Abstract
Loss-of-function mutations in the sphingomyelin phosphodiesterase 1 (SMPD1) gene are associated with decreased catalytic activity of acid sphingomyelinase (ASM) and are the cause of the autosomal recessive lysosomal storage disorder Niemann-Pick disease (NPD) types A and B. Currently, >100 missense mutations in SMPD1 are listed in the Human Gene Mutation Database. However, not every sequence variation in SMPD1 is detrimental and gives rise to NPD. We have analysed several alleged SMPD1 missense mutations mentioned in a recent publication and found them to be common variants of SMPD1 that give rise to normal in vivo and in vitro ASM activity. (Comment on Manshadi et al. Int. J. Mol. Sci. 2015, 16, 6668–6676).
Keywords: gene variant, missense mutation, Niemann-Pick disease, polymorphism, sphingomyelin phosphodiesterase
1. To the Editor
Loss-of-function mutations in the sphingomyelin phosphodiesterase 1 (SMPD1) gene are associated with decreased catalytic activity of acid sphingomyelinase (ASM) and are the cause of the autosomal recessive lysosomal storage disorder Niemann-Pick disease types A and B (NPD). Currently, >100 missense mutations in SMPD1 are listed in the Human Gene Mutation Database. However, not every sequence variation in SMPD1 is detrimental and gives rise to NPD.
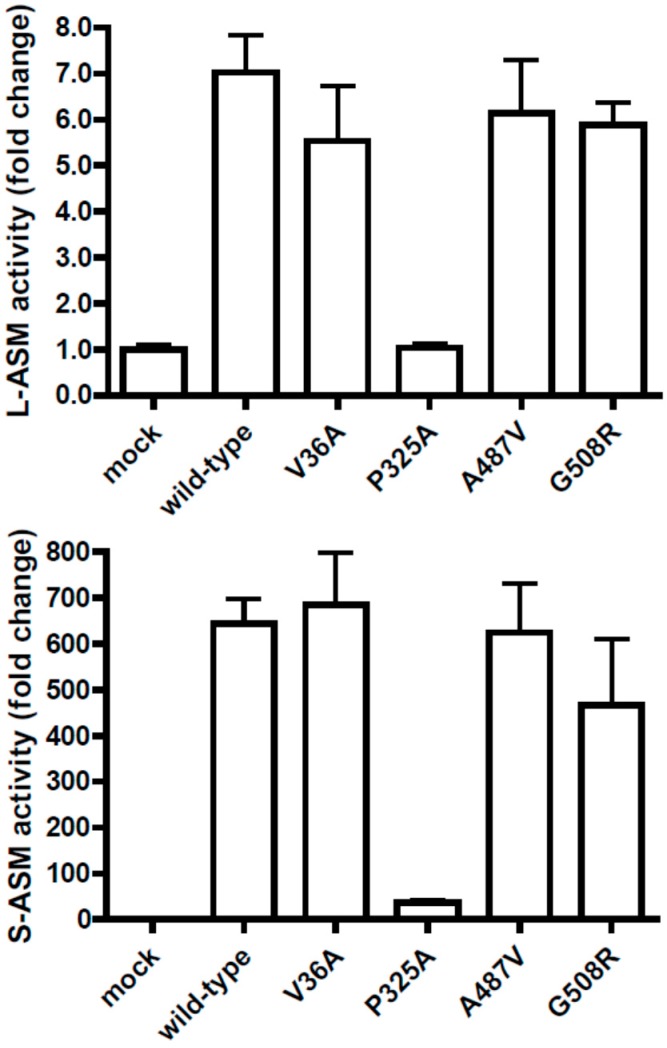
In order to identify mutations in the SMPD1 gene that potentially explain increased ASM activity associated with major depressive disorder [1] we performed a re-sequencing analysis of the six exons of SMPD1. This region includes a 1896 bp open reading frame that encodes a 631 amino acid protein according to NCBI Reference Sequence NM_000543.4. We identified three non-synonymous single nucleotide polymorphisms (SNP)-c.973C>G (p.P325A), c.1460C>T (p.A487V) and c.1522G>A (p.G508R; rs1050239)-as well as a bipartite polymorphic site including the non-synonymous SNP c.107T>C (p.V36A; rs1050228), which is located adjacent to a polymorphic region composed of a variable number of hexanucleotide sequences. Two of these variants, p.P325A and p.A487V, were previously reported to constitute loss-of-function mutations of SMPD1 [2], whereas the other two, p.G508R and the hexanucleotide repeat, an association with NPD was ruled out [3,4]. To analyze the functional implications of these polymorphisms in detail, we first conducted genotype-phenotype correlations in different human samples (e.g., a comparison of individual SMPD1 genotypes with the respective in vivo ASM activities) and, second, cloned the respective cDNAs for transient transfection studies. Hereby, we were able to demonstrate that the carriers of c.1460C>T (minor allele frequency ~1%) displayed secreted (S-) and lysosomal (L-) ASM activities in the normal range [5]. Moreover, transient transfection of c.1460T-cDNA into HeLa and MDCK cells resulted in S- and L-ASM activities similar to wild-type ASM [5]. The genotype-phenotype comparison for c.1522G>A (minor allele frequency ~27%), on the other hand, revealed a significant association especially with the S-ASM activity, which decreased with the number of A alleles in a gene-dosage dependent manner [6]. S-ASM activity in subjects homozygous for c.1522G>A was 50% decreased compared to subjects homozygous for the major allele c.1522G [6]. In sharp contrast, NPD patients and carriers of NPD alleles were reported to display S-ASM activities <3% and about 20% of normal values, respectively [7]. Thus, the only moderately decreased S-ASM activity in subjects homozygous for c.1522A and the high allele frequency argue strongly against the notion that c.1522G>A constitutes a loss-of-function mutation causing NPD. In addition, a transient transfection of a c.1522A-construct into HeLa and MCDK cells resulted in L- and S-ASM activities comparable to wild-type ASM (Figure 1). The functional relevance of c.107T>C was analyzed together with that of the adjacent polymorphic hexanucleotide repeat. While we obtained data showing that less repeats of the hexanucleotide motif are associated with slightly decreased S-ASM activity in vivo and in vitro [8], we did not observe a significant reduction of ASM activity associated with c.107T>C, neither in vivo nor in vitro (Figure 1). In contrast, transient transfection of a c.973G-construct into HeLa and MDCK cells did not increase ASM activity compared to control cells, thus confirming that c.973C>G is a NPD mutation (Figure 1).
