Abstract
The detection of biomarkers plays a central role in our effort to establish whether there is, or was, life beyond Earth. In this review, we address the importance of considering mineralogy in relation to the selection of locations and biomarker detection methodologies with characteristics most promising for exploration. We review relevant mineral-biomarker and mineral-microbe interactions. The local mineralogy on a particular planet reflects its past and current environmental conditions and allows a habitability assessment by comparison with life under extreme conditions on Earth. The type of mineral significantly influences the potential abundances and types of biomarkers and microorganisms containing these biomarkers. The strong adsorptive power of some minerals aids in the preservation of biomarkers and may have been important in the origin of life. On the other hand, this strong adsorption as well as oxidizing properties of minerals can interfere with efficient extraction and detection of biomarkers. Differences in mechanisms of adsorption and in properties of minerals and biomarkers suggest that it will be difficult to design a single extraction procedure for a wide range of biomarkers. While on Mars samples can be used for direct detection of biomarkers such as nucleic acids, amino acids, and lipids, on other planetary bodies remote spectrometric detection of biosignatures has to be relied upon. The interpretation of spectral signatures of photosynthesis can also be affected by local mineralogy. We identify current gaps in our knowledge and indicate how they may be filled to improve the chances of detecting biomarkers on Mars and beyond. Key Words: DNA—Lipids—Photosynthesis—Extremophiles—Mineralogy—Subsurface. Astrobiology 15, 492–507.
1. Introduction
Certain questions have always intrigued humankind: How did life evolve on Earth? Are we alone in the Universe, or is there life elsewhere? Does this life resemble life on Earth? These are also fundamental scientific questions. The discovery of organisms in extreme environments on Earth has fostered the search for these answers and provided further motivation for the exploration for life beyond Earth. Life, some of it surprisingly complex, has been and is being found in places on our own planet that were once considered to be uninhabitable. The cold dry valleys of Antarctica harbor diverse, specialized microbial communities (Pointing et al., 2009), while multicellular eukaryotes have been encountered deep (>1 km) in the subsurface (Borgonie et al., 2011). Life can thrive under conditions of extreme radiation, oxidative stress, pressure, temperature, pH, aridity, and salinity and with scarce nutrient availability (Satyanarayana et al., 2005). The potential for long-term (>250 million years) preservation of life-forms (Vreeland et al., 2000) and their components (Brocks et al., 1999) provides further motivation for the search for past and present life on other planets, or their moons, and exoplanets.
Limitations in technology and distances are critical constraints in the search for life in planetary exploration programs. The search for habitable planetary bodies will often be limited to the remote spectrometric detection of signatures of possible life (Hanslmeier, 2009). However, several planetary bodies are physically accessible. Mars is the most promising candidate for detecting (extinct) life (Irwin and Schulze-Makuch, 2001) and remains the major target for planetary exploration in the near future.
A search for life requires consideration of how life may reveal itself elsewhere and how life or biomarkers indicative for life may be detected. A biomarker refers to a measurable indicator of some biological state or condition, such as cellular components or isotopic fractionation. Considerable effort is being spent in the development and optimization of high-quality scientific instruments to measure biomarkers. However, sensitive detection also requires the efficient extraction of biomarkers from various substrates and the selection of sampling locations with characteristics most promising for successful exploration. The aim of this review is to address the importance of considering mineralogy in relation to the selection of sampling locations and to the methodology of detection of biomarkers by providing a review of relevant mineral-biomarker and microbe-mineral interactions. We will focus on three types of organic biomarkers of high interest to in situ detection: amino acids, lipids, and nucleic acids. We will first describe how martian mineralogy informs on current and past environmental conditions and whether they are, or were, compatible with life as we know it on Earth. Next, we will review research that indicates that on Earth the abundances and identities of the organisms producing these biomarkers are influenced by the type of mineral present in their local growth environment. Chemical characteristics of certain minerals have also played an important role in the evolution of life and the preservation of biomarkers, which is related to strong adsorption and accumulation of biomarkers on these minerals. Subsequently, we will indicate that this adsorption interferes with efficient extraction of biomarkers for detection and that minerals may contribute to the destruction of organic biomarkers during attempts to detect them. As only a few planetary bodies are accessible for in situ detection, we will also address the remote detection of biomarkers and how mineralogy influences their detection. We will conclude with suggestions for future research.
2. Molecular Biomarkers
Ideally, we would prefer to directly observe extraterrestrial life-forms on other (exo)planets. However, with regard to Mars, it is obvious that this planet is not teeming with life. Even on Earth, most of the living biomass consists of microorganisms and is invisible to the human eye (Whitman et al., 1998). Therefore, the detection of biomarkers is likely more rewarding and central to planetary life-detection missions (Hanslmeier, 2009).
Life on Earth uses carbon-based molecules for structural and metabolic functions. In principle, life elsewhere could be based on a completely different chemistry. Silicon, an abundant element in many minerals, has often been proposed as an alternative for carbon (Pace, 2001; Zhang et al., 2003). However, it cannot form the types of chemical bonds with many of the elements with which carbon does, which limits the flexibility needed for biological metabolism (Pace, 2001; Rizzotti, 2009). Furthermore, carbon chemistry is ubiquitous; a wide variety of complex carbon-based molecules have been identified in the interstellar medium and on comets and meteorites (Ehrenfreund and Charnley, 2000). Organic building blocks of the common factors of life are in general relatively easily formed and joined into polymers by chemical reactions (Ehrenfreund et al., 2006). Organic matter delivered by meteoritic impacts to planetary bodies also comprises a source of diverse amino acids and nucleobases (Sephton, 2002; Martins et al., 2008). These factors may have contributed to the rapid establishment of life on Earth soon after cooling of its crust, 3.8 billion years ago (Derenne et al., 2008; Westall, 2009), and warrant the extraterrestrial search for organic biomarkers.
Several classes of organic biomarkers have been proposed based on a number of biological features that are common to all known life-forms on Earth, such as a compartmentalized shape, energy-producing and storage mechanisms, and the use of biomolecules to store hereditary information (McKay, 1997; Parnell et al., 2007; Summons et al., 2008). These biomarkers comprise relatively simple monomers that constitute the building blocks of cells, such as L-amino acids, D-sugars, pigments, and lipids (e.g., isoprenoids, hopanoids), and complex biopolymers such as nucleic acids.
The implicit assumption in proposing particular biomarkers is an expected commonality of chemistry in life's processes (Pace, 2001). However, although the core classes of molecules in extraterrestrial life-forms might be similar to those of life on Earth, their overall structural makeup could be different in some aspects. Therefore, planetary missions aim to address a wide range of biomarkers in order to provide the strongest evidence for extraterrestrial life (Parnell et al., 2007). The search should be broad enough to include detection of biomarkers that are not necessarily completely identical to those used in Earth's biology. In this review, we will focus on three different types of biomarkers that, taken together, cover these aspects: amino acids, membrane lipids, and nucleic acids.
Amino acids and lipids are building blocks of life, which are considered high-priority molecular targets in the search for life on Mars because of their great chemical stability and potential for persisting for billions of years in the geological record (Brocks et al., 1999; Aubrey et al., 2006; Kminek and Bada, 2006). Amino acids consist of an amine group, a carboxylic acid, and a structural group, which is different in each amino acid and partially determines its characteristics. More than 500 different amino acids have been detected (Wagner and Musso, 1983). Of these, the α-amino acids play an important role in life on Earth. These amino acids have an amine and carboxylic acid group attached to the first carbon (α) atom and have an organic substituent as the functional side chain (Fig. 1). The α-amino acids include the 23 proteinogenic amino acids, which are linked together by the protein synthesis machinery into peptides resulting in the formation of proteins in all domains of life. Amino acids are relatively easily formed abiotically and have been detected in extraterrestrial meteorites (Sephton, 2002; Martins et al., 2007), which can be used as an argument for their potential universal use in life. Amino acids are rapidly degraded when exposed to ionizing radiation and other oxidizing conditions at the surface (ten Kate et al., 2006; Dartnell, 2011; Pavlov et al., 2012), but when buried at a depth of 2 m, and in the absence of enzymatic degradation, they can persist up to 3.5 billion years (Aubrey et al., 2006; Kminek and Bada, 2006).
FIG. 1.
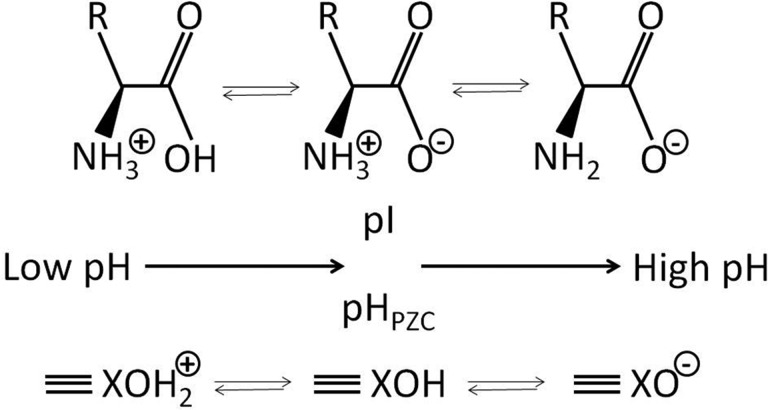
General molecular structure of L-α-amino acids, revealing their zwitterionic characteristics and the dependence of the charge of their carboxylic acid and α-amino groups on pH. At a pH equal to the isoelectric point (pI), the net charge is zero. R is the side chain specific to each amino acid. The bottom part shows a hydroxide mineral, which reveals a similar impact of pH on charge, with pHpzc indicating the pH corresponding to the point of zero charge (pzc).
The abiotic formation of amino acids appears to undermine their usefulness as unambiguous biomarkers. However, most amino acids display isomerism, appearing as levorotatory (L-) and dextrorotatory (D-) enantiomers, which are each other's non-superimposable mirror image. Life on Earth primarily uses amino acids in the L-configuration (Fig. 1). Amino acid mixtures become racemic (i.e., the two different enantiomers are present in equal amounts) over time due to natural processes. Deviations from racemic mixture hints at a biological origin, which provides additional motivation to target amino acids as indicators for life (Meierhenrich, 2008). However, it is becoming increasingly clear that abiotic processes can also introduce enantiomer excesses, so care must be taken when using these excesses as an exclusive indication of biotic origin (Jorissen and Cerf, 2002; Glavin et al., 2013). Carbon isotope composition can also reveal the biological or abiotic origin of individual amino acids (Ehrenfreund et al., 2001).
Lipids are the major component of cell membranes in life-forms on Earth and play an essential role in membrane permeability, as protein anchor, and in energy conservation (Madigan et al., 2012). Eight classes of lipids are distinguished. An example of phospholipids is shown in Fig. 2. These lipids contain a polar head that interacts with water and hydrophobic tails. Lipids can self-assemble to form double-layered membranes with heads facing an aqueous phase and tails facing each other. The ability for self-assembly into double-layered membranes has also been observed for abiotic molecules identified in meteorites; however, these molecules were much shorter than those observed in organisms (Deamer, 1985; Naraoka et al., 1999).
FIG. 2.
