Abstract
Purpose of review
Detection of early HIV infections (EHIs), including acute HIV infection (AHI), is important for individual health, prevention of HIV transmission, and measurement of HIV incidence. We describe markers of EHI, diagnostic strategies for detecting these markers, and ways to incorporate these strategies into diagnostic and HIV incidence algorithms.
Recent findings
For individual diagnosis in the United States and Europe, laboratory-based diagnostic algorithms increasingly incorporate fourth-generation HIV antigen tests, allowing for earlier detection. In some sub-Saharan African settings, symptom-based screening is being explored to identify subsets of persons at high risk for AHI. Point-of-care diagnostics designed for AHI detection are in the pipeline and, if validated, represent an opportunity for real-time AHI diagnosis. At the population level, multiassay algorithms are promising new strategies for estimating HIV incidence on the basis of several assays applied to cross-sectional samples. These algorithms can be developed to optimize performance, in addition to cost and logistical considerations.
Summary
There are important recent advances in detection of EHIs at the individual and population levels. Applying optimal combinations of tests in diagnostic and HIV incidence algorithms is urgently needed to support the multiple goals derived from enhanced detection and discrimination of EHIs.
Keywords: acute HIV infection, early HIV infection, fourth generation, multiassay algorithm, point of care
Introduction
Early HIV infection (EHI) is the period from HIV acquisition through the establishment of chronic infection. EHI encompasses the eclipse phase (when HIV is not detectable in the plasma) [1], acute HIV infection (AHI) (when HIV is detectable, but the antibody response is not), and recent infection (when HIV antibodies are detectable by sensitive diagnostic assays, but the immune response is immature). Together these phases last approximately 3–6 months. EHI ends when a stable viral ‘set point’ is established, a key determinant of subsequent disease progression [2] and chronic transmissibility. Directed laboratory testing can locate individuals within this spectrum. One common system proposed in 2003 by Fiebig et al. [1] defined six EHI stages on the basis of the maturation of viremia and antibody responses using then-current diagnostics. With substantial advances in EHI diagnostics, efforts are now underway to improve on this staging system [3••].
At the individual level, EHI detection is important for proper diagnosis. When EHI clinical manifestations occur, they are almost universally misdiagnosed as another endemic illness, such as malaria or influenza, resulting in inappropriate subsequent testing and treatment [4,5••,6]. More rarely, severe manifestations of EHI, such as meningitis, encephalitis, or esophagitis, can occur, which must be distinguished from both non-HIV causes and manifestations of AIDS.
EHI detection is also important for infection management. Detecting and correctly staging EHI enables rapid initiation of treatment with potential for long-term clinical benefits [7,8•]. Progressive changes occur over the first few weeks of infection: irreversible depletion of CD4 lymphocytes in the gut [9], mucosal damage allowing gastrointestinal bacterial products access to the circulation [10], replication in the central nervous system [11•], and the establishment of latent HIV reservoirs, which to date has rendered HIV incurable [7,12]. Initiation of antiretroviral therapy (ART) during EHI improves host immune control of viral replication [13,14•,15] and has been associated with improvements in CD4 cell counts over time [16•,17], reduced systemic inflammation [18], preserved cognitive function [19•], and a reduced latent reservoir [8•]. In the United States, immediate treatment for persons with EHI has been recommended [20•].
Detection of EHI is also important for HIV prevention. Rapid viral replication results in elevated viral load in blood and genital secretions, making the host extremely infectious. Infectiousness is amplified during EHI by viral variants that are more infectious than viruses from the same individual later in infection [7]. Because of increased infectiousness during EHI, most mathematical models suggest that EHI is responsible for a disproportionate share of HIV transmissions [21–24]. Accordingly, ART during EHI can be expected to make the host less infectious [13,14•,15]. Detection of EHI may also facilitate behavior change, as some persons who become aware that they are HIV-infected, including those with AHI, report declines in unprotected sex [25•,26,27].
Finally, for HIV surveillance, discriminating EHI from established infection can be used to estimate HIV incidence. Information from such surveys is becoming increasingly important for measuring impact of prevention interventions in cluster randomized trials [28•] and for monitoring the impact of biomedical interventions in populations as they are brought to scale.
In this review, we discuss the markers of EHI, strategies for detecting these markers, and how these strategies have been combined into algorithms for individual EHI case detection and HIV incidence estimation.
Clinical Markers of Acute HIV Infection
During the first several weeks of HIV infection, many persons display an acute retroviral syndrome with symptoms, such as fever, diarrhea, and rashes [29]. Because these symptoms are nonspecific, they cannot reliably identify AHI independently. Recently, three studies of outpatient clinic populations in Uganda, Mozambique, and Kenya have reported results of targeted AHI testing based on patients presenting with fever or suspected malaria [4,5••,6]; all three reported high AHI prevalence confused with more common diseases. In the recent Kenyan study, fever was just as likely to be AHI, as it was to be malaria [5••]. These studies demonstrate that far from being a rare event, AHI is likely a common clinical syndrome in HIV endemic parts of sub-Saharan Africa. These results have yet to translate into clinical practice or guidance recommending widespread AHI testing in general medical settings in sub-Saharan Africa [30•,31•]. However, this may change with improved, cheaper AHI diagnostics that are now becoming available.
Symptoms, along with behavioral or demographic characteristics, can also be used to identify a subset of persons more likely to have AHI, who then receive laboratory screening. For instance, a risk score developed for AHI in Malawi reduced the proportion of sexually transmitted disease clinic attendees who needed laboratory screening by 60%, but identified 95% of persons with detectable AHI [32]. Targeted, symptom-based screening has been replicated in emergency departments and outpatient facilities in developed and developing countries, with a wide range of risk factors being predictive of AHI [5••,33•,34•,35].
