Abstract
Tea (Camellia sinensis L.) is a perennial woody plant that is widely cultivated to produce a popular non-alcoholic beverage; this beverage has received much attention due to its pleasant flavor and bioactive ingredients, particularly several important secondary metabolites. Due to the significant changes in the metabolite contents of the buds and the young expanding leaves of tea plants, high-performance liquid chromatography (HPLC) analysis and isobaric tags for relative and absolute quantitation (iTRAQ) analysis were performed. A total of 233 differentially expressed proteins were identified. Among these, 116 proteins were up-regulated and 117 proteins were down-regulated in the young expanding leaves compared with the buds. A large array of diverse functions was revealed, including roles in energy and carbohydrate metabolism, secondary metabolite metabolism, nucleic acid and protein metabolism, and photosynthesis- and defense-related processes. These results suggest that polyphenol biosynthesis- and photosynthesis-related proteins regulate the secondary metabolite content of tea plants. The energy and antioxidant metabolism-related proteins may promote tea leaf development. However, reverse transcription quantitative real-time PCR (RT-qPCR) showed that the protein expression levels were not well correlated with the gene expression levels. These findings improve our understanding of the molecular mechanism of the changes in the metabolite content of the buds and the young expanding leaves of tea plants.
Keywords: Camellia sinensis L., proteome, iTRAQ
1. Introduction
Tea (Camellia sinensis L.) is a perennial woody plant that is widely cultivated to produce a popular non-alcoholic beverage; this beverage has received much attention due to its pleasant flavor and bioactive ingredients, particularly several key secondary metabolites [1]. Tea leaves contain important secondary metabolites, including polyphenols (catechins, flavones, anthocyanidin and phenolic acid), alkaloids (theobromine, theophylline and caffeine), and theanine, which not only contribute to tea quality but also have important human health benefits [2].
The changes in the chemical composition of the buds and the young expanding leaves of tea have been extensively studied. A previous study showed that during seeding development, total catechins, epigallocatechin gallate (EGCG) and epicatechin gallate (ECG) decreased, whereas the epigallocatechin (EGC) content increased [3]. As the shoots matured, the total flavonol glycoside and myricetin contents increased, but the kaempferol content decreased [4]. Purine alkaloid metabolism also appears to be closely associated with leaf development and aging in tea seedlings. In addition, the expression levels of several genes related to metabolite synthesis in tea leaves were analyzed. A positive correlation was found between the catechin concentration and the expression of flavanone 3-hydroxylase (F3H) in tea leaves at different developmental stages [5]. A study has shown that most catechins accumulate to higher levels in the shoots than in the mature leaves; similarly, the genes involved in catechin synthesis, including phenylalanine ammonia-lyase 1 (PAL1), chalcone synthase (CHS), dihydroflavonol 4-reductase (DFR), leucoanthocyanidin reductase (LCR), and F3H are more highly expressed in the shoots than in the mature leaves [6]. Zhang et al. also found that the content of non-galloylated catechins—except gallocatechin (GC)—as well as the activity of DFR and anthocyanidin reductase (ANR), gradually increased from the buds to the mature leaves [7]. An analysis of purine alkaloids in different parts of the seedlings showed that the caffeine and theobromine content was greater in young leaves and decreased with increasing leaf maturity, and the levels of tea caffeine synthase (TCS) transcripts were also highest in young leaves and declined markedly during leaf development [8,9]. Different levels of metabolites in tea leaves are likely characterized by diverse gene and protein expression profiles at each developmental stage.
Despite studies on the metabolite synthesis-related genes in tea plants, the molecular mechanisms underlying the changes in metabolite content have not yet been examined in detail. In this study, isobaric tags for relative and absolute quantitation (iTRAQ) analysis were first used to separate the differentially expressed proteins. In addition, the content of a set of important metabolites was studied, and the expression of the genes associated with the differentially expressed proteins was also measured. The purpose of this study is to provide an improved understanding of the molecular mechanisms behind the change in the metabolite content between the apical buds and the young expanding leaves of tea plants.
2. Results
2.1. Analysis of Metabolite Profiles
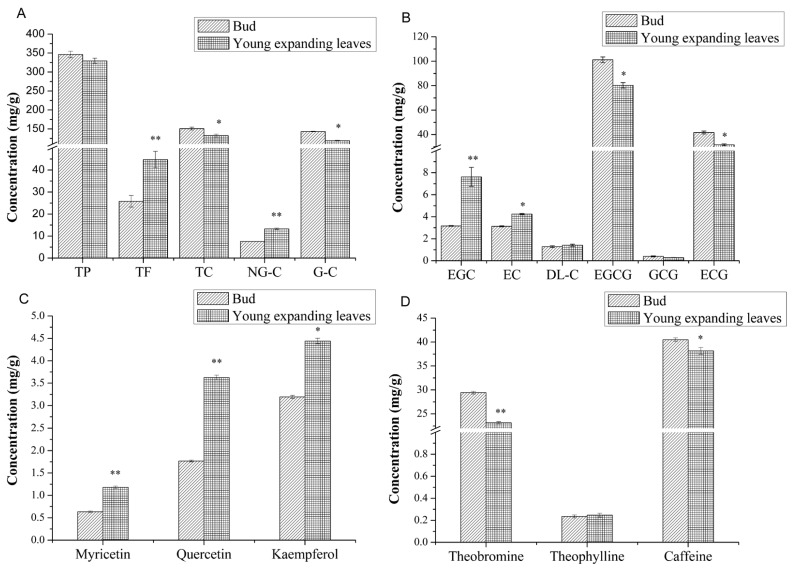
To further investigate the important changes in metabolite content, the polyphenol, catechin, and flavonoid contents of the buds and the young expanding leaves of tea plants were analyzed (Figure 1). As shown in Figure 1A, the concentration of total catechin in young expanding leaves (132.507 ± 3.889 mg/g) was 0.839-fold lower (p < 0.05) than that in the buds (150.851 ± 3.640 mg/g). The total polyphenol content of the young expanding leaves (329.395 ± 6.984 mg/g) was 0.951-fold lower than that of the buds (346.219 ± 8.609 mg/g), but this difference was not significant (p > 0.05). However, the total flavonoid content of the young expanding leaves (44.754 ± 3.731 mg/g) was 1.734-fold higher than that of the buds (25.803 ± 2.619 mg/g) (p < 0.01).
Figure 1.
Changes in the levels of secondary metabolites in the buds and the young expanding leaves of tea. (A) Total polyphenols (TP), total flavonoids (TF), total catechins (TC), non-galloylated catechins (NG-C) and galloylated catechins (G-C); (B) Individual catechins; (C) Myicetin, quercetin and kaempferol; and (D) Individual alkaloids. Statistical significance: * p < 0.05 and ** p < 0.01.
The levels of non-galloylated catechins, including EGC, epicatechin (EC) and DL-catechin (DL-C), were significantly greater in the young expanding leaves (13.280 ± 0.338 mg/g) than in the buds (7.574 ± 0.053 mg/g) (p < 0.01). However, the contents of galloylated catechins, including EGCG, GCG and ECG, were significantly lower in the young expanding leaves (119.226 ± 0.997 mg/g) than in the buds (143.277 ± 0.823 mg/g) (p < 0.05) (Figure 1A). In both the buds and the young expanding leaves the most abundant individual catechin was EGCG, and the least abundant individual catechin was GCG (gallocatechin gallate). The relative concentrations of each individual catechins in both the buds and the young expanding leaves were EGCG > ECG > EGC > EC > DL-C > GCG. The concentrations of EGC and EC in the young expanding leaves (EGC: 7.626 ± 0.859 mg/g, EC: 4.244 ± 0.060 mg/g) were greater than those in the buds (EGC: 3.167 ± 0.034 mg/g, EC: 3.127 ± 0.044 mg/g) (p < 0.01 for EGC and p < 0.05 for EC), and the level of DL-C was slightly higher (p > 0.05) in the young expanding leaves (1.410 ± 0.095 mg/g) than in the buds (1.280 ± 0.081 mg/g). However, the concentrations of EGCG and ECG were lower in the young expanding leaves (EGCG: 80.292 ± 2.216 mg/g, ECG: 38.646 ± 0.769 mg/g) than in the buds (EGCG: 101.169 ± 2.343 mg/g, ECG: 41.705 ± 1.204 mg/g) (p < 0.05), and the GCG level was also slightly lower (p > 0.05) in the young expanding leaves (0.288 ± 0.008 mg/g) than in the buds (0.403 ± 0.051 mg/g) (Figure 1B). In the young expanding leaves, the levels of individual flavonols, including myricetin, quercetin and kaempferol, were all greater than those in the buds (1.181 ± 0.026 mg/g myricetin, 3.627 ± 0.051 mg/g quercetin, and 4.441 ± 0.063 mg/g kaempferol in the leaves compared with 0.635 ± 0.017 mg/g myricetin, 1.767 ± 0.021 mg/g quercetin, and 3.193 ± 0.038 mg/g kaempferol in the buds, p < 0.01 for myricetin and quercetin, and p < 0.05 for kaempferol) (Figure 1C). Three types of alkaloids, including theobromine, theophylline and caffeine, were also detected via HPLC analysis. The theobromine and caffeine levels were lower in the young expanding leaves than in the buds (theobromine: 23.165 ± 0.213 mg/g in leaves and 29.418 ± 0.299 mg/g in buds, p < 0.01; caffeine: 38.167 ± 0.704 mg/g in leaves and 40.484 ± 0.396 mg/g in buds, p < 0.05), and the theophylline levels were slightly higher in the young expanding leaves (0.247 ± 0.017 mg/g) compared with the buds (0.235 ± 0.013 mg/g) (p > 0.05) (Figure 1D). Due to the significant changes in the metabolite contents of the buds and the young expanding leaves of tea plants, iTRAQ analysis was performed to determine the molecular mechanisms behind this change.
2.2. Protein Identification
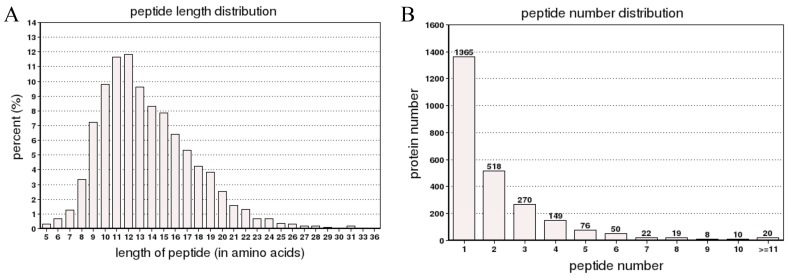
To explore the correlation between the proteomic and metabolite profiles of buds and young expanding leaves, samples were analyzed by iTRAQ proteomics coupled with LC-MS/MS. A total of 60,820 spectra were generated from the iTRAQ experiment and the data were analyzed using Mascot software. A total of 8015 spectra were matched to known spectra, 6974 spectra were matched to unique spectra, 4746 were matched to peptides, 4260 were matched to unique peptides and 2507 were matched to proteins (Figure 2A). The distribution of the number of peptides defining each protein is shown in Figure 2B; over 55% of the proteins were represented by at least two peptides.
Figure 2.
The spectra, peptides, and proteins, as well as the number of peptides in the iTRAQ proteomic analysis identified as matching proteins. The spectra, peptides and proteins were identified by searching against a database (A); and The number of peptides matched to proteins using MASCOT (B).
2.3. Functional Classification of the Differentially Expressed Proteins
The proteins whose levels changed more than 1.5-fold and had a p-values of less than 0.05 were considered differentially expressed. Based on these two criteria, 233 proteins were differentially expressed between the buds and the young expanding leaves, and these proteins were isolated and quantified using comparative proteomics via iTRAQ. Of the 233 differentially expressed proteins, 116 were more abundant and 117 were less abundant in the young expanding leaves compared with the buds. GO analysis revealed that the differentially expressed proteins participated in several biological processes (p < 0.05), as shown in Table S1. KEGG enrichment analysis suggested that the differentially expressed proteins are involved in several pathways (p < 0.05), including phenylalanine metabolism (Table S2).