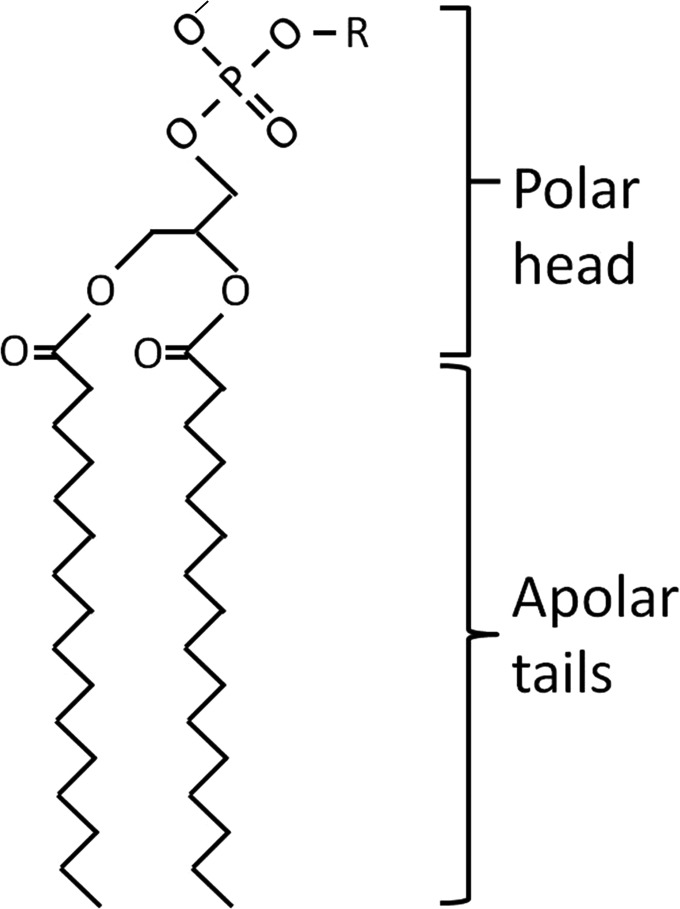
Example of a lipid: structure of a membrane phospholipid, containing a diglyceride, a phosphate group, and an organic molecule R. The diglyceride contains two saturated C16 fatty acid chains, which form a hydrophobic tail. The fatty acids are covalently bound to glycerol through ester linkages. The glycerol backbone and phosphate group provide a hydrophilic, charged polar head.
Membranes in living organisms are generally characterized by a specific distribution profile of lipids, with a strong dominance of lipids with an even number of 14–20 carbons (Georgiou and Deamer, 2014). Many lipids, such as isoprenoids, have no known abiotic source and are derived from unique structural building blocks (e.g., isoprene units) specific to life (Summons et al., 2008). Biological lipids and their derivatives can survive for billions of years in the geological record, such as in the 2.7 billion-year-old shales from the Pilbara Craton, Australia (Brocks et al., 1999). Altogether, this makes lipids an obvious target for life detection (Summons et al., 2008).
Compared to amino acids and lipids, the detection of biopolymers, such as molecules storing hereditary information, would be far more compelling evidence for life due to their high complexity. On Earth, life-forms store their hereditary information in nucleic acids—in DNA or RNA (Madigan et al., 2012). These nucleic acids are composed of nucleotide monomers containing a sugar, a phosphate, and a nucleobase (Fig. 3). The most common nucleobases found in nucleic acids of organisms on Earth are adenine, thymine, cytosine, uracil, and guanine. DNA has deoxyribose as the sugar group, and RNA has ribose. The presence of DNA- and RNA-like molecules in extraterrestrial life is plausible since building blocks of nucleic acids also have been delivered from space to Earth. Nucleobases encountered in carbonaceous chondritic meteorites include guanine, hypoxanthine, xanthine, adenine, and uracil (Martins et al., 2008; Callahan et al., 2011). Riboses have been identified in the gaseous phases of interstellar medium (Cocinero et al., 2012).
FIG. 3.
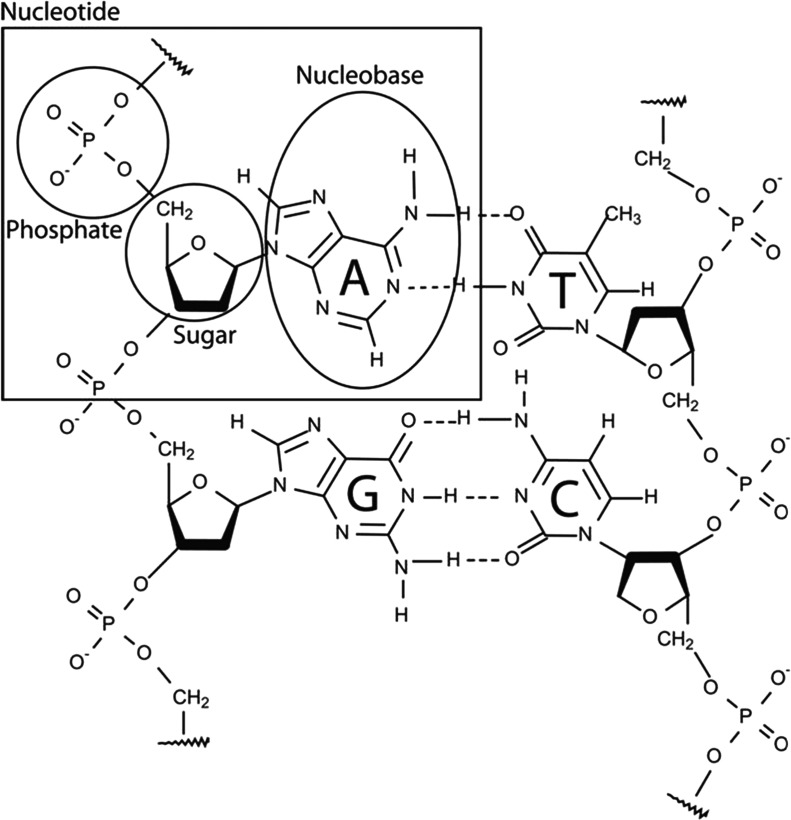
The double-stranded DNA structure and its four nucleotides. DNA is composed of nucleotide monomers containing a phosphate, a sugar (deoxyribose), and a nucleobase. In DNA, the nucleobase adenine (A) pairs with thymine (T), and guanine (G) pairs with cytosine (C). In RNA, thymine (T) is replaced by uracil (U), and the sugar is ribose.
A drawback to nucleic acids as biomarkers is their relative instability in comparison to amino acids and lipids; the theoretical life span of nucleic acids is 50,000–100,000 years (Lindahl, 1997). Still, there are reports of ancient DNA and even living microorganisms (thus containing intact DNA) being recovered from samples that are millions of years old (Vreeland et al., 2000; Panieri et al., 2010). Nucleic acids rapidly degrade under the strong UV radiation and oxidizing conditions of Mars' surface (Martins, 2011). However, in the subsurface they are more protected from these destructive forces, and martian low temperatures and dryness may help preserve remnants of life even better than on Earth (Sephton, 2010).
3. Mineralogy of Mars in Relation to Conditions for Life
Mars is the major destination of current astrobiological missions. Studies of martian meteorites (e.g., Bouvier et al., 2005) and orbiter, lander, and rover missions have provided detailed insight on its mineralogy and therewith its evolution and habitability (e.g., Hanslmeier, 2009; Arvidson et al., 2014; Grotzinger et al., 2014). While current conditions on the surface of Mars are hostile to life and the persistence of biomarkers, conditions in the past were more compatible with life. Three major climatic stages have been proposed for the geological history of Mars (Bibring et al., 2005). During its first 0.7–0.9 billion years, Mars was likely water-rich and possessed a magnetic field and thicker atmosphere (Walter and Des Marais, 1993; Kasting, 1997). Physicochemical conditions of the surface of Mars were mainly controlled by a global hydrological cycle on its surface, and in its subsurface, by volcanic activity (Walter and Des Marais, 1993), and by meteoritic impacts delivering organics (Flynn, 1996; Grady, 2008). These conditions were rather similar to those on early Earth where life evolved in this period, soon after cooling of the Earth's crust, 3.8 billion years ago (Poulet et al., 2005; Bénilan and Cottin, 2007). The presence of evaporite minerals and hydrated silicate minerals is indicative of past aqueous activity (Meslin et al., 2013).
Martian mineralogy is diverse and spatially variable, with iron oxides (magnetite and hematite), iron oxyhydroxides (goethite and ferrihydrite), iron sulfides, silicates (feldspar, olivine, pyroxene, and plagioclase), evaporites (sulfates, e.g., jarosite and gypsum), carbonates, clay minerals (phyllosilicates; e.g., montmorillonite and kaolinite), and quartz (e.g., Squyres et al., 2004a, 2004b; Poulet et al., 2005; Grady, 2008; Mustard et al., 2008; Bish et al., 2013; Arvidson et al., 2014; Grotzinger et al., 2014; Vaniman et al., 2014). Mineral assemblages such as jarosite suggest that acidic, oxidizing, and saline conditions once prevailed at Gusev Crater and Meridiani Planum (Hurowitz and McLennan, 2007). Smectite signatures associated with fine-grained, layered rocks containing spherules indicate aqueous environments of slightly acidic to circumneutral pH in the Endeavour Crater, which would have been more favorable for prebiotic chemistry and microorganisms than those recorded by younger sulfate-rich rocks at Meridiani Planum (Arvidson et al., 2014). The mineralogy at Yellowknife Bay, Gale Crater, indicates a habitable fluviolacustrine environment characterized by neutral pH and low salinity and variable redox states of both sulfur and iron species (Grotzinger et al., 2014).
A climate shift occurred on Mars about 3.6–3.8 billion years ago, which led to a drastic decline in global temperature and water availability (Fairén et al., 2010). During this second stage, a snowball Mars arose, with greatly diminished surface runoff and large areas becoming ice-covered, as has also been hypothesized to have occurred on Earth at least once (Fairén et al., 2010). The third and current stage of Mars is characterized by extremely arid and cold conditions (average surface temperature is about −60°C, varying from −130°C at the poles in winter to +30°C during daytime at the equator in summer), with a thin atmosphere (∼6 mbar) composed of ∼95% CO2. Surface exposure to galactic cosmic radiation and solar energetic particles is high (Pavlov et al., 2002; Dartnell et al., 2007; Pavlov et al., 2012; Hassler et al., 2014), and it has been speculated that radiation generates strong oxidants on the martian soil surface (Yen et al., 2000). Indeed, perchlorates, salts with strong oxidizing properties, especially when heated, appear to be globally distributed on Mars (Hecht et al., 2009; Glavin et al., 2013; Leshin et al., 2013).
Analogues that emulate several of the physicochemical conditions during these three geological stages of Mars can be found on Earth, such as phyllosilicate-rich field locations, acidic aqueous environments, permafrost, and hyperarid deserts (Fairén et al., 2010). Investigation of these Mars analogues has revealed their habitability. However, the combination of all surface conditions on Mars is not found anywhere on current Earth. On present-day Mars, the continuously high radiation is expected to destroy life and its biomarkers on, and close to, the surface (Pavlov et al., 2002; Dartnell et al., 2007; Pavlov et al., 2012; Hassler et al., 2014). It has been suggested that after surface conditions on Mars became hostile for life, life may have found a refuge in the martian subsurface (Boston et al., 1992). Organics may also have been preserved buried underneath the oxidizing surface of Mars in, for example, evaporites that filter radiation effectively (Kanavarioti and Mancinelli, 1990; Vreeland et al., 2000). Even on Earth, the majority of microorganisms are found in the subsurface, and the microbial biomass in the subsurface has been estimated to roughly equal or even outweigh the plant biomass on the surface of Earth (Whitman et al., 1998). Evidence for long-term (>1 million years) sustained life in Earth's subsurface that does not depend on solar energy and products of photosynthesis is accumulating (Lin et al., 2006). Radiolysis and hydrogen-generating reactions between iron-containing minerals and water may fuel this subsurface life (Pedersen, 2000).
The occurrence of methane on Mars was for a long time debated (Webster et al., 2013), but the Sample Analysis at Mars (SAM) instrument on the Curiosity rover recently detected methane (Webster et al., 2015). Assuming a biotic origin for previously reported methane concentrations on Mars (Formisano et al., 2004), Tung et al. (2005) estimated that Earth-like methanogens may be present at just around one cell per milliliter, if distributed uniformly over a 10 m thick layer at a temperature of about 0°C. This temperature occurs between 150 and 8 km depth on Mars. This low abundance and the remote location provide challenges for detecting life on Mars. As already briefly alluded to above, when we described reactions between minerals and water fueling subsurface life, the relationships between mineralogy and microorganisms and their associated biomarkers need to be taken into account in order for the odds of detecting extinct or extant life on Mars to be as high as possible.
4. Microbe-Mineral Interactions
The numbers of microorganisms associated with solid surfaces in subsurface environments on Earth are typically more than one order of magnitude higher than those of the free-living microorganisms in pore water, per volume unit (Albrechtsen and Winding, 1992; Holm et al., 1992). The identities and metabolic potentials of attached microorganisms differ from those in the nearby liquid phase (Albrechtsen et al., 1996; Crump et al., 1999; Röling et al., 2001).