Figure 1.
Analysis of sphingomyelin phosphodiesterase 1 (SMPD1) sequence variations by transient transfection studies. The full-length SMPD1 cDNA was cloned into the FLAG-N2 expression vector, and the SMPD1 variants p.V36A, p.P325A, p.A487V and p.G508R were generated by site-directed mutagenesis. The variant cDNAs and the empty FLAG-N2 vector (mock) were transiently transfected into MDCK cells, and acid sphingomyelinase activity was determined from cell lysates (L-ASM activity; upper panel) and supernatants (S-ASM activity; lower panel). Representative results of a typical experiment with three replicates are given as fold increase over the mock-transfected control. Error bars indicate the standard deviation. With the exception of p.P325A, all mutants increased acid sphingomyelinase activity by >fivefold, similarly to SMPD1 wild-type. Methods are described in detail in [5].
2. Conclusions
Our results strongly support the notion that p.V36A, p.A487V and p.G508R are frequent polymorphisms in SMPD1. These polymorphisms might increase the susceptibility for common diseases such as allergy [6], but they do not constitute loss-of-function mutations that are responsible for the occurrence of NPD. Since the alleged loss-of function mutations in 12 out of 15 NPD patients reported by Manshadi et al. [9] are common polymorphisms, a critical molecular, biochemical and clinical evaluation of the mentioned patients is recommended.
Author Contributions
Cosima Rhein, Johannes Kornhuber and Martin Reichel conceived and designed the experiments; Cosima Rhein, Christiane Mühle and Martin Reichel performed the experiments; Cosima Rhein and Martin Reichel analyzed the data; Martin Reichel wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kornhuber J., Medlin A., Bleich S., Jendrossek V., Henkel A.W., Wiltfang J., Gulbins E. High activity of acid sphingomyelinase in major depression. J. Neural Transm. 2005;112:1583–1590. doi: 10.1007/s00702-005-0374-5. [DOI] [PubMed] [Google Scholar]
- 2.Simonaro C.M., Desnick R.J., McGovern M.M., Wasserstein M.P., Schuchman E.H. The demographics and distribution of type B Niemann-Pick disease: Novel mutations lead to new genotype/phenotype correlations. Am. J. Hum. Genet. 2002;71:1413–1419. doi: 10.1086/345074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuchman E.H., Levran O., Suchi M., Desnick R.J. An MspI polymorphism in the human acid sphingomyelinase gene (SMPD1) Nucleic Acids Res. 1991;19:3160. doi: 10.1093/nar/19.11.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan Q., Schuchman E.H. A novel polymorphism in the human acid sphingomyelinase gene due to size variation of the signal peptide region. Biochim. Biophys. Acta. 1995;1270:207–210. doi: 10.1016/0925-4439(95)00050-E. [DOI] [PubMed] [Google Scholar]
- 5.Rhein C., Naumann J., Mühle C., Zill P., Adli M., Hegerl U., Hiemke C., Mergl R., Moller H.J., Reichel M., et al. The acid sphingomyelinase sequence variant p.A487V is not associated with decreased levels of enzymatic activity. JIMD Rep. 2013;8:1–6. doi: 10.1007/8904_2012_147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichel M., Richter-Schmidinger T., Mühle C., Rhein C., Alexopoulos P., Schwab S.G., Gulbins E., Kornhuber J. The common acid sphingomyelinase polymorphism p.G508R is associated with self-reported allergy. Cell. Physiol. Biochem. 2014;34:82–91. doi: 10.1159/000362986. [DOI] [PubMed] [Google Scholar]
- 7.He X., Chen F., Dagan A., Gatt S., Schuchman E.H. A fluorescence-based, high-performance liquid chromatographic assay to determine acid sphingomyelinase activity and diagnose types A and B Niemann-Pick disease. Anal. Biochem. 2003;314:116–120. doi: 10.1016/S0003-2697(02)00629-2. [DOI] [PubMed] [Google Scholar]
- 8.Rhein C., Reichel M., Mühle C., Rotter A., Schwab S.G., Kornhuber J. Secretion of acid sphingomyelinase is affected by its polymorphic signal peptide. Cell. Physiol. Biochem. 2014;34:1385–1401. doi: 10.1159/000366345. [DOI] [PubMed] [Google Scholar]
- 9.Manshadi M.D., Kamalidehghan B., Keshavarzi F., Aryani O., Dadgar S., Arastehkani A., Tondar M., Ahmadipour F., Meng G.Y., Houshmand M. Four novel p.N385K, p.V36A, c.1033–1034insT and c.1417–1418delCT mutations in the sphingomyelin phosphodiesterase 1 (SMPD1) gene in patients with types A and B Niemann-Pick disease (NPD) Int. J. Mol. Sci. 2015;16:6668–6676. doi: 10.3390/ijms16046668. [DOI] [PMC free article] [PubMed] [Google Scholar]