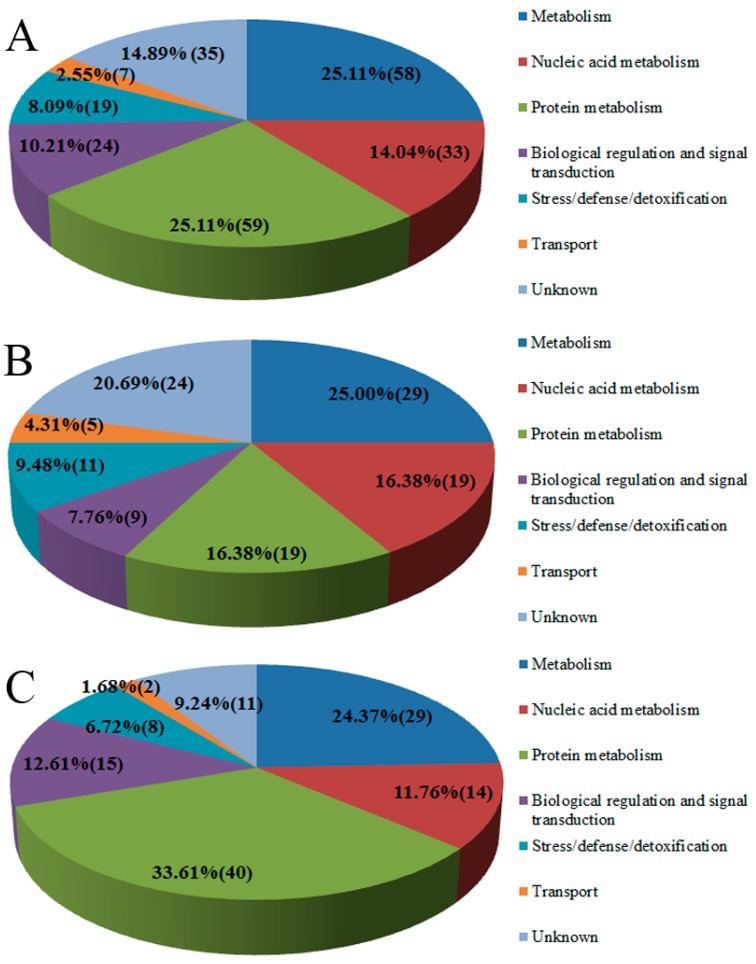
The proteins were classified into seven functional categories based on their functional biological properties and pathways: metabolism (58, 25.11%), nucleic acid metabolism (33, 14.04%), protein metabolism (59, 25.11%), biological regulation and signal transduction (24, 10.21%), stress/defense/detoxification (19, 8.09%), transport (7, 2.55%), and unknown function (35, 14.89%) (Figure 3A). Of the up-regulated proteins, 25.00% (29 proteins) function in metabolism, 16.38% (19 proteins) function in nucleic acid metabolism, 16.38% (19 proteins) are involved in protein metabolism, 7.76% (nine proteins) have biological regulation and signal transduction function, 9.58% (11 proteins) function in stress/defense/detoxification, 4.31% (5 proteins) are involved in transport and 20.69% of them (24 proteins) were of unknown function (Figure 3B). Among the down-regulated proteins, 24.37% (29 proteins) function in metabolism, 11.76% (14 proteins) function in nucleic acid metabolism, 33.61% (40 proteins) have a role in protein metabolism, 12.61% (15 proteins) are involved in biological regulation and signal transduction, 6.72% (8 proteins) are involved in stress/defense/detoxification, 1.68% (two proteins) function in transport and 9.24% (11 proteins) were of unknown function (Figure 3C). More detailed information can be found in Table 1.
Figure 3.
Functional classification of the differentially expressed proteins. Functional groups and the numbers of proteins of all 233 differentially expressed proteins that fall into each group (A); categorization of the 116 up-regulated proteins (B); and categorization of the 117 down-regulated proteins (C). The number in each functional category represents the number of proteins in that category.
Table 1.
List of proteins that are differentially expressed between the buds and the young expanding leaves of tea plants.
| Accession Number | Proteins Name and Species | Score | Mass (Da) | Coverage | Peptide Count | Fold Change (Leaves/Bud) | Function |
|---|---|---|---|---|---|---|---|
| gi|350536667| | Dihydrolipoamide dehydrogenase precursor [Solanum lycopersicum] | 202 | 68,166 | 13.9 | 5 | 2.164 | Metabolism |
| gi|15081610| | Xyloglucan endotransglycosylase XET2 [Vitis vinifera] | 137 | 39,952 | 12.4 | 3 | 1.614 | Metabolism |
| gi|76786311| | Flavonol synthase [Camellia sinensis] | 288 | 45,768 | 27.9 | 6 | 1.788 | Metabolism |
| gi|225458243| | PREDICTED: isoflavone reductase homolog P3 [Vitis vinifera] | 315 | 37,529 | 31.6 | 7 | 2.649 | Metabolism |
| gi|359491464| | PREDICTED: lysosomal α-mannosidase [Vitis vinifera] | 111 | 38,176 | 12.7 | 3 | 1.825 | Metabolism |
| gi|71535021| | α-glucosidase [Medicago sativa] | 204 | 86,320 | 6 | 4 | 2.353 | Metabolism |
| gi|255578100| | Dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase, putative [Ricinus communis] | 57 | 38,166 | 6.9 | 2 | 1.878 | Metabolism |
| gi|225426623| | PREDICTED: 2-keto-3-deoxy-l-rhamnonate aldolase-like [Vitis vinifera] | 78 | 21,742 | 10.2 | 1 | 2.281 | Metabolism |
| gi|193290728| | Putative pyruvate dehydrogenase E3 subunit [Capsicum annuum] | 74 | 42,069 | 8.3 | 2 | 1.567 | Metabolism |
| gi|255566959| | NADH-cytochrome B5 reductase, putative [Ricinus communis] | 32 | 11,336 | 11.4 | 1 | 1.58 | Metabolism |
| sp|Q9SVG4| | Reticuline oxidase-like protein [Spinacia oleracea] | 108 | 30,376 | 6.9 | 1 | 1.729 | Metabolism |
| sp|Q9M069| | Glucan endo-1,3-β-glucosidase 7 [Arabidopsis thaliana] | 139 | 40,171 | 16.2 | 3 | 1.568 | Metabolism |
| gi|147767550| | Hypothetical protein VITISV_013343 [Vitis vinifera] | 159 | 20,700 | 22.4 | 2 | 1.542 | Metabolism |
| gi|193290702| | Putative 3-isopropylmalate dehydrogenase small subunit [Capsicum annuum] | 354 | 25,595 | 36.2 | 4 | 1.681 | Metabolism |
| gi|225451235| | PREDICTED: cysteine synthase isoform 2 [Vitis vinifera] | 321 | 47,206 | 14.9 | 4 | 2.216 | Metabolism |
| gi|225454278| | PREDICTED: cysteine synthase, chloroplastic/chromoplastic isoform 1 [Vitis vinifera] | 60 | 40,113 | 10.5 | 2 | 1.905 | Metabolism |
| sp|P50246| | Adenosylhomocysteinase [Medicago sativa] | 186 | 65,326 | 11.9 | 5 | 1.551 | Metabolism |
| sp|P47999| | Cysteine synthase, chloroplastic/chromoplastic [Arabidopsis thaliana] | 111 | 51,056 | 14.5 | 4 | 1.856 | Metabolism |
| sp|P94170| | Carbonic anhydrase [Nostoc sp.] | 191 | 12,938 | 27.6 | 2 | 2.527 | Metabolism |
| sp|Q42876| | Leucine aminopeptidase 2, chloroplastic [Solanum lycopersicum] | 124 | 74,254 | 3.9 | 2 | 1.852 | Metabolism |
| gi|75755999| | TO87b-13 [Taraxacum officinale] | 260 | 83,317 | 6 | 3 | 2.18 | Metabolism |
| gi|330318698| | Light-inducible protein atls1 [Camellia sinensis] | 115 | 18,370 | 8.8 | 1 | 2.68 | Metabolism |
| gi|224098154| | predicted protein [Populus trichocarpa] | 145 | 29,956 | 28.8 | 5 | 1.504 | Metabolism |
| gi|225433426| | PREDICTED: 3-ketoacyl-CoA thiolase 2, peroxisomal isoform 2 [Vitis vinifera] | 197 | 7506 | 51.9 | 2 | 1.568 | Metabolism |
| gi|225462452| | PREDICTED: GDSL esterase/lipase At5g45670 [Vitis vinifera] | 250 | 22,819 | 30.5 | 4 | 1.79 | Metabolism |
| sp|Q93YW8| | GDSL esterase/lipase At4g18970 [Arabidopsis thaliana] | 250 | 22,819 | 30.5 | 4 | 1.79 | Metabolism |
| sp|Q9SYF0| | GDSL esterase/lipase 2 [Arabidopsis thaliana] | 316 | 46,565 | 14.2 | 5 | 2.203 | Metabolism |
| sp|Q9LZS7| | GDSL esterase/lipase At5g03610 [Arabidopsis thaliana] | 80 | 45,316 | 11.8 | 3 | 3.399 | Metabolism |
| gi|296088201| | Unnamed protein product [Vitis vinifera] | 73 | 30,392 | 9.5 | 2 | 1.55 | Metabolism |
| gi|18140567| | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit [Camellia japonica] | 518 | 52,867 | 15.8 | 6 | 0.357 | Metabolism |
| gi|156106226| | Rubisco activase [Camellia sinensis] | 646 | 43,205 | 31.4 | 7 | 0.564 | Metabolism |
| gi|20257362| | Ribulose 1,5-bisphosphate carboxylase/oxygenase, partial (chloroplast) [Schima superba] | 303 | 24,090 | 10.3 | 2 | 0.465 | Metabolism |
| gi|255553993| | Phosphoenolpyruvate carboxylase, putative [Ricinus communis] | 108 | 32,666 | 14.7 | 3 | 0.469 | Metabolism |
| gi|169807676| | NADP-dependent glyceraldehyde-3-phosphate dehydrogenase [Platanus x acerifolia] | 322 | 69,081 | 17 | 6 | 0.543 | Metabolism |
| gi|356524319| | PREDICTED: probable glycerophosphoryl diester phosphodiesterase 1-like [Glycine max] | 212 | 56,841 | 8.8 | 3 | 0.552 | Metabolism |
| gi|2266947| | Phosphoenolpyruvate carboxylase 1 [Gossypium hirsutum] | 173 | 94,629 | 7.7 | 5 | 0.477 | Metabolism |
| gi|255581778| | chlorophyll A/B binding protein, putative [Ricinus communis] | 96 | 41,261 | 3.8 | 1 | 0.664 | Metabolism |
| sp|P81833| | Thylakoid lumenal 29 kDa protein, chloroplastic (Fragment) [Spinacia oleracea] | 176 | 43,057 | 22.2 | 4 | 0.651 | Metabolism |
| sp|Q8H1Q1| | Thylakoid lumenal protein At1g12250, chloroplastic [Spinacia oleracea] | 254 | 35,331 | 21.5 | 4 | 0.655 | Metabolism |
| sp|O04138| | Chitinase 4 [Oryza sativa subsp. Japonica] | 223 | 30,457 | 19.4 | 3 | 0.466 | Metabolism |
| sp|Q9FKK7| | Xylose isomerase [Spinacia oleracea] | 172 | 63,511 | 13 | 4 | 0.575 | Metabolism |
| gi|27804768| | Sedoheptulose-1,7-bisphosphatase precursor [Oryza sativa Indica Group] | 268 | 54,158 | 8.7 | 3 | 0.58 | Metabolism |
| gi|380508822| | Putative hydroxycinnamoyl-CoA:shikimate/quinate hydroxycinnamoyltransferase [Camellia sinensis] | 48 | 7531 | 32.7 | 2 | 0.493 | Metabolism |
| gi|330318804| | Photosystem I reaction center subunit XI [Camellia sinensis] | 132 | 27,128 | 20.5 | 3 | 0.63 | Metabolism |
| gi|357521691| | Atypical receptor-like kinase MARK [Medicago truncatula] | 94 | 45,630 | 9.6 | 3 | 0.562 | Metabolism |
| gi|357494517| | Calcium dependent protein kinase [Medicago truncatula] | 118 | 14,910 | 21.8 | 2 | 0.422 | Metabolism |
| gi|297744280| | Unnamed protein product [Vitis vinifera] | 82 | 49,263 | 13.9 | 3 | 0.47 | Metabolism |
| sp|Q56YA5| | Serine--glyoxylate aminotransferase [Spinacia oleracea] | 124 | 32,097 | 7 | 1 | 0.251 | Metabolism |
| sp|P45726| | Phenylalanine ammonia-lyase [Camellia sinensis] | 463 | 90,257 | 19.2 | 11 | 0.63 | Metabolism |
| gi|71480741| | β-1,3-glucanase [Camellia sinensis] | 126 | 60,244 | 2.4 | 1 | 0.565 | Metabolism |
| sp|P46637| | Arginase [Spinacia oleracea] | 470 | 39,198 | 21 | 6 | 0.47 | Metabolism |
| sp|Q6AUR2| | Nitrogen regulatory protein P-II homolog [Oryza sativa subsp. Japonica] | 101 | 28,420 | 12.8 | 3 | 0.648 | Metabolism |
| gi|302566881| | Lipoxygenase [Camellia sinensis] | 72 | 59,000 | 10.2 | 3 | 0.54 | Metabolism |