Attachment to minerals can help microorganisms protect themselves against extreme environmental conditions and physicochemical stresses (Decho, 2000) or against predators (Wey et al., 2008). Nutrient availability in minerals is also an important reason for attachment (Roberts, 2004; Carson et al., 2009). Electrostatic interactions between charged minerals and cell surface components play an important role in attachment (Roberts, 2004; Walker et al., 2004). Microorganisms may also become associated with minerals due to entrapment as the result of mineral precipitation (Fortin et al., 1997; Southam and Saunders, 2005).
The distribution and abundances of mineral-associated microorganisms are affected by the type of mineral (Boyd et al., 2007; Mauck and Roberts, 2007) and their particle sizes (Albrechtsen, 1994; Sessitsch et al., 2001). These factors, and associated differences in pore sizes (Ruamps et al., 2011), pore connectivity (Carson et al., 2010), and other physicochemical properties (Killham et al., 1993), may contribute to heterogeneous and complex microbial communities over short distances, on a centimeter scale (Franklin et al., 2002), and thus are important to consider in relation to astrobiological missions and sampling extraterrestrial soils for biomarker detection.
Elemental mineral composition plays a major role in attachment and the abundances of microorganisms (Banfield et al., 1999; Roberts, 2004; Mauck and Roberts, 2007). Essential elements for growth (such as P and K) are often scarce in groundwater (Banfield et al., 1999). Silicate weathering by microorganisms is determined by nutrient requirements. Microorganisms can display a preference for attachment to, and dissolution of, minerals containing growth-limiting nutrients (Rogers et al., 1998; Bennett et al., 2001). Weathering of minerals like apatite provides phosphate required for microbial activity and growth (Bennett et al., 2001). Dissolution of sulfide minerals and iron-magnesium silicates supplies enzyme cofactors like Cr, Cu, Fe, Mg, Mo, Ni, and Zn (Banfield et al., 1999; Rogers and Bennett, 2004).
Microorganisms can also use redox-reactive elements in minerals for their energy metabolism (Edwards et al., 2003; Lovley et al., 2004). Generally, large quantities of these elements are required for a chemotrophic lifestyle. In particular, Fe- and S-bearing minerals are utilized as electron donors, such as Fe(II)- or sulfide-containing minerals (Edwards et al., 2003), or as electron acceptors, such as sulfur and Fe(III) (Lovley et al., 2004). A wide range of iron (oxyhydr)oxides and clays can be utilized for Fe(III) reduction or Fe(II) oxidation (Shelobolina et al., 2004; Dong et al., 2009). Anaerobic microbial iron oxidation and reduction has been hypothesized to be one of the most primordial microbial metabolisms on Earth and is proposed as a possible metabolism for other iron-mineral-rich planets like Mars (Weber et al., 2006). Geochemical, phylogenetic, and physiological data suggest that anaerobic Fe(III) reduction by thermophilic microorganisms may have been the first electron-accepting process on Earth (Vargas et al., 1998). The postulated variable redox states of both sulfur and iron species, in association with neutral pH and low salinity, may have once supported a chemolithoautotrophy-fueled biosphere at Yellowknife Bay in the Gale Crater of Mars (Grotzinger et al., 2014).
On the other hand, minerals can also have antimicrobial properties. Natural antibacterial clays containing nanoscale illite-smectite and reduced iron phases buffer conditions that promote iron solubility, leading to subsequent intracellular iron accumulation, oxidation, and precipitation, and the formation of iron-associated deadly radicals (Williams et al., 2011). Aluminum, such as that found in the phyllosilicate kaolinite (Al2Si2O5(OH)4), is potentially toxic to some bacteria (Roberts, 2004).
Thus, mineralogy affects the abundances of microorganisms and thereby the abundances of their biomarkers. It also impacts the type and diversity of biomarkers, as mineralogy also influences the structure and diversity of bacterial communities associated with geological substrates incubated in situ. Most of these observations are based on studies that involved only a limited number (∼3–6) of minerals or other growth supports (Reardon et al., 2004; Boyd et al., 2007; Mauck and Roberts 2007; Carson et al., 2009; Direito et al., 2012; Mitchell et al., 2013) exposed to a variety of incubation procedures, ranging from direct incubation of minerals in soils (Carson et al., 2009) to the use of different solid supports in aquifers (Mauck and Roberts, 2007). These studies included anaerobic subsurface environments (Mauck and Roberts, 2007; Direito et al., 2012), which are of relevance with respect to detecting life on Mars. In an iron-reducing aquifer, a positive correlation between Fe content of minerals and attached biomass was observed (Mauck and Roberts, 2007). Recently, the effect of bedrock mineral composition on microbial abundances and diversity in a subglacial environment was determined. This study demonstrated that Fe- and S-containing minerals, especially pyrite, can have a large influence on bacterial community structure and biomass density (Mitchell et al., 2013). Reported biomass per gram mineral was about tenfold higher on Fe- and S-containing minerals compared to quartz. Fe(III) is present in a wide range of martian minerals. When Fe(III)-containing minerals are the sole source of electron acceptor in anaerobic settings, different forms of these minerals may support different communities, as has been shown experimentally (Lin et al., 2007).
Mechanistic understanding on the distribution and abundances of microbial species in relation to mineralogy is rudimentary. Next to physiological drivers, microbial attachment to mineral surfaces is frequently governed by differences in the surface charge of cell membranes and minerals. Electrostatic interactions were found to dominate microbial colonization on positively charged oxide surfaces irrespective of mineral composition, while on negatively charged silicate minerals, the solid phase composition determined the extent of microbial colonization and diversity (Roberts, 2004).
5. Adsorption of Organics to Minerals: The Origin of Life and Preservation
Mineral type not only affects the abundances and diversity of microorganisms but also the abundances and diversity of organic biomarkers attached to minerals. Crystalline surfaces of minerals are postulated to have contributed to the origin of life on Earth by participating in the synthesis, selection, concentration, and organization of organic molecules in the transition from geochemistry to biochemistry (Bernal, 1951; Hazen and Sverjensky, 2010). Transition metal sulfides and oxides can promote a variety of organic reactions, such as nitrogen reduction, hydroformylation, and amination. Specific amino acids, sugars, and other molecules are concentrated on the surfaces of oxides, silicates, and carbonates, which can also enhance their stability. Clay minerals and hydroxides have the capacity to facilitate self-organization and condensation polymerization. Clays tend to adsorb organic molecules strongly (e.g., Saeki and Sakai, 2009), and this contributes to their ability to catalyze a diversity of organic reactions, such as RNA formation and elongation on montmorillonite clay (Ferris et al., 1996). Illite and hydroxylapatite can act as scaffolds in the oligomerization of amino acids (Ferris et al., 1996; Lambert, 2008). Minerals may even have contributed to homochirality, because chiral surfaces of a range of minerals, including quartz, alkali feldspars, and clinopyroxene, can separate L- and R-configured molecules, such as L- and D-amino acids (Hazen and Sverjensky, 2010).
Minerals implicated in the origin of life on Earth are also of interest to astrobiological missions because biomarkers may be protected by adsorption to them. For instance, DNA binding to clay minerals protects against enzymatic digestion (Aardema et al., 1983; Scappini et al., 2004), UV radiation (Scappini et al., 2004), and X-rays (Ciaravella et al., 2004).
The strength of the adsorption of biomarkers to minerals depends on properties of both. Minerals that adsorb one type of biomarker strongly may adsorb other biomarkers weakly. Hematite adsorbs a range of amino acids (Bentaleb et al., 1994), but DNA hardly at all (Direito et al., 2012). Minerals adsorb amino acids with charged side groups much more than those with uncharged side groups (Zaia, 2004). Also the structure of biomolecules affects their adsorption; linear DNA binds more strongly than supercoiled DNA, likely by having a continuous line of contact with the surface (Melzak et al., 1996).
The mechanisms of adsorption of biomarkers to minerals are diverse, again relating to properties of both minerals and biomarkers and to local physicochemical conditions, such as solution chemistry and pH. Adsorption of organic molecules on oxide surfaces is affected by complex surface speciation in relation to mineral structure, with inner and outer sphere species varying in relative importance over a range of pH, ionic strength, and surface coverage (Hazen and Sverjensky, 2010). The substitution of the inorganic interlayer cations that occur naturally in smectite clays by small organic cations alters the adsorption characteristics of clays for amino acids (Fraser et al., 2011). The differences in side chains between amino acids have an effect on their charge and polarity, although most amino acids are readily soluble in water. Electrostatic interactions generally play a dominant role in the adsorption of amino acids to minerals (Churchill et al., 2004). The net charge of mineral surfaces is a function of pH; the net charge is zero when the pH of the solution equals the so-called “point of zero charge,” pHpzc. When the pH of the solution is below this pHpzc, the net surface charge of the mineral is positive, and it is negative when the pH of the solution is above pHpzc (Fig. 1). The charge of an amino acid is similarly affected by solution pH. Amino acids are zwitterions; they contain both acid and base groups (Fig. 1). The charge of an amino acid is zero when solution pH equals the “isoelectric point,” pI, which is determined by the side group of an amino acid (Fig. 1). Both pI and pHpzc differ strongly among amino acids and among minerals, respectively, ranging from acidic to alkaline. Adsorption between minerals and amino acids is stronger when their respective pHpzc and pI values differ more from each other (Churchill et al., 2004). However, other factors besides net electrostatic interactions play a significant role in selective adsorption and its strength (Bentaleb et al., 1994; Churchill et al., 2004), such as the geometrical distribution of positive and negative charge centers associated with specific mineral surfaces and individual amino acids (Churchill et al., 2004).
Clays are of great interest to astrobiology because of their surface electrostatic charges and their fine-grained particles with large reactive surface areas, which enable them to absorb strongly polar biomarkers such as DNA and RNA (Novinscak and Filion, 2011; Direito et al., 2012). Saeki and Kunito (2010) suggested that DNA adsorption is more likely to depend on mineral surface charges than on mineral surface area. Adsorption is proposed to occur at the edges of sheets containing aluminum(III) groups that coordinate with water molecules (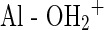 ), promoting acidity and causing negatively charged substances, such as the DNA phosphate groups, to bind (Ferris, 2005; Saeki and Sakai, 2009). DNA molecules have low affinity for silica (SiOx); however, DNA adsorption is enhanced by the presence of cations (Saeki and Kunito, 2010). Monovalent cations, like Na+, reduce the electrostatic barrier between DNA and silica, while divalent cations, like Ca2+ and Mg2+, neutralize charges by binding to the DNA phosphate backbone and binding to Si-OH groups of the silica, reducing electrostatic repulsion and forming bridges between mineral and DNA (Nguyen and Elimelech, 2007). Preferential adsorption of small DNA fragments to soils and clays may be due to size exclusion or mechanisms related to the kinetics of diffusion (Ogram et al., 1994).
), promoting acidity and causing negatively charged substances, such as the DNA phosphate groups, to bind (Ferris, 2005; Saeki and Sakai, 2009). DNA molecules have low affinity for silica (SiOx); however, DNA adsorption is enhanced by the presence of cations (Saeki and Kunito, 2010). Monovalent cations, like Na+, reduce the electrostatic barrier between DNA and silica, while divalent cations, like Ca2+ and Mg2+, neutralize charges by binding to the DNA phosphate backbone and binding to Si-OH groups of the silica, reducing electrostatic repulsion and forming bridges between mineral and DNA (Nguyen and Elimelech, 2007). Preferential adsorption of small DNA fragments to soils and clays may be due to size exclusion or mechanisms related to the kinetics of diffusion (Ogram et al., 1994).
The distribution of a biomarker on a mineral phase and in a liquid phase is related to its concentration. Relatively more biomarker will adsorb to minerals when the overall biomarker concentration decreases. These adsorption characteristics can be described by Freundlich or Langmuir isotherms (Benaziz et al., 2001), but they have been little studied in the context of life detection. Up to now, adsorption has been studied at most with a combination of a few molecules and a few minerals. Recently, a systematic study on the adsorption of DNA to a wide range of pure minerals, of significance to Mars, was conducted (Direito et al., 2012). Not unexpectedly, clays were found to adsorb DNA strongly; more than 99% of added DNA was adsorbed and could not be recovered with conventional methods for DNA isolation. However, other minerals, such as jarosite and the silica-bearing minerals olivine, diopside, labradorite, and apatite also strongly adsorbed DNA. In contrast, quartz and iron oxides show relatively limited adsorption (<50%). The low adsorption by iron (oxyhydr)oxides is a promising result considering that these minerals are of interest in the search for life on Mars. Besides DNA, RNA can also adsorb strongly to clays (Novinscak and Filion, 2011).
