Abstract
Background: Few studies have focused on prognostic factors among adolescents and young adults (AYAs) 15 to 39 years of age when diagnosed with differentiated thyroid cancer (DTC). Our study expands upon prior work by including an evaluation of survival among AYA men and by neighborhood socioeconomic status, health insurance, and clinical factors to identify subgroups of young DTC patients at higher risk of mortality.
Methods: Data for 16,827 AYA DTC patients diagnosed between 1988 and 2010 were obtained from the California Cancer Registry. Survival, through 2010, by sociodemographic and clinical factors was analyzed using Cox proportional hazards regression.
Results: Of the 2.1% of AYAs who died, 16.7% died from thyroid cancer and 21.4% died from a subsequent cancer. In multivariate analyses, older AYAs 35 to 39 year of age (versus 15- to 29-year-olds), men (hazard ratio [HR] 2.77, 95% confidence interval [CI] 1.62–4.72), and AYAs of African American or Hispanic race/ethnicity (versus non-Hispanic whites) had worse thyroid cancer specific survival. In addition, residing in low socioeconomic status neighborhoods (HR 3.11 [CI 1.28–7.56]) and nonmetropolitan areas (HR 5.53 [CI 2.07–14.78]) was associated with worse thyroid cancer–specific survival among AYA men, but not AYA women.
Conclusions: Despite the generally good prognosis among AYAs with DTC, we identified subgroups of AYA patients at risk for poor outcomes. Further study of the factors underlying these associations, including possible barriers to receiving high-quality treatment and follow-up care, as well as lifestyle factors, are critical to reducing these disparities.
Introduction
Thyroid cancer is one of the most common cancers in adolescent and young adults (AYA) between the ages of 15 and 39, ranking as the second or third most common cancer from 1975 to 2011 within this age group (1). The incidence of this cancer overall has been increasing for the last three decades (2), with a particularly dramatic increase since 2001 (1). Further, the rise in incidence rates have been observed in men and women of all race/ethnicities (3). Thyroid cancer is typically divided into differentiated and undifferentiated forms, with papillary and follicular thyroid carcinoma classified together as differentiated thyroid cancer (DTC) (4). Temporal increases have been greatest for papillary carcinomas, the most common histologic type accounting for about 80% of all thyroid cancers in the United States, with smaller but significant increases observed for follicular carcinomas, which account for about 10% of all thyroid cancers (2,5,6).
While five-year relative survival after diagnosis of DTC is high among AYAs (>98%) (1,6) and better than survival among older DTC patients (7–9), recently it was shown that survival was worse in AYA women diagnosed with distant stage disease, follicular histology, or subsequent primary cancers, as well as among AYA women who were not married or of African American race/ethnicity (versus non-Hispanic [NH] whites) (8). As age is an effect modifier of survival (8) and few studies have focused on factors associated with prognosis within AYAs (7,8,10), it remains unclear whether additional factors, such as neighborhood socioeconomic status (SES) and health insurance status, are associated with prognosis, or whether the observed survival associations in AYA women (8) apply to AYA men as well. Our study expands upon prior work by including an evaluation of survival among AYA men and by neighborhood SES, health insurance status, and detailed clinical factors to identify subgroups of young patients at higher risk of mortality from this cancer that is, otherwise, generally associated with excellent prognosis.
Materials and Methods
Study population
We obtained data from the California Cancer Registry (CCR), which contributes approximately half of the data in the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program and is estimated to include more than 99% of all invasive cancers diagnosed in the state of California. We included patients diagnosed with a first, invasive thyroid carcinoma (International Classification of Disease for Oncology, third edition, site codes C73.9) between 15 and 39 years of age during the period January 1, 1998, to December 31, 2010. Included were patients with one of the following histologic types: classical papillary (International Classification of Disease for Oncology, third edition, histology codes 8050, 8260, 8340, 8341, 8343, and 8450), diffuse sclerosing variant of papillary (histology code 8350), tall cell variant of papillary (histology codes 8344), or follicular (histology codes 8290, 8330, 8331, 8332, and 8335); we hereafter refer to these patients as having DTC even though we have not included Hürthle cell carcinomas. After excluding 35 AYAs who were diagnosed by death certificate/autopsy only, the final study population included 16,827 patients. Ethics approval for human-subjects research was obtained from the California Prevention Institute of California Institutional Review Board. As the analysis was based on state-mandated cancer registry data, the study was conducted in accordance with the waivers of individual informed consent and Health Insurance Portability and Accountability Act authorization.
Sociodemographic and clinical characteristics
For each thyroid cancer case, we obtained cancer registry information routinely abstracted from the medical record (11) on age at diagnosis, race/ethnicity (Hispanic, NH white, NH black, and NH Asian/Pacific Islander [API], hereafter referred to as “Hispanic,” “White,” “African American,” and “API,” respectively), marital status (never married, married, previously married, unknown), histology (papillary and its variants—hereafter referred to as “papillary”—and follicular, as defined above), SEER summary stage at diagnosis (localized, regional, distant, and unknown), tumor size (≤1, >1–2, >2–3, >3–4, and >4 cm), lymph node involvement (none, regional/distant, unknown), extension of the primary tumor (intrathyroidal, extrathyroidal, unknown), and focality (solitary, multifocal, unknown). Lymph node dissection was determined by ≥4 lymph nodes examined or positive lateral neck or mediastinal lymph node involvement (N1b). We also obtained registry information on initial course of treatment (recorded separately for surgery, chemotherapy, hormone, and radiation therapy), the development of any subsequent primary cancers, and vital status as of December 31, 2010 (routinely determined by the CCR through hospital follow-up and database linkages including the Social Security Administration), and, for deceased patients, the underlying cause of death.
We also obtained information on health insurance, defined as the primary source of payment at the time of initial diagnosis and/or treatment, which is routinely abstracted by the CCR for patients diagnosed from 2001 forward. Health insurance was grouped into public insurance (Medicaid and other government-assisted programs), private insurance (health maintenance organizations, preferred provider organizations, and managed care not otherwise specified) military care, no insurance and insurance status unknown (12).
As information on patient education or other individual-level measures of SES are not collected by the CCR, we assigned a multicomponent measure of neighborhood SES based on patients' residential census-block group at diagnosis using a previously described index that incorporates 2000 U.S. Census (for cases diagnosed through 2005) and 2006–2010 American Community Survey data (for cases diagnosed in 2006 forward) on education, occupation, unemployment, household income, poverty, rent, and house values (13). Each cancer case was then assigned to a neighborhood SES quintile based on the distribution of SES across all census block groups in California and then into two categories: lower SES (quintiles 1 and 2) and higher SES (quintiles 3, 4, and 5). Based upon the population density of census blocks (14,15), we defined urbanization level as metropolitan (metropolitan urban and metropolitan suburban blocks) and nonmetropolitan (city, town and rural blocks).
Statistical analyses
To evaluate differences in survival by sociodemographic and clinical factors, we conducted survival analyses with Cox proportional hazards regression to estimate hazard ratios (HR) and associated 95% confidence intervals (CI) among all AYAs and for males and females separately. For deceased patients, survival time was measured in days from the date of diagnosis to the date of death from any cause for overall survival or to the date of death from thyroid cancer for thyroid cancer–specific survival. Patients who died from other causes were censored at the time of death for analyses of thyroid cancer–specific survival. Competing risk analysis was conducted among all AYAs using the same vital status and cause of death information to classify deaths from other (nonthyroid) cancers and deaths from all noncancer causes. Patients alive at the study end date (December 31, 2010) were censored at this time or at date of last follow-up (i.e., last known contact); 92% of all censored patients had a follow-up date within 2 years of the study end date.