Detection of Acute Viremia (Preantibody Phase)
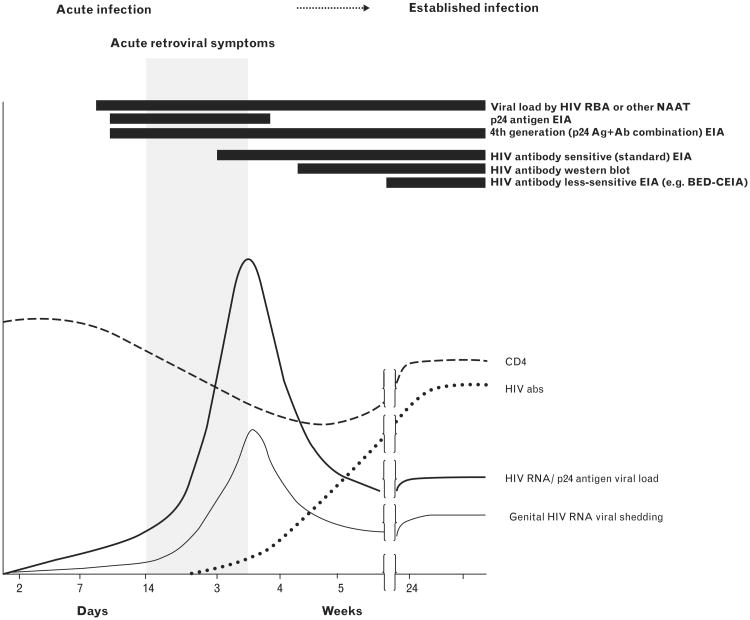
HIV RNA and p24 antigen, components of circulating virions in plasma, are detectable before HIV antibodies are formed (Fig. 1). HIV RNA appears first, approximately 7–10 days after HIV infection, and can be detected using PCR or other nucleic acid amplification technologies (NAATs). Currently, RNA testing is performed in well resourced laboratories. Samples can be tested individually, or multiple samples can be tested at once in ‘pools,’ with only samples from positive pools subsequently tested individually [36,37]. Optimal pooling strategies for different conditions have been described [38] with substantial reductions in cost, but with increases in time and complexity.
Figure 1.
Timing of early HIV infection biomarkers and symptoms. This figure depicts the trajectories of CD4 count, the appearance of HIV antibodies and symptoms, and blood and genital shedding soon after HIV infection. Black bars indicate timing of detection. Reproduced from [35].
The next biomarker to appear is p24 antigen, which is detectable approximately 5–7 days after HIV RNA. P24 can be detected by ‘fourth-generation’, antigen-antibody ‘combo’ assays with very good performance characteristics [39•,40,41]. Fourth-generation laboratory immunoassays have become routine in European countries and have replaced HIV antibody-only tests for HIV screening in many US laboratories [42]. Unfortunately, neither fourth-generation laboratory immunoassays nor traditional HIV NAATs are suitable for rapid, point-of-care diagnosis in the clinic settings in which AHI is common and the potential for efficient AHI case-finding the greatest.
Notably, two first-generation rapid NAAT devices for use in AHI diagnosis have been introduced quite recently. The Alere q HIV-1/2 Detect (Alere Technologies, Jena, Germany) is designed for both point-of-care early infant diagnosis and potentially adult AHI diagnosis. The first assessment shows excellent performance characteristics in infants [43•], but field test results for adult AHI are not available. The SAMBA HIV semiquantitative point-of-care test was developed primarily for point-of-care viral load monitoring, and secondarily to detect persons with AHI, but to date it has not been evaluated for AHI either [44•].
Previous efforts to deploy rapid tests for AHI have been mixed: the Alere Determine HIV-1/2 Ag/Ab is a lateral flow-based rapid test designed to detect and differentiate established HIV infection (through detection of HIV antibodies) and EHI (through detection of p24 antigen) at the point of care using plasma, serum, or whole blood. The antibody portion of the test has performed exceptionally well, detecting antibodies sooner than other rapid tests, but the antigen portion has not. In clinic-based evaluations on whole blood in Malawi, France, the United Kingdom, Australia, and South Africa, the p24 antigen portion of the assay failed to detect any of the AHI cases [45–48,49•,50]. Sensitivity for detection of p24 varied widely in laboratory assessments of stored serum and plasma with the p24 well displaying lower sensitivity than other fourth-generation assays [51•–54•,55–59]. These concerns notwithstanding, the test has provisional US FDA approval for use in clinical laboratories with quality assurance programs [60]. Development and field testing of a reliable point-of-care assay for AHI detection remains an important goal that merits investment.
Detecting Postantibody Early HIV Infection
Approximately 1 week after the appearance of p24, immunoglobulin M antibodies are detectable through third-generation immunoassays, several weeks earlier than first or second-generation immunoassays. Although third-generation assays detect HIV earlier, they classify very few persons with EHI when used in combination with fourth-generation assays, due to the very brief ‘window period.’ Identifying persons with EHI in the postseroconversion period is also important, as it allows for brief early interventions for treatment or prevention.
In the past, results from supplemental Western blot tests extended the EHI period by several weeks, and could be used to stage persons in the early postseroconversion period [61]. However, the Western blot is no longer being performed in most clinical laboratories. Among confirmatory tests replacing the Western blot are the INNO-LIA HIV I/II Score (Innogenetics, Gent, Belgium) [62] and a newer rapid test, the Bio-Rad™ Geenius HIV 1/2 Assay (Bio-Rad Laboratories, Inc.). These diagnostics provide similar semiquantitative information and can distinguish EHI from long-standing infections [55,63•] if such information is sought. Updated staging systems, which rely on diagnostics currently in use, are urgently needed.
Additional assays rely on the evolving nature of the antibody response following HIV acquisition with respect to quantity, proportion, avidity, or isotope. These antibody-testing approaches, referred to as ‘incidence assays’ because of their use in HIV surveillance, have been developed to classify EHI among antibody-positive individuals. These include the serologic testing algorithm for recent seroconversion, the BED capture enzyme immunoassay (BED-CEIA), and limiting-antigen (LAg) avidity assays (discussed in more detail below).