Studies on the interactions of minerals with lipids in relation to the origin of life are relatively limited, in spite of their high utility as unique biomarkers with high conservation potential. Possibly this relates to their largely hydrophobic, uncharged molecular characteristics, on the basis of which one would expect low adsorption to minerals. Adsorption studies with lipids appear to have mainly focused on the interaction between anionic fatty acids and cationic mineral surface sites. Surprisingly, an increasing affinity for carbonate surfaces was found with increasing alkyl chain length for these fatty acids (Zullig and Morse, 1988; Thomas et al., 1993). Adsorption is largely irreversible in aqueous systems (Thomas et al., 1993). Adsorption isotherms that describe the amount of surface-bound lipid as a function of the lipid present in solution are S-shaped. Roughly three regions can be distinguished: in the first and third regions, a relatively slow increase in bound lipid is observed with increasing lipid in solution, compared to the second region. In the first region, chemisorption between the charged groups of lipids and minerals is important, but in the second phase hydrophobic bonding between alkyl chains dominates adsorption behavior, while in the third phase the mineral is covered by a monolayer that prevents further adsorption of lipids (Zullig and Morse, 1988).
6. Impact of Mineralogy on the Extraction of Biomarkers
On the one hand, certain minerals favor the attachment of microorganisms and the adsorption and preservation of organic biomarkers, while on the other hand, the strong adsorption potential and other properties of minerals may interfere with the efficient extraction and detection of biomarkers. The degree of interference is influenced by the method of biomarker extraction, for which a large variety of approaches exist.
Conventional methods to characterize biomarkers in samples on Earth generally employ solvents to extract amino acids, nucleic acids, and lipids. These liquid extracts are subsequently analyzed. Use of equipment on Mars that requires solvents for the detection of biomarkers is avoided because it poses a risk of contamination. Viking 1 and 2, the first robotic landers on Mars, were equipped with instrumentation employing thermovolatilization, or pyrolysis, to search for life: soil samples were directly heated, and the gaseous phase was analyzed by gas chromatography (GC) followed by mass spectrometry (MS) (Klein, 1978). Since August 2012, NASA's Curiosity rover has been investigating the habitability of the martian surface, employing the SAM instrument, which conducts thermal volatilization GC-MS and can chemically derivatize organics to make them more volatile. The 2018 ExoMars mission will include the Mars Organic Molecule Analyzer (MOMA), which is able to detect and identify organic molecules at low concentrations (ppb to ppt) with an ion-trap mass spectrometer. Two major ion sources are employed. The first is classical GC in which solid soil samples are pyrolyzed and GC separates the volatile fraction, including separation based on chirality. The second is laser desorption/ionization (LDI) where larger and likely less volatile molecules are ionized by short laser pulses. LDI has an additional advantage in that it can also provide mineral signatures (Ehrenfreund et al., 2011).
We will deal first, and especially, with solvent-based extraction, as most research on the relation between minerals and biomarker extraction efficiency has been obtained by using this approach. Furthermore, solvent-based extraction is essential when one wants to target more specific and complex biomarkers as indicators of past or present life on Mars. The Life Marker Chip (LMC) instrument, which in the end was not selected for the payload of the ExoMars 2018 mission, has this potential. It relies on antibody microarray technology, in which immobilized antibodies are used as receptors to capture specific biomarkers (Parnell et al., 2007).
The three types of biomarkers central to this review are extractable by solvents, but while aqueous solutions are generally used for amino and nucleic acids, organic solvents are typically employed for lipids. Subsequently GC-MS allows detection and quantification of chemically derivatized amino acids and their chirality, and lipids. GC-MS is not possible for complex nucleic acids, but detection of even one single nucleic acid molecule can be achieved by in vitro amplification (Mullis et al., 1986). Novel nonselective amplification methods such as multiple displacement amplification offer advantages in astrobiology over the conventional polymerase chain reaction, which requires a priori sequence information (Direito et al., 2014). These techniques could even be adapted to detect hereditable information that deviates from the nucleic acids utilized in life on Earth (Direito et al., 2014).
Solvent extraction methods currently considered in relation to space missions (subcritical water extraction, solvent extraction using ultrasonication) are predicted to be unable to fully recover biomarkers because recovery efficiencies among biomarkers differ and because destruction of biomarkers may occur (Skelley et al., 2005; Court et al., 2010). Specific combinations of biomarkers and minerals may present methodological problems, as observed for the SAM (Stalport et al., 2012) and LMC instruments (Fornaro et al., 2013). For instance, the extraction procedure for the LMC instrument is optimized for aliphatic biomarkers, employing sonication in a solvent system of 20:80 methanol:water, with 1.5 mg/mL Tween, a polysorbate detergent. However, this procedure results in low extraction of nucleobases adsorbed to magnesium oxide (Fornaro et al., 2013). The SubCritical Water Extractor (SCWE) is one of the components of the Urey instrument, now de-scoped from the ExoMars mission. It has been applied to extract amino acids from mineral matrices but might be employed to extract other organic compounds (Aubrey et al., 2008). The use of water as a solvent to extract DNA would be ideal for planetary missions instead of a more hazardous organic solvent. However, a temperature of 100°C damages the DNA and negatively influences its detection (Direito et al., 2012).
Solvent extraction methods have been intensively investigated in the field of microbial ecology, with respect to representative extraction of biomarkers indicative of microbial community composition. The choice of extraction method has a strong influence on the final outcome, irrespective of whether one addresses polar lipid fatty acids (Nielsen and Petersen, 2000) or DNA (Frostegård et al., 1999) to profile communities. DNA extraction procedures include a cell lysing step that can be performed in several ways: by bead beating, heating or detergent treatment, grinding–freezing–thawing, or sonicating a sample directly or indirectly after separating cells from their environmental matrix (Robe et al., 2003). Indirect cell extraction and the use of either differential centrifugation approaches or chemicals to dissolve the mineral matrix generally lead to severe loss of cells or damage their biomarkers (Robe et al., 2003; Direito et al., 2012). On the other hand, during direct DNA extraction, DNA is released from cells and can adhere to minerals. This adsorption also has a detrimental impact on microbial community studies. Samples from the Mars Desert Research Station (MDRS) in the Utah desert revealed a wide variety of putative extremophiles and all three domains of life (Archaea, Bacteria, and Eukarya), but these were not detected in all samples (Direito et al., 2011). Large differences over short distances, even on the centimeter scale, were uncovered in the occurrence and diversity of microorganisms. Spiking with defined quantities of a gene fragment showed that this heterogeneity may relate to low DNA recovery from some samples, due to strong adsorption or degradation (Direito et al., 2011).
Understanding the strength of adsorption and mechanisms contributing to adsorption enables a more rational design of methods for the efficient extraction of biomarkers and their subsequent detection. We have been able to improve DNA recoveries by up to a hundredfold by modifying the chemical composition of extraction solutions (Direito et al., 2012). A high concentration of phosphate was applied to compete with the phosphate in DNA (Fig. 3) for adsorption to minerals. A high temperature (85°C) was also applied. The phosphate was dissolved in a solution of 15% ethanol. Ethanol helps to disrupt cell membranes, while it makes DNA change conformation at concentrations of 15% or higher, promoting DNA desorption from mineral surfaces (Fang et al., 1999). A relatively low concentration of 15% avoids precipitation of DNA. The introduction of this step in extraction resulted in a much higher DNA yield for several mineral samples, both clay and non-clays, while maintaining reproducible microbial community profiles (Direito et al., 2012). The latter was in line with expectation, as nucleobases are not involved in the adsorption to clays (Pietramellara et al., 2001).
Modification of extraction solutions for other biomarkers may also help enhance their recovery. The use of a mixed organic-aqueous solvent to provide both ionic replacements, in the form of orthophosphate, and solubility for lipids, helped increase the yield of lipids (Zullig and Morse, 1988). The amount of lipid biomarkers was not strongly affected when vegetative cells were extracted with a variety of methods; however, pressurized hot solvent extraction yielded significantly higher amounts of lipids from spores than other methods (Macnaughton et al., 1997).
DNA is very sensitive to chemical and physical conditions; however, other biomarkers are much less sensitive. Hydrofluoric acid treatment can dissolve the mineral matrix while leaving cell morphology intact. For example, proteins were shown to be recovered from inorganic material by dissolving mineral matrices when using 10% hydrofluoric acid (Schulze et al., 2005). The large differences in mechanisms of adsorption and in properties of minerals and biomarkers suggest that it will be difficult to design a single solvent-based extraction procedure for a wide range of biomarkers.
Thermovolatilization of biomarkers in soil samples avoids the use of liquid solvents. Research on the impact of adsorption to minerals on thermovolatilization-based detection of biomarkers appears to be absent from the scientific literature. The presence of oxidizing minerals such as sulfates and perchlorate may compromise the detection of organic biomarkers (Navarro-González et al., 2010; Lewis et al., 2015). The failure of the Viking gas chromatograph–mass spectrometer to detect organics at the part-per-billion level in the top few centimeters of the martian soil (Biemann et al., 1976) is now assumed to have been due to chemical oxidation of organics by perchlorate at high temperatures in the oven of the gas chromatograph instrument (Navarro-González et al., 2010). Perchlorates likely also influenced analyses by SAM (Leshin et al., 2013; Ming et al., 2014).
Laser desorption/ionization avoids the problem of oxidation of organic compounds by minerals at high temperature (Getty et al., 2012). However, a systematic evaluation of LDI efficiency in extracting biomarkers appears to be lacking. A few studies have addressed how minerals may act as a geological matrix that aids desorption and ionization of amino acids, fatty acids, and small proteins by a laser, comparable to matrix-assisted laser desorption/ionization (MALDI) (Yan et al., 2007; Kotler et al., 2008; Richardson et al., 2008). The minerals impact detection; desorption and ionization of the biomarker is dependent on the energy of the laser absorbed by the mineral matrix and converted to heat. Evaporates like halite and jarosite yielded a signal, but hematite did not (Yan et al., 2007; Kotler et al., 2008; Richardson et al., 2008). Amino acids incorporated within halite yielded a better signal-to-noise ratio than those applied to its surface (Yan et al., 2007). The properties of biomarkers affect detection and fragmentation and may complicate their identification (Yan et al., 2007; Richardson et al., 2008). MALDI itself is not effective when biomarkers are strongly adsorbed to minerals, as dissolution of biomarkers in MALDI solvents is required for detection (Yan et al., 2007). Biomarkers were also not detectable when directly exposed to LDI, without a matrix (Yan et al., 2007; Richardson et al., 2008).
7. Remote Detection of Indicators for Life on Planets and Exoplanets
Mars is one of only a few planetary bodies that are accessible for direct assessment of biomarkers in the near future. However, for the very large majority of planets and exoplanets, we will have to resort to remote detection in which spectrometry will play a key role. Spectrometric biosignatures are spectral characteristics that can be remotely detected and inform on the habitability of a planetary environment or are indicative of life itself. Most attention has so far been given to atmospheric biosignatures (e.g., detection of molecular oxygen) (Kaltenegger et al., 2012), even though surface biosignatures are a more specific indicator of life. In particular, two surface biosignatures relating to photosynthesis are of high interest: the “red edge” and circular polarization indicating homochirality.
Photosynthesis, employing (bacterio)chlorophylls, is the major driving factor of life on Earth since at least 2.5 Ga (Madigan et al., 2012). Bacterial photosynthesis likely developed soon after the emergence of life on Earth (Xiong and Bauer, 2002; Blankenship, 2010). For other (exo)planets, we may also expect that photosynthesis is essential in sustaining life over long periods of time (Seager et al., 2005; Kiang et al., 2007). The selective use of light energy produces spectral biosignatures in land plants: the “green bump” caused by absorbance of blue and red light by chlorophyll and the “red edge” caused by the contrast between absorbance in the red by chlorophyll and its reflectance in the near IR. The red edge is a very strong, specific signal that is employed for remote spectrometric sensing of vegetation cover on Earth (Joint and Groom, 2000). The red edge has not only been observed for plants but also for several photosynthetic bacteria and algae (Kiang et al., 2007). A second important and likely universal characteristic of life is that almost all biological molecules display homochirality. Scattering of light by biological molecules of photosynthetic organisms can result in circular polarization by circular dichroism, that is, the differential absorption of left and right circular polarized light. This circular polarization is also detectable remotely (Sparks et al., 2009a).