The proportional hazards assumption was examined by statistically evaluating the correlation between weighted Schoenfeld residuals and logarithmically transformed survival time. Violations of the assumption were observed only for stage at diagnosis, so stage at diagnosis was included as a stratifying variable in the analyses and is not included in Tables 2, 3, or 4. Multivariate Cox regression models included the variables significant at p<0.1 in univariate models for overall survival [i.e., age at diagnosis, subsequent cancer, sex, marital status, urbanization level, neighborhood SES, radioactive iodine, tumor size, extension, and lymph node dissection] or with a priori hypotheses for inclusion [i.e., year of diagnosis, race/ethnicity, histology subtype (papillary, follicular), total thyroidectomy, and hormone therapy]. Components of stage at diagnosis, including tumor size and extension, were included in the multivariable models to control for the residual effects of stage. Having a subsequent cancer was strongly correlated with other cancer–specific survival and was not included in the multivariate model for this survival outcome.
Table 2.
Overall, Thyroid Cancer–Specific, Other Cancer–Specific, and Noncancer-Specific Survival Among Adolescents and Young Adults 15–39 Years of Age When Diagnosed with Thyroid Cancer, California, 1988–2010
| Overall survival | Thyroid cancer–specific survival | Other cancer–specific survival | Noncancer-specific survival | |||||
|---|---|---|---|---|---|---|---|---|
| Sociodemographic andclinical characteristics | No. deaths (N=359) | HR (95% CI) | No. deaths (N=60) | HR (95% CI) | No. deaths (N=77) | HR (95% CI) | No. deaths (N=222) | HR (95% CI) |
| Sex | ||||||||
| Male | 135 | 2.68 (2.14–3.34) | 26 | 2.77 (1.62–4.72) | 13 | 0.82 (0.45–1.51) | 96 | 3.31 (2.52–4.36) |
| Female | 224 | Reference | 34 | Reference | 64 | Reference | 126 | Reference |
| Age at diagnosis | ||||||||
| 15–29 | 106 | Reference | 17 | Reference | 21 | Reference | 68 | Reference |
| 30–34 | 107 | 1.53 (1.16–2.01) | 15 | 1.27 (0.62–2.59) | 22 | 1.65 (0.89–3.05) | 70 | 1.77 (1.26–2.49) |
| 35–39 | 146 | 2.01 (1.54–2.62) | 28 | 2.50 (1.32–4.75) | 34 | 2.53 (1.43–4.48) | 84 | 2.18 (1.56–3.05) |
| Race/ethnicity | ||||||||
| Non-Hispanic white | 187 | Reference | 16 | Reference | 44 | Reference | 127 | Reference |
| African American | 19 | 1.35 (0.83–2.20) | 6 | 6.70 (2.46–18.23) | <5 | ∼ | 10 | 1.11 (0.57–2.16) |
| Hispanic | 110 | 1.14 (0.88–1.47) | 30 | 3.60 (1.87–6.92) | 14 | 0.56 (0.30–1.07) | 66 | 1.03 (0.75–1.41) |
| Asian/Pacific Islander | 41 | 1.02 (0.72–1.44) | 8 | 2.08 (0.86–5.07) | 16 | 1.58 (0.86–2.87) | 17 | 0.63 (0.38–1.06) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Marital status at diagnosis | ||||||||
| Married | 165 | Reference | 37 | Reference | 41 | Reference | 87 | Reference |
| Not married | 183 | 1.78 (1.43–2.23) | 19 | 0.76 (0.42–1.37) | 35 | 1.57 (0.98–2.53) | 129 | 2.37 (1.79–3.15) |
| Unknown | 11 | 1.77 (0.94–3.33) | <5 | ∼ | <5 | ∼ | 6 | 1.53 (0.65–3.59) |
| Socioeconomic status | ||||||||
| Low (quintile 1–2) | 163 | 1.85 (1.48–2.31) | 27 | 1.25 (0.72–2.17) | 34 | 2.01 (1.24–3.26) | 102 | 1.89 (1.42–2.50) |
| High (quintile 3–5) | 196 | Reference | 33 | Reference | 43 | Reference | 120 | Reference |
| Urbanization level | ||||||||
| Metropolitan | 208 | Reference | 32 | Reference | 45 | Reference | 131 | Reference |
| Nonmetropolitan | 110 | 1.29 (1.01–1.64) | 23 | 2.17 (1.22–3.87) | 23 | 1.33 (0.78–2.27) | 64 | 1.04 (0.76–1.42) |
| Unknown | 41 | 1.27 (0.90–1.81) | 5 | 1.10 (0.40–3.05) | 9 | 1.50 (0.71–3.19) | 27 | 1.31 (0.85–2.02) |
| Histology | ||||||||
| Papillary | 320 | Reference | 45 | Reference | 73 | Reference | 202 | Reference |
| Follicular | 39 | 1.10 (0.77–1.56) | 15 | 4.60 (2.38–8.89) | <5 | ∼ | 20 | 1.01 (0.62–1.64) |
| Lymph node dissection | ||||||||
| No | 204 | Reference | 35 | Reference | 45 | Reference | 124 | Reference |
| Yes | 56 | 0.77 (0.54–1.08) | 14 | 0.57 (0.28–1.17) | 9 | 0.99 (0.43–2.28) | 33 | 0.90 (0.57–1.40) |
| Unknown | 99 | 1.24 (0.72–2.13) | 11 | 0.53 (0.17–1.71) | 23 | 0.85 (0.32–2.25) | 65 | 2.96 (1.30–6.72) |
| Subsequent cancer | ||||||||
| No | 284 | Reference | 55 | Reference | ± | 216 | Reference | |
| Yes | 75 | 4.68 (3.59–6.11) | 5 | 1.73 (0.67–4.49) | 6 | 0.44 (0.19–1.01) | ||
| Total thyroidectomy | ||||||||
| No | 106 | Reference | 9 | Reference | 27 | Reference | 70 | Reference |
| Yes | 250 | 1.10 (0.86–1.41) | 50 | 2.66 (1.23–5.75) | 49 | 0.78 (0.47–1.31) | 151 | 0.95 (0.70–1.29) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Radioactive iodine | ||||||||
| No | 194 | Reference | 29 | Reference | 41 | Reference | 124 | Reference |
| Yes | 165 | 0.67 (0.53–0.84) | 31 | 0.55 (0.31–0.96) | 36 | 0.71 (0.43–1.17) | 98 | 0.64 (0.48–0.85) |
| Hormone therapy | ||||||||
| No | 238 | Reference | 35 | Reference | 43 | Reference | 160 | Reference |
| Yes | 119 | 0.91 (0.72–1.15) | 24 | 1.16 (0.66–2.05) | 34 | 1.53 (0.96–2.45) | 61 | 0.72 (0.53–0.98) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Insurance status, limited to patients who were diagnosed after 2001 | ||||||||
| Private/military insurance | 38 | Reference | 6 | Reference | 9 | Reference | 23 | Reference |
| Public insurance/no insurance/unknown | 35 | 2.56 (1.39–4.71) | 6 | 1.61 (0.34–7.69) | <5 | ∼ | 25 | 2.56 (1.39–4.71) |
All Cox models were stratified by stage at diagnosis (localized, regional, metastasized, unknown), included the variables presented in the tables, and additionally adjusted for year at diagnosis (continuous), tumor size (<1.0 cm, >1.0 to ≤2.0 cm, <2.0 to ≤4.0 cm, >4.0 cm, unknown), extension (intrathyroidal, extrathyroidal, unknown), and block effect by block group.
“∼” Symbol indicates data not shown.
“±” Symbol indicates variable not included in model.
Table 3.