| gi|194466253| | N-acetyltransferase [Arachis hypogaea] | 103 | 29,065 | 12 | 2 | 0.605 | Metabolism |
| sp|Q9LZ72| | 3-ketoacyl-CoA synthase 21 [Spinacia oleracea] | 120 | 62,270 | 7.7 | 3 | 0.532 | Metabolism |
| sp|Q570B4| | 3-ketoacyl-CoA synthase 10 [Spinacia oleracea] | 53 | 38,242 | 3.8 | 1 | 0.455 | Metabolism |
| sp|Q9FJ41| | GDSL esterase/lipase At5g45950 [Spinacia oleracea] | 138 | 50,893 | 9.3 | 2 | 0.619 | Metabolism |
| sp|Q9LEB4| | Polyadenylate-binding protein RBP45 [Nicotiana plumbaginifolia] | 135 | 56,285 | 10.6 | 4 | 1.53 | Nucleic acid metabolism |
| sp|Q43349| | 29 kDa ribonucleoprotein, chloroplastic [Spinacia oleracea] | 43 | 34,389 | 7.1 | 2 | 1.624 | Nucleic acid metabolism |
| sp|P43333| | U2 small nuclear ribonucleoprotein A´ [Spinacia oleracea] | 135 | 40,620 | 14.3 | 3 | 1.574 | Nucleic acid metabolism |
| gi|307940738| | G-strand specific single-stranded telomere-binding protein 1 [Nicotiana tabacum] | 250 | 22,819 | 30.5 | 4 | 1.79 | Nucleic acid metabolism |
| sp|Q84L31| | Putative DNA repair protein RAD23-3 [Spinacia oleracea] | 174 | 42,346 | 15.4 | 4 | 1.533 | Nucleic acid metabolism |
| gi|255603771| | DNA binding protein, putative [Ricinus communis] | 250 | 22,819 | 30.5 | 4 | 1.79 | Nucleic acid metabolism |
| gi|255603771| | DNA binding protein, putative [Ricinus communis] | 177 | 41,602 | 16.9 | 3 | 2.491 | Nucleic acid metabolism |
| sp|Q9S7C9| | Putative DNA-binding protein ESCAROLA [Spinacia oleracea] | 250 | 38,185 | 17 | 4 | 1.664 | Nucleic acid metabolism |
| gi|79596510| | AT hook motif DNA-binding family protein [Spinacia oleracea] | 137 | 29,457 | 21.1 | 4 | 2.576 | Nucleic acid metabolism |
| sp|Q9S7C9| | Putative DNA-binding protein ESCAROLA [Spinacia oleracea] | 250 | 38,185 | 17 | 4 | 1.664 | Nucleic acid metabolism |
| gi|45533923| | Glycine-rich RNA-binding protein RGP-1c [Nicotiana sylvestris] | 487 | 21,422 | 28.1 | 4 | 2.945 | Nucleic acid metabolism |
| sp|Q9SVM8| | Glycine-rich RNA-binding protein 2, mitochondrial [Spinacia oleracea] | 410 | 20,091 | 17.1 | 2 | 1.506 | Nucleic acid metabolism |
| gi|225440996| | PREDICTED: histone deacetylase HDT1-like [Vitis vinifera] | 99 | 37,638 | 12.2 | 3 | 3.522 | Nucleic acid metabolism |
| sp|Q9XI36| | Methyl-CpG-binding domain-containing protein 10 [Spinacia oleracea] | 285 | 42,453 | 37.6 | 7 | 1.364 | Nucleic acid metabolism |
| gi|225457458| | PREDICTED: transcription factor BTF3 [Vitis vinifera] | 170 | 25,374 | 35.6 | 4 | 1.829 | Nucleic acid metabolism |
| gi|297723091| | Os04g0385700 [Oryza sativa Japonica Group] | 56 | 34,525 | 4.3 | 1 | 2.34 | Nucleic acid metabolism |
| gi|296081863| | Unnamed protein product [Vitis vinifera] | 177 | 38,697 | 15.7 | 3 | 2.094 | Nucleic acid metabolism |
| gi|297744195| | Unnamed protein product [Vitis vinifera] | 155 | 29,045 | 22.4 | 3 | 2.146 | Nucleic acid metabolism |
| gi|255642098| | Unknown [Glycine max] | 119 | 51,914 | 10.3 | 4 | 2.23 | Nucleic acid metabolism |
| sp|Q9LFN6| | DEAD-box ATP-dependent RNA helicase 56 [Spinacia oleracea] | 220 | 54,506 | 18.6 | 5 | 0.649 | Nucleic acid metabolism |
| sp|Q84UQ1| | DEAD-box ATP-dependent RNA helicase 42 [Oryza sativa subsp. Japonica] | 98 | 120,162 | 2.2 | 2 | 0.325 | Nucleic acid metabolism |
| sp|B6EUA9| | Pre-mRNA-processing protein 40A [Spinacia oleracea] | 242 | 83,063 | 9.7 | 5 | 0.233 | Nucleic acid metabolism |
| sp|O22315| | Pre-mRNA-splicing factor SF2 [Spinacia oleracea] | 126 | 7308 | 35.7 | 2 | 0.432 | Nucleic acid metabolism |
| gi|374095609| | Spliceosomal-like protein [Camellia sinensis] | 23 | 4551 | 17.1 | 1 | 0.385 | Nucleic acid metabolism |
| sp|Q9S709| | Splicing factor U2af small subunit A [Spinacia oleracea] | 71 | 29,349 | 7.9 | 1 | 0.637 | Nucleic acid metabolism |
| sp|P81766| | Nucleoside diphosphate kinase 3 [Spinacia oleracea] | 61 | 31,625 | 7.5 | 2 | 0.508 | Nucleic acid metabolism |
| gi|224117596| | Predicted protein [Populus trichocarpa] | 368 | 54,505 | 13.4 | 5 | 0.398 | Nucleic acid metabolism |
| gi|225462994| | PREDICTED: DNA replication licensing factor mcm5-A-like [Vitis vinifera] | 259 | 94,881 | 13.6 | 8 | 0.582 | Nucleic acid metabolism |
| sp|O04716| | DNA mismatch repair protein MSH6 [Spinacia oleracea] | 159 | 50,441 | 4.6 | 1 | 0.276 | Nucleic acid metabolism |
| gi|359386142| | RNA recognition motif protein 1 [Citrus sinensis] | 155 | 14,712 | 42.7 | 3 | 0.492 | Nucleic acid metabolism |
| gi|195626496| | Glycine-rich RNA-binding protein 2 [Zea mays] | 318 | 21,175 | 33.1 | 4 | 0.435 | Nucleic acid metabolism |
| sp|Q9FLH0| | PUTATIVE nuclear matrix constituent protein 1-like protein [Spinacia oleracea] | 69 | 78,347 | 5.6 | 2 | 0.66 | Nucleic acid metabolism |
| gi|385213056| | 20S proteasome β2 subunit, partial [Oryza brachyantha] | 163 | 40,674 | 14.1 | 4 | 2.297 | Protein metabolism |
| gi|49175785| | 26S proteasome β subunit [Pisum sativum] | 187 | 35,781 | 16 | 4 | 1.632 | Protein metabolism |
| gi|16225442| | 26S proteasome regulatory subunit S12 isolog-like protein [Castanea sativa] | 144 | 38,542 | 10.6 | 3 | 2.263 | Protein metabolism |
| gi|225431100| | PREDICTED: 26S proteasome non-ATPase regulatory subunit 4 [Vitis vinifera] | 73 | 8946 | 16.9 | 1 | 1.553 | Protein metabolism |
| gi|24473796| | 60s acidic ribosomal protein [Prunus dulcis] | 208 | 14,983 | 15.8 | 2 | 2.924 | Protein metabolism |
| gi|330318716| | 60S acidic ribosomal protein p2 [Camellia sinensis] | 156 | 15,062 | 16.2 | 2 | 4.223 | Protein metabolism |
| sp|Q8LEQ0| | 60S acidic ribosomal protein P1-3 [Spinacia oleracea] | 185 | 19,758 | 10.1 | 1 | 1.928 | Protein metabolism |
| sp|Q9SVZ6| | 60S acidic ribosomal protein P3-1 [Spinacia oleracea] | 666 | 15,391 | 13.7 | 1 | 1.866 | Protein metabolism |
| gi|255574159| | Proteasome subunit β type 6,9, putative [Ricinus communis] | 368 | 31,544 | 22.1 | 5 | 1.644 | Protein metabolism |
| gi|255564428| | Elongation factor 1-β, putative [Ricinus communis] | 62 | 33,531 | 5.7 | 1 | 1.899 | Protein metabolism |
| gi|255539639| | Cucumisin precursor, putative [Ricinus communis] | 86 | 56,932 | 2.8 | 1 | 1.535 | Protein metabolism |
| gi|14594919| | Putative α5 proteasome subunit [Nicotiana tabacum] | 170 | 30,889 | 13.3 | 3 | 2.021 | Protein metabolism |
| gi|356549495| | PREDICTED: heat shock 70 kDa protein, mitochondrial-like [Glycine max] | 62 | 12,864 | 10.9 | 1 | 1.605 | Protein metabolism |
| gi|272716096| | Disulfide isomerase-like protein [Gloeospermum blakeanum] | 87 | 43,279 | 12.1 | 2 | 1.781 | Protein metabolism |
| gi|272716065| | Disulfide isomerase [Gloeospermum blakeanum] | 250 | 22,819 | 30.5 | 4 | 1.79 | Protein metabolism |
| sp|Q8VX13| | Protein disulfide isomerase-like 1-3 [Spinacia oleracea] | 165 | 80,245 | 10.1 | 5 | 1.625 | Protein metabolism |
| sp|O65351| | SUBTILISIN-like protease [Spinacia oleracea] | 128 | 32,198 | 16.7 | 3 | 1.556 | Protein metabolism |
| gi|359473000| | PREDICTED: aspartic proteinase nepenthesin-1-like [Vitis vinifera] | 250 | 22,819 | 30.5 | 4 | 1.79 | Protein metabolism |
| sp|P81898| | Peptide-N4-( N-acetyl-β-glucosaminyl)asparagine amidase A [Prunus dulcis] | 49 | 28,569 | 5.3 | 1 | 1.823 | Protein metabolism |
| gi|7141245| | 26S proteasome regulatory ATPase subunit S10b [Vitis riparia] | 164 | 54,246 | 13.8 | 4 | 0.539 | Protein metabolism |
| gi|56481167| | 40S ribosomal protein S3a [Pseudotsuga menziesii var. menziesii] | 119 | 40,753 | 17.6 | 3 | 0.437 | Protein metabolism |
| sp|Q9SCM3| | 40S ribosomal protein S2-4 [Spinacia oleracea] | 253 | 38,711 | 12.9 | 3 | 0.548 | Protein metabolism |
| gi|241865275| | 40S RPS3B [Sonneratia alba] | 150 | 30,886 | 27.4 | 5 | 0.536 | Protein metabolism |
| gi|255569736| | 40S ribosomal protein S6, putative [Ricinus communis] | 88 | 41,992 | 8.8 | 2 | 0.574 | Protein metabolism |
| gi|330318726| | 40S ribosomal protein s9 [Camellia sinensis] | 126 | 28,301 | 14 | 3 | 0.513 | Protein metabolism |
| gi|357444481| | 40S ribosomal protein S18 [Medicago truncatula] | 223 | 25,387 | 24.2 | 3 | 0.414 | Protein metabolism |
| gi|255544840| | 40S ribosomal protein S2, putative [Ricinus communis] | 202 | 35,538 | 13.4 | 3 | 0.64 | Protein metabolism |
| gi|255549228| | 40S ribosomal protein S4, putative [Ricinus communis] | 277 | 39,687 | 25.6 | 6 | 0.415 | Protein metabolism |
| gi|241865275| | 40S RPS3B [Sonneratia alba] | 209 | 31,709 | 27.4 | 5 | 0.538 | Protein metabolism |
| sp|Q9ZNS1| | 40S ribosomal protein S7 [Avicennia marina] | 58 | 32,180 | 12.8 | 3 | 0.61 | Protein metabolism |
| sp|O80360| | 50S ribosomal protein L3, chloroplastic (Fragment) [Nicotiana tabacum] | 179 | 36,343 | 19.5 | 4 | 0.591 | Protein metabolism |