Photosynthetic organisms are highly diverse and widespread on present-day Earth (Kiang et al., 2007; Madigan et al., 2012). Two major photosynthetic processes occur: anoxygenic and oxygenic photosynthesis. Anoxygenic photosynthetic bacteria are considered to have been the ancestors of oxygenic photosynthetic organisms (Xiong and Bauer, 2002; Blankenship 2010). Anoxygenic photosynthetic bacteria are phylogenetically and physiologically diverse, using organic compounds, hydrogen, ferrous iron, sulfur, or sulfide as reductant via a variety of bacteriochlorophylls (Konhauser, 2007; Madigan et al., 2012). The type of anoxygenic photosynthetic microorganism that evolved first is still a subject of debate (Xiong and Bauer, 2002; Kiang et al., 2007; Blankenship, 2010). Cyanobacteria are considered to have been the first oxygenic photosynthetic organisms, oxygenating Earth's atmosphere by using light to energize the oxidation of water to oxygen gas. There are a large variety of chlorophylls and bacteriochlorophylls that mediate photosynthesis, each with its unique absorption spectrum and light reflection characteristics. Different biological species produce different (bacterio)chlorophyll-binding proteins and accessory pigments (e.g., carotenoids, phycobilins) that affect light absorption properties (Madigan et al., 2012). Photosynthesis on Earth occurs under a wide range of environmental conditions, such as at extreme low and high temperatures, low and high pH, and in arid and saline settings (Zettler et al., 2002; Satyanarayana et al., 2005; Konhauser, 2007; Pointing et al., 2009).
Research on remote detection of the red edge and, in particular, homochirality, has so far been limited to a small set of organisms and conditions, in particular plants and algae under “normal” environmental conditions (aerobic, circumneutral pH, ambient temperature, etc.) (Kiang et al., 2007; Sparks et al., 2009a, 2009b; Martin et al., 2010). This hardly covers the wide range of conditions under which photosynthesis occurs on Earth. If the organisms that thrive in extreme environments and the organisms that represent the evolution of photosynthesis on Earth also consistently reveal a specific reflectance signal and can be detected on the basis of homochirality, this would provide larger possibilities to indicate possible life on other (exo)planets, that is, planets with general conditions that are at the limits for life on Earth or planets that are in an earlier evolutionary phase.
It will again be important to consider the influence of minerals, soils, and rocks on the spectrometric signals. Firstly, minerals also reveal spectrometric signals, mainly caused by crystal field effects (Hunt, 1977). Minerals absorb and reflect light, and while most minerals investigated so far have shown reflection spectra clearly different from microorganisms, some minerals reveal edges at visible and near-IR wavelengths that might prove difficult to distinguish from biological signatures (Seager et al., 2005). Similarly, the few minerals investigated in relation to remote detection of homochirality did reveal circular dichroism, but at low amplitude and different from organisms (Sparks et al., 2009a). Secondly, microorganisms often grow in biofilms on mineral surfaces, or are encrusted by minerals under some extreme conditions, such as photosynthetic endoliths in arid environments (Wynn-Williams, 2000). Finally, anoxygenic photosynthetic microorganisms using Fe(II) produce solid iron minerals (Ehrenreich and Widdel, 1994). Shielding by minerals may interfere with remote detection of photosynthetic microorganisms.
8. Conclusions and Perspective
Studies of mineral-molecule and microbe-mineral interactions are still in their infancy when compared to the wide range of extreme environments, minerals, and biomarkers on Earth that are of relevance to astrobiology. Yet the importance of mineralogy with respect to detecting extraterrestrial life is evident. The type of mineral has a big impact on the potential abundances and types of biomarkers and microbes associated with these minerals and influences extractability and thus detection. The impact of mineral type on microbial abundances informs in particular on current life, but one may postulate that a soil that once supported a large living biomass may have a higher chance of retaining biomarkers over long periods of time than a mineral soil that supported less biomass, in particular if the soil has a high capacity to adsorb released biomarkers. Other direct and indirect interactions between minerals and microorganisms or their biomarkers are also of importance to biomarker detection: secondary minerals produced by microorganisms may also be good biomarkers (Weber et al., 2006), and compounds released as the result of microbial activity on minerals may help to stabilize biomarkers; for example, sulfurization can protect biomarkers against biodegradation (Summons et al., 2008). Hence, local mineralogy is a factor that should be taken into account when selecting sampling locations for the detection of life and designing biomarker-detection protocols.
More systematic studies on mineral-biomarker interactions and understanding the mechanisms that contribute to adsorption are needed. This will aid in a more rational design of methods for high extraction efficiency of a wide range of biomarkers. We advocate the approach that we recently applied in the case of nucleic acid biomarkers (Direito et al., 2012): spike “clean” minerals (i.e., minerals free of biomarkers) with known quantities of biomarkers and then quantify their recovery by using extraction protocols optimized on the basis of mechanistic insight on the adsorption of biomarkers to minerals.
Despite the significant improvements we made for nucleic acid extraction, extraction from some minerals, clays in particular, remained suboptimal, as “only” 1% of added DNA could be extracted (Direito et al., 2012). Therefore, it is also important to include internal controls in life-detection strategies, that is, spike samples retrieved from extreme environments on Earth or from planetary bodies with known quantities of distinctive markers prior to extraction in order to quantify their recovery (Direito et al., 2011, 2012). This information will be essential to better interpret negative results: Are these due to the absence of biomarkers (that is, biomarkers are present below the detection limit) in the investigated sample or due to strong loss of biomarkers due to adsorption to minerals or destruction during the extraction procedure? This is an especially important issue when investigating low-biomass environments such as are expected on Mars. On the other hand, it is important to avoid false positives due to contamination of equipment with the biomarkers used as spikes.
Life-detection strategies should be robust and recover biomarkers of high quality with great efficiency from samples. For this, it is important also to retrieve biomarkers that are present in cells, spores, and fossils. The astrobiology community pays relatively little attention to these intracellular biomarkers, which are considered in microbial ecology as we indicated in relation to the use of nucleic acids to study microbial communities in extreme environments.
Differences in mineral composition over short distances can lead to large differences in microbial community and biomarker composition, and indicate that future sampling missions should employ high-density sampling at any location of interest to maximize the chance of finding life (Direito et al., 2011). Similarly, mineralogy is of high importance in relation to the remote detection of spectral features of photosynthesis. More knowledge is required on the in situ interaction between minerals and microorganisms and the implication for biomarker detection. The unavailability of extraterrestrial samples, with the exception of a few available meteorites (with possible contamination risks), makes it essential to further study the interaction between minerals and microbes and their biomarkers in extreme environments on Earth.
As Mars is a prime target in the near future, the environments to study should include terrestrial representatives of the three geological stages of Mars and ideally be linked to available data on biota and their abiotic environment, as such information can benefit experimental design and data interpretation. Current extant martian life, if it exists, would face challenges from low temperature, aridity, and high radiation. The McMurdo Dry Valleys and MDRS are analogues to this situation. The McMurdo Dry Valleys, in Antarctica, constitute a cold hyperarid polar desert. Despite their very extreme and stressful conditions, they contain a highly specialized microbial diversity, revealing radiation- and desiccation-tolerant microorganisms, including photosynthetic endoliths (Pointing et al., 2009). MDRS, in Utah, is an arid, oxidizing environment with high temperatures during the day and low temperatures at night. MDRS has a mineralogy comparable to that found on Mars and a patchy distribution of biomarkers and microorganisms (Clarke and Stoker, 2011; Direito et al., 2011; Ehrenfreund et al., 2011; Martins et al., 2011). Parts of Mars may have been highly acidic and saline in the past, resembling conditions currently found at Río Tinto, Spain. This acidic (pH 2–3), iron-rich river system is produced from rock-water-biology interactions. Pyrite effectively provides the energy for the development of the Río Tinto ecosystem, which is surprisingly rich (Fernández-Remolar et al., 2005). Mars' early history resembled that of early Earth: moist and warm. Hydrothermal systems have been suggested as suitable environments for the appearance of life on early Earth (Russell and Hall, 1997) and were likely active in the crust of Mars.
Studying natural environments where life had time to evolve and adapt to multiple stressors will contribute to the optimization of sampling strategies and life-detection methodologies and will also indicate the type of organisms and biomarkers to expect in such environments (e.g., Cockell et al., 2005). We will obtain more insightful information on mineral-microbe-biomarker interactions from these natural environments than from relative short-term incubations in Mars simulation chambers (e.g., Osman et al., 2008), for which survival and growth of microorganisms might be insufficient to establish meaningful relationships between minerals, microbes, and biomarkers. Nevertheless, simulation chambers could be useful to determine protective properties of minerals on the conservation of biomarkers under simulated martian conditions.
Studying the interactions between minerals, biomarkers, and microbes is not only relevant for astrobiology but also for other research fields such as microbial ecology, evolutionary sciences, forensic sciences, and bioarchaeology. These research areas can, and should, influence each other mutually to contribute new methodologies and mechanistic insight on microbe-mineral-biomarker relationships.
Abbreviations Used
- GC
gas chromatography
- LDI
laser desorption/ionization
- LMC
Life Marker Chip
- MALDI
matrix-assisted laser desorption/ionization
- MDRS
Mars Desert Research Station
- MS
mass spectrometry
- SAM
Sample Analysis at Mars
Acknowledgments
This work was supported by grants from the User Support Programme Space Research (grants GO-PL/04 and ALW-GO/13-09) and the Planetary and Exoplanetary Science Programme (PEPSci) (grant 648.001.004) of the Netherlands Organisation for Scientific Research (NWO). P.E. acknowledges support from the NASA Astrobiology Institute.