Overall and Thyroid Cancer–Specific Survival Among Adolescents and Young Adults 15–39 Years of Age When Diagnosed with Thyroid Cancer, by Sex, California, 1988–2010
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall survival | Thyroid cancer–specific survival | Overall survival | Thyroid cancer–specific survival | |||||
| Sociodemographic and clinical characteristics | No. deaths (n=135) | HR (95% CI) | No. deaths (n=26) | HR (95% CI) | No. deaths (n=224) | HR (95% CI) | No. deaths (n=34) | HR (95% CI) |
| Age at diagnosis | ||||||||
| 15–29 | 38 | Reference | 7 | Reference | 68 | Reference | 10 | Reference |
| 30–34 | 42 | 1.74 (1.10–2.75) | 6 | 1.20 (0.35–4.11) | 65 | 1.40 (0.99–2.00) | 9 | 1.38 (0.54–3.53) |
| 35–39 | 55 | 2.37 (1.52–3.69) | 13 | 2.95 (1.08–8.03) | 91 | 1.87 (1.34–2.61) | 15 | 2.20 (0.93–5.23) |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 76 | Reference | 7 | Reference | 111 | Reference | 9 | Reference |
| African American | 5 | 1.05 (0.42–2.65) | <5 | ∼ | 14 | 1.76 (0.99–3.15) | <5 | ∼ |
| Hispanic | 42 | 1.12 (0.74–1.68) | 13 | 4.63 (1.66–12.94) | 68 | 1.16 (0.84–1.62) | 17 | 3.86 (1.58–9.45) |
| Asian/Pacific Islander | 10 | 0.68 (0.35–1.34) | <5 | ∼ | 31 | 1.18 (0.78–1.78) | <5 | ∼ |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Marital status at diagnosis | ||||||||
| Married | 51 | Reference | 16 | Reference | 114 | Reference | 21 | Reference |
| Not married | 80 | 2.33 (1.61–3.39) | 9 | 0.88 (0.35–2.23) | 103 | 1.51 (1.14–2.00) | 10 | 0.72 (0.32–1.61) |
| Unknown | <5 | ∼ | <5 | ∼ | 7 | 1.59 (0.72–3.50) | <5 | ∼ |
| Socioeconomic Status (SES) | ||||||||
| Low (Quintile 1–2) | 67 | 2.60 (1.81–3.73) | 15 | 3.11 (1.28–7.56) | 96 | 1.48 (1.11–1.98) | 12 | 0.61 (0.28–1.32) |
| High (Quintile 3–5) | 68 | Reference | 11 | Reference | 128 | Reference | 22 | Reference |
| Urbanization level | ||||||||
| Metropolitan | 77 | Reference | 10 | Reference | 131 | Reference | 22 | Reference |
| Non-metropolitan | 45 | 1.41 (0.96–2.07) | 13 | 5.53 (2.07–14.78) | 65 | 1.24 (0.91–1.68) | 10 | 1.32 (0.59–2.94) |
| Unknown | 13 | 1.10 (0.59–2.05) | <5 | ∼ | 28 | 1.37 (0.89–2.11) | <5 | ∼ |
| Histology | ||||||||
| Papillary | 118 | Reference | 19 | Reference | 202 | Reference | 26 | Reference |
| Follicular | 17 | 1.40 (0.80–2.43) | 7 | 10.25 (3.13–33.62) | 22 | 0.92 (0.58–1.46) | 8 | 3.23 (1.33–7.82) |
| Lymph node dissection | ||||||||
| No | 74 | Reference | 14 | Reference | 130 | Reference | 21 | Reference |
| Yes | 29 | 0.72 (0.42–1.22) | 8 | 0.77 (0.23–2.53) | 27 | 0.75 (0.47–1.20) | 6 | 0.36 (0.13–1.01) |
| Unknown | 32 | 0.94 (0.39–2.26) | <5 | ∼ | 67 | 1.69 (0.83–3.43) | 7 | 1.12 (0.19–6.74) |
| Subsequent cancer | ||||||||
| No | 122 | Reference | 26 | Reference | 162 | Reference | 29 | Reference |
| Yes | 13 | 2.74 (1.49–5.03) | 0 | ∼ | 62 | 5.79 (4.26–7.86) | 5 | 2.72 (1.01–7.33) |
| Total thyroidectomy | ||||||||
| No | 34 | Reference | <5 | Reference | 72 | Reference | 6 | Reference |
| Yes | 100 | 1.32 (0.86–2.03) | 23 | 4.60 (1.17–18.05) | 150 | 0.96 (0.71–1.31) | 27 | 1.60 (0.60–4.23) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Radioactive iodine | ||||||||
| No | 69 | Reference | 15 | Reference | 125 | Reference | 14 | Reference |
| Yes | 66 | 0.60 (0.41–0.88) | 11 | 0.23 (0.09–0.57) | 99 | 0.72 (0.54–0.96) | 20 | 0.93 (0.43–2.02) |
| Hormone therapy | ||||||||
| No | 91 | Reference | 16 | Reference | 147 | Reference | 19 | Reference |
| Yes | 42 | 0.84 (0.57–1.24) | 9 | 1.30 (0.49–3.47) | 77 | 0.98 (0.74–1.31) | 15 | 1.25 (0.59–2.64) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ | 0 | ∼ |
| Insurance status, limited to patients who were diagnosed from 2001–2010 | ||||||||
| Private/military insurance | 17 | Reference | <5 | Reference | 21 | Reference | <5 | Reference |
| Public insurance/no insurance/unknown | 15 | 1.59 (0.57–4.44) | <5 | ∼ | 20 | 2.71 (1.29–5.70) | <5 | ∼ |
All Cox models were stratified by stage at diagnosis (localized, regional, metastasized, unknown), included the variables presented in the tables, and additionally adjusted for year at diagnosis (continuous), tumor size (<1.0 cm, >1.0 to ≤2.0 cm, <2.0 to ≤4.0 cm, >4.0 cm, unknown), extension (intrathyroidal, extrathyroidal, unknown), and block effect by block group.
“∼” Symbol indicates data not shown.
Table 4.