Genetic information can also be used to stage EHI. Within a single host, the HIV genome is typically homogenous following acquisition and diversifies over time into complex quasispecies, making viral diversity another potential measure of EHI duration. Some of these methods include ‘molecular clock’ models [64], high-resolution melting technologies implemented manually or through automated platforms [65–67,68••], HIV sequencing techniques [69], and Pairwise Alignment Positional Nucleotide Counting, a novel computational method [70••,71]. These assays are currently used for research purposes and some have been incorporated into HIV incidence estimation activities.
Evolving Practice in Early HIV Infection Detection and Staging
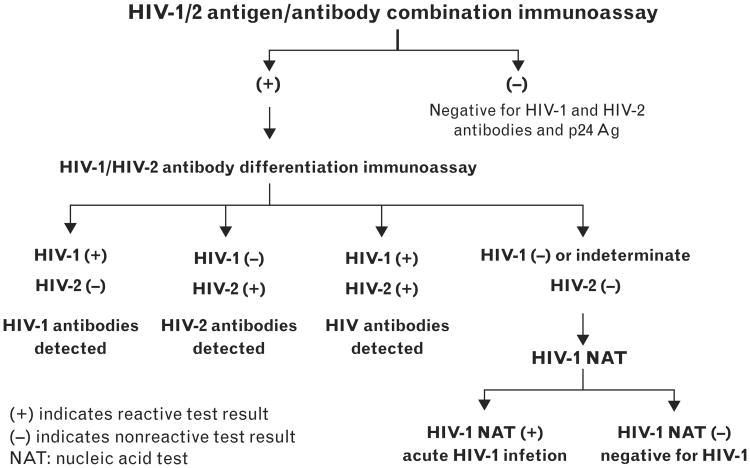
In 2014, nearly all well resourced countries have adopted fourth-generation immunoassays as first-line screening tests for HIV, on a variety of automated and manual platforms. In 2014, the Centers for Disease Control and Prevention (CDC) issued a new laboratory algorithm for use in the United States with a sequence of US FDA-approved HIV assays designed to detect persons in either EHI or chronic HIV infection, and to differentiate HIV-1 from HIV-2 [72,73,74••] (Fig. 2). The algorithm begins with a highly sensitive fourth-generation test, and, for reactive samples, an immunoglobulin G immunoassay capable of differentiating HIV-1 from HIV-2. Antibody-nonreactive specimens can be tested individually for HIV-1 RNA to identify patients who were recently infected. Use of fourth-generation and NAAT technology has increased detection of AHI in US settings where this has been implemented [36,75,76•–79•].
Figure 2.
Updated testing algorithm for US HIV diagnosis. This figure depicts the algorithm issued by the Centers for Disease Control and Prevention for HIV diagnostic testing in the United States in 2014. It distinguishes HIV-1 from HIV-2 and acute HIV infection from established HIV infection. Reproduced from [74••].
In many European countries, including the United Kingdom, France, and Italy, modified ‘incidence assays’ are used routinely to complete the staging of new infections as being in EHI versus long-standing HIV infection. Assay results are provided to guide patient management, in addition to the information they contribute to surveillance. In the United States, negative, indeterminate, or evolving results of supplemental antibody tests are often used to make the diagnosis of EHI, but the use of supplemental incidence assays has not been embraced. In 2014, however, the US CDC issued a new surveillance case definition, which, for the first time, includes EHI as a reportable category referred to as ‘stage 0’ HIV infection [80••]. This guideline, along with the arrival of new supplemental assays capable of EHI staging, may lead to more postseroconversion EHI diagnosis in the United States.
Evolving Practice in HIV Incidence Estimation
HIV incidence estimation, which also relies on the detection of EHI, can be measured directly in cohorts of HIV-uninfected persons followed longitudinally for HIV seroconversion. However, longitudinal methods require large cohorts, followed at frequent intervals, over long periods with minimal, nondifferential loss to follow-up [81]. Additionally, the process of frequent HIV testing can itself affect HIV acquisition risk [82].
Back-projection techniques were developed for incidence estimation in surveillance data, using assumptions about the timing between HIV acquisition and HIV testing. However, due to inevitable time lags, these techniques were inadequate for measuring recent trends, and estimates were often imprecise due to assumptions about the duration between infection and diagnosis [83–86].
Cross-sectional HIV incidence estimation can measure recent HIV incidence trends with one or more biomarker at a single time point. The Serologic Testing Algorithm for Recent Seroconversion relies on the maturity of the antibody response [87,88], but variability between HIV subtypes makes this approach unreliable [89,90]. The BED-CEIA was designed to detect multiple HIV-1 subtypes and differentiate recent from long-standing seroconversion based on the proportion of anti-HIV immunoglobulin G, but misclassifies persons with advanced disease and immunosuppression as having EHI, leading to overestimates [91–94]. The newer LAg assay measures the avidity of antibody binding to low concentrations of a multisubtype peptide derived from an immunodominant region of gp41 [95,96]. However, this assay suffers from similar challenges of misclassifying persons with advanced HIV disease, elite controllers, and persons with viral suppression and partial seroreversion following ART as having EHI [96,97••].
The key recent advance in cross-sectional HIV incidence estimation is the development, refinement, and validation of multiassay algorithms (MAAs) [28•,98••,99•,100•,101••,102,103•,104]. MAAs apply a sequential, hierarchical set of assays to classify confirmed seropositive samples into recent versus long-standing infection categories. MAAs first screen in persons with possible EHI, then screen out those with risk factors for false-recent misclassification, such as ARV-treated individuals and elite controllers. As with the effort to develop clinical uses of EHI tests, the central challenge remains reducing false-recent results without over-shortening the window period [105].