| gi|255551787| | 60S ribosomal protein L22, putative [Ricinus communis] | 119 | 22,528 | 22.1 | 3 | 0.615 | Protein metabolism |
| gi|148466442| | 60S ribosomal protein L21 [Paeonia suffruticosa] | 56 | 26,661 | 9.8 | 2 | 0.467 | Protein metabolism |
| sp|P51413| | 60S ribosomal protein L17-2 [Spinacia oleracea] | 48 | 28,359 | 5.1 | 1 | 0.525 | Protein metabolism |
| sp|Q6UNT2| | 60S ribosomal protein L5 [Cucumis sativus] | 90 | 44,735 | 6.7 | 2 | 0.661 | Protein metabolism |
| sp|Q9SPB3| | 60S ribosomal protein L10 [Vitis riparia] | 189 | 33,718 | 12.9 | 3 | 0.532 | Protein metabolism |
| sp|P30707| | 60S ribosomal protein L9 [Pisum sativum] | 184 | 33,248 | 26.4 | 4 | 0.641 | Protein metabolism |
| gi|225427377| | PREDICTED: 60S ribosomal protein L37a-like [Vitis vinifera] | 93 | 15,986 | 16.3 | 1 | 0.64 | Protein metabolism |
| gi|330318574| | Ribosomal petrp-like protein [Camellia sinensis] | 48 | 28,359 | 5.1 | 1 | 0.525 | Protein metabolism |
| gi|3885519| | Similar to ribosomal protein L32 [Medicago sativa] | 86 | 23,581 | 13.9 | 2 | 0.363 | Protein metabolism |
| gi|209922600| | Elongation factor 1-α [Prunus persica] | 351 | 80,605 | 24.2 | 11 | 0.624 | Protein metabolism |
| gi|225452282| | PREDICTED: elongation factor Tu, chloroplastic-like isoform 1 [Vitis vinifera] | 313 | 57,834 | 26.6 | 8 | 0.593 | Protein metabolism |
| gi|356524672| | PREDICTED: eukaryotic translation initiation factor 3 subunit C-like [Glycine max] | 58 | 6862 | 27.3 | 1 | 0.545 | Protein metabolism |
| gi|71534902| | Histidyl-tRNA synthetase [Medicago sativa] | 71 | 41,277 | 10.8 | 2 | 0.603 | Protein metabolism |
| sp|P31542| | ATP-dependent Clp protease ATP-binding subunit clpA homolog CD4B, chloroplastic [Solanum lycopersicum] | 497 | 55,091 | 24.1 | 8 | 0.562 | Protein metabolism |
| gi|356516495| | PREDICTED: chaperone protein ClpC, chloroplastic-like [Glycine max] | 497 | 55,091 | 24.1 | 8 | 0.562 | Protein metabolism |
| gi|52075839| | Putative chloroplast protease [Oryza sativa Japonica Group] | 340 | 85,534 | 15.4 | 8 | 0.523 | Protein metabolism |
| sp|Q8VY06| | Presequence protease 2, chloroplastic/mitochondrial [Spinacia oleracea] | 85 | 35,368 | 13.8 | 3 | 0.622 | Protein metabolism |
| sp|Q75GT3| | Chaperone protein ClpB2, chloroplastic [Oryza sativa subsp. Japonica] | 385 | 130,278 | 16.2 | 11 | 0.504 | Protein metabolism |
| gi|225431090| | PREDICTED: proteasome subunit α type-7 [Vitis vinifera] | 292 | 35,407 | 21.6 | 4 | 0.646 | Protein metabolism |
| gi|225457058| | PREDICTED: T-complex protein 1 subunit gamma [Vitis vinifera] | 354 | 76,271 | 13.4 | 6 | 0.552 | Protein metabolism |
| gi|225459806| | PREDICTED: T-complex protein 1 subunit β [Vitis vinifera] | 975 | 60,327 | 38.8 | 11 | 0.482 | Protein metabolism |
| gi|255567297| | chaperonin containing t-complex protein 1, α subunit, tcpa, putative [Ricinus communis] | 84 | 28,459 | 18.8 | 3 | 0.622 | Protein metabolism |
| sp|P32955| | Cysteine proteinase 2 (Fragment) [Carica candamarcensis] | 385 | 130,278 | 16.2 | 11 | 0.504 | Protein metabolism |
| sp|P35016| | Endoplasmin homolog [Catharanthus roseus] | 416 | 123,589 | 18.5 | 13 | 0.657 | Protein metabolism |
| sp|P38661| | Probable protein disulfide-isomerase A6 [Medicago sativa] | 213 | 54,232 | 24.9 | 8 | 0.665 | Protein metabolism |
| sp|Q5Z974| | ATP-dependent zinc metalloprotease FTSH 1, chloroplastic [Oryza sativa subsp. Japonica] | 281 | 42,007 | 19.7 | 4 | 0.427 | Protein metabolism |
| gi|147766666| | Hypothetical protein VITISV_035841 [Vitis vinifera] | 177 | 44,924 | 17.6 | 4 | 0.557 | Protein metabolism |
| gi|224141163| | Predicted protein [Populus trichocarpa] | 60 | 36,379 | 10.7 | 2 | 0.56 | Protein metabolism |
| gi|59797458| | Superoxide dismutase [Lilium hybrid cultivar] | 223 | 21,087 | 29.1 | 3 | 1.849 | Stress/defense/detoxification |
| sp|Q93VQ9| | Thioredoxin O2, mitochondrial [Spinacia oleracea] | 80 | 25,925 | 12 | 2 | 1.907 | Stress/defense/detoxification |
| gi|536838| | NADPH thioredoxin reductase, partial [Helianthus annuus] | 207 | 45,192 | 17.4 | 4 | 1.778 | Stress/defense/detoxification |
| sp|Q9LS40| | protein aspartic protease in guard cell 1 [Rabidopsis thaliana] | 193 | 48,663 | 17.6 | 5 | 1.635 | Stress/defense/detoxification |
| sp|Q96520| | Peroxidase 12 [Spinacia oleracea] | 132 | 41,132 | 15.2 | 3 | 1.899 | Stress/defense/detoxification |
| gi|3201547| | Endochitinase [Persea americana] | 79 | 18,633 | 4.3 | 1 | 1.971 | Stress/defense/detoxification |
| sp|Q06015| | Endochitinase 3 (Fragment) [Arachis hypogaea] | 167 | 39,946 | 13.3 | 3 | 1.691 | Stress/defense/detoxification |
| gi|215398978| | Dehydrin [Camellia sinensis] | 44 | 20,578 | 11 | 2 | 5.811 | Stress/defense/detoxification |
| gi|15637350| | Glutaredoxin [Tilia platyphyllos] | 150 | 18,171 | 10.9 | 1 | 1.74 | Stress/defense/detoxification |
| sp|P13240| | Disease resistance response protein 206 [Pisum sativum] | 167 | 39,946 | 13.3 | 3 | 1.691 | Stress/defense/detoxification |
| sp|O80934| | Uncharacterized protein At2g37660, chloroplastic [rabidopsis thaliana] | 161 | 35,389 | 21.8 | 4 | 1.963 | Stress/defense/detoxification |
| gi|75138338| | Peroxiredoxin Q, chloroplastic [Gentiana triflora] | 95 | 27,886 | 11.1 | 3 | 0.595 | Stress/defense/detoxification |
| sp|O23044| | Peroxidase 3 [Spinacia oleracea] | 241 | 36,174 | 19.8 | 5 | 0.584 | Stress/defense/detoxification |
| sp|A7NY33| | Peroxidase 4 [Vitis vinifera] | 119 | 33,610 | 21.9 | 4 | 0.599 | Stress/defense/detoxification |
| sp|P22242| | Desiccation-related protein PCC13-62 [Craterostigma plantagineum] | 695 | 21,349 | 33.3 | 4 | 0.615 | Stress/defense/detoxification |
| gi|270064305| | Abscisic stress ripening [Musa ABB Group] | 243 | 26,152 | 15.3 | 2 | 0.265 | Stress/defense/detoxification |
| sp|Q41328| | Pto-interacting protein 1 [Solanum lycopersicum] | 52 | 42,570 | 13.3 | 3 | 0.63 | Stress/defense/detoxification |
| sp|Q9FM19| | Hypersensitive-induced response protein 1 [Spinacia oleracea] | 124 | 37,917 | 9.1 | 2 | 0.517 | Stress/defense/detoxification |
| sp|P85524| | kirola [Actinidia deliciosa] | 136 | 24,392 | 19.3 | 3 | 0.599 | Stress/defense/detoxification |
| gi|15637165| | Voltage-dependent anion channel [β vulgaris] | 340 | 39,615 | 13.9 | 4 | 2.321 | Transport |
| gi|225439482| | PREDICTED: importin subunit β-1 [Vitis vinifera] | 65 | 90,956 | 3.9 | 2 | 2.014 | Transport |
| gi| 526118004| | Probable E3 ubiquitin-protein ligase HERC1 [Vitis vinifera] | 106 | 55,419 | 8 | 2 | 1.845 | Transport |
| gi|147859669| | Hypothetical protein VITISV_026572 [Vitis vinifera] | 105 | 32,300 | 9.1 | 2 | 1.988 | Transport |
| gi|147842983| | Hypothetical protein VITISV_024360 [Vitis vinifera] | 41 | 29,785 | 4 | 1 | 3.304 | Transport |
| sp|Q41009| | Translocase of chloroplast 34 [Pisum sativum] | 42 | 12,006 | 28.9 | 2 | 0.599 | Transport |
| gi|87247471| | Putative glutathione S-transferase [Populus x canadensis] | 295 | 31,506 | 16.9 | 2 | 0.577 | Transport |
| gi|8896066| | FtsZ1 [Tagetes erecta] | 71 | 30,227 | 11.2 | 2 | 2.163 | Biological regulation and signal transduction |
| gi|71535005| | Zinc finger Glo3-like protein [Medicago sativa] | 151 | 53,757 | 9.9 | 3 | 1.887 | Biological regulation and signal transduction |
| sp|P93508| | Calreticulin [Ricinus communis] | 250 | 22,819 | 30.5 | 4 | 1.79 | Biological regulation and signal transduction |
| gi|255562771| | STS14 protein precursor, putative [Ricinus communis] | 165 | 20,709 | 22.1 | 3 | 4.166 | Biological regulation and signal transduction |
| gi|40807639| | Cystatin [Actinidia eriantha] | 250 | 22,819 | 30.5 | 4 | 1.79 | Biological regulation and signal transduction |
| gi|359497545| | PREDICTED: leucine-rich repeat receptor-like serine/threonine-protein kinase BAM1-like [Vitis vinifera] | 250 | 22,819 | 30.5 | 4 | 1.79 | Biological regulation and signal transduction |
| sp|Q8H100| | Probable ADP-ribosylation factor GTPase-activating protein AGD8 [Spinacia oleracea] | 382 | 58,424 | 15.4 | 5 | 2.137 | Biological regulation and signal transduction |
| gi|359495838| | PREDICTED: uncharacterized protein LOC100264206 [Vitis vinifera] | 58 | 30,115 | 10.4 | 2 | 3.438 | Biological regulation and signal transduction |
| sp|O23193| | CBS domain-containing protein CBSX1, chloroplastic [Spinacia oleracea] | 138 | 27,995 | 20.6 | 3 | 1.672 | Biological regulation and signal transduction |
| sp|P93654| | Syntaxin-22 [Spinacia oleracea] | 35 | 30,780 | 8.7 | 2 | 0.522 | Biological regulation and signal transduction |