Other Cancer–Specific and Noncancer-Specific Survival Among Adolescents and Young Adults 15–39 Years of Age When Diagnosed with Thyroid Cancer, by Sex, California, 1988–2010
| Malesa | Females | |||||
|---|---|---|---|---|---|---|
| Noncancer-specific survival | Other cancer–specific survival | Noncancer-specific survival | ||||
| Sociodemographic and clinical characteristics | No. deaths (n=96) | HR (95% CI) | No. deaths (n=64) | HR (95% CI) | No. deaths (n=126) | HR (95% CI) |
| Age at diagnosis | ||||||
| 15–29 | 27 | Reference | 17 | Reference | 41 | Reference |
| 30–34 | 34 | 2.18 (1.29–3.69) | 20 | 1.87 (0.96–3.66) | 36 | 1.53 (0.97–2.43) |
| 35–39 | 35 | 2.41 (1.41–4.11) | 27 | 2.44 (1.29–4.61) | 49 | 2.15 (1.39–3.31) |
| Race/ethnicity | ||||||
| Non-Hispanic White | 59 | Reference | 34 | Reference | 68 | Reference |
| African-American | <5 | ∼ | <5 | ∼ | 7 | 1.43 (0.64–3.19) |
| Hispanic | 28 | 1.02 (0.63–1.66) | 13 | 0.66 (0.33–1.31) | 38 | 1.03 (0.67–1.58) |
| Asian/Pacific Islander | <5 | ∼ | 14 | 1.77 (0.92–3.41) | 13 | 0.83 (0.45–1.52) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Marital status at diagnosis | ||||||
| Married | 29 | Reference | 35 | Reference | 58 | Reference |
| Not married | 64 | 3.32 (2.10–5.26) | 28 | 1.52 (0.90–2.57) | 65 | 1.91 (1.32–2.76) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Socioeconomic status (SES) | ||||||
| Low (quintile 1–2) | 47 | 2.40 (1.57–3.69) | 29 | 1.94 (1.14–3.29) | 55 | 1.61 (1.10–2.36) |
| High (quintile 3–5) | 49 | Reference | 35 | Reference | 71 | Reference |
| Urbanization level | ||||||
| Metropolitan | 59 | Reference | 37 | Reference | 72 | Reference |
| Nonmetropolitan | 28 | 0.99 (0.62–1.57) | 19 | 1.37 (0.76–2.45) | 36 | 1.12 (0.74–1.69) |
| Unknown | 9 | 0.96 (0.46–2.02) | 8 | 1.67 (0.75–3.75) | 18 | 1.58 (0.91–2.72) |
| Histology | ||||||
| Papillary | 86 | Reference | 60 | Reference | 116 | Reference |
| Follicular | 10 | 1.16 (0.57–2.36) | <5 | ∼ | 10 | 0.83 (0.43–1.62) |
| Lymph node dissection | ||||||
| No | 52 | Reference | 37 | Reference | 72 | Reference |
| Yes | 18 | 0.69 (0.36–1.32) | 6 | 0.86 (0.32–2.31) | 15 | 1.10 (0.58–2.08) |
| Unknown | 26 | 3.06 (0.77–12.14) | 21 | 1.04 (0.35–3.04) | 39 | 3.21 (1.15–8.99) |
| Subsequent cancer | ||||||
| No | 94 | Reference | ± | 122 | Reference | |
| Yes | <5 | ∼ | <5 | ∼ | ||
| Total thyroidectomy | ||||||
| No | 26 | Reference | 22 | Reference | 44 | Reference |
| Yes | 69 | 1.07 (0.65–1.77) | 41 | 0.86 (0.49–1.52) | 82 | 0.88 (0.59–1.32) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Radioactive iodine | ||||||
| No | 48 | Reference | 35 | Reference | 76 | Reference |
| Yes | 48 | 0.67 (0.43–1.04) | 29 | 0.72 (0.42–1.24) | 50 | 0.63 (0.42–0.93) |
| Hormone therapy | ||||||
| No | 67 | Reference | 35 | Reference | 93 | Reference |
| Yes | 28 | 0.78 (0.49–1.24) | 29 | 1.63 (0.97–2.72) | 33 | 0.70 (0.46–1.05) |
| Unknown | <5 | ∼ | <5 | ∼ | <5 | ∼ |
| Insurance status, limited to patients who were diagnosed from 2001–2010 | ||||||
| Private/military insurance | 12 | Reference | 6 | Reference | 25 | Reference |
| Public insurance/no insurance/unknown | 11 | 1.27 (0.37–4.38) | <5 | ∼ | 11 | 3.68 (1.45–9.36) |
All Cox models were stratified by stage at diagnosis (localized, regional, metastasized, unknown), included the variables presented in the tables, and additionally adjusted for year at diagnosis (continuous), tumor size (<1.0 cm, >1.0 to ≤2.0 cm, <2.0 to ≤4.0 cm, >4.0 cm, unknown), extension (intrathyroidal, extrathyroidal, unknown), and block effect by block group.
“∼” Symbol indicates data not shown. “±” Symbol indicates variable not included in model.
The few other cancer–specific causes of death among males (n=13) precluded conducting separate analyses.
Source of health insurance coverage was included in subanalyses for patients diagnosed after 2001; due to the small number of events among uninsured AYAs (<5) and consistent with prior studies that suggest the small percentage of AYA cancer patients who were uninsured likely reflect retroactive enrollment in Medicaid at the time of cancer diagnosis (16), we considered publicly insured and uninsured together in the survival analyses. Because focality was not available for most patients, it was not included in the multivariate models. Finally, because the effect of marital status on survival may vary by age, we repeated our analyses, removing those under 25 years of age, and included interaction terms between age and marital status in our survival models (p>0.10); in these sensitivity analyses, the associations between marital status and survival remained and were more pronounced for overall survival (data not shown). Cells with fewer than five cases are not shown for privacy purposes. Analyses were carried out using SAS software version 9.3 (SAS Institute, Cary, North Carolina). All p values reported are two-sided.
Results
In this cohort of 16,827 AYAs diagnosed with DTC, we had up to 23 years of follow-up and a mean follow-up time of 10.3 years (standard deviation=6.7 years). Most AYA patients were female (83%), white (52%) or Hispanic (30%), living in a high-SES neighborhood at diagnosis (47% in the two highest quintiles), and had a total thyroidectomy (76%) or received radioactive iodine (57%) (Table 1). Sixty-two percent of AYAs who had a total thyroidectomy and 33% of AYAs without a total thyroidectomy received radioactive iodine. Among AYAs diagnosed after 2001 (i.e., for whom insurance data is available), most (74%) had private health insurance. Males were much more likely than females to be diagnosed at a regional/ distant stage of disease (47% of males versus 35% of females). Among the 2.1% of patients who died, 38% died from either their thyroid or a subsequent cancer.
Table 1.
Sociodemographic and Clinical Characteristics for Adolescents and Young Adults 15–39 Years of Age When Diagnosed with Invasive Thyroid Cancer by Sex, California, 1988–2010
| Sociodemographic and clinical characteristic | Total(n=16,827) | Males(n=2885) | Females(n=13,942) |
|---|---|---|---|
| Age at diagnosis | |||
| 15–29 | 6554 (38.9%) | 998 (34.6%) | 5557 (39.9%) |