MAAs are evolving rapidly and large-scale efforts to systematically evaluate the full range of new EHI tests are now underway [106••]. To determine the optimal MAA for each setting, many possible algorithms are systematically applied to the same group of samples in different orders and with a range of cut-points [101••,103•]. For example, in the United States, primarily a subtype B epidemic, the optimal MAA consisted of CD4 more than 200 cells/μl, BED-CEIA optical density less than 1.0, avidity less than 80%, and HIV viral load more than 400 copies/ml [101••]. A significant milestone in the MAA concept occurred with NIMH Project Accept (HPTN 043), which used an MAA as its primary outcome measure [28•]. This MAA used the same tests, but in a different sequence with different cut-points: BED-CEIA optical density less than 1.2, avidity index less than 90%, CD4 cell count more than 200 cells/μl, and viral load more than 400 copies/ml [28•]. MAAs can also be developed to meet specific cost or logistical constraints. For example, MAAs using high-resolution melting technology, rather than CD4 count, can be conducted entirely on stored serum or plasma [68••]. Still others, such as those using the LAg assay, can be performed on stored serum or plasma, collected via dried blood spots, and performed with fewer assays [97••].
Conclusion
Careful study of the natural history of EHI has resulted in an array of clinical, virologic, immunologic, and genetic markers that can be used to identify EHI cases and classify them into different stages. The brevity of each stage and the difficulty to identify them and distinguish them from established HIV infection at the point of care remain major challenges. Nonetheless, substantial progress has been achieved at the individual and population levels. As more persons with prevalent HIV infection learn their HIV status, a larger share of those in need of HIV diagnosis will be those with EHI, making its detection increasingly important. Ever better strategies for simple, efficient detection of EHI and measurement of HIV incidence are critical public health priorities.
Key Points.
In the United States and Europe, laboratory-based diagnostic algorithms increasingly incorporate fourth-generation HIV antigen tests, allowing for earlier HIV detection.
In some sub-Saharan African settings, symptom-based screening has been explored to identify subsets of persons at high risk for AHI.
Point-of-care diagnostics designed for AHI detection are in the pipeline and, if validated, represent an exciting opportunity for diagnosis in real time.
MAAs are a promising new strategy for estimating HIV incidence at the population level.
Acknowledgments
None.
Financial support and sponsorship: N.E.R. is supported by the UNC Center for AIDS Research, an NIH funded program (P30 AI50410) and a UNC Hopkins Morehouse Tulane Fogarty Global Health Fellows Program (R25 TW009340).
C.D.P. is supported by grants from NIH/NIMH (R34MH096606) and has received grant funding from Bio-rad.
Footnotes
Conflicts of interest: M.S.C. has consulted with Roche Molecular Systems, the Bill and Melinda Gates Foundation, and Janssen Global Services.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Lavreys L, Baeten JM, Chohan V, et al. Higher set point plasma viral load and more-severe acute HIV type 1 (HIV-1) illness predict mortality among high-risk HIV-1-infected African women. Clin Infect Dis. 2006;42:1333–1339. doi: 10.1086/503258. [DOI] [PubMed] [Google Scholar]
- 3••.Ananworanich J, Fletcher JL, Pinyakorn S, et al. A novel acute HIV infection staging system based on 4th generation immunoassay. Retrovirology. 2013;10:56. doi: 10.1186/1742-4690-10-56. Fourth-generation immunoassays can be used as part of an AHI staging system, distinguishing different groups of recently infected persons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bebell LM, Pilcher CD, Dorsey G, et al. Acute HIV-1 infection is highly prevalent in Ugandan adults with suspected malaria. AIDS. 2010;24:1945–1952. doi: 10.1097/QAD.0b013e32833bb732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Sanders EJ, Mugo P, Prins HA, et al. Acute HIV-1 infection is as common as malaria in young febrile adults seeking care in coastal Kenya. AIDS. 2014;28:1357–63. doi: 10.1097/QAD.0000000000000245. AHI was as common as malaria in young febrile adults, suggesting an AHI detection strategy targeting febrile young adults is feasible and important. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serna-Bolea C, Munoz J, Almeida JM, et al. High prevalence of symptomatic acute HIV infection in an outpatient ward in southern Mozambique: identification and follow-up. AIDS. 2010;24:603–608. doi: 10.1097/QAD.0b013e328335cda3. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Smith MK, Rutstein SE, Powers KA, et al. The detection and management of early HIV infection: a clinical and public health emergency. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S187–S199. doi: 10.1097/QAI.0b013e31829871e0. Synthesizes evidence on the use of immediate ART to treat EHI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun TW, Engel D, Berrey MM, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Peluso MJ, Meyerhoff DJ, Price RW, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207:1703–1712. doi: 10.1093/infdis/jit088. Early neuronal injury occurs in a subset of persons with EHI through mechanisms involving central nervous system inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMichael AJ, Borrow P, Tomaras GD, et al. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan CM, Degruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012;205:87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Spartac Trial Investigators. Fidler S, Porter K, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368:207–217. doi: 10.1056/NEJMoa1110039. ART in patients with primary HIV infection delayed disease progression, although not significantly longer than the duration of the treatment. There was no evidence of adverse effects of ART interruption on the clinical outcome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grijsen ML, Steingrover R, Wit FW, et al. No treatment versus 24 or 60 weeks of antiretroviral treatment during primary HIV infection: the randomized Primo-SHM trial. PLoS Med. 2012;9:e1001196. doi: 10.1371/journal.pmed.1001196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. A spontaneous restoration of CD4+ T cells occurs in the 4 months after HIV infection. ART initiation during this period is associated with enhanced likelihood of CD4 recovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hecht FM, Wang L, Collier A, et al. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J Infect Dis. 2006;194:725–733. doi: 10.1086/506616. [DOI] [PubMed] [Google Scholar]