| gi|350534900| | 14-3-3 protein 3 [Solanum lycopersicum] | 368 | 38,590 | 27.9 | 7 | 0.599 | Biological regulation and signal transduction |
| gi|359492889| | PREDICTED: 14-3-3 protein [Vitis vinifera] | 244 | 40,230 | 20.7 | 6 | 0.525 | Biological regulation and signal transduction |
| sp|Q9FM65| | Fasciclin-like arabinogalactan protein 1 [Spinacia oleracea] | 247 | 36,736 | 18.8 | 3 | 0.559 | Biological regulation and signal transduction |
| gi|95116526| | Ethylene inducible protein hever [Theobroma cacao] | 131 | 37,972 | 15.2 | 4 | 0.619 | Biological regulation and signal transduction |
| sp|A1Y2B7| | Protein suppressor of gene SILENCING 3 homolog [Zea mays] | 86 | 51,127 | 7.8 | 2 | 0.602 | Biological regulation and signal transduction |
| gi|255587170| | Minichromosome maintenance protein, putative [Ricinus communis] | 63 | 16,167 | 7.5 | 1 | 0.547 | Biological regulation and signal transduction |
| gi|255571471| | Systemin receptor SR160 precursor, putative [Ricinus communis] | 200 | 108,992 | 6.6 | 5 | 0.483 | Biological regulation and signal transduction |
| gi|359494860| | PREDICTED: protein MOR1-like, partial [Vitis vinifera] | 102 | 17,742 | 8.8 | 1 | 0.603 | Biological regulation and signal transduction |
| gi|359495954| | PREDICTED: syntaxin-51-like [Vitis vinifera] | 318 | 19,583 | 10.5 | 1 | 0.243 | Biological regulation and signal transduction |
| sp|Q94KK7| | Syntaxin-52 [Spinacia oleracea] | 40 | 31,732 | 6.3 | 1 | 0.594 | Biological regulation and signal transduction |
| gi|359493650| | PREDICTED: early nodulin-like protein 2-like [Vitis vinifera] | 214 | 26,770 | 23 | 4 | 0.538 | Biological regulation and signal transduction |
| gi|225456479| | PREDICTED: signal recognition particle 68 kDa protein [Vitis vinifera] | 52 | 30,059 | 4.6 | 1 | 0.623 | Biological regulation and signal transduction |
| sp|O22126| | Fasciclin-like arabinogalactan protein 8 [Spinacia oleracea] | 256 | 45,342 | 16.4 | 5 | 0.51 | Biological regulation and signal transduction |
| gi|388491766| | Unknown [Lotus japonicus] | 171 | 15,992 | 18.3 | 1 | 0.538 | Biological regulation and signal transduction |
| gi|255545216| | Conserved hypothetical protein [Ricinus communis] | 85 | 37,085 | 13 | 3 | 2.209 | Unknown biological processes |
| gi|255547524| | Conserved hypothetical protein [Ricinus communis] | 50 | 41,524 | 3.3 | 1 | 2.09 | Unknown biological processes |
| sp|Q6YYB0| | UNCHARACTERIZED protein Os08g0359500 [Oryza sativa subsp. Japonica] | 56 | 15,660 | 12.7 | 1 | 1.541 | Unknown biological processes |
| gi|225423539| | PREDICTED: uncharacterized protein LOC100262861 [Vitis vinifera] | 152 | 24,072 | 16 | 2 | 2.042 | Unknown biological processes |
| gi|330318602| | Hypothetical protein [Camellia sinensis] | 40 | 19,541 | 6 | 1 | 1.809 | Unknown biological processes |
| gi|224070853| | Predicted protein [Populus trichocarpa] | 104 | 23,465 | 22.3 | 3 | 1.788 | Unknown biological processes |
| gi|224144195| | Predicted protein [Populus trichocarpa] | 93 | 28,934 | 6.2 | 1 | 1.668 | Unknown biological processes |
| gi|147818671| | Hypothetical protein VITISV_014852 [Vitis vinifera] | 43 | 14,360 | 10.5 | 1 | 1.715 | Unknown biological processes |
| gi|359488537| | PREDICTED: uncharacterized protein LOC100853981 [Vitis vinifera] | 71 | 21,254 | 8.3 | 1 | 1.921 | Unknown biological processes |
| gi|147818796| | Hypothetical protein VITISV_021596 [Vitis vinifera] | 625 | 66,920 | 20.2 | 9 | 1.795 | Unknown biological processes |
| gi|225452887| | PREDICTED: uncharacterized protein At5g39570 [Vitis vinifera] | 141 | 28,611 | 31.3 | 4 | 1.902 | Unknown biological processes |
| gi|359491847| | PREDICTED: uncharacterized protein LOC100240982 [Vitis vinifera] | 78 | 22,451 | 15.1 | 2 | 1.893 | Unknown biological processes |
| gi|225463725| | PREDICTED: uncharacterized protein LOC100261025 [Vitis vinifera] | 79 | 82,239 | 7.5 | 3 | 1.632 | Unknown biological processes |
| gi|147818796| | Hypothetical protein VITISV_021596 [Vitis vinifera] | 625 | 66,920 | 20.2 | 9 | 1.795 | Unknown biological processes |
| gi|356551464| | PREDICTED: uncharacterized protein LOC100807412 [Glycine max] | 53 | 57,807 | 3 | 1 | 2.315 | Unknown biological processes |
| gi|298205066| | Unnamed protein product [Vitis vinifera] | 293 | 59,849 | 8 | 3 | 1.506 | Unknown biological processes |
| gi|358249210| | Uncharacterized protein LOC100818758 [Glycine max] | 74 | 26,221 | 15.1 | 2 | 2.26 | Unknown biological processes |
| gi|224089721| | Predicted protein [Populus trichocarpa] | 64 | 16,865 | 21 | 2 | 1.824 | Unknown biological processes |
| gi|225443833| | PREDICTED: uncharacterized protein LOC100253185 [Vitis vinifera] | 155 | 13,410 | 33 | 3 | 1.815 | Unknown biological processes |
| gi|297743302| | Unnamed protein product [Vitis vinifera] | 269 | 40,524 | 24.2 | 5 | 1.913 | Unknown biological processes |
| gi|296081618| | Unnamed protein product [Vitis vinifera] | 280 | 16,151 | 51.6 | 4 | 2.009 | Unknown biological processes |
| gi|225459322| | PREDICTED: uncharacterized protein LOC100260886 isoform 2 [Vitis vinifera] | 80 | 43,415 | 11.6 | 3 | 1.733 | Unknown biological processes |
| sp|Q6ID70| | Uncharacterized protein At3g03773 [Spinacia oleracea] | 189 | 28,352 | 12.6 | 2 | 1.772 | Unknown biological processes |
| gi|225451915| | PREDICTED: uncharacterized protein LOC100244706 [Vitis vinifera] | 181 | 28,654 | 12.2 | 2 | 2.492 | Unknown biological processes |
| gi|297738842| | Unnamed protein product [Vitis vinifera] | 68 | 67,341 | 4.9 | 2 | 0.657 | Unknown biological processes |
| gi|359489218| | PREDICTED: uncharacterized protein LOC100232913 [Vitis vinifera] | 95 | 31,024 | 10.9 | 2 | 0.565 | Unknown biological processes |
| gi|17863981| | Unknown [Davidia involucrata] | 150 | 95,611 | 9.6 | 6 | 0.598 | Unknown biological processes |
| gi|224056457| | Predicted protein [Populus trichocarpa] | 43 | 32,607 | 7.2 | 1 | 0.25 | Unknown biological processes |
| gi|359488731| | PREDICTED: uncharacterized protein LOC100264617 [Vitis vinifera] | 111 | 34,149 | 5.2 | 1 | 0.446 | Unknown biological processes |
| gi|359476152| | PREDICTED: uncharacterized protein LOC100260975 [Vitis vinifera] | 142 | 40,178 | 10.4 | 3 | 0.374 | Unknown biological processes |
| gi|297742161| | Unnamed protein product [Vitis vinifera] | 141 | 34,984 | 19.3 | 3 | 0.436 | Unknown biological processes |
| gi|225449483| | PREDICTED: uncharacterized protein LOC100244410 [Vitis vinifera] | 73 | 23,689 | 7.1 | 1 | 0.65 | Unknown biological processes |
| gi|225463406| | PREDICTED: uncharacterized protein LOC100250442 [Vitis vinifera] | 85 | 20,570 | 7.9 | 1 | 0.631 | Unknown biological processes |
| gi|351722061| | Uncharacterized protein LOC100305495 precursor [Glycine max] | 236 | 23,212 | 10.4 | 1 | 0.52 | Unknown biological processes |
| gi|224141949| | Predicted protein [Populus trichocarpa] | 101 | 18,222 | 19 | 1 | 0.514 | Unknown biological processes |
2.4. RT-qPCR Analysis and Enzyme Activity Assay
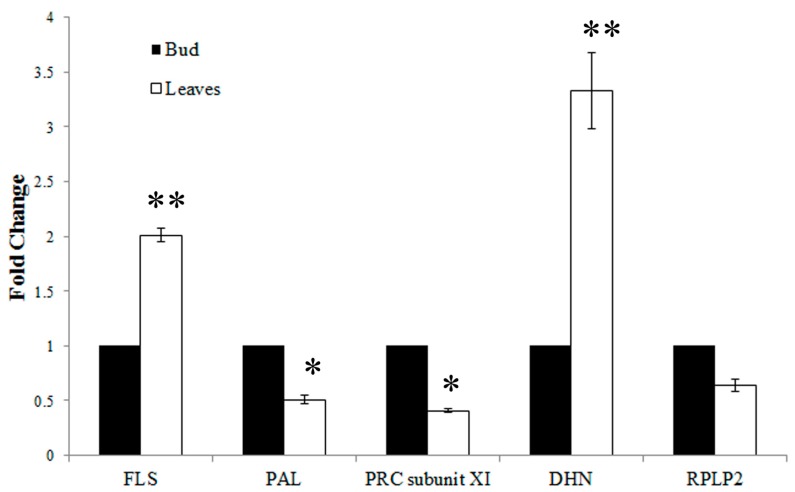
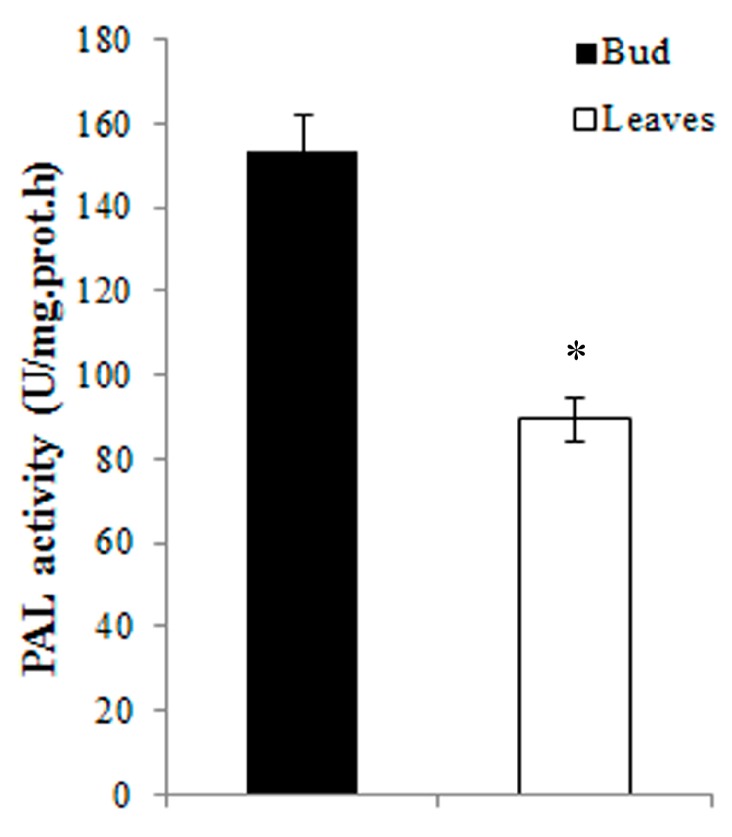
To evaluate the iTRAQ results, RT-qPCR analysis and enzyme activity assays were performed. Five proteins were selected for RT-qPCR analysis; three were up-regulated (flavonol synthase, FLS; dehydrin, DHN; and 60S acidic ribosomal protein p2, RPLP2), and two were down-regulated (phenylalanine ammonia-lyase, PAL; photosystem I reaction center subunit XI, PRC subunit XI) in the young expanding leaves compared with the buds. As shown in Figure 4, the expression levels of FLS and DHN were significantly up-regulated in the young leaves compared with the buds (FLS: 2.01 ± 0.06-fold, p < 0.01. DHN: 3.33 ± 0.34-fold, p < 0.01). However, the expression levels of PAL and PRC subunit XI were significantly down-regulated in the young leaves compared with the buds (PAL: 0.51 ± 0.04-fold, p < 0.05. PRC subunit XI: 0.41 ± 0.02-fold, p < 0.05). The expression of RPLP2 was also down-regulated in the young expanding leaves compared with the buds (0.64 ± 0.05-fold), but no significant difference was observed (p > 0.05). The transcription levels of FLS, DHN, PAL and PRC subunit XI were closely correlated with the levels of their translation products in the buds and the young expanding leaves, whereas the RPLP2 transcript levels did not correspond with those of its translation products. As shown in Figure 5, PAL activity was significantly lower in young expanding leaves than in buds, which is consistent with its gene and protein expression levels in the buds and the young expanding leaves of tea plants.