| 30–34 | 4812 (28.6%) | 872 (30.2%) | 3940 (28.3%) |
| 35–39 | 5461 (32.5%) | 1015 (35.2%) | 4446 (31.9%) |
| Race/ethnicity | |||
| Non-Hispanic white | 8725 (51.9%) | 1645 (57.0%) | 7080 (50.8%) |
| African American | 490 (2.9%) | 83 (2.9%) | 407 (2.9%) |
| Hispanic | 4995 (29.7%) | 718 (24.9%) | 4277 (30.7%) |
| Asian/Pacific Islander | 2442 (14.5%) | 411 (14.2%) | 2031 (14.6%) |
| Unknown | 175 (1.0%) | 28 (1.0%) | 147 (1.1%) |
| Marital status at diagnosis | |||
| Married | 9439 (56.1%) | 1527 (52.9%) | 7912 (56.7%) |
| Never married | 6150 (36.5%) | 1167 (40.5%) | 4983 (35.7%) |
| Previously married | 836 (5.0%) | 114 (4.0%) | 722 (5.2%) |
| Unknown | 402 (2.4%) | 77 (2.7%) | 325 (2.3%) |
| Neighborhood socioeconomic status | |||
| Quintile 1 (low) | 2405 (14.3%) | 334 (11.6%) | 2071 (14.9%) |
| Quintile 2 | 2930 (17.4%) | 486 (16.8%) | 2444 (17.5%) |
| Quintile 3 | 3619 (21.5%) | 618 (21.4%) | 3002 (21.5%) |
| Quintile 4 | 3941 (23.4%) | 705 (24.4%) | 3236 (23.2%) |
| Quintile 5 (high) | 3932 (23.4%) | 742 (25.7%) | 3190 (22.9%) |
| Urbanization level | |||
| Metropolitan | 10,893 (64.7%) | 1897 (65.8%) | 8996 (64.5%) |
| Nonmetropolitan | 4865 (28.9%) | 813 (28.2%) | 4052 (29.1%) |
| Unknown | 1069 (6.4%) | 175 (6.1%) | 894 (6.4%) |
| Histology | |||
| Classic papillary | 15,328 (91.1%) | 2604 (90.3%) | 12,724 (91.3%) |
| Papillary, diffuse sclerosing variant | 47 (0.3%) | 6 (0.2%) | 41 (0.3%) |
| Papillary, tall cell variant | 52 (0.3%) | 10 (0.3%) | 42 (0.3%) |
| Follicular | 1400 (8.3%) | 265 (9.2%) | 1135 (8.1%) |
| Tumor stage | |||
| Localized | 10,169 (60.4%) | 1463 (50.7%) | 8706 (62.4%) |
| Regional | 5746 (34.1%) | 1208 (41.9%) | 4538 (32.5%) |
| Distant | 556 (3.3%) | 155 (5.4%) | 401 (2.9%) |
| Unknown | 356 (2.1%) | 59 (2.0%) | 297 (2.1%) |
| Tumor size | |||
| 0–1.00 cm | 3565 (21.2%) | 485 (16.8%) | 3080 (22.1%) |
| 1.01–2.00 cm | 4849 (28.8%) | 644 (22.3%) | 4205 (30.2%) |
| 2.01–4.00 cm | 4992 (29.7%) | 903 (31.3%) | 4089 (29.3%) |
| >4.00 cm | 1612 (9.6%) | 476 (16.5%) | 1136 (8.1%) |
| Unknown/missing | 1809 (10.8%) | 377 (13.1%) | 1432 (10.3%) |
| Lymph node dissection | |||
| No | 12,365 (73.5%) | 1895 (65.7%) | 10,470 (75.1%) |
| Yes | 2742 (16.3%) | 659 (22.8%) | 2083 (14.9%) |
| Unknown | 1720 (10.2%) | 331 (11.5%) | 1389 (10.0%) |
| Lymph node involvement | |||
| None | 9100 (54.1%) | 1273 (44.1%) | 7827 (56.1%) |
| Regional involvement | 4806 (28.6%) | 1072 (37.2%) | 3734 (26.8%) |
| Distant involvement | 110 (0.7%) | 32 (1.1%) | 78 (0.6%) |
| Unknown | 2811 (16.7%) | 508 (17.6%) | 2303 (16.5%) |
| Focality, limited to patients who were diagnosed after 2004 (n=6688) | |||
| Solitary | 3985 (59.6%) | 638 (57.4%) | 3347 (60.0%) |
| Multifocal | 2556 (38.2%) | 445 (40.1%) | 2111 (37.9%) |
| Unknown | 147 (2.2%) | 28 (2.5%) | 119 (2.1%) |
| Extension | |||
| Intrathyroidal | 12,099 (71.9%) | 1906 (66.1%) | 10,193 (73.1%) |
| Extrathyroidal | 2402 (14.3%) | 532 (18.4%) | 1870 (13.4%) |
| Unknown | 2326 (13.8%) | 447 (15.5%) | 1879 (13.5%) |
| Surgery | |||
| No | 254 (1.5%) | 62 (2.1%) | 192 (1.4%) |
| Surgery, but not total thyroidectomy | 3728 (22.2%) | 545 (18.9%) | 3183 (22.8%) |
| Total thyroidectomy | 12,816 (76.2%) | 2272 (78.8%) | 10,544 (75.6%) |
| Surgery not otherwise specified or unknown | 29 (0.2%) | 6 (0.2%) | 23 (0.2%) |
| Chemotherapy | |||
| No | 16,751 (99.5%) | 2876 (99.7%) | 13,875 (99.5%) |
| Yes | 43 (0.3%) | <5 | 39 (0.3%) |
| Unknown | 33 (0.2%) | 5 (0.2%) | 28 (0.2%) |
| Hormone therapy | |||
| No | 10,062 (59.8%) | 1714 (59.4%) | 8348 (59.9%) |
| Yes | 6577 (39.1%) | 1144 (39.7%) | 5433 (39.0%) |
| Unknown | 188 (1.1%) | 27 (0.9%) | 161 (1.2%) |
| Radiation therapy | |||
| No | 7177 (42.7%) | 1064 (36.9%) | 6113 (43.8%) |
| Yes | 9644 (57.3%) | 1821 (63.1%) | 7823 (56.1%) |
| Radioactive iodine | 9178 (95.2) | 1730 (95.0%) | 7448 (95.2%) |
| External beam radiation | 220 (1.3%) | 47 (1.6%) | 173 (1.2%) |
| Other radiation therapy | 466 (4.8%) | 91 (5.0%) | 375 (4.8%) |
| Unknown | 6 (0.0%) | <5 | 6 (0.0%) |
| Subsequent cancer | |||
| No | 16,233 (96.5%) | 2809 (97.4%) | 13,424 (96.3%) |
| Yes* | 594 (3.5%) | 76 (2.6%) | 518 (3.7%) |
| Breast | <5 | 151 (29.2%) | |
| Melanoma | <5 | 42 (8.1%) | |
| Thyroid | <5 | 34 (6.6%) | |
| Uterus | <5 | 32 (6.2%) | |
| Colorectal | <5 | 25 (4.8%) | |
| Lymphoma | <5 | 17 (3.3%) | |
| Head and neck | <5 | 13 (2.5%) | |
| Kidney | 8 (10.5%) | 6 (1.2%) | |
| Ovary | <5 | 11 (2.1%) | |
| Prostate | 10 (13.5%) | ||
| Stomach | <5 | 7 (1.4%) | |
| Lung | <5 | 7 (1.4%) | |
| Others | 21 (27.6%) | 46 (8.9%) | |
| Unknown | 17 (22.3%) | 127 (24.5%) | |
| Insurance status, limited to patients who were diagnosed after 2001 (n=8929) | |||
| Private, health maintenance organizations | 1848 (20.7%) | 296 (19.8%) | 1552 (20.9%) |
| Private, preferred provider organizations | 1310 (14.7%) | 252 (16.9%) | 1058 (14.2%) |
| Private, other | 3417 (38.3%) | 557 (37.3%) | 2860 (38.5%) |
| Military | 230 (2.6%) | 51 (3.4%) | 179 (2.4%) |
| Public insurance | 1064 (11.9%) | 133 (8.9%) | 931 (12.5%) |
| No insurance | 259 (2.9%) | 50 (3.4%) | 209 (2.8%) |
| Unknown | 801 (9.0%) | 153 (10.3%) | 648 (8.7%) |
| Vital status | |||
| Alive | 16,468 (97.9%) | 2750 (95.3%) | 13,718 (98.4%) |
| Deceased | 359 (2.1%) | 135 (4.7%) | 224 (1.6%) |
| Cause of death among deceased | |||
| Thyroid cancer | 60 (16.7%) | 26 (19.3%) | 34 (15.2%) |
| Other cancers* | 77 (21.4%) | 13 (9.6%) | 64 (28.6%) |
| Breast | <5 | 14 (21.9%) | |
| Leukemia | <5 | 9 (14.1%) | |
| Lung | <5 | 8 (12.5%) | |
| Ovary | <5 | 5 (7.8%) | |
| Other | 10 (76.9%) | 28 (43.8%) | |
| Heart/cerebrovascular disease | 34 (9.5%) | 19 (14.1%) | 15 (6.7%) |
| Accidents/intentional self-harm/assault | 47 (13.1%) | 26 (19.3%) | 21 (9.4%) |
| Other causes | 99 (27.6%) | 40 (29.6%) | 59 (26.3%) |
| State death certificate not available yet | 42 (11.7%) | 11 (8.1%) | 31 (13.8%) |
Data on type of subsequent cancer and other cancer cause of death are presented for males and females only in order to protect confidentiality, as many cancer types included <5 adolescent and young adults.