- 18.Burdo TH, Lentz MR, Autissier P, et al. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after antiretroviral therapy. J Infect Dis. 2011;204:154–163. doi: 10.1093/infdis/jir214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Spudich SS. CROI 2014: neurologic complications of HIV infection. Top Antivir Med. 2014;22:594–601. Very early HIV diagnosis and treatment may protect the central nervous system from HIV-related injury. [PMC free article] [PubMed] [Google Scholar]
- 20•.Marrazzo JM, del Rio C, Holtgrave DR, et al. HIV prevention in clinical care settings: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312:390–409. doi: 10.1001/jama.2014.7999. International Antiviral Society experts delineate recommendations for HIV prevention in clinical care settings based on current evidence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eaton JW, Hallett TB, Garnett GP. Concurrent sexual partnerships and primary HIV infection: a critical interaction. AIDS Behav. 2011;15:687–692. doi: 10.1007/s10461-010-9787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 23.Powers KA, Ghani AC, Miller WC, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinkerton SD. Probability of HIV transmission during acute infection in Rakai, Uganda. AIDS Behav. 2008;12:677–684. doi: 10.1007/s10461-007-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Rosenberg NE, Pettifor AE, Bruyn GD, et al. HIV Testing and Counseling Leads to Immediate Consistent Condom Use Among South African Stable HIV-Discordant Couples. J Acquir Immune Defic Syndr. 2013;62:226–233. doi: 10.1097/QAI.0b013e31827971ca. When persons learn that they are in HIV-discordant relationships, they report substantial declines in unprotected sex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research. Am J Public Health. 1999;89:1397–1405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steward WT, Remien RH, Higgins JA, et al. Behavior change following diagnosis with acute/early HIV infection-a move to serosorting with other HIV-infected individuals. The NIMH Multisite Acute HIV Infection Study: III. AIDS Behav. 2009;13:1054–1060. doi: 10.1007/s10461-009-9582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Laeyendecker O, Kulich M, Donnell D, et al. Development of methods for cross-sectional HIV incidence estimation in a large, community randomized trial. PLoS One. 2013;8:e78818. doi: 10.1371/journal.pone.0078818. HIV incidence estimates and intervention effects can be estimated from cross-sectional surveys using MAAs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 30•.Prins HA, Mugo P, Wahome E, et al. Diagnosing acute and prevalent HIV-1 infection in young African adults seeking care for fever: a systematic review and audit of current practice. Int Health. 2014;6:82–92. doi: 10.1093/inthealth/ihu024. Young febrile adults in sub-Saharan Africa presenting to care are rarely screened for established or AHI, representing a missed opportunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31•.Powers KA, Cohen MS. Acute HIV-1 infection in sub-Saharan Africa: a common occurrence overlooked. AIDS. 2014;28:1365–1367. doi: 10.1097/QAD.0000000000000277. This letter makes the case that AHI is common but overlooked in sub-Saharan Africa. [DOI] [PubMed] [Google Scholar]
- 32.Powers KA, Miller WC, Pilcher CD, et al. Improved detection of acute HIV-1 infection in sub-Saharan Africa: development of a risk score algorithm. AIDS. 2007;21:2237–2242. doi: 10.1097/QAD.0b013e3282f08b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Wahome E, Fegan G, Okuku HS, et al. Evaluation of an empiric risk screening score to identify acute and early HIV-1 infection among MSM in Coastal Kenya. AIDS. 2013;27:2163–2166. doi: 10.1097/QAD.0b013e3283629095. It is possible to identify AHI by screening symptoms among men who have sex with men in a generalized epidemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Mlisana K, Sobieszczyk M, Werner L, et al. Challenges of diagnosing acute HIV-1 subtype C infection in African women: performance of a clinical algorithm and the need for point-of-care nucleic-acid based testing. PLoS One. 2013;8:e62928. doi: 10.1371/journal.pone.0062928. Using a clinical algorithm can facilitate AHI detection with important prevention implications. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facente SN, Pilcher CD, Hartogensis WE, et al. Performance of risk-based criteria for targeting acute HIV screening in San Francisco. PLoS One. 2011;6:e21813. doi: 10.1371/journal.pone.0021813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilcher CD, Fiscus SA, Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 37.Klausner JD, Grant RM, Kent CK. Detection of acute HIV infections. N Engl J Med. 2005;353:631–633. doi: 10.1056/NEJM200508113530620. [DOI] [PubMed] [Google Scholar]
- 38.Westreich DJ, Hudgens MG, Fiscus SA, Pilcher CD. Optimizing screening for acute human immunodeficiency virus infection with pooled nucleic acid amplification tests. J Clin Microbiol. 2008;46:1785–1792. doi: 10.1128/JCM.00787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Kfutwah A, Lemee V, Ngono HV, et al. Field evaluation of the Abbott ARCHITECT HIV Ag/Ab Combo immunoassay. J Clin Virol. 2013;58(Suppl 1):e70–e75. doi: 10.1016/j.jcv.2013.08.015. ARCHITECT HIV Ag/Ab assay is adapted to the wide genetic diversity of viruses circulating in West Central Africa. [DOI] [PubMed] [Google Scholar]