Figure 4.
RT-qPCR analysis of the transcript levels of the differentially expressed proteins. FLS: flavonol synthase; PAL: phenylalanine ammonia-lyase; PRC subunit XI: photosystem I reaction center subunit XI; DHN: dehydrin; RPLP2: 60S acidic ribosomal protein p2. Statistical significance: * p < 0.05 and ** p < 0.01.
Figure 5.
PAL activity in the buds and in young expanding leaves. Statistical significance: * p < 0.05.
3. Discussion
A previous study used subtractive cDNA library analysis to reveal the genes involved in the production of polyphenols and other secondary metabolites that are relatively abundant in young leaves [2]. However, because of post-transcriptional regulation, protein expression levels cannot always be predicted from quantitative mRNA data; the mRNA level does not always correlate with the protein level [10]. Therefore, proteomic analysis could improve our understanding of the molecular mechanisms underlying the change in the metabolite contents of the apical buds and the young expanding leaves of tea plants.
3.1. Changes in Secondary Metabolites
Tea leaves contain large amounts of flavonoids, including flavanones, flavones, flavonols, flavan-3-ols, and anthocyanidins. The predominant flavonoid in tea is catechin, which distinguishes tea from other plants and is an important determinant of tea quality and taste. A previous study showed that the concentrations of total catechins and polyphenols in tea leaves declined with leaf age, but changes in individual catechins varied [11]. Our HPLC analysis showed that EGCG and ECG were the most abundant catechins in both the buds and the young expanding leaves. These compounds exist in the green parts of tea seedlings but were not detected in the roots or cotyledons [12]. The catechins index [(EGCG + ECG)/EGC] was positively correlated with the sensory evaluation of brewed green tea [13]. Based on HPLC results, the green tea quality indexes of the buds and the young leaves were 45.11 and 15.59, respectively. These results were consistent with previous research [7,11]. Historically, tea has been valued for its purine alkaloids, including theobromine, theophylline and caffeine [14]. Theobromine is formed as part of the caffeine biosynthetic pathway and is produced in abundance if the methylation pathway of caffeine biosynthesis is absent [14]. An analysis of purine alkaloids in different tea seedling organs showed that more than 99% of the caffeine was in the leaves, with older leaves containing more per gram of fresh weight. Theobromine was found only in the younger leaves, and theophylline was either not present or present only in trace amounts [8]. Our study showed that the concentrations of theobromine and caffeine were lower in young expanding leaves, but no significant difference in theophylline levels was observed. Purine alkaloid metabolism also appears to be closely associated with leaf development and aging in tea seedlings [9,15]. The major biosynthetic route for caffeine is thought to be xanthosine→7-methyxanthosine→7-methylxanthine→theobromine →caffeine, and previous studies have indicated that caffeine biosynthesis was primarily controlled by the first N-methyl-transfer reaction, which is catalyzed by 7-methylxanthosine synthase [16,17]. Hence, the relatively lower caffeine and theobromine contents of young expanding leaves found in this study may be attributable either to a smaller supply of xanthosine for caffeine biosynthesis or to the lower activity of 7-methylxanthosine synthase in young expanding tea leaves.
3.2. Proteins Involved in Carbohydrate and Energy Metabolism
In plants, glycolysis and the tricarboxylic acid (TCA) cycle provide not only energy and cofactors but also important substrates for the synthesis of metabolites, as well as feedback signals [18]. Dynamic proteomic analysis revealed that the levels of glycolysis- and TCA cycle-related proteins increased during early-stage seed development in rice [19]. Our present results show that a subset of the differentially expressed proteins were involved in glycolysis and TCA, such as NADP-dependent glyceraldehyde-3-phosphate dehydrogenase (NADP-dependent GAPDH), dihydrolipoyl dehydrogenase (DLD), pyruvate dehydrogenase E3 subunit (PDE3), dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase (DLST) and phosphoenolpyruvate carboxylase (PEPC); these proteins were present at higher levels in the young, expanding leaves than in the buds. These results indicated that glycolysis and the TCA cycle increased in the young, expanding leaves and that more energy and substrates were required during the developmental stage at which young, expanding leaves are present.
3.3. Proteins Related to Secondary Metabolism
Polyphenols are the most important chemical compounds in tea plants, and have received increasing attention in recent years because of their benefits to human health [20,21,22,23]. The polyphenols in tea are predominantly members of three subclasses: flavanols, flavones and flavonols [24]. Four major catechins (flavanols), (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC), and (−)-epigallocatechin gallate (EGCG), constitute approximately one-third of the dry weight of green tea [25]. Quercetin, kaempferol, myricetin and their glycosides (flavonols), as well as apigenin glycosides (flavones), are also present, but at much lower concentrations [24]. Several proteins related to polyphenol biosynthesis were differentially expressed between the buds and the young, expanding leaves. Flavonol synthase (FLS), a dioxygenase that converts dihydroflavonols into flavonols, was initially found in parsley and was shown to require 2-oxoglutarare and Fe/ascorbate for full activity [26]. In FLS-silenced tobacco, there was a 25%–93% reduction in the flavonoid (quercetin) content and an increase in the catechin and epicatechin content [27,28]. Our previous study also indicated that FLS expression was a negative regulator of catechin biosynthesis, and especially of ECG and EGCG [29]. In our proteomic analysis, the expression of FLS was increased at the stage of young, expanding leaves, which indicated that at this stage, flavonol biosynthesis was enhanced and catechin biosynthesis was inhibited. These results also agree with our metabolic data, which show that compared with the buds, the flavonol content was greater and the total catechin content was lower in the young, expanding leaves. Isoflavone reductase homolog P3 belongs to the NmrA-type oxidoreductase family and the isoflavone reductase subfamily. Isoflavone reductase (IFR) specifically recognizes isoflavones and catalyzes a stereospecific, NADPH-dependent reduction to (3R)-isoflavanone [30]. In tea plants, IFR catalyzes the conversion of leucocyanidin and leucodelphinidin to (+)-catechin and (+)-gallocatechin, respectively. In our proteomic analysis, the expression of IFR homolog P3, which is involved in the accumulation of high levels of catechins, was more highly expressed in the buds compared with the young, expanding leaves. Phenylalanine ammonia-lyase (PAL) is an enzyme that catalyzes the conversion of l-phenylalanine to ammonia and trans-cinnamic acid [31]. PAL resides at a metabolically important position, linking secondary metabolism to primary metabolism. PAL is part of the first committed step in the phenylpropanoid pathway and is a key enzyme in the allocation of significant amounts of carbon from phenylalanine into the biosynthesis of several important secondary metabolites, such as lignins, flavonoids, and coumarins [32,33]. The overall flux into phenylpropanoid metabolism has been suggested to be regulated by PAL, which acts as a rate-limiting enzyme [34]. Park et al. found that PAL gene expression and catechin content were also reduced in mature leaves compared with young leaves [2]. A positive correlation between catechin content and the gene expression of PAL was observed under drought stress, after wounding and after abscisic acid treatment [35]. In the present study, the expression of both the PAL gene and protein were inhibited, and the catechin content was also reduced in young, expanding leaves. These results indicated that the carbon flux from phenylalanine into the biosynthesis of secondary metabolites was inhibited in the young, expanding leaves compared with the buds. Hydroxycinnamoyl-CoA: shikimate/quinate hydroxycinnamoyltransferase (HCT), which converts p-coumarate from CoA to shikimate/quinate esters, has been described as reversible enzyme [36]. It is involved in a step in lignin synthesis, and its down-regulation affects lignin content and composition [37,38]. In our proteomic analysis, the expression level of HCT was lower in the young, expanding leaves than in the buds. Arabidopsis plants in which HCT is silenced or lignin is repressed direct the metabolic flux into flavonoids through chalcone synthase [39], which may explain why the non-galloylated catechin content increased in the young, expanding tea leaves.
3.4. Photosynthetic Proteins
Photosynthesis is a key biological process in plant growth and development. In the present study, the abundance of several proteins involved in photosynthesis differed between the buds and the young expanding leaves. These proteins include ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and its large subunit (RubiscoL), sedoheptulose-1,7-bisphosphatase (SBPase) precursor, photosystem I reaction center subunit XI (PS I-E), thylakoid lumenal 29 kDa protein (TL29), peroxiredoxin Q (PRXQ) and chlorophyll A/B binding protein (CitCAB1,2). Several studies have shown that during leaf development, photosynthetic activity gradually increases, and photosynthetic enzymes slowly accumulate [40,41,42,43]. Correlations between the photosynthetic rate and the catechin content of the leaves of tea plants showed that there was a positive correlation between the photosynthetic rate and the EC and GCG contents but a negative correlation between the photosynthetic rate, the total catechin content and the galloylated catechin content [44]. A study focusing on the relationship between the synthesis and accumulation of phenolics and flavonoids and the photosynthetic rate in ginger showed that when photosynthesis decreased, the synthesis of flavonoids such as quercetin, catechin, epicatechin and naringenin increased, and the soluble carbohydrates and plant biomass decreased [45]. The results of our proteomic analysis also showed that the expression of photosynthetic proteins was down-regulated in the buds compared with the young, expanding leaves. We infer that in the buds, the rate of photosynthesis is lower, so the carbon flow shifts from photosynthesis to the shikimic acid pathway, thereby producing more phenolics and flavonoids.
3.5. Defense-Related Proteins
The cellular antioxidant system consists of different enzymes. In our proteomic analysis, antioxidant proteins, such as superoxide dismutase (SOD), thioredoxin O2 (TO2), NADPH thioredoxin reductase (NADPH-TR), and glutaredoxin (GRX), were more abundant in young, expanding leaves than in buds. The activity of antioxidant enzymes, such as SOD also increased at early stages of leaf expansion and was sustained throughout leaf expansion [46,47]. Therefore, the proteins involved in the antioxidant system may be related to leaf expansion. Another study also indicated that a certain concentration of reactive oxygen species (ROS) is necessary for leaf elongation, but it could not be determined if H2O2 or other ROS are the active agents [48]. We suggest that the accumulation of antioxidant proteins could dissipate excess excitation energy and protect leaves against photodamage, which can be caused by a certain levels of ROS in expanding tea leaves.
4. Experimental Section
4.1. Plant Materials
Tea plants were grown in the experimental tea garden of Hunan Agricultural University in Changsha, China. The apical buds and the first unfolding leaves were plucked from the same plants at different stages of development, briefly washed with sterile water, immediately frozen in liquid nitrogen and stored at −80 °C prior to analysis (Figure 6).
Figure 6.

The buds and young expanding leaves of tea plants.