References
- Aardema B.W., Lorenz M.G., and Krumbein W.E. (1983) Protection of sediment-adsorbed transforming DNA against enzymatic inactivation. Appl Environ Microbiol 46:417–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechtsen H.J. (1994) Distribution of bacteria, estimated by a viable count method, and heterotrophic activity in different size fractions of aquifer sediment. Geomicrobiol J 12:253–264 [Google Scholar]
- Albrechtsen H.J. and Winding A. (1992) Microbial biomass and activity in subsurface sediments from Vejen, Denmark. Microb Ecol 23:303–317 [DOI] [PubMed] [Google Scholar]
- Albrechtsen H.J., Smith P.M., Nielsen P., and Christensen T.H. (1996) Significance of biomass support particles in laboratory studies on microbial degradation of organic chemicals in aquifers. Water Res 30:2977–2984 [Google Scholar]
- Arvidson R.E., Squyres S.W., Bell J.F., Catalano J.G., Clark B.C., Crumpler L.S., de Souza P.A., Fairén A.G., Farrand W.H., Fox V.K., Gellert R., Ghosh A., Golombek M.P., Grotzinger J.P., Guinness E.A., Herkenhoff K.E., Jolliff B.L., Knoll A.H., Li R., McLennan S.M., Ming D.W., Mittlefehldt D.W., Moore J.M., Morris R.V., Murchie S.L., Parker T.J., Paulsen G., Rice J.W., Ruff S.W., Smith M.D., and Wolff M.J. (2014) Ancient aqueous environments at Endeavour Crater, Mars. Science 343, doi: 10.1126/science.1248097 [DOI] [PubMed] [Google Scholar]
- Aubrey A., Cleaves H.J., Chalmers J.H., Skelley A.M., Mathies R.A., Grunthaner F.J., Ehrenfreund P., and Bada J.L. (2006) Sulfate minerals and organic compounds on Mars. Geology 34:357–360 [Google Scholar]
- Aubrey A.D., Chalmers J.H., Bada J.L., Grunthaner F.J., Amashukeli X., Willis P., Skelley A.M., Mathies R.A., Quinn R.C., Zent A.P., Ehrenfreund P., Amundson R., Glavin D.P., Botta O., Barron L., Blaney D.L., Clark B.C., Coleman M., Hofmann B.A., Josset J.L., Rettberg P., Ride S., Robert F., Sephton M.A., and Yen A. (2008) The Urey instrument: an advanced in situ organic and oxidant detector for Mars exploration. Astrobiology 8:583–595 [DOI] [PubMed] [Google Scholar]
- Banfield J.F., Barker W.W., Welch S.A., and Taunton A. (1999) Biological impact on mineral dissolution: application of the lichen model to understanding mineral weathering in the rhizosphere. Proc Natl Acad Sci USA 96:3404–3411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaziz L., Barroug A., Legrouri A., Rey C., and Lebugle A. (2001) Adsorption of O-phospho-L-serine and L-serine onto poorly crystalline apatite. J Colloid Interface Sci 238:48–53 [DOI] [PubMed] [Google Scholar]
- Bénilan Y. and Cottin H. (2007) Comets, Titan and Mars: Astrobiology and Space Projects Lectures in Astrobiology, edited by Gargaud M., Martin H., and Claeyss P., Springer, Berlin, pp 347–428 [Google Scholar]
- Bennett P.C., Rogers J.R., Choi W.J., and Hiebert F. (2001) Silicates, silicate weathering, and microbial ecology. Geomicrobiol J 18:3–19 [Google Scholar]
- Bentaleb A., Vera P., Delgado A.V., and Gallardo V. (1994) Electrokinetic studies of monodisperse hematite particles—effects of inorganic electrolytes and amino-acids Mater Chem Phys 37:68–75 [Google Scholar]
- Bernal J.D. (1951) The Physical Basis of Life, Routledge and Paul, London [Google Scholar]
- Bibring J.P., Langevin Y., Gendrin A., Gondet B., Poulet F., Berthe M., Soufflot A., Arvidson R., Mangold N., Mustard J., Drossart P., and the OMEGA team. (2005) Mars surface diversity as revealed by the OMEGA/Mars Express observations. Science 307:1576–1581 [DOI] [PubMed] [Google Scholar]
- Biemann K., Oro J., Toulmin P., Orgel L.E., Nier A.O., Anderson D.M., Simmonds P.G., Flory D., Diaz A.V., Rushneck D.R., and Biller J.A. (1976) Search for organic and volatile inorganic compounds in two surface samples from the Chryse Planitia Region of Mars. Science 194:72–76 [DOI] [PubMed] [Google Scholar]
- Bish D.L., Blake D.F., Vaniman D.T., Chipera S.J., Morris R.V., Ming D.W., Treiman A.H., Sarrazin P., Morrison S.M., Downs R.T., Achilles C.N., Yen A.S., Bristow T.F., Crisp J.A., Morookian J.M., Farmer J.D., Rampe E.B., Stolper E.M., Spanovich N., and the MSL science team.(2013) X-ray diffraction results from Mars Science Laboratory: mineralogy of Rocknest at Gale Crater. Science 341, doi: 10.1126/science.1238932 [DOI] [PubMed] [Google Scholar]
- Blankenship R.E. (2010) Early evolution of photosynthesis. Plant Physiol 154:434–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgonie G., Garcia-Moyano A., Litthauer D., Bert W., Bester A., van Heerden E., Moller C., Erasmus M., and Onstott T.C. (2011) Nematoda from the terrestrial deep subsurface of South Africa. Nature 474:79–82 [DOI] [PubMed] [Google Scholar]
- Boston P.J., Ivanov M.V., and McKay C.P. (1992) On the possibility of chemosynthetic ecosystems in subsurface habitats on Mars. Icarus 95:300–308 [DOI] [PubMed] [Google Scholar]
- Bouvier A., Blichert-Toft J., Vervoort J.D., and Albarède F. (2005) The age of SNC meteorites and the antiquity of the martian surface. Earth Planet Sci Lett 240:221–233 [Google Scholar]
- Boyd E.S., Cummings D.E., and Geesey G.G. (2007) Mineralogy influences structure and diversity of bacterial communities associated with geological substrata in a pristine aquifer. Microb Ecol 54:170–182 [DOI] [PubMed] [Google Scholar]
- Brocks J.J., Logan G.A., Buick R., and Summons R.E. (1999) Archean molecular fossils and the early rise of eukaryotes. Science 285:1033–1036 [DOI] [PubMed] [Google Scholar]
- Callahan M.P., Smith K.E., Cleaves H.J., Ruzicka J., Stern J.C., Glavin D.P., House C.H., and Dworkin J.P. (2011) Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc Natl Acad Sci USA 108:13995–13998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson J.K., Campbell L., Rooney D., Clipson N., and Gleeson D.B. (2009) Minerals in soil select distinct bacterial communities in their microhabitats. FEMS Microbiol Ecol 67:381–388 [DOI] [PubMed] [Google Scholar]
- Carson J.K., Gonzalez-Quinones V., Murphy D.V., Hinz C., Shaw J.A., and Gleeson D.B. (2010) Low pore connectivity increases bacterial diversity in soil. Appl Environ Microbiol 76:3936–3942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill H., Teng H., and Hazen R.M. (2004) Correlation of pH-dependent surface interaction forces to amino acid adsorption: implications for the origin of life. Am Mineral 89:1048–1055 [Google Scholar]
- Ciaravella A., Scappini F., Franchi M., Cecchi-Pestellini C., Barbera M., Candia R., Gallori E., and Micela G. (2004) Role of clays in protecting adsorbed DNA against X-ray radiation. International Journal of Astrobiology 3:31–35 [Google Scholar]
- Clarke J.D.A. and Stoker C.R. (2011) Concretions in exhumed and inverted channels near Hanksville Utah: implications for Mars. International Journal of Astrobiology 10:161–175 [Google Scholar]
- Cocinero E.J., Lesarri A., Écija P., Basterretxea F.J., Grabow J.-U., Fernández J.A., and Castaño F. (2012) Ribose found in the gas phase. Angew Chem Int Ed Engl 51:3119–3124 [DOI] [PubMed] [Google Scholar]
- Cockell C.S., Schuerger A.C., Billi D., Friedmann E.I., and Panitz C. (2005) Effects of a simulated martian UV flux on the cyanobacterium, Chroococcidiopsis sp 029. Astrobiology 5:127–140 [DOI] [PubMed] [Google Scholar]
- Court R.W., Baki A.O., Sims M.R., Cullen D., and Sephton M.A. (2010) Novel solvent systems for in situ extraterrestrial sample analysis. Planet Space Sci 58:1470–1474 [Google Scholar]
- Crump B.C., Armbrust E.V., and Baross J.A. (1999) Phylogenetic analysis of particle-attached and free-living bacterial communities in the Columbia River, its estuary, and the adjacent coastal ocean. Appl Environ Microbiol 65:3192–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnell L.R. (2011) Ionizing radiation and life. Astrobiology 11:551–582 [DOI] [PubMed] [Google Scholar]
- Dartnell L.R., Desorgher L., Ward J.M., and Coates A.J. (2007) Modelling the surface and subsurface martian radiation environment: implications for astrobiology. Geophys Res Lett 34, doi: 10.1029/2006GL027494 [DOI] [Google Scholar]
- Deamer D.W. (1985) Boundary structures are formed by organic components of the Murchison carbonaceous chondrite. Nature 317:792–794 [Google Scholar]
- Decho A.W. (2000) Microbial biofilms in intertidal systems: an overview. Cont Shelf Res 20:1257–1273 [Google Scholar]
- Derenne S., Robert F., Skrzypczak-Bonduelle A., Gourier D., Binet L., and Rouzaud J.N. (2008) Molecular evidence for life in the 3.5 billion year old Warrawoona chert. Earth Planet Sci Lett 272:476–480 [Google Scholar]
- Direito S.O.L., Ehrenfreund P., Marees A., Staats M., Foing B., and Röling W.F.M. (2011) A wide variety of putative extremophiles and large beta-diversity at the Mars Desert Research Station (Utah). International Journal of Astrobiology 10:191–207 [Google Scholar]
- Direito S.O.L., Marees A., and Röling W.F.M. (2012) Sensitive life detection strategies for low-biomass environments: optimizing extraction of nucleic acids adsorbing to terrestrial and Mars analogue minerals. FEMS Microbiol Ecol 81:111–123 [DOI] [PubMed] [Google Scholar]
- Direito S.O.L., Zaura E., Little M., Ehrenfreund P., and Röling W.F.M. (2014) Systematic evaluation of bias in microbial community profiles induced by whole genome amplification. Environ Microbiol 16:643–657 [DOI] [PubMed] [Google Scholar]
- Dong H.L., Jaisi D.P., Kim J., and Zhang G.X. (2009) Microbe-clay mineral interactions. Am Mineral 94:1505–1519 [Google Scholar]
- Edwards K.J., McCollom T.M., Konishi H., and Buseck P.R. (2003) Seafloor bioalteration of sulfide minerals: results from in situ incubation studies. Geochim Cosmochim Acta 67:2843–2856 [Google Scholar]
- Ehrenfreund P. and Charnley S.B. (2000) Organic molecules in the interstellar medium, comets, and meteorites: a voyage from dark clouds to the early Earth. Annu Rev Astron Astrophys 38:427–483 [Google Scholar]
- Ehrenfreund P., Glavin D.P., Botta O., Cooper G., and Bada J.L. (2001) Extraterrestrial amino acids in Orgueil and Ivuna: tracing the parent body of CI type carbonaceous chondrites. Proc Natl Acad Sci USA 98:2138–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenfreund P., Rasmussen S., Cleaves J., and Chen L.H. (2006) Experimentally tracing the key steps in the origin of life: the aromatic world. Astrobiology 6:490–520 [DOI] [PubMed] [Google Scholar]
- Ehrenfreund P., Röling W.F.M., Thiel C.S., Quinn R., Sephton M.A., Stoker C., Kotler J.M., Direito S.O.L., Martins Z., Orzechowska G.E., Kidd R.D., van Sluis C.A., and Foing B.H. (2011) Astrobiology and habitability studies in preparation for future Mars missions: trends from investigating minerals, organics and biota. International Journal of Astrobiology 10:239–253 [Google Scholar]
- Ehrenreich A. and Widdel F. (1994) Anaerobic oxidation of ferrous iron by purple bacteria, a new-type of phototrophic metabolism. Appl Environ Microbiol 60:4517–4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairén A.G., Davila A.F., Lim D., Bramall N., Bonaccorsi R., Zavaleta J., Uceda E.R., Stoker C., Wierzchos J., Dohm J.M., Amils R., Andersen D., and McKay C.P. (2010) Astrobiology through the ages of Mars: the study of terrestrial analogues to understand the habitability of Mars. Astrobiology 10:821–843 [DOI] [PubMed] [Google Scholar]