- 40.Ly TD, Martin L, Daghfal D, et al. Seven human immunodeficiency virus (HIV) antigen-antibody combination assays: evaluation of HIV seroconversion sensitivity and subtype detection. J Clin Microbiol. 2001;39:3122–3128. doi: 10.1128/JCM.39.9.3122-3128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ly TD, Ebel A, Faucher V, et al. Could the new HIV combined p24 antigen and antibody assays replace p24 antigen specific assays? J Virol Methods. 2007;143:86–94. doi: 10.1016/j.jviromet.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Jurriaans S, Back NK, Wolthers KC. Ten years of HIV testing with fourth generation assays: the Amsterdam experience. J Clin Virol. 2011;52(Suppl 1):S67–S69. doi: 10.1016/j.jcv.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 43•.Jani IV, Meggi B, Mabunda N, et al. Accurate Early Infant HIV Diagnosis in Primary Health Clinics Using a Point-Of-Care Nucleic Acid Test. J Acquir Immune Defic Syndr. 2014;67:e1–4. doi: 10.1097/QAI.0000000000000250. An NAAT point-of-care test displayed excellent performance characteristics for early infant diagnosis. [DOI] [PubMed] [Google Scholar]
- 44•.Ritchie AV, Ushiro-Lumb I, Edemaga D, et al. SAMBA HIV semi-quantitative test, a new point-of-care viral load monitoring assay for resource-limited settings. J Clin Microbiol. 2014;52:3377–83. doi: 10.1128/JCM.00593-14. A point-of-care viral load test distinguished patients with viral loads above and below certain thresholds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg NE, Kamanga G, Phiri S, et al. Detection of acute HIV infection: a field evaluation of the determine(R) HIV-1/2 Ag/Ab combo test. J Infect Dis. 2012;205:528–534. doi: 10.1093/infdis/jir789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones CB, Kuldanek K, Muir D, et al. Clinical evaluation of the Determine HIV-1/2 Ag/Ab Combo test. J Infect Dis. 2012;206:1947–1949. doi: 10.1093/infdis/jis617. author reply 9–50. [DOI] [PubMed] [Google Scholar]
- 47.Taegtmeyer M, MacPherson P, Jones K, et al. Programmatic evaluation of a combined antigen and antibody test for rapid HIV diagnosis in a community and sexual health clinic screening programme. PLoS One. 2011;6:e28019. doi: 10.1371/journal.pone.0028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pavie J, Rachline A, Loze B, et al. Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting. PLoS One. 2010;5:e11581. doi: 10.1371/journal.pone.0011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Conway DP, Holt M, McNulty A, et al. Multi-Centre Evaluation of the Determine HIV Combo Assay when Used for Point of Care Testing in a High Risk Clinic-Based Population. PLoS One. 2014;9:e94062. doi: 10.1371/journal.pone.0094062. The Determine HIV-1/2 Ag/Ab Combo test p24 well did not effectively detect p24 at the point of care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chetty V, Moodley D, Chuturgoon A. Evaluation of a 4th generation rapid HIV test for earlier and reliable detection of HIV infection in pregnancy. J Clin Virol. 2012;54:180–184. doi: 10.1016/j.jcv.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 51•.Pilcher CD, Louie B, Facente S, et al. Performance of rapid point-of-care and laboratory tests for acute and established HIV infection in San Francisco. PLoS One. 2013;8:e80629. doi: 10.1371/journal.pone.0080629. The Determine HIV-1/2 Ag/Ab Combo Ag well was able to detect p24 in approximately half of plasma samples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Faraoni S, Rocchetti A, Gotta F, et al. Evaluation of a rapid antigen and antibody combination test in acute HIV infection. J Clin Virol. 2013;57:84–87. doi: 10.1016/j.jcv.2013.01.007. The Determine HIV-1/2 Ag/Ab Combo test p24 antigen well was only capable of detecting a fraction acute HIV cases when used in serum. [DOI] [PubMed] [Google Scholar]
- 53•.Masciotra S, Luo W, Youngpairoj AS, et al. Performance of the Alere Determine HIV-1/2 Ag/Ab Combo Rapid Test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. J Clin Virol. 2013;58(Suppl 1):e54–8. doi: 10.1016/j.jcv.2013.07.002. On seroconversion panels, Determine HIV-1/2 Ag/Ab Combo test performed between third and fourth-generation laboratory tests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Brauer M, De Villiers JC, Mayaphi SH. Evaluation of the Determine fourth generation HIV rapid assay. J Virol Methods. 2013;189:180–183. doi: 10.1016/j.jviromet.2013.01.017. The Determine HIV-1/2 Ag/Ab Combo test p24 antigen well was only capable of detecting a fraction acute HIV cases on serum. [DOI] [PubMed] [Google Scholar]
- 55.Pandori MW, Louie B, Pilcher C. Assessing the sensitivities of laboratory-based and point-of-care HIV antigen-antibody combination tests using a panel of specimens from recently and acutely infected individuals. HIV Diagn Conf. 2010 [Google Scholar]
- 56.Laperche S, Leballais L, Ly TD, Plantier JC. Failures in the detection of HIV p24 antigen with the Determine HIV-1/2 Ag/Ab Combo rapid test. J Infect Dis. 2012;206:1946–1947. doi: 10.1093/infdis/jis616. [DOI] [PubMed] [Google Scholar]
- 57.Fox J, Dunn H, O'Shea S. Low rates of p24 antigen detection using a fourth-generation point of care HIV test. Sex Transm Infect. 2011;87:178–179. doi: 10.1136/sti.2010.042564. [DOI] [PubMed] [Google Scholar]
- 58.Kilembe W, Keeling M, Karita E, et al. Failure of a novel, rapid antigen and antibody combination test to detect antigen-positive HIV infection in African adults with early HIV infection. PLoS One. 2012;7:e37154. doi: 10.1371/journal.pone.0037154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beelaert G, Fransen K. Evaluation of a rapid and simple fourth-generation HIV screening assay for qualitative detection of HIV p24 antigen and/or antibodies to HIV-1 and HIV-2. J Virol Methods. 2010;168:218–222. doi: 10.1016/j.jviromet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Food and Drug Administration. Approval letter – Alere Determine HIV-1/2 Ag/Ab Combo 2013. 2014 Jul 8; http://www.fda.gov/biologicsbloodvaccines/bloodbloodproducts/approvedproducts/%20premarketapprovalspmas/ucm364653.htm.