4.2. Metabolic Analysis of Tea Samples
Total polyphenols, catechins and alkaloids were extracted from the samples and analyzed as previously described with a slight modification [29]; a total of 0.20 g of freeze-dried, ground leaves was accurately weighed and extracted twice with 5 mL of a 75:25 (v/v) ethanol:water solution at 80 °C for 15 min. The extract was filtered through filter paper and then diluted to 50 mL. The total polyphenol and flavonoid content in the sample was determined using the ferrous tartrate method [49] and the aluminum trichloride method [50]. The catechin and alkaloid contents were determined with high-performance liquid chromatography (HPLC) according to Wang et al. [51] with slight modifications. A Shimadzu HPLC system (Shimadzu, Tokyo, Japan) with 10AD dual pumps was used with a reversed-phase column (Welchorm C18 200 × 4.6, 5 μm), a mobile phase of distilled water (A) and a mobile phase (B) of 40% N,N-dimethylformamide, 2% methanol and 1.5% acetic acid. The gradient was as follows: 0.01–13.00 min, linear gradient from 14% to 23% B; 13.00–25.00 min, linear gradient from 23% to 36% B; 25.00–28.00 min, 36% B; 28.00–30.00 min, linear gradient from 36%–14% B; 30.00–34.00 min, 14% B. The samples were eluted at 35 °C and at a flow rate of 1.00 mL/min. The chromatograms were recorded at 278 nm. The peaks were identified by comparing the retention times of the sample to those of authentic standards. The extraction for flavone hydrolysis was carried out as follows: plant material (0.5 g dry weight) was mixed with 20 mL methanol and 2.0 mL HCl (6 M). After refluxing at 95 °C for 1.5 h, the hydrolyzed solution was filtered through filter paper, then diluted to 25 mL with methanol. Flavonols were detected with the following HPLC method [52]: the mobile phase consisted of 30% acetonitrile in 0.025 M KH2PO4 buffer solution (v/v); the pH of the mobile phase was adjusted to 2.5 using H3PO4. The samples were eluted at 35 °C at a flow rate of 1.00 mL/min and were monitored at 370 nm. The peaks were identified by comparing the retention times of the sample to those of authentic standards. All experiments included three separate biological replicates.
4.3. Protein Extraction
Leaf samples were weighed and ground in liquid nitrogen, then suspended in lysis buffer [7 M urea, 2 M thiourea, 4% 3-[(3-Cholamidopropyl)dimethylammonio]propanesulfonate (CHAPS), 40 mM Tris-HCl, pH 8.5, 1 mM Phenylmethanesulfonyl fluoride (PMSF), 2 mM Ethylene Diamine Tetraacetic Acid (EDTA), 10 mM dl-Dithiothreitol (DTT)] and kept in an ice bath for 2 h. After this 2 h lysis, the samples were sonicated in an ice bath for 15 min and were clarified by centrifugation at 25,000× g. The supernatant was collected, and the protein concentration was determined with a 2D quantification kit (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
4.4. iTRAQ Analysis
iTRAQ analysis was performed at the Beijing Genomics Institute (BGI, Shenzhen, China). Protein samples were reduced with 10 mM DTT, alkylated with 55 mM iodoacetamide, digested using sequencing-grade trypsin (Promega, Madison, WI, USA), and labeled using an iTRAQ Reagent Multiplex Kit (AB SCIEX, Foster City, CA, USA) according to the manufacturer’s protocol. The bud and leaf samples were labeled with 114 and 117 Da, respectively. After labeling, all samples were pooled and purified using a strong cation exchange chromatography (SCX) column (Phenomenex, Torrance, CA, USA) with an LC-20AB HPLC system (Shimadzu, Tokyo, Japan). The labeled peptides were separated with mobile phase B (2% water, 98% acetonitrile and 0.1% formic acid) at a flow rate of 300 nL/min, 0%–5% over 1 min, 5%–35% over 40 min and 35%–80% over 5 min on a nanoACQuity system (Waters, Milford, MA, USA). The LC fractions were analyzed using a Triple TOF 5600 mass spectrometer (AB SCIEX, Foster City, CA, USA) fitted with a Nanospray Ⅲ source (AB SCIEX, Concord, MA, USA) and a pulled quartz tip (New Objectives, Woburn, MA, USA). The data were acquired using an ion spray voltage of 2.5 kV and an interface heater temperature of 150 °C. Curtain gas and nebulizer gas were delivered at 30 pounds per square inch (PSI) and 15 PSI, respectively. For information-dependent acquisition (IDA), survey scans were acquired in 250 ms, and once the detection of ions with a 2+ to 5+ charge state crossed a threshold of 150 counts per second, as many as 35 product ion scans were collected. The total cycle time was fixed at 2.5 s. A rolling collision energy setting was applied to all precursor ions for collision-induced dissociation (CID). Two independent biological experiments with three technical replicates each were performed.
4.5. Data Analysis
MS/MS data acquisition was performed with Analyst QS 2.0 software (AB SCIEX, Foster City, CA, USA). For protein identification, MS/MS data were searched against the “plant” subset of the National Center for Biotechnology Information Non-redundant protein sequences (NCBInr) database using Mascot version 2.3.02 (Matrix Science, London, UK). The search parameters were as follows: a peptide mass tolerance of 10 ppm was allowed for intact peptides and ± 0.05 Da for fragmented ions; a maximum of one missed cleavage was allowed in the trypsin digests; cysteine carbamidomethylation was considered a fixed modification; glutamine pyrophosphorylation variable oxidation of methionine and iTRAQ labeling of tyrosine were set as variable modifications; carbamidomethylation of cysteine and iTRAQ labeling of lysines and the N-terminal amino group of peptides were set as fixed modifications. Only peptides with significance scores greater than “identity score” were considered identified, and a protein was considered identified if at least one such unique peptide match was apparent for the protein. For protein quantitation, the peptide to be quantified was automatically selected using the Pro Group algorithm to calculate the reporter peak area, the error factor (EF), and the p-value. Proteins with a fold change of >1.5 and a two-tailed p-value of less than 0.05 were considered to have significantly different expression.
4.6. Bioinformatic Analysis of Proteins
Differentially expressed proteins were mapped to Gene Ontology Terms (GO) using a local Bell Labs Layered Space-Time (BLAST) against a reference database downloaded from the website (GO-Annotation@EBI). The Clusters of Orthologous Groups of Proteins system (COG) can be used to functionally annotate genes from new genomes and for research on genome evolution [53]. The Kyoto Encyclopedia of Genes and Genomes (KEGG) is an updated system that computerizes current knowledge on biochemical pathways and other types of molecular interactions and can be used as a reference for the systematic interpretation of sequencing data [54]. To augment the biological and functional properties of differentially expressed proteins, the proteins were further analyzed using the COG (http://www.ncbi.nlm.nih.gov/COG/) and KEGG databases (http://www. genome.jp/kegg/pathway.html).
4.7. Real-time Quantitative PCR Analysis
Total RNA for RT-qPCR analysis was extracted from leaves at the two developmental stages using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) and an RNase-Free DNase Set (Qiagen, Hilden, Germany). cDNA was synthesized from the total RNA (1 μg) using oligo(dT)18 primers and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) according to the manufacturer’s instructions. The primers used for RT-qPCR (Table 2) were designed using Beacon Designer 7.0 software (Premier Biosoft, Palo Alto, CA, USA) and were based on the cDNA sequences. The reactions were carried out with a Rotor-Gene Q 6200 real-time PCR system (Qiagen, Hilden, Germany) using three-step cycling conditions of 95 °C for 10 min followed by 45 cycles of 95 °C for 10 s, 56 °C for 15 s and 72 °C for 20 s. The reaction mixture (20 μL) contained 1 μL of cDNA solution, 10 μL of Platinum SYBR® Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA, USA) and primers at a concentration of 6 μM each. For each RT-qPCR sample, there were three biological replicates with three technical replicates. The GAPDH gene was used as an internal standard for the normalization of gene expression, and the tea buds were used as a reference sample whose value was set to 1. The relative gene expression was evaluated using the comparative cycle threshold method [55].
Table 2.
Primers used in RT-qPCR analysis.
| Gene Name | Accession Number | Primer Sequence (5′–3′) |
|---|---|---|
| Flavonol synthase | DQ198089 | Forward: ggagaacagcaaggctatcg |
| Reverse: tctcctcctgtgggagctta | ||
| Phenylalanine ammonia-lyase | D26596 | Forward: tccgatcatcgacaaaatca |
| Reverse: agctcagagaattgggcaaa | ||
| Photosystem I reaction center subunit XI | HM003371 | Forward: tcaaagaaggagagccatcg |
| Reverse: gcaagaaataggcccaaatg | ||
| Dehydrin | FJ436978 | Forward: gaggagaggaccaacagcag |
| Reverse: acgacaccgacacacacatt | ||
| 60S acidic ribosomal protein p2 | HM003314 | Forward: gggtgctattgcagtgacct |
| Reverse: attgggggagaaagaaggaa |
4.8. PAL Extraction and Enzyme Assays
Tea samples (1 g) were ground into a fine powder with a mortar and pestle in liquid N2. The powder was extracted with 5 mL of extraction buffer 50 mM Tris-HCl pH 8.9, 10 μM leupeptin, 5 mM EDTA, 15 mM β-mercaptoethanol, 5 mM Vc, 1 mM PMSF, 0.15% Polyvinylpyrrolidone (PVP)], and then was stirred on ice for 10 min. Subsequently, the mixture was centrifuged at 30,000× g for 30 min at 20 °C. The supernatant was stirred on Dowex (1 × 2) in the chloride (Cl) form for 30 min to remove residual phenolics. The cleared supernatant was used in a PAL enzyme assay. The protein concentrations in the enzyme extract were measured with a 2D quantification kit (GE Healthcare, Chalfont St Giles, Buckinghamshire, UK). PAL activity was assayed using the method of Solecka and Kacperska [29].
4.9. Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences software (SPSS; Chicago, IL, USA). ANOVA and Student’s t-tests were used to determine significant differences between different groups. A p-value <0.05 was considered significant.
5. Conclusions
The quantitative protein expression data presented in this study provide a global overview of a set of proteins that are expressed in the buds and the young, expanding leaves of tea. A total of 233 proteins were identified as being differentially expressed between the buds and the young leaves. A large array of diverse functions, including energy metabolism and the metabolism of carbohydrates, secondary metabolites, nucleic acids and proteins, as well as photosynthesis and defense-related processes, were revealed. Based on these results, we infer that the proteins involved in polyphenol biosynthesis and photosynthesis may also mediate the secondary metabolite content in tea plants. The proteins related to energy and antioxidant metabolism may promote tea leaf development. However, the RT-qPCR results showed that the protein expression levels did not closely correlate with their gene expression levels. Overall, these findings improve our understanding of the molecular mechanisms underlying the change in the metabolite content from the buds to the young, expanding leaves of tea plants.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (31200522 and 31470692), National “Five-twelfth” Plan for Science & Technology Support (2011BAD01B01), Program for Changjiang Scholar and Innovative Research Team in University (IRT0963), the Ministry of Science and Technology of China (NCET-11-0969), the Natural Science Foundation of Hunan Province (13JJ4067), Scientific Research fund of Hunan Agricultural University (13YJ13) and the “1515 Talent Project” of Hunan Agricultural University.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/16/06/14007/s1.