- Fang Y., Spisz T.S., and Hoh J.H. (1999) Ethanol-induced structural transitions of DNA on mica. Nucleic Acids Res 27:1943–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Remolar D.C., Morris R.V., Gruener J.E., Amils R., and Knoll A.H. (2005) The Río Tinto basin, Spain: mineralogy, sedimentary geobiology, and implications for interpretation of outcrop rocks at Meridiani Planum, Mars. Earth Planet Sci Lett 240:149–167 [Google Scholar]
- Ferris J.P. (2005) Mineral catalysis and prebiotic synthesis: montmorillonite-catalyzed formation of RNA. Elements 1:145–149 [Google Scholar]
- Ferris J.P., Hill A.R., Liu R.H., and Orgel L.E. (1996) Synthesis of long prebiotic oligomers on mineral surfaces. Nature 381:59–61 [DOI] [PubMed] [Google Scholar]
- Flynn G. (1996) The delivery of organic matter from asteroids and comets to the early surface of Mars. Earth Moon Planets 72:469–474 [DOI] [PubMed] [Google Scholar]
- Formisano V., Atreya S., Encrenaz T., Ignatiev N., and Giuranna M. (2004) Detection of methane in the atmosphere of Mars. Science 306:1758–1761 [DOI] [PubMed] [Google Scholar]
- Fornaro T., Brucato J.R., Pucci A., and Branciamore S. (2013) Development of extraction protocols for life detection biosensor-based instruments. Planet Space Sci 86:75–79 [Google Scholar]
- Fortin D., Ferris F.G., and Beveridge T.J. (1997) Surface-mediated mineral development by bacteria. Reviews in Mineralogy and Geochemistry 35:161–180 [Google Scholar]
- Franklin R.B., Blum L.K., McComb A.C., and Mills A.L. (2002) A geostatistical analysis of small-scale spatial variability in bacterial abundance and community structure in salt marsh creek bank sediments. FEMS Microbiol Ecol 42:71–80 [DOI] [PubMed] [Google Scholar]
- Fraser D.G., Fitz D., Jakschitz T., and Rode B.M. (2011) Selective adsorption and chiral amplification of amino acids in vermiculite clay—implications for the origin of biochirality. Phys Chem Chem Phys 13:831–838 [DOI] [PubMed] [Google Scholar]
- Frostegård A., Courtois S., Ramisse V., Clerc S., Bernillon D., Le Gall F., Jeannin P., Nesme X., and Simonet P. (1999) Quantification of bias related to the extraction of DNA directly from soils. Appl Environ Microbiol 65:5409–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou C.D. and Deamer D.W. (2014) Lipids as universal biomarkers of extraterrestrial life. Astrobiology 14:541–549 [DOI] [PubMed] [Google Scholar]
- Getty S.A., Brinckerhoff W.B., Cornish T., Ecelberger S., and Floyd M. (2012) Compact two-step laser time-of-flight mass spectrometer for in situ analyses of aromatic organics on planetary missions. Rapid Commun Mass Spectrom 26:2786–2790 [DOI] [PubMed] [Google Scholar]
- Glavin D.P., Freissinet C., Miller K.E., Eigenbrode J.L., Brunner A.E., Buch A., Sutter B., Archer P.D., Jr., Atreya S.K., Brinckerhoff W.B., Cabane M., Coll P., Conrad P.G., Coscia D., Dworkin J.P., Franz H.B., Grotzinger J.P., Leshin L.A., Martin M.G., McKay C., Ming D.W., Navarro-González R., Pavlov A., Steele A., Summons R.E., Szopa C., Teinturier S., and Mahaffy P.R. (2013) Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J Geophys Res Planets 118:1955–1973 [Google Scholar]
- Grady M.M. (2008) Astrobiology of the terrestrial planets, with emphasis on Mars. In Complete Course in Astrobiology, edited by Horneck G. and Rettberg P., Wiley-VCH, Weinheim, Germany, pp 203–222 [Google Scholar]
- Grotzinger J.P., Sumner D.Y., Kah L.C., Stack K., Gupta S., Edgar L., Rubin D., Lewis K., Schieber J., Mangold N., Milliken R., Conrad P.G., Des Marais, D., Farmer J., Siebach K., Calef F., III, Hurowitz J., McLennan S.M., Ming D., Vaniman D., Crisp J., Vasavada A., Edgett K.S., Malin M., Blake D., Gellert R., Mahaffy P., Wiens R.C., Maurice S., Grant J.A., Wilson S., Anderson R.C., Beegle L., Arvidson R., Hallet B., Sletten R.S., Rice M., Bell J., III, Griffes J., Ehlmann B., Anderson R.B., Bristow T.F., Dietrich W.E., Dromart G., Eigenbrode J., Fraeman A., Hardgrove C., Herkenhoff K., Jandura L., Kocurek G., Lee S., Leshin L.A., Leveille R., Limonadi D., Maki J., McCloskey S., Meyer M., Minitti M., Newsom H., Oehler D., Okon A., Palucis M., Parker T., Rowland S., Schmidt M., Squyres S., Steele A., Stolper E., Summons R., Treiman A., Williams R., Yingst A., and the MSL science team. (2014) A habitable fluvio-lacustrine environment at Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1242777 [DOI] [PubMed] [Google Scholar]
- Hanslmeier A. (2009) The search for extraterrestrial life. In Habitability and Cosmic Catastrophes, edited by Hanslmeier A., Springer, Berlin, pp 187–208 [Google Scholar]
- Hassler D.M., Zeitlin C., Wimmer-Schweingruber R.F., Ehresmann B., Rafkin S., Eigenbrode J.L., Brinza D.E., Weigle G., Bottcher S., Bohm E., Burmeister S., Guo J., Köhler J., Martin C., Reitz G., Cucinotta F.A., Kim M.H., Grinspoon D., Bullock M.A., Posner A., Gómez-Elvira J., Vasavada A., Grotzinger J.P., and the MSL science team. (2014) Mars' surface radiation environment measured with the Mars Science Laboratory's Curiosity rover. Science 343, doi: 10.1126/science.1244797 [DOI] [PubMed] [Google Scholar]
- Hazen R.M. and Sverjensky D.A. (2010) Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harb Perspect Biol 2, doi: 10.1101/cshperspect.a002162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M.H., Kounaves S.P., Quinn R.C., West S.J., Young S.M.M., Ming D.W., Catling D.C., Clark B.C., Boynton W.V., Hoffman J., Deflores L.P., Gospodinova K., Kapit J., and Smith P.H. (2009) Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science 325:64–67 [DOI] [PubMed] [Google Scholar]
- Holm P.E., Nielsen P.H., Albrechtsen H.J., and Christensen T.H. (1992) Importance of unattached bacteria and bacteria attached to sediment in determining potentials for degradation of xenobiotic organic contaminants in an aerobic aquifer. Appl Environ Microbiol 58:3020–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt G.R. (1977) Spectral signatures of particulate minerals in the visible and near infrared. Geophysics 42:501–513 [Google Scholar]
- Hurowitz J.A. and McLennan S.M. (2007) A similar to 3.5 Ga record of water-limited, acidic weathering conditions on Mars. Earth Planet Sci Lett 260:432–443 [Google Scholar]
- Irwin L.N. and Schulze-Makuch D. (2001) Assessing the plausibility of life on other worlds. Astrobiology 1:143–160 [DOI] [PubMed] [Google Scholar]
- Joint I. and Groom S.B. (2000) Estimation of phytoplankton production from space: current status and future potential of satellite remote sensing. J Exp Mar Bio Ecol 250:233–255 [DOI] [PubMed] [Google Scholar]
- Jorissen A. and Cerf C. (2002) Asymmetric photoreactions as the origin of biomolecular homochirality: a critical review. Orig Life Evol Biosph 32:129–142 [DOI] [PubMed] [Google Scholar]
- Kaltenegger L., Miguel Y., and Rugheimer S. (2012) Rocky exoplanet characterization and atmospheres. International Journal of Astrobiology 11:297–307 [Google Scholar]
- Kanavarioti A. and Mancinelli R.L. (1990) Could organic matter have been preserved on Mars for 3.5 billion years? Icarus 84:196–202 [DOI] [PubMed] [Google Scholar]
- Kasting J.F. (1997) Warming early Earth and Mars. Science 276:1213–1215 [DOI] [PubMed] [Google Scholar]
- Kiang N.Y., Siefert J., Govindjee, and Blankenship R.E. (2007) Spectral signatures of photosynthesis. I. Review of Earth organisms. Astrobiology 7:222–251 [DOI] [PubMed] [Google Scholar]
- Killham K., Amato M., and Ladd J.N. (1993) Effect of substrate location in soil and soil pore-water regime on carbon turnover. Soil Biol Biochem 25:57–62 [Google Scholar]
- Klein H.P. (1978) The Viking biological experiments on Mars. Icarus 34:666–674 [Google Scholar]
- Kminek G. and Bada J.L. (2006) The effect of ionizing radiation on the preservation of amino acids on Mars. Earth Planet Sci Lett 245:1–5 [Google Scholar]
- Konhauser K. (2007) Introduction to Geomicrobiology, Blackwell Publishing, Oxford, UK [Google Scholar]
- Kotler J.M., Hinman N.W., Yan B., Stoner D.L., and Scott J.R. (2008) Glycine identification in natural jarosites using laser desorption Fourier transform mass spectrometry: implications for the search for life on Mars. Astrobiology 8:253–266 [DOI] [PubMed] [Google Scholar]
- Lambert J.-F. (2008) Adsorption and polymerization of amino acids on mineral surfaces: a review. Orig Life Evol Biosph 38:211–242 [DOI] [PubMed] [Google Scholar]
- Leshin L.A., Mahaffy P.R., Webster C.R., Cabane M., Coll P., Conrad P.G., Archer P.D., Jr., Atreya S.K., Brunner A.E., Buch A., Eigenbrode J.L., Flesch G.J., Franz H.B., Freissinet C., Glavin D.P., McAdam A.C., Miller K.E., Ming D.W., Morris R.V., Navarro-González R., Niles P.B., Owen T., Pepin R.O., Squyres S., Steele A., Stern J.C., Summons R.E., Sumner D.Y., Sutter B., Szopa C., Teinturier S., Trainer M.G., Wray J.J., Grotzinger J.P., and the MSL science team. (2013) Volatile, isotope, and organic analysis of martian fines with the Mars Curiosity rover. Science 341, doi: 10.1126/science.1238937 [DOI] [PubMed] [Google Scholar]
- Lewis J.M.T., Watson J.S., Najorka J., Luong D., and Sephton M.A. (2015) Sulfate minerals: a problem for the detection of organic compounds on Mars? Astrobiology 15:247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B., Hyacinthe C., Bonneville S., Braster M., Van Cappellen, P., and Röling W.F.M. (2007) Phylogenetic and physiological diversity of dissimilatory ferric iron reducers in sediments of the polluted Scheldt estuary, Northwest Europe. Environ Microbiol 9:1956–1968 [DOI] [PubMed] [Google Scholar]
- Lin L.H., Wang P.L., Rumble D., Lippmann-Pipke J., Boice E., Pratt L.M., Lollar B.S., Brodie E.L., Hazen T.C., Andersen G.L., DeSantis T.Z., Moser D.P., Kershaw D., and Onstott T.C. (2006) Long-term sustainability of a high-energy, low-diversity crustal biome. Science 314:479–482 [DOI] [PubMed] [Google Scholar]
- Lindahl T. (1997) Facts and artifacts of ancient DNA. Cell 90:1–3 [DOI] [PubMed] [Google Scholar]
- Lovley D.R., Holmes D.E., and Nevin K.P. (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286 [DOI] [PubMed] [Google Scholar]
- Macnaughton S.J., Jenkins T.L., Wimpee M.H., Cormier M.R., and White D.C. (1997) Rapid extraction of lipid biomarkers from pure culture and environmental samples using pressurized accelerated hot solvent extraction. J Microbiol Methods 31:19–27 [Google Scholar]
- Madigan M., Martinko J., Stahl D., and Clark D. (2012) Brock Biology of Microorganisms, Pearson, San Fransisco, CA [Google Scholar]
- Martin W.E., Hesse E., Hough J.H., Sparks W.B., Cockell C.S., Ulanowski Z., Germer T.A., and Kaye P.H. (2010) Polarized optical scattering signatures from biological materials. J Quant Spectrosc Radiat Transf 111:2444–2459 [Google Scholar]
- Martins Z. (2011) In situ biomarkers and the Life Marker Chip. Astronomy & Geophysics 52:1.34–1.35 [Google Scholar]
- Martins Z., Alexander C.M.O'D., Orzechowska G.E., Fogel M.L., and Ehrenfreund P. (2007) Indigenous amino acids in primitive CR meteorites. Meteorit Planet Sci 42:2125–2136 [Google Scholar]
- Martins Z., Botta O., Fogel M.L., Sephton M.A., Glavin D.P., Watson J.S., Dworkin J.P., Schwartz A.W., and Ehrenfreund P. (2008) Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet Sci Lett 270:130–136 [Google Scholar]
- Martins Z., Sephton M.A., Foing B.H., and Ehrenfreund P. (2011) Extraction of amino acids from soils close to the Mars Desert Research Station (MDRS), Utah. International Journal of Astrobiology 10:231–238 [Google Scholar]