- 61.Hecht FM, Wellman R, Busch MP, et al. Identifying the early post-HIV antibody seroconversion period. J Infect Dis. 2011;204:526–533. doi: 10.1093/infdis/jir304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schupbach J, Bisset LR, Gebhardt MD, et al. Diagnostic performance of line-immunoassay based algorithms for incident HIV-1 infection. BMC Infect Dis. 2012;12:88. doi: 10.1186/1471-2334-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Mor O, Mileguir F, Michaeli M, et al. Evaluation of the Bio-Rad Geenius HIV 1/2 Assay as an Alternative to the INNO-LIA HIV 1/2 Assay for Confirmation of HIV Infection. J Clin Microbiol. 2014;52:2677–2679. doi: 10.1128/JCM.01184-14. The Geenius assay detected more cases and detected them sooner compared with the INNO-LIA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gay C, Dibben O, Anderson JA, et al. Cross-sectional detection of acute HIV infection: timing of transmission, inflammation and antiretroviral therapy. PLoS One. 2011;6:e19617. doi: 10.1371/journal.pone.0019617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Towler WI, James MM, Ray SC, et al. Analysis of HIV diversity using a high-resolution melting assay. AIDS Res Hum Retroviruses. 2010;26:913–918. doi: 10.1089/aid.2009.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cousins MM, Laeyendecker O, Beauchamp G, et al. Use of a high resolution melting (HRM) assay to compare gag, pol, and env diversity in adults with different stages of HIV infection. PLoS One. 2011;6:e27211. doi: 10.1371/journal.pone.0027211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cousins MM, Swan D, Magaret CA, et al. Analysis of HIV using a high resolution melting (HRM) diversity assay: automation of HRM data analysis enhances the utility of the assay for analysis of HIV incidence. PLoS One. 2012;7:e51359. doi: 10.1371/journal.pone.0051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.Cousins MM, Konikoff J, Laeyendecker O, 3rd, et al. HIV diversity as a biomarker for HIV incidence estimation: including a high-resolution melting diversity assay in a multiassay algorithm. J Clin Microbiol. 2014;52:115–121. doi: 10.1128/JCM.02040-13. An MAA that includes a high-resolution melting diversity assay for HIV incidence estimation is an important development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ragonnet-Cronin M, Aris-Brosou S, Joanisse I, et al. Genetic diversity as a marker for timing infection in HIV-infected patients: evaluation of a 6-month window and comparison with BED. J Infect Dis. 2012;206:756–764. doi: 10.1093/infdis/jis411. [DOI] [PubMed] [Google Scholar]
- 70••.Shao W, Kearney MF, Boltz VF, et al. PAPNC, a novel method to calculate nucleotide diversity from large scale next generation sequencing data. J Virol Methods. 2014;203:73–80. doi: 10.1016/j.jviromet.2014.03.008. The authors developed a novel method for calculating genetic diversities for large-scale datasets from next generation sequencing. It can be implemented easily with software programs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shankarappa R, Margolick JB, Gange SJ, et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J Virol. 1999;73:10489–10502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Branson BM, Mermin J. Establishing the diagnosis of HIV infection: new tests and a new algorithm for the United States. J Clin Virol. 2011;52(Suppl 1):S3–S4. doi: 10.1016/j.jcv.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 73.Branson BM, Stekler JD. Detection of acute HIV infection: we can't close the window. J Infect Dis. 2012;205:521–524. doi: 10.1093/infdis/jir793. [DOI] [PubMed] [Google Scholar]
- 74••.Centers for Disease Control and Prevention. Laboratory testing for the diagnosis of HIV infection: updated recommendations. 2014 Jun 27; Provides updated recommendations for HIV testing by laboratories in the United States, including an algorithm for AHI detection. [Google Scholar]
- 75.Patel P, Klausner JD, Bacon OM, et al. Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr. 2006;42:75–79. doi: 10.1097/01.qai.0000218363.21088.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Centers for Disease Control and Prevention. Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm-United States, 2011–2013. MMWR Morbidity and mortality weekly report. 2013;62:489–494. Algorithms that include AHI detection in the United States can have high diagnostic yields. [PMC free article] [PubMed] [Google Scholar]
- 77•.Goodhue T, Kazianis A, Werner BG, et al. 4th generation HIV screening in Massachusetts: a partnership between laboratory and program. J Clin Virol. 2013;58(Suppl 1):e13–e18. doi: 10.1016/j.jcv.2013.08.019. The Massachusetts Department of Health laboratory identified eight acute infections in its first year using a fourth-generation screening assay as part of a revised diagnostic algorithm. [DOI] [PubMed] [Google Scholar]
- 78•.Emerson B, Plough K. Detection of acute HIV-1 infections utilizing NAAT technology in Dallas, Texas. J Clin Virol. 2013;58(Suppl 1):e48–e53. doi: 10.1016/j.jcv.2013.08.005. In Dallas County Public Health Department many cases of AHI were detected through routine screening, as well as through contact tracing. [DOI] [PubMed] [Google Scholar]
- 79•.Christopoulos KA, Zetola NM, Klausner JD, et al. Leveraging a rapid, round-the-clock HIV testing system to screen for acute HIV infection in a large urban public medical center. J Acquir Immune Defic Syndr. 2013;62:e30–e38. doi: 10.1097/QAI.0b013e31827a0b0d. Patients undergoing HIV testing in emergency rooms and urgent care clinics may benefit from being screened for AHI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80••.Centers for Disease Control and Prevention. Revised surveillance case definition for HIV infection-United States, 2014. MMWR Recommendations and reports: Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2014 Apr 11;63:1–10. The CDC updated its surveillance guidelines to include a case definition for EHI: stage 0. [PubMed] [Google Scholar]
- 81.Reniers G, Eaton J. Refusal bias in HIV prevalence estimates from nationally representative seroprevalence surveys. AIDS. 2009;23:621–629. doi: 10.1097/QAD.0b013e3283269e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Amici D, Klersy C, Ramajoli F, et al. Impact of the Hawthorne effect in a longitudinal clinical study: the case of anesthesia. Control Clin Trials. 2000;21:103–114. doi: 10.1016/s0197-2456(99)00054-9. [DOI] [PubMed] [Google Scholar]
- 83.Cui J, Becker NG. Estimating HIV incidence using dates of both HIV and AIDS diagnoses. Stat Med. 2000;19:1165–1177. doi: 10.1002/(sici)1097-0258(20000515)19:9<1165::aid-sim419>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 84.Becker NG, Lewis JJ, Li Z, McDonald A. Age-specific back-projection of HIV diagnosis data. Stat Med. 2003;22:2177–2190. doi: 10.1002/sim.1406. [DOI] [PubMed] [Google Scholar]
