stored in α-granules of unstimulated platelets and when platelets become activated, CD154 translocates to the platelet surface. Then CD154 is cleaved by MMP-9 and later released into blood circulation as sCD154 [30]. Afterwards, sCD154 binds to its receptor CD40 in different cells, such as endothelial cells mononuclear phagocytes triggers the expression of various proinflammatory mediators, such as the interleukin (IL)-1, IL-6, IL-12, TNF-alpha, and interferon-gamma [23]. In addition, sCD154 could have prothrombotic effects via induction of TF [24,25,26,27] and binding to the glycoprotein IIb/IIIa platelet receptor [28,29]. Interestingly, we report for the first time a positive association of circulating levels of sCD154 with TF and TNF-alpha in patients with severe MMCAI patients. The association between serum levels of sCD154 and TNF-alpha, a pro-inflammatory cytokine, could involve a higher inflammatory state in those patients with higher serum sCD154 levels and higher risk of death. The association between serum sCD154 and plasma TF levels, a prothrombotic factor, could facilitate that patients with higher circulating sCD154 levels are associated with the development of vascular thrombosis, brain ischemia and death of the patient.
Another interesting findings of our study were that we found lower platelet count in non-surviving MMCAI patients than in surviving patients. These findings are in agreement with those of the study by D’Erasmo et al. [31]. In that study, the authors found an association between early platelet count reduction and infarct extension and clinical outcome in patients with ischemic cerebral infarction; and they believed that the results demonstrate that the platelet consumption and/or accumulation in the infarct area, expressed by circulating platelet decrease, is related to the severity of neurological involvement, infarct size and poor clinical outcome.
The administration of modulators of sCD154 activity could have a potential beneficial effect in MMCAI patients. In several studies the use of statins has been found to decrease circulating sCD154 levels in patients with coronary artery disease [32,33,34]. In addition, in a meta-analysis the use of statin therapy at stroke onset was associated with improved outcome and reduced fatality [35].
Some limitations of our study should be recognized. First, we did not report data of circulating sCD154 during follow-up. Second, the determination of other inflammatory cytokines and coagulation biomarker could be interesting. Third, we found that serum sCD154 levels were associated with mortality controlling for GCS score and age; however other factors could have played a role in mortality, and thus, larger series of patients including more variables in a single multiple logistic regression analysis are needed to confirm our findings.
We believe, that according to the results of our study, the determination of serum sCD154 levels at the moment of the MMCAI diagnosis could be used to prediction the outcome and could generate interest for research into the use of modulator agents of sCD154 activity in these patients.
5. Conclusions
The novel and most important finding of our study is that serum sCD154 levels in MMCAI patients are associated with mortality.
Acknowledgments
This study was supported by funding from the Fundación Canaria de Investigación Sanitaria (FUNCANIS) (La Laguna, Tenerife, Spain).
Author Contributions
Leonardo Lorente was responsible for the conception, design and coordination of the study, made substantial contributions to acquisition of data, analysis and interpretation of data, and drafted the manuscript. María M. Martín, Luis Ramos, Mónica Argueso, Juan J. Cáceres and Jordi Solé-Violán have made substantial contributions to acquisition of data and provided useful suggestions. Agustín F. González-Rivero and Juan M. Borreguero-León carried out the determination of sCD154, TNF-α and TF and have made substantial contributions to analysis and interpretation of data. Alejandro Jiménez has made substantial contributions to analysis and interpretation of data. All authors revised the manuscript critically for important intellectual content and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Adams H.P., Jr., del Zoppo G., Alberts M.J., Bhatt D.L., Brass L., Furlan A., Grubb R.L., Higashida R.T., Jauch E.C., Kidwell C., et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 2.Waid D.M., Schreiner T., Vaitaitis G., Carter J.R., Corboy J.R., Wagner D.H., Jr. Defining a new biomarker for the autoimmune component of Multiple Sclerosis: Th40 cells. J. Neuroimmunol. 2014;270:75–85. doi: 10.1016/j.jneuroim.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang B., Wu T., Chen M., Zhou Y., Yi D., Guo R. The CD40/CD40L system: A new therapeutic target for disease. Immunol. Lett. 2013;153:58–61. doi: 10.1016/j.imlet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Oberheiden T., Nguyen X.D., Fatar M., Elmas E., Blahak C., Morper N., Dempfle C.E., Hennerici M., Borggrefe M., Kälsch T. Platelet and monocyte activation in acute ischemic stroke—Is there a correlation with stroke etiology? Clin. Appl. Thromb. Hemost. 2012;18:87–91. doi: 10.1177/1076029611412359. [DOI] [PubMed] [Google Scholar]