Overall survival
After adjusting for sociodemographic and cancer characteristics, AYA men were over two times more likely than women of the same age to die from any cause after a diagnosis of thyroid cancer (HR 2.68 [CI 2.14–3.34]) (Table 2). AYAs diagnosed at 30 years of age and older has worse survival than younger AYAs. There were no racial/ethnic differences in overall survival; however, residing in lower SES neighborhoods, residing in a nonmetropolitan area, being unmarried, and having a subsequent cancer were associated with poorer overall survival. Having received radioactive iodine therapy was associated with better overall survival. In addition, AYA patients diagnosed during 2001–2010 with public or no medical insurance also experienced worse overall survival (HR 2.56 [CI 1.39–4.71]).
In analyses stratified by sex (Table 3), sociodemographic and clinical factors associated with survival were generally similar for AYA men and women. However, the worse survival associated with residing in a low-SES neighborhood was stronger in AYA men than women (p for interaction=0.03) and the worse survival associated with a subsequent primary cancer was stronger in AYA women than men (p for interaction<0.01).
Thyroid cancer–specific survival
AYA men were over two times more likely to die from thyroid cancer than were women of the same age (HR 2.77 [CI 1.62–4.72]) (Table 2). Older AYAs 35–39 years of age had worse survival than AYAs diagnosed between the ages of 15 and 29 years, and African American and Hispanic AYAs were over six and three times, respectively, more likely to die than White AYAs. Residing in lower SES neighborhoods was not significantly associated with thyroid cancer–specific survival, but living in a nonmetropolitan area was associated with poorer survival (HR 2.17 [CI 1.22–3.87]). AYA patients with follicular histology or who received a total thyroidectomy experienced worse thyroid cancer–specific survival. Radioactive iodine therapy was associated with better thyroid cancer–specific survival.
Many associations between age, race/ethnicity and thyroid-specific survival were similar for AYA men and women (Table 3). However, the worse survival associated with residing in low-SES neighborhoods (p for interaction with sex<0.01) and nonmetropolitan areas (p for interaction with sex=0.05) was limited to AYA men. In addition, the beneficial effect of radioactive iodine was somewhat stronger in AYA men than women (p for interaction with sex=0.06).
Other cancer–specific survival
AYA patients who were older, unmarried (borderline significant) or resided in lower SES neighborhoods (HR 2.01 [CI 1.24–3.26]) experienced worse other cancer–specific survival (Table 2). Radioactive iodine therapy was not associated with other cancer–specific survival (Table 2). Results were similar when we considered only females (Table 4); the few other cancer–specific causes of death among males (n=13) precluded conducting separate analyses for males.
Noncancer-specific survival
In analyses of noncancer causes of death (62% of all deaths), we found similar associations to those reported above for overall survival (Table 2). Additionally, we found that hormone therapy was associated with better noncancer-specific survival. Associations were similar when men and women were considered separately (Table 4).
Histology
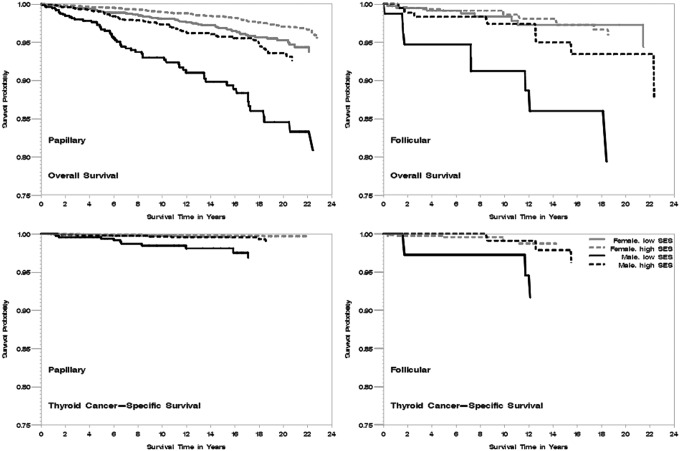
In analysis of AYAs with follicular histology, we found similar findings to those presented for DTC (Fig. 1; multivariate Cox regression analyses not shown). Specifically, we did not observe significant interactions between histology and the sociodemographic and clinical factors considered in these analyses for any of the survival outcomes, with the exception of total thyroidectomy, which was associated with worse overall survival among AYAs with follicular (HR 2.26 [CI 1.04–4.91]), but not papillary (HR 0.96 [CI 0.74–1.25]), histology (p for interaction=0.01).
FIG. 1.
Kaplan-Meier curve of overall and thyroid cancer-specific survival by histologic subtypes (papillary, follicular), neighborhood socioeconomic status (SES; low, high) and sex in adolescent and young adult patients, California 1988–2011. The vertical axis represents survival probability; the horizontal axis represents survival time in years. All p log-ranks <0.01.
Discussion
Despite the overall very good outcome for DTC (1,6), our population-based study of 16,827 AYAs diagnosed with DTC in California found sociodemographic disparities in survival. After taking into account factors, including stage at diagnosis, histology, and initial treatment, we found that older AYAs 35 to 39 years of age, men, and AYAs of African American or Hispanic race/ethnicity had worse thyroid cancer–specific survival. In addition, residing in low-SES neighborhoods and nonmetropolitan areas were associated with worse thyroid cancer–specific survival among AYA men, but not AYA women. Because most AYAs die from other causes, we also considered competing causes of death and found that men and AYAs with public or no medical insurance were more likely to die from other, noncancer causes of death and AYAs who were unmarried or residing in low-SES neighborhoods were more likely to die from nonthyroid cancer–specific causes of death. Our study identified populations of DTC cancer survivors at higher risk for mortality after diagnosis with the most common, best prognosis DTC tumors. Identifying these patient subgroups may help in developing more effective treatment decisions and tailored survivorship guidelines for the highest risk individuals.
AYA men were over two and one-half times more likely to die from thyroid cancer than AYA women diagnosed with DTC. Many, but not all (17), studies have found poorer survival in men with DTC (9,10,18,19). While a previous study concluded that the apparent survival disadvantage among men overall resulted from a larger percentage of men diagnosed with advanced stage disease, in young men there was a small, statistically significant survival disadvantage for stage 1 papillary thyroid cancer and suggestive survival disadvantage in stage 2 papillary thyroid cancer (18). When we considered survival by stage and histology in sensitivity analyses, we found sex disparities across all stage and histology groups (data not shown), suggesting that these factors do not explain these sex survival differences in our study.
In our study, radioactive iodine therapy was associated with better survival, particularly in AYA men. In addition, we found that radioactive iodine was associated with better survival across all stages of disease (localized, regional, or distant stage), except among AYA women with localized disease where radioactive iodine was not associated with survival (data not shown). Few studies have considered the impact of radioactive iodine therapy on survival in AYAs (10,20). In one SEER cancer registry analysis, radiation therapy was not associated with overall or thyroid-specific survival in young women with DTC (8), while another SEER cancer registry analysis found radioactive iodine to be associated with better survival in women, but not men, of all ages with DTC (9). Furthermore, among AYAs with first or second primary thyroid cancer in a National Cancer Database study, radioactive iodine therapy was associated with better overall survival (10). Given its higher use among AYAs than older adults (21), the influence of radioactive iodine on health and survival outcomes in AYAs should be considered in more detail in future studies.