- 85.Wand H, Yan P, Wilson D, et al. Increasing HIV transmission through male homosexual and heterosexual contact in Australia: results from an extended back-projection approach. HIV Med. 2010;11:395–403. doi: 10.1111/j.1468-1293.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 86.Mallitt KA, Wilson DP, McDonald A, Wand H. Is back-projection methodology still relevant for estimating HIV incidence from national surveillance data? Open AIDS J. 2012;6:108–111. doi: 10.2174/1874613601206010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Le Vu S, Pillonel J, Semaille C, et al. Principles and uses of HIV incidence estimation from recent infection testing: a review. Euro Surveill. 2008;13:18969. [PubMed] [Google Scholar]
- 88.Murphy G, Parry JV. Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill. 2008;13:18966. [PubMed] [Google Scholar]
- 89.Kothe D, Byers RH, Caudill SP, et al. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J Acquir Immune Defic Syndr. 2003;33:625–634. doi: 10.1097/00126334-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 90.Parekh BS, Hu DJ, Vanichseni S, et al. Evaluation of a sensitive/less-sensitive testing algorithm using the 3A11-LS assay for detecting recent HIV seroconversion among individuals with HIV-1 subtype B or E infection in Thailand. AIDS Res Hum Retroviruses. 2001;17:453–458. doi: 10.1089/088922201750102562. [DOI] [PubMed] [Google Scholar]
- 91.Mastro TD, Kim AA, Hallett T, et al. Estimating HIV Incidence in Populations Using Tests for Recent Infection: Issues, Challenges and the Way Forward. J HIV AIDS Surveill Epidemiol. 2010;2:1–14. [PMC free article] [PubMed] [Google Scholar]
- 92.Busch MP, Pilcher CD, Mastro TD, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS. 2010;24:2763–2771. doi: 10.1097/QAD.0b013e32833f1142. [DOI] [PubMed] [Google Scholar]
- 93.Karita E, Price M, Hunter E, et al. Investigating the utility of the HIV-1 BED capture enzyme immunoassay using cross-sectional and longitudinal seroconverter specimens from Africa. AIDS. 2007;21:403–408. doi: 10.1097/QAD.0b013e32801481b7. [DOI] [PubMed] [Google Scholar]
- 94.McDougal JS, Parekh BS, Peterson ML, et al. Comparison of HIV type 1 incidence observed during longitudinal follow-up with incidence estimated by cross-sectional analysis using the BED capture enzyme immunoassay. AIDS Res Hum Retroviruses. 2006;22:945–952. doi: 10.1089/aid.2006.22.945. [DOI] [PubMed] [Google Scholar]
- 95.Wei X, Liu X, Dobbs T, et al. Development of two avidity-based assays to detect recent HIV type 1 seroconversion using a multisubtype gp41 recombinant protein. AIDS Res Hum Retroviruses. 2010;26:61–71. doi: 10.1089/aid.2009.0133. [DOI] [PubMed] [Google Scholar]
- 96.Duong YT, Qiu M, De AK, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One. 2012;7:e33328. doi: 10.1371/journal.pone.0033328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97••.Konikoff J, Brookmeyer R, Longosz AF, et al. Performance of a limiting-antigen avidity enzyme immunoassay for cross-sectional estimation of HIV incidence in the United States. PLoS One. 2013;8:e82772. doi: 10.1371/journal.pone.0082772. The LAg-Avidity assay did not perform well in a single-assay format, but did in combination with Bio-Rad-Avidity assays, as well as with viral load and CD4 cell count. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98••.Brookmeyer R, Konikoff J, Laeyendecker O, Eshleman SH. Estimation of HIV incidence using multiple biomarkers. Am J Epidemiol. 2013;177:264–272. doi: 10.1093/aje/kws436. Describes the process of selecting optimal MAAs based on accuracy, cost, and logistical considerations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99•.Brookmeyer R, Laeyendecker O, Donnell D, Eshleman SH. Cross-sectional HIV incidence estimation in HIV prevention research. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S233–S239. doi: 10.1097/QAI.0b013e3182986fdf. MAAs provide a practical, accurate, and cost-effective approach for cross-sectional HIV incidence estimation that can be used for HIV prevention research and epidemic monitoring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100•.Eshleman SH, Hughes JP, Laeyendecker O, et al. Use of a multifaceted approach to analyze HIV incidence in a cohort study of women in the United States: HIV Prevention Trials Network 064 Study. J Infect Dis. 2013;207:223–231. doi: 10.1093/infdis/jis658. Cross-sectional HIV incidence estimates obtained using the MAA were similar to the longitudinal estimate based on HIV seroconversion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101••.Laeyendecker O, Brookmeyer R, Cousins MM, et al. HIV incidence determination in the United States: a multiassay approach. J Infect Dis. 2013;207:232–239. doi: 10.1093/infdis/jis659. An MAA for HIV incidence that includes the BED-CEIA, an antibody avidity assay, HIV load, and CD4+ T cell count provides a powerful tool for cross-sectional HIV incidence estimation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laeyendecker O, Brookmeyer R, Mullis CE, et al. Specificity of four laboratory approaches for cross-sectional HIV Incidence determination: analysis of samples from adults with known nonrecent HIV infection from five African countries. AIDS Res Hum Retroviruses. 2012;28:1177–1183. doi: 10.1089/aid.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103•.Laeyendecker O, Piwowar-Manning E, Fiamma A, et al. Estimation of HIV incidence in a large, community-based, randomized clinical trial: NIMH project accept (HIV Prevention Trials Network 043) PLoS One. 2013;8:e68349. doi: 10.1371/journal.pone.0068349. HIV incidence and intervention effects can be accurately estimated from cross-sectional surveys using an MAA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laeyendecker O, Latimore A, Eshleman SH, et al. The effect of sample handling on cross sectional HIV incidence testing results. PLoS One. 2011;6:e25899. doi: 10.1371/journal.pone.0025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Welte A, McWalter TA, Laeyendecker O, Hallett TB. Using tests for recent infection to estimate incidence: problems and prospects for HIV. Euro Surveill. 2010;15:19589. [PMC free article] [PubMed] [Google Scholar]
- 106••.Kassanjee R, McWalter TA, Welte A. Short Communication: Defining optimality of a test for recent infection for HIV incidence surveillance. AIDS Res Hum Retroviruses. 2014;30:45–49. doi: 10.1089/aid.2013.0113. For population level estimates, accuracy and precision are more important than the individual level measures of sensitivity and specificity. For HIV incidence estimation, the important parameter inputs are long duration of recent infection and a low false-recent rate. [DOI] [PMC free article] [PubMed] [Google Scholar]