- 5.Cha J.K., Jeong M.H., Jang J.Y., Bae H.R., Lim Y.J., Kim J.S., Kim S.H., Kim J.W. Serial measurement of surface expressions of CD63, P-selectin and CD40 ligand on platelets in atherosclerotic ischemic stroke. A possible role of CD40 ligand on platelets in atherosclerotic ischemic stroke. Cerebrovasc. Dis. 2003;16:376–382. doi: 10.1159/000072560. [DOI] [PubMed] [Google Scholar]
- 6.Wang J.H., Zhang Y.W., Zhang P., Deng B.Q., Ding S., Wang Z.K., Wu T., Wang J. CD40 ligand as a potential biomarker for atherosclerotic instability. Neurol. Res. 2013;35:693–700. doi: 10.1179/1743132813Y.0000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao D.J., Guo R.Y., Tang Y.C., Zang Y.H. Expression of sCD40L in peripheral blood and NF-κBp65 in PBMC of patients with acute progressive cerebral infarction. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011;27:177–179. [PubMed] [Google Scholar]
- 8.Ding S., Zhang M., Zhao Y., Chen W., Yao G., Zhang C., Zhang P., Zhang Y. The role of carotid plaque vulnerability and inflammation in the pathogenesis of acute ischemic stroke. Am. J. Med. Sci. 2008;336:27–31. doi: 10.1097/MAJ.0b013e31815b60a1. [DOI] [PubMed] [Google Scholar]
- 9.Tuttolomondo A., di Raimondo D., di Sciacca R., Casuccio A., Bivona G., Bellia C., Barreca L., Serio A., D’Aguanno G., Ciaccio M., et al. Fetuin-A and CD40 L plasma levels in acute ischemic stroke: Differences in relation to TOAST subtype and correlation with clinical and laboratory variables. Atherosclerosis. 2010;208:290–296. doi: 10.1016/j.atherosclerosis.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B., Chen M., Yang H., Wu T., Song C., Guo R. Evidence for involvement of the CD40/CD40L system in post-stroke epilepsy. Neurosci. Lett. 2014;567:6–10. doi: 10.1016/j.neulet.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Lukasik M., Dworacki G., Michalak S., Kufel-Grabowska J., Watala C., Kozubski W. Chronic hyper-reactivity of platelets resulting in enhanced monocyte recruitment in patients after ischaemic stroke. Platelets. 2012;23:132–142. doi: 10.3109/09537104.2011.597528. [DOI] [PubMed] [Google Scholar]
- 12.Novo S., Basili S., Tantillo R., Falco A., Davì V., Novo G., Corrado E., Davì G. Soluble CD40L and cardiovascular risk in asymptomatic low-grade carotid stenosis. Stroke. 2005;36:673–675. doi: 10.1161/01.STR.0000154878.58398.14. [DOI] [PubMed] [Google Scholar]
- 13.Davì G., Tuttolomondo A., Santilli F., Basili S., Ferrante E., di Raimondo D., Pinto A., Licata G. CD40 ligand and MCP-1 as predictors of cardiovascular events in diabetic patients with stroke. J. Atheroscler. Thromb. 2009;16:707–713. doi: 10.5551/jat.1537. [DOI] [PubMed] [Google Scholar]
- 14.Tsai N.W., Chang W.N., Shaw C.F., Jan C.R., Chang H.W., Huang C.R., Chen S.D., Chuang Y.C., Lee L.H., Wang H.C., et al. Levels and value of platelet activation markers in different subtypes of acute non-cardio-embolic ischemic stroke. Thromb. Res. 2009;124:213–218. doi: 10.1016/j.thromres.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Lukasik M., Dworacki G., Kufel-Grabowska J., Watala C., Kozubski W. Upregulation of CD40 ligand and enhanced monocyte-platelet aggregate formation are associated with worse clinical outcome after ischaemic stroke. Thromb. Haemost. 2012;107:346–355. doi: 10.1160/TH11-05-0345. (See comment in PubMed Commons below) [DOI] [PubMed] [Google Scholar]
- 16.Teasdale G., Jennett B. Assessement of coma and impaired consciousness: A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/S0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 17.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Varo N., de Lemos J.A., Libby P., Morrow D.A., Murphy S.A., Nuzzo R., Gibson C.M., Cannon C.P., Braunwald E., Schonbeck U. Soluble CD40L: Risk prediction after acute coronary syndromes. Circulation. 2003;108:1049–1052. doi: 10.1161/01.CIR.0000088521.04017.13. [DOI] [PubMed] [Google Scholar]