We also observed worse thyroid cancer–specific survival among AYA men residing in low-SES neighborhoods and nonmetropolitan areas, but did not observe these associations in AYA women. However, for other cancer–specific and noncancer-specific causes of death, we found that poorer survival was observed for both AYA men and women residing in low-SES neighborhoods. Ours is the first study to observe survival differences by these neighborhood characteristics. These findings suggest that patients of low-SES, as well as men in nonmetropolitan areas, may experience barriers (e.g., lack of health insurance, financial burden, long travel time to healthcare facilities) (22–24) to obtaining high-quality treatment and follow-up care that result in poorer outcomes. It is also possible that AYA women receive more routine health care, regardless of SES, than AYA men because of increased physician visits to the obstetrician/gynecologist during their reproductive years.
In the subset of patients diagnosed after 2000, we found that public or no medical insurance was associated with worse noncancer causes of death, findings that are consistent with recent studies of young adults (25,26). In an analysis of nearly 40,000 20- to 40-year-olds diagnosed with cancer, those who were younger, male, nonwhite, and unmarried were more likely to be uninsured, as were patients who were from rural or low-SES regions (25). Furthermore, being uninsured was associated with presentation of metastatic disease, being undertreated and worse all-cause survival (25). A recent CCR analysis of DTC patients of all ages similarly found that nonwhite, low-SES, uninsured/Medicaid-insured and male patients were more likely to be diagnosed with later stage disease at diagnosis (19). While we considered health insurance at diagnosis, we did not have this information for patients diagnosed before 2001 or information on changes in health insurance after initial treatment, a factor that likely influences subsequent care and outcomes.
In our study, African American and Hispanic AYAs were more likely to die from thyroid cancer than non-Hispanic whites. In a SEER analyses of women under 40 years of age, African Americans were over two times more likely to die from any cause and over three times more likely to die from noncancer causes of death (8). In the same study of young women, Hispanics and APIs had suggestively worse overall survival (8) and, in AYAs with primary or secondary thyroid cancer, Hispanics experienced worse overall survival (10). Previous studies in patients of all ages have also found African Americans, but not Hispanics or APIs, to have worse survival than non-Hispanic whites (9,19). Prior studies in thyroid cancer patients of all ages suggest that these racial/ethnic disparities in survival may result from minority patients receiving inadequate surgical care (27) and being less likely to be operated on by a high-volume, experienced surgeon (28), factors that can contribute to a higher risk of complications and poorer outcomes.
In addition, our study shows that nonmarried AYAs generally experienced poorer survival outcomes compared with married patients, an observation consistent with previous findings for many cancers (8,25,29,30). These findings likely relate to married patients having stronger social support from spouses, which results in better adherence with prescribed treatments (31), fewer psychological difficulties (32), and greater likelihood of having health insurance (25) than nonmarried cancer patients. The findings in our study that older AYAs 35–39 years of age had worse survival is consistent with a large, population-based cancer registry analysis that considered over 42,000 patients diagnosed with papillary and follicular thyroid cancer (4), as well as a number of other studies (9). In particular, Oyer et al. found that survival began to decline in patients >35 years of age and continued steadily declining with further advances in age (4). Declining survival by age may reflect the inherent increase in mortality as age increases (9) or differences in disease biology between younger and older patients (7). As in prior studies (4), follicular histology was associated with much worse thyroid cancer–specific survival. Even with differences in prognosis, we observed similar associations between sociodemographic and clinical characteristics and survival by histology in our study.
The occurrence of second primary cancers in our study was associated with worse other cancer–specific survival, consistent with prior studies (8,33). As with our study, others have found that thyroid cancer is associated with subsequent primary cancers of many different organs (34,35), with one study finding increased risks of salivary gland, stomach, colon, breast, kidney, brain and central nervous system, thyroid, and adrenal gland cancers in patients diagnosed with first primary thyroid cancer before the age of 40 years (34). Although the risk of second primaries after thyroid cancer has been found to be comparable among men and women and higher among those treated with radioactive iodine for certain cancer types (i.e., stomach cancer and leukemia) (34,35), we found that AYA women were more likely to die from subsequent cancers than AYA men, and radioactive iodine for the first primary thyroid cancer was not associated with other cancer–specific survival. Our finding that AYA women were more likely to die from subsequent cancers may be consistent with a cancer predisposition and should be explored further.
Our study is one of the first to simultaneously consider the impact of small-area neighborhood SES, health insurance, marital status, diagnosis of subsequent cancers, and a number of tumor characteristics on survival after DTC in AYAs. Because thyroid cancer is the only cancer that includes age as part of the American Joint Committee on Cancer (AJCC) staging criteria (no patient <45 years of age can have stage 3 or 4 disease) (4,9), we accounted for stage by stratifying our survival models by SEER summary stage at diagnosis, which does not follow the AJCC age-specific staging guidelines, and additionally controlled for lymph node dissection, tumor size, and extension in our analyses. This population-based study included a large diverse AYA population of DTC cancer patients who received their care across all types of institutions, thus increasing the generalizability of these findings. Furthermore, having a mean follow-up of over 10 years allowed us to consider factors associated with competing causes of death in patients with this usually indolent malignancy.
On the other hand, we were unable to consider additional factors that could contribute to the survival disparities we observed in our study, including differences in genetic susceptibility, disease biology, access to high-quality care, treatment adherence, lifestyle behaviors, comorbidities, obesity, environmental exposures, and follow-up care (19,22). For comorbidities, however, we did not observe racial/ethnic differences in noncancer-specific causes of death, as found previously (9), suggesting that competing risk from comorbid conditions may play less of a role in our population. The potential misclassification of race/ethnicity needs to be considered, although excellent overall agreement with self-reported race/ethnicity has been shown for whites and blacks, and good agreement has been shown for Hispanics and Asians (36,37); as a result, differential recording of race/ethnicity at the time of diagnosis is unlikely to be related to survival. While we considered the first course of cancer-directed treatment, we did not have details on treatment such as dosing, or treatment received after this period, including treatment for subsequent malignancies; therefore, our findings could be subject to residual confounding from incomplete treatment data in the cancer registry (38). We also lacked information about treatment failure or recurrence and molecular factors that could influence prognosis (7,17). Furthermore, our study did not have individual-level measures of SES to consider separately or with our neighborhood measure. While neighborhood and individual SES are associated, neighborhood SES has been found to underestimate associations observed with individual-level SES (39).
Conclusion
Despite the good prognosis among AYAs diagnosed with DTC, we identified subgroups of AYA patients at risk for poor outcomes, including men, African American and Hispanic patients, publicly insured or uninsured patients, unmarried patients and patients residing in low-SES neighborhoods and non-metropolitan areas. These subgroups of patients are also less likely to have medical insurance, a factor that influences stage at diagnosis, treatment, and outcomes (25). Further study of the factors underlying these associations, including possible barriers to receiving high-quality treatment and follow-up care, as well as lifestyle factors, are critical to further understanding and reducing these disparities.