Author Contributions
Qin Li carried out the iTRAQ, HPLC and RT-qPCR experiments. Juan Li participated in the HPLC analysis. Shuoqian Liu participated in the design of this paper. Jianan Huang participated in the optimization of HPLC and RT-qPCR protocol. Haiyan Lin participated in the optimization of HPLC protocol. Kunbo Wang participated in the HPLC experiments. Xiaomei Cheng participated in the iTRAQ experiments. Zhonghua Liu conceived of the study, and participated in its design and coordination. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Khan N., Mukhtar H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013;19:6141–6147. doi: 10.2174/1381612811319340008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park J.S., Kim J.B., Hahn B.S., Kim K.H., Ha S.H., Kim Y.H. EST analysis of genes involved in secondary metabolism in Camellia sinensis (tea), using suppression subtractive hybridization. Plant Sci. 2004;166:953–961. doi: 10.1016/j.plantsci.2003.12.010. [DOI] [Google Scholar]
- 3.Shan Y., Li W., Wang Y., Liu Y., Wang H., Wang X., Lu Z., Tian Y., Gao L., Xia T. Catechin synthesis and accumulation in tea seedlings at different development stages. J. Anhui Agr. Univ. 2011;38:600–605. (In Chinese) [Google Scholar]
- 4.Li J., Zhao M., Zhang G., Ding X., Hu Y., Shen X., Shao W. HPLC analysis of myricetin, quercetin and kaempferol in fresh shoots of “Zijuan” tea, a new cultivar of Camellia sinensis var. Assamica. J. Yunnan Agri. Univ. 2012;27:235–240. (In Chinese) [Google Scholar]
- 5.Singh K., Rani A., Kumar S., Sood P., Mahajan M., Yadav S.K., Singh B., Ahuja P.S. An early gene of the flavonoid pathway, flavanone 3-hydroxylase, exhibits a positive relationship with the concentration of catechins in tea (Camellia sinensis) Tree Physiol. 2008;28:1349–1356. doi: 10.1093/treephys/28.9.1349. [DOI] [PubMed] [Google Scholar]
- 6.Eungwanichayapant P.D., Popluechai S. Accumulation of catechins in tea in relation to accumulation of mRNA from genes involved in catechin biosynthesis. Plant Physiol. Biochem. 2009;47:94–97. doi: 10.1016/j.plaphy.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Gao L., Xia T., Liu Y., Gao K. Study on the changes of non-galloylated catechins and relative enzymes in tea shoots. J. Tea Sci. 2009;29:365–371. [Google Scholar]
- 8.Suzuki T., Ashihara H., Waller G.R. Purine and purine alkaloid metabolism in Camellia and Coffea plants. Phytochemistry. 1992;31:2575–2584. doi: 10.1016/0031-9422(92)83590-U. [DOI] [Google Scholar]
- 9.Kato M., Kitao N., Ishida M., Morimoto H., Irino F., Mizuno K. Expression for caffeine biosynthesis and related enzymes in Camellia sinensis. Z. Naturforsch C. 2010;65:245–256. doi: 10.1515/znc-2010-3-413. [DOI] [PubMed] [Google Scholar]
- 10.Yan S.P., Zhang Q.Y., Tang Z.C., Su W.A., Sun W.N. Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol. Cell. Proteomics. 2006;5:484–496. doi: 10.1074/mcp.M500251-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Mamati G.E., Liang Y., Lu J. Expression of basic genes involved in tea polyphenol synthesis in relation to accumulation of catechins and total tea polyphenols. J. Sci. Food Agric. 2006;86:459–464. doi: 10.1002/jsfa.2368. [DOI] [Google Scholar]
- 12.Ashihara H., Deng W.W., Mullen W., Crozier A. Distribution and biosynthesis of flavan-3-ols in Camellia sinensis seedlings and expression of genes encoding biosynthetic enzymes. Phytochemistry. 2010;71:559–566. doi: 10.1016/j.phytochem.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Liang Y.R., Liu Z.S., Xu Y.R., Hu Y.L. A study on chemical composition of two special green teas (Camellia sinensis) J. Sci. Food Agric. 1990;53:541–548. doi: 10.1002/jsfa.2740530411. [DOI] [Google Scholar]
- 14.Harbowy M.E., Balentine D.A., Davies A.P., Cai Y. Tea chemistry. Crit. Rev. Plant Sci. 1997;16:415–480. doi: 10.1080/07352689709701956. [DOI] [Google Scholar]
- 15.Ashihara H., Kubota H. Patterns of adenine metabolism and caffeine biosynthesis in different parts of tea seedlings. Physiol. Plant. 1986;68:275–281. doi: 10.1111/j.1399-3054.1986.tb01926.x. [DOI] [Google Scholar]
- 16.Deng W.-W., Li Y., Ogita S., Ashihara H. Fine control of caffeine biosynthesis in tissue cultures of Camellia sinensis. Phytochem. Lett. 2008;1:195–198. doi: 10.1016/j.phytol.2008.09.009. [DOI] [Google Scholar]
- 17.Ashihara H., Sano H., Crozier A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry. 2008;69:841–856. doi: 10.1016/j.phytochem.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez L., van Wuytswinkel O., Castelain M., Bellini C. Combined networks regulating seed maturation. Trends Plant Sci. 2007;12:294–300. doi: 10.1016/j.tplants.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Ge P., Ma C., Wang S., Gao L., Li X., Guo G., Ma W., Yan Y. Comparative proteomic analysis of grain development in two spring wheat varieties under drought stress. Anal. Bioanal. Chem. 2012;402:1297–1313. doi: 10.1007/s00216-011-5532-z. [DOI] [PubMed] [Google Scholar]
- 20.McKay D.L., Blumberg J.B. The role of tea in human health: An update. J. Am. Coll. Nutr. 2002;21:1–13. doi: 10.1080/07315724.2002.10719187. [DOI] [PubMed] [Google Scholar]
- 21.Cabrera C., Artacho R., Gimenez R. Beneficial effects of green tea--a review. J. Am. Coll. Nutr. 2006;25:79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 22.Kao Y.-H., Chang H.-H., Lee M.-J., Chen C.-L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006;50:188–210. doi: 10.1002/mnfr.200500109. [DOI] [PubMed] [Google Scholar]
- 23.Boon N. Health potential for functional green teas? Int. J. Vitam. Nutr. Res. 2008;78:275–281. doi: 10.1024/0300-9831.78.6.275. [DOI] [PubMed] [Google Scholar]
- 24.Beecher G.R., Warden B.A., Merken H. Analysis of tea polyphenols. Proc. Soc. Exp. Biol. Med. 1999;220:267–270. doi: 10.3181/00379727-220-44377A. [DOI] [PubMed] [Google Scholar]
- 25.Balentine D.A., Wiseman S.A., Bouwens L.C.M. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 26.Lin G.-Z., Lian Y.-J., Ryu J.-H., Sung M.-K., Park J.-S., Park H.-J., Park B.K., Shin J.-S., Lee M.-S., Cheon C.-I. Expression and purification of His-tagged flavonol synthase of Camellia sinensis from Escherichia coli. Protein Expr. Purif. 2007;55:287–292. doi: 10.1016/j.pep.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 27.Mahajan M., Ahuja P.S., Yadav S.K. Post-transcriptional silencing of flavonol synthase mRNA in tobacco leads to fruits with arrested seed set. PLoS ONE. 2011;6:e28315. doi: 10.1371/journal.pone.0028315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahajan M., Joshi R., Gulati A., Yadav S.K. Increase in flavan-3-ols by silencing flavonol synthase mRNA affects the transcript expression and activity levels of antioxidant enzymes in tobacco. Plant Biol. 2012;14:725–733. doi: 10.1111/j.1438-8677.2011.00550.x. [DOI] [PubMed] [Google Scholar]
- 29.Xiong L., Li J., Li Y., Yuan L., Liu S., Huang J.A., Liu Z. Dynamic changes in catechin levels and catechin biosynthesis-related gene expression in albino tea plants (Camellia sinensis L.) Plant Physiol. Biochem. 2013;71:132–143. doi: 10.1016/j.plaphy.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Wang X., He X., Lin J., Shao H., Chang Z., Dixon R.A. Crystal structure of isoflavone reductase from alfalfa (Medicago sativa L.) J. Mol. Biol. 2006;358:1341–1352. doi: 10.1016/j.jmb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 31.Camm E.L., Towers G.H.N. Phenylalanine ammonia lyase. Phytochemistry. 1973;12:961–973. doi: 10.1016/0031-9422(73)85001-0. [DOI] [Google Scholar]
- 32.Fritz R.R., Hodgins D.S., Abell C.W. Phenylalanine ammonia-lyase. Induction and purification from yeast and clearance in mammals. J. Biol. Chem. 1976;251:4646–4650. [PubMed] [Google Scholar]
- 33.Tanaka Y., Matsuoka M., Yamanoto N., Ohashi Y., Kano-Murakami Y., Ozeki Y. Structure and characterization of a cDNA clone for phenylalanine ammonia-lyase from cut-injured roots of sweet potato. Plant Physiol. 1989;90:1403–1407. doi: 10.1104/pp.90.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bate N.J., Orr J., Ni W., Meromi A., Nadler-Hassar T., Doerner P.W., Dixon R.A., Lamb C.J., Elkind Y. Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Proc. Natl. Acad. Sci. USA. 1994;91:7608–7612. doi: 10.1073/pnas.91.16.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh K., Kumar S., Rani A., Gulati A., Ahuja P.S. Phenylalanine ammonia-lyase (PAL) and cinnamate 4-hydroxylase (C4H) and catechins (flavan-3-ols) accumulation in tea. Funct. Integr. Genomics. 2009;9:125–134. doi: 10.1007/s10142-008-0092-9. [DOI] [PubMed] [Google Scholar]
- 36.Whetten R.W., MacKay J.J., Sederoff R.R. Recent advances in understanding lignin biosynthesis. Annu. Rev. Plant Biol. 1998;49:585–609. doi: 10.1146/annurev.arplant.49.1.585. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann L., Maury S., Martz F., Geoffroy P., Legrand M. Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J. Biol. Chem. 2003;278:95–103. doi: 10.1074/jbc.M209362200. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann L., Besseau S., Geoffroy P., Ritzenthaler C., Meyer D., Lapierre C., Pollet B., Legrand M. Silencing of hydroxycinnamoyl-coenzyme a shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell. 2004;16:1446–1465. doi: 10.1105/tpc.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Besseau S., Hoffmann L., Geoffroy P., Lapierre C., Pollet B., Legrand M. Flavonoid accumulation in arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell. 2007;19:148–162. doi: 10.1105/tpc.106.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maayan I., Shaya F., Ratner K., Mani Y., Lavee S., Avidan B., Shahak Y., Ostersetzer-Biran O. Photosynthetic activity during olive (Olea europaea) leaf development correlates with plastid biogenesis and Rubisco levels. Physiol. Plant. 2008;134:547–558. doi: 10.1111/j.1399-3054.2008.01150.x. [DOI] [PubMed] [Google Scholar]
- 41.Makino A., Mae T., Ohira K. Photosynthesis and ribulose 1,5-bisphosphate carboxylase in rice leaves: Changes in photosynthesis and enzymes involved in carbon assimilation from leaf development through senescence. Plant Physiol. 1983;73:1002–1007. doi: 10.1104/pp.73.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy R., Johnson D. Changes in photosynthetic characteristics during leaf development in apple. Photosynth. Res. 1981;2:213–223. doi: 10.1007/BF00032360. [DOI] [PubMed] [Google Scholar]
- 43.Roper T.R., Kennedy R.A. Photosynthetic characteristics during leaf development in bing sweet cherry. J. Am. Soc. Hortic. Sci. 1986;111:938–941. [Google Scholar]
- 44.Tu L., Lin Y., Yang Z., Huang Z., Zhao W., Sun P. The study of the correlation between the net photosynthetic rate and catechins components of tea plant leaves. Chin. Agric. Sci. Bull. 2012;28:227–233. (In Chinese) [Google Scholar]
- 45.Ghasemzadeh A., Jaafar H.Z., Rahmat A. Synthesis of phenolics and flavonoids in ginger (Zingiber officinale roscoe) and their effects on photosynthesis rate. Int. J. Mol. Sci. 2010;11:4539–4555. doi: 10.3390/ijms11114539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perl-Treves R., Galun E. The tomato Cu, Zn superoxide dismutase genes are developmentally regulated and respond to light and stress. Plant Mol. Biol. 1991;17:745–760. doi: 10.1007/BF00037058. [DOI] [PubMed] [Google Scholar]
- 47.Jiang C.-D., Li P.-M., Gao H.-Y., Zou Q., Jiang G.-M., Li L.-H. Enhanced photoprotection at the early stages of leaf expansion in field-grown soybean plants. Plant Sci. 2005;168:911–919. doi: 10.1016/j.plantsci.2004.11.004. [DOI] [Google Scholar]
- 48.Rodriguez A.A., Grunberg K.A., Taleisnik E.L. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol. 2002;129:1627–1632. doi: 10.1104/pp.001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turkmen N., Sari F., Velioglu Y.S. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and folin–ciocalteu methods. Food Chem. 2006;99:835–841. doi: 10.1016/j.foodchem.2005.08.034. [DOI] [Google Scholar]
- 50.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 51.Wang Z., Tong X., Zhu S. Quantitative analysis of catechins with HPLC. J. Tea Sci. 1991;11:93–99. (In Chinese) [Google Scholar]
- 52.Li Y., Li J., Gong X., Liu Z. Simultaneous determination of eight catechins, three purine alkaloids and gallic acid in tea by high-performance liquid chromatography. Food Sci. 2011;32:214–217. [Google Scholar]
- 53.Tatusov R.L., Fedorova N.D., Jackson J.D., Jacobs A.R., Kiryutin B., Koonin E.V., Krylov D.M., Mazumder R., Mekhedov S.L., Nikolskaya A.N., et al. The COG database: An updated version includes eukaryotes. BMC Bioinform. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanehisa M., Goto S., Sato Y., Furumichi M., Tanabe M. Kegg for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Livak K., Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.