- 19.Lorente L., Martín M.M., Varo N., Borreguero-León J.M., Solé-Violán J., Blanquer J., Labarta L., Díaz C., Jiménez A., Pastor E., et al. Association between serum soluble CD40 ligand levels and mortality in patients with severe sepsis. Crit. Care. 2011;15:R97. doi: 10.1186/cc10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez de Lizarrondo S., Roncal C., Calvayrac O., Rodríguez C., Varo N., Purroy A., Lorente L., Rodríguez J.A., Doeuvre L., Hervás-Stubbs S., et al. Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2012;32:1477–1487. doi: 10.1161/ATVBAHA.112.248773. [DOI] [PubMed] [Google Scholar]
- 21.Lorente L., Martín M.M., González-Rivero A.F., Ramos L., Argueso M., Cáceres J.J., Solé-Violán J., Serrano N., Rodríguez S.T., Jiménez A., et al. Serum soluble CD40 Ligand levels are associated with severity and mortality of brain trauma injury patients. Thromb. Res. 2014;134:832–836. doi: 10.1016/j.thromres.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 22.Noelle R.J., Roy M., Shepherd D.M., Stamenkovic I., Ledbetter J.A., Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc. Natl. Acad. Sci. USA. 1992;9:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mach F., Schönbeck U., Sukhova G.K., Bourcier T., Bonnefoy J.Y.M., Pober J.S., Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40-CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L., Stordeur P., de Lavareille A., Thielemans K., Capel P., Goldman M., Pradier O. CD40 engagement on endothelial cells promotes tissue factor-dependent procoagulant activity. Thromb. Haemost. 1998;79:1025–1028. [PubMed] [Google Scholar]
- 25.Hezi-Yamit A., Wong P.W., Bien-Ly N., Komuves L.G., Prasad K.S., Phillips D.R., Sinha U. Synergistic induction of tissue factor by coagulation factor Xa and TNF: Evidence for involvement of negative regulatory signaling cascades. Proc. Natl. Acad. Sci. USA. 2005;102:12077–12082. doi: 10.1073/pnas.0504526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller D.L., Yaron R., Yellin M.J. CD40L-CD40 interactions regulate endothelial cell surface tissue factor and thrombomodulin expression. J. Leukoc. Biol. 1998;63:373–379. doi: 10.1002/jlb.63.3.373. [DOI] [PubMed] [Google Scholar]
- 27.Slupsky J.R., Kalbas M., Willuweit A., Henn V., Kroczek R.A., Müller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb. Haemost. 1998;80:1008–1014. [PubMed] [Google Scholar]
- 28.Prasad K.S., Andre P., He M., Bao M., Manganello J., Phillips D.R. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc. Natl. Acad. Sci. USA. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.André P., Prasad K.S., Denis C.V., He M., Papalia J.M., Hynes R.O., Phillips D.R., Wagner D.D. CD40L stabilizes arterial thrombi by a beta3 integrin—Dependent mechanism. Nat. Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 30.Menchén L., Marín-Jiménez I., Arias-Salgado E.G., Fontela T., Hernández-Sampelayo P., Rodríguez M.C., Butta N.V. Matrix metalloproteinase 9 is involved in Crohn’s disease-associated platelet hyperactivation through the release of soluble CD40 ligand. Gut. 2009;58:920–992. doi: 10.1136/gut.2008.150318. [DOI] [PubMed] [Google Scholar]
- 31.D’Erasmo E., Acca M., Pisani D., Volpe M.S. Neurological state, infarct size and clinical outcome are related to early platelet count decrease in stroke. Gerontology. 1993;39:276–279. doi: 10.1159/000213543. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Zhao S.P., Peng D.Q., Xu Z.M., Zhou H.N. Early effect of pravastatin on serum soluble CD40L, matrix metalloproteinase-9, and C-reactive protein in patients with acute myocardial infarction. Clin. Chem. 2004;50:1696–1699. doi: 10.1373/clinchem.2003.030940. [DOI] [PubMed] [Google Scholar]
- 33.Hamdan R., Hajj F., Kadry Z., Kassab R., Salame E., Aboujaoude S., Azar R., Badaoui G. Benefit and tolerability of the coadministration of ezetimibe and atorvastatin in acute coronary syndrome patients. J. Med. Liban. 2011;59:65–69. [PubMed] [Google Scholar]
- 34.Han S.H., Koh K.K., Quon M.J., Lee Y., Shin E.K. The effects of simvastatin, losartan, and combined therapy on soluble CD40 ligand in hypercholesterolemic, hypertensive patients. Atherosclerosis. 2007;190:205–211. doi: 10.1016/j.atherosclerosis.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 35.Ní Chróinín D., Asplund K., Åsberg S., Callaly E., Cuadrado-Godia E., Díez-Tejedor E., di Napoli M., Engelter S.T., Furie K.L., Giannopoulos S., et al. Statin therapy and outcome after ischemic stroke: Systematic review and meta-analysis of observational studies and randomized trials. Stroke. 2013;44:448–456. doi: 10.1161/STROKEAHA.112.668277. [DOI] [PubMed] [Google Scholar]