Acknowledgments
This work was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885 and the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute (NCI) at the National Institutes of Health under contract HHSN2612010000140C awarded to the Cancer Prevention Institute of California (THMK, LT) and the Stanford Cancer Institute (THMK). RHG was supported by the NCI award K12CA139160. HMP, MGW, KO, and PLH did not receive financial support for this project.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology, and End Results Program under contract HHSN2612010000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement 1U58 DP000807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Health Services, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. (eds) SEER cancer statistics review, 1975–2011. National Cancer Institute, Bethesda, MD: Available at http://seer.cancer.gov/csr/1975_2011/ (accessed September11, 2014) [Google Scholar]
- 2.Davies L, Welch HG. 2014. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140:317–322 [DOI] [PubMed] [Google Scholar]
- 3.Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, Angelos P, Grogan RH. 2013. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Oncol 20:2746–2753 [DOI] [PubMed] [Google Scholar]
- 4.Oyer SL, Smith VA, Lentsch EJ. 2012. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg 147:221–226 [DOI] [PubMed] [Google Scholar]
- 5.Horn-Ross PL, Lichtensztajn DY, Clarke CA, Dosiou C, Oakley-Girvan I, Reynolds P, Gomez SL, Nelson DO. 2014. Continued rapid increase in thyroid cancer incidence in california: trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol Biomarkers Prev 23:1067–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. 2014. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr 164:1481–1485 [DOI] [PubMed] [Google Scholar]
- 7.Vriens MR, Moses W, Weng J, Peng M, Griffin A, Bleyer A, Pollock BH, Indelicato DJ, Hwang J, Kebebew E. 2011. Clinical and molecular features of papillary thyroid cancer in adolescents and young adults. Cancer 117:259–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroup AM, Harrell CJ, Herget KA. 2012. Long-term survival in young women: hazards and competing risks after thyroid cancer. J Cancer Epidemiol 2012:641372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston LE, Tran Cao HS, Chang DC, Bouvet M. 2012. Sociodemographic predictors of survival in differentiated thyroid cancer: results from the SEER database. ISRN Endocrinol 2012:384707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldfarb M, Freyer DR. 2014. Comparison of secondary and primary thyroid cancer in adolescents and young adults. Cancer 120:1155–1161 [DOI] [PubMed] [Google Scholar]
- 11.California Cancer Registry 2009 Cancer reporting in California: abstracting and coding procedures for hospitals. California Cancer Registry. Volume I, Data Standards and Data Dictionary. Ninth edition. Available at www.ccrcal.org/Vol_1_2009_html/Vol_01_09.htm (Accessed July28, 2010)
- 12.Smith EC, Ziogas A, Anton-Culver H. 2012. Association between insurance and socioeconomic status and risk of advanced stage Hodgkin lymphoma in adolescents and young adults. Cancer 118:6179–6187 [DOI] [PubMed] [Google Scholar]
- 13.Yost K, Perkins C, Cohen R, Morris C, Wright W. 2001. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 12:703–711 [DOI] [PubMed] [Google Scholar]
- 14.Reynolds P, Hurley SE, Quach AT, Rosen H, Von Behren J, Hertz A, Smith D. 2005. Regional variations in breast cancer incidence among California women, 1988–1997. Cancer Causes Control 16:139–150 [DOI] [PubMed] [Google Scholar]
- 15.Urayama KY, Von Behren J, Reynolds P, Hertz A, Does M, Buffler PA. 2009. Factors associated with residential mobility in children with leukemia: implications for assigning exposures. Ann Epidemiol 19:834–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg AR, Kroon L, Chen L, Li CI, Jones B. 2014. Insurance status and risk of cancer mortality among adolescents and young adults. Cancer [Epub ahead of print]; DOI: 10.1002/cncr.29187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OM, Angelos P, Kaplan EL, Schechter RB. 2013. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 154:1436–1446; discussion 1446–1437. [DOI] [PubMed] [Google Scholar]
- 18.Oyer SL, Smith VA, Lentsch EJ. 2013. Sex is not an independent risk factor for survival in differentiated thyroid cancer. Laryngoscope 123:2913–2919 [DOI] [PubMed] [Google Scholar]
- 19.Harari A, Li N, Yeh MW. 2014. Racial and socioeconomic disparities in presentation and outcomes of well-differentiated thyroid cancer. J Clin Endocrinol Metab 99:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel SS, Goldfarb M. 2013. Well-differentiated thyroid carcinoma: the role of post-operative radioactive iodine administration. J Surg Oncol 107:665–672 [DOI] [PubMed] [Google Scholar]
- 21.Goldfarb M, Sener SF. 2014. Comparison of radioiodine utilization in adolescent and young adult and older thyroid cancer patients. Endocr Pract 20:405–411 [DOI] [PubMed] [Google Scholar]
- 22.Golden SH, Brown A, Cauley JA, Chin MH, Gary-Webb TL, Kim C, Sosa JA, Sumner AE, Anton B. 2012. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab 97:E1579–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirchhoff AC, Lyles CR, Fluchel M, Wright J, Leisenring W. 2012. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer 118:5964–5972 [DOI] [PubMed] [Google Scholar]
- 24.Bleyer A, Ulrich C, Martin S. 2012. Young adults, cancer, health insurance, socioeconomic status, and the Patient Protection and Affordable Care Act. Cancer 118:6018–6021 [DOI] [PubMed] [Google Scholar]
- 25.Aizer AA, Falit B, Mendu ML, Chen MH, Choueiri TK, Hoffman KE, Hu JC, Martin NE, Trinh QD, Alexander BM, Nguyen PL. 2014. Cancer-specific outcomes among young adults without health insurance. J Clin Oncol 32:2025–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbins AS, Lerro CC, Barr RD. 2014. Insurance status and distant-stage disease at diagnosis among adolescent and young adult patients with cancer aged 15 to 39 years: National Cancer Data Base, 2004 through 2010. Cancer 120:1212–1219 [DOI] [PubMed] [Google Scholar]
- 27.Sosa JA, Mehta PJ, Wang TS, Yeo HL, Roman SA. 2007. Racial disparities in clinical and economic outcomes from thyroidectomy. Ann Surg 246:1083–1091 [DOI] [PubMed] [Google Scholar]
- 28.Epstein AJ, Gray BH, Schlesinger M. 2010. Racial and ethnic differences in the use of high-volume hospitals and surgeons. Arch Surg 145:179–186 [DOI] [PubMed] [Google Scholar]
- 29.Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Hu JC, Nguyen PL. 2013. Marital status and survival in patients with cancer. J Clin Oncol 31:3869–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson NJ, Backlund E, Sorlie PD, Loveless CA. 2000. Marital status and mortality: the national longitudinal mortality study. Ann Epidemiol 10:224–238 [DOI] [PubMed] [Google Scholar]
- 31.Cohen SD, Sharma T, Acquaviva K, Peterson RA, Patel SS, Kimmel PL. 2007. Social support and chronic kidney disease: an update. Adv Chronic Kidney Dis 14:335–344 [DOI] [PubMed] [Google Scholar]
- 32.Goldzweig G, Andritsch E, Hubert A, Brenner B, Walach N, Perry S, Baider L. 2010. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol 21:877–883 [DOI] [PubMed] [Google Scholar]
- 33.Lang BH, Lo CY, Wong IO, Cowling BJ. 2010. Impact of second primary malignancy on outcomes of differentiated thyroid carcinoma. Surgery 148:1191–1196; discussion 1196–1197. [DOI] [PubMed] [Google Scholar]
- 34.Ronckers CM, McCarron P, Ron E. 2005. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer 117:281–288 [DOI] [PubMed] [Google Scholar]
- 35.Brown AP, Chen J, Hitchcock YJ, Szabo A, Shrieve DC, Tward JD. 2008. The risk of second primary malignancies up to three decades after the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab 93:504–515 [DOI] [PubMed] [Google Scholar]
- 36.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, Schwartz SM, Bernstein L, Chen VW, Goodman MT, Gomez SL, Graff JJ, Lynch CF, Lin CC, Edwards BK. 2007. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control 18:177–187 [DOI] [PubMed] [Google Scholar]
- 37.Gomez SL, Glaser SL. 2006. Misclassification of race/ethnicity in a population-based cancer registry (United States). Cancer Causes Control 17:771–781 [DOI] [PubMed] [Google Scholar]
- 38.Giordano SH, Kuo YF, Duan Z, Hortobagyi GN, Freeman J, Goodwin JS. 2008. Limits of observational data in determining outcomes from cancer therapy. Cancer 112:2456–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krieger N. 1992. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 82:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]