- Mauck B.S. and Roberts J.A. (2007) Mineralogic control on abundance and diversity of surface-adherent microbial communities. Geomicrobiol J 24:167–177 [Google Scholar]
- McKay C.P. (1997) The search for life on Mars. Orig Life Evol Biosph 27:263–289 [PubMed] [Google Scholar]
- Meierhenrich U. (2008) Amino Acids and the Assymetry of Life, Springer, Berlin [Google Scholar]
- Melzak K.A., Sherwood C.S., Turner R.F.B., and Haynes C.A. (1996) Driving forces for DNA adsorption to silica in perchlorate solutions. J Colloid Interface Sci 181:635–644 [Google Scholar]
- Meslin P.Y., Gasnault O., Forni O., Schroeder S., Cousin A., Berger G., Clegg S.M., Lasue J., Maurice S., Sautter V., Le Mouélic, S., Wiens R.C., Fabre C., Goetz W., Bish D., Mangold N., Ehlmann B., Lanza N., Harri A.M., Anderson R., Rampe E., McConnochie T.H., Pinet P., Blaney D., Léveillé R., Archer D., Barraclough B., Bender S., Blake D., Blank J.G., Bridges N., Clark B.C., DeFlores L., Delapp D., Dromart G., Dyar M.D., Fisk M., Gondet B., Grotzinger J., Herkenhoff K., Johnson J., Lacour J.L., Langevin Y., Leshin L., Lewin E., Madsen M.B., Melikechi N., Mezzacappa A., Mischna M.A., Moores J.E., Newsom H., Ollila A., Perez R., Renno N., Sirven J.B., Tokar R., de la Torre, M., d'Uston L., Vaniman D., Yingst A., and the MSL science team. (2013) Soil diversity and hydration as observed by ChemCam at Gale Crater, Mars. Science 341, doi: 10.1126/science.1238670 [DOI] [PubMed] [Google Scholar]
- Ming D.W., Archer P.D., Jr., Glavin D.P., Eigenbrode J.L., Franz H.B., Sutter B., Brunner A.E., Stern J.C., Freissinet C., McAdam A.C., Mahaffy P.R., Cabane M., Coll P., Campbell J.L., Atreya S.K., Niles P.B., Bell J.F., III, Bish D.L., Brinckerhoff W.B., Buch A., Conrad P.G., Des Marais D.J., Ehlmann B.L., Fairén A.G., Farley K., Flesch G.J., Francois P., Gellert R., Grant J.A., Grotzinger J.P., Gupta S., Herkenhoff K.E., Hurowitz J.A., Leshin L.A., Lewis K.W., McLennan S.M., Miller K.E., Moersch J., Morris R.V., Navarro-González R., Pavlov A.A., Perrett G.M., Pradler I., Squyres S.W., Summons R.E., Steele A., Stolper E.M., Sumner D.Y., Szopa C., Teinturier S., Trainer M.G., Treiman A.H., Vaniman D.T., Vasavada A.R., Webster C.R., Wray J.J., Yingst R.A., and the MSL science team. (2014) Volatile and organic compositions of sedimentary rocks in Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1245267 [DOI] [PubMed] [Google Scholar]
- Mitchell A.C., Lafreniere M.J., Skidmore M.L., and Boyd E.S. (2013) Influence of bedrock mineral composition on microbial diversity in a subglacial environment. Geology 41:855–858 [Google Scholar]
- Mullis K., Faloona F., Scharf S., Saiki R., Horn G., and Erlich H. (1986) Specific enzymatic amplification of DNA in vitro: the polymerase chain reaction. Cold Spring Harb Symp Quant Biol 51(Pt 1):263–273 [DOI] [PubMed] [Google Scholar]
- Mustard J.F., Murchie S.L., Pelkey S.M., Ehlmann B.L., Milliken R.E., Grant J.A., Bibring J.P., Poulet F., Bishop J., Dobrea E.N., Roach L., Seelos F., Arvidson R.E., Wiseman S., Green R., Hash C., Humm D., Malaret E., McGovern J.A., Seelos K., Clancy T., Clark R., Des Marais, D., Izenberg N., Knudson A., Langevin Y., Martin T., McGuire P., Morris R., Robinson M., Roush T., Smith M., Swayze G., Taylor H., Titus T., and Wolff M. (2008) Hydrated silicate minerals on Mars observed by the Mars Reconnaissance Orbiter CRISM instrument. Nature 454:305–309 [DOI] [PubMed] [Google Scholar]
- Naraoka H., Shimoyama A., and Harada K. (1999) Molecular distribution of monocarboxylic acids in Asuka carbonaceous chondrites. Orig Life Evol Biosph 29:187–201 [DOI] [PubMed] [Google Scholar]
- Navarro-González R., Vargas E., de la Rosa, J., Raga A.C., and McKay C.P. (2010) Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars. J Geophys Res 115, doi: 10.1029/2010JE003599 [DOI] [Google Scholar]
- Nguyen T.H. and Elimelech M. (2007) Plasmid DNA adsorption on silica: kinetics and conformational changes in monovalent and divalent salts. Biomacromolecules 8:24–32 [DOI] [PubMed] [Google Scholar]
- Nielsen P. and Petersen S.O. (2000) Ester-linked polar lipid fatty acid profiles of soil microbial communities: a comparison of extraction methods and evaluation of interference from humic acids. Soil Biol Biochem 32:1241–1249 [Google Scholar]
- Novinscak A. and Filion M. (2011) Effect of soil clay content on RNA isolation and on detection and quantification of bacterial gene transcripts in soil by quantitative reverse transcription-PCR. Appl Environ Microbiol 77:6249–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogram A.V., Mathot M.L., Harsh J.B., Boyle J., and Pettigrew C.A. (1994) Effects of DNA polymer length on its adsorption to soils. Appl Environ Microbiol 60:393–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman S., Peeters Z., La Duc M.T., Mancinelli R., Ehrenfreund P., and Venkateswaran K. (2008) Effect of shadowing on survival of bacteria under conditions simulating the martian atmosphere and UV radiation. Appl Environ Microbiol 74:959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace N.R. (2001) The universal nature of biochemistry. Proc Natl Acad Sci USA 98:805–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panieri G., Lugli S., Manzi V., Roveri M., Schreiber B.C., and Palinska K.A. (2010) Ribosomal RNA gene fragments from fossilized cyanobacteria identified in primary gypsum from the late Miocene, Italy. Geobiology 8:101–111 [DOI] [PubMed] [Google Scholar]
- Parnell J., Cullen D., Sims M.R., Bowden S., Cockell C.S., Court R., Ehrenfreund P., Gaubert F., Grant W., Parro V., Rohmer M., Sephton M., Stan-Lotter H., Steele A., Toporski J., and Vago J. (2007) Searching for life on Mars: selection of molecular targets for ESA's Aurora ExoMars mission. Astrobiology 7:578–604 [DOI] [PubMed] [Google Scholar]
- Pavlov A.A., Vasilyev G., Ostryakov V.M., Pavlov A.K., and Mahaffy P.R. (2012) Degradation of the organic molecules in the shallow subsurface of Mars due to irradiation by cosmic rays. Geophys Res Lett 39, doi: 10.1029/2012GL052166 [DOI] [Google Scholar]
- Pavlov A.K., Blinov A.V., and Konstantinov A.N. (2002) Sterilization of martian surface by cosmic radiation. Planet Space Sci 50:669–673 [Google Scholar]
- Pedersen K. (2000) Exploration of deep intraterrestrial microbial life: current perspectives. FEMS Microbiol Lett 185:9–16 [DOI] [PubMed] [Google Scholar]
- Pietramellara G.P., Franchi M.F., Gallori E.G., and Nannipieri P.N. (2001) Effect of molecular characteristics of DNA on its adsorption and binding on homoionic montmorillonite and kaolinite. Biol Fertil Soils 33:402–409 [Google Scholar]
- Pointing S.B., Chan Y., Lacap D.C., Lau M.C.Y., Jurgens J.A., and Farrell R.L. (2009) Highly specialized microbial diversity in hyper-arid polar desert. Proc Natl Acad Sci USA 106:19964–19969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulet F., Bibring J.P., Mustard J.F., Gendrin A., Mangold N., Langevin Y., Arvidson R.E., Gondet B., and Gomez C. (2005) Phyllosilicates on Mars and implications for early martian climate. Nature 438:623–627 [DOI] [PubMed] [Google Scholar]
- Reardon C.L., Cummings D.E., Petzke L.M., Kinsall B.L., Watson D.B., Peyton B.M., and Geesey G.G. (2004) Composition and diversity of microbial communities recovered from surrogate minerals incubated in an acidic uranium-contaminated aquifer. Appl Environ Microbiol 70:6037–6046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C.D., Hinman N.W., McJunkin T.R., Kotler J.M., and Scott J.R. (2008) Exploring biosignatures associated with thenardite by geomatrix-assisted laser desorption/ionization Fourier transform ion cyclotron resonance mass spectrometry (GALDI-FTICR-MS). Geomicrobiol J 25:432–440 [Google Scholar]
- Rizzotti M. (2009) The Origin and Evolution of Early Life, Vol. 2 of Biological Science Fundamentals and Systematics, edited by Minelli A. and Contrafatto G., Encyclopedia of Life Support Systems (EOLSS), Oxford, UK [Google Scholar]
- Robe P., Nalin R., Capellano C., Vogel T.M., and Simonet P. (2003) Extraction of DNA from soil. Eur J Soil Biol 39:183–190 [Google Scholar]
- Roberts J.A. (2004) Inhibition and enhancement of microbial surface colonization: the role of silicate composition. Chem Geol 212:313–327 [Google Scholar]
- Rogers J.R. and Bennett P.C. (2004) Mineral stimulation of subsurface microorganisms: release of limiting nutrients from silicates. Chem Geol 203:91–108 [Google Scholar]
- Rogers J.R., Bennett P.C., and Choi W.J. (1998) Feldspars as a source of nutrients for microorganisms. Am Mineral 83:1532–1540 [Google Scholar]
- Röling W.F.M., van Breukelen B.M., Braster M., Lin B., and van Verseveld H.W. (2001) Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer. Appl Environ Microbiol 67:4619–4629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruamps L.S., Nunan N., and Chenu C. (2011) Microbial biogeography at the soil pore scale. Soil Biol Biochem 43:280–286 [Google Scholar]
- Russell M.J. and Hall A.J. (1997) The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J Geol Soc London 154:377–402 [DOI] [PubMed] [Google Scholar]
- Saeki K. and Kunito T. (2010) Adsorptions of DNA molecules by soils and variable-charged soil constituents. In Current Research Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, edited by Mendez-Vilas A., Formatex Research Center, Badajoz, Spain, pp 188–195 [Google Scholar]
- Saeki K. and Sakai M. (2009) The influence of soil organic matter on DNA adsorptions on andosols. Microbes Environ 24:175–179 [DOI] [PubMed] [Google Scholar]
- Satyanarayana T., Raghukumar C., and Shivaji S. (2005) Extremophilic microbes: diversity and perspectives. Current Science 89:78–90 [Google Scholar]
- Scappini F., Casadei F., Zamboni R., Franchi M., Gallori E., and Monti S. (2004) Protective effect of clay minerals on adsorbed nucleic acid against UV radiation: possible role in the origin of life. International Journal of Astrobiology 3:17–19 [Google Scholar]
- Schulze W.X., Gleixner G., Kaiser K., Guggenberger G., Mann M., and Schulze E.-D. (2005) A proteomic fingerprint of dissolved organic carbon and of soil particles. Oecologia 142:335–343 [DOI] [PubMed] [Google Scholar]
- Seager S., Turner E.L., Schafer J., and Ford E.B. (2005) Vegetation's red edge: a possible spectroscopic biosignature of extraterrestrial plants. Astrobiology 5:372–390 [DOI] [PubMed] [Google Scholar]
- Sephton M.A. (2002) Organic compounds in carbonaceous meteorites. Nat Prod Rep 19:292–311 [DOI] [PubMed] [Google Scholar]
- Sephton M.A. (2010) Organic geochemistry and the exploration of Mars. The Journal of Cosmology 5:1141–1149 [Google Scholar]
- Sessitsch A., Weilharter A., Gerzabek M.H., Kirchmann H., and Kandeler E. (2001) Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl Environ Microbiol 67:4215–4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelobolina E.S., Anderson R.T., Vodyanitskii Y.N., Sivtsov A.V., Yuretich R., and Lovley D.R. (2004) Importance of clay size minerals for Fe(III) respiration in a petroleum-contaminated aquifer. Geobiology 2:67–76 [Google Scholar]
- Skelley A.M., Scherer J.R., Aubrey A.D., Grover W.H., Ivester R.H.C., Ehrenfreund P., Grunthaner F.J., Bada J.L., and Mathies R.A. (2005) Development and evaluation of a microdevice for amino acid biomarker detection and analysis on Mars. Proc Natl Acad Sci USA 102:1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam G. and Saunders J.A. (2005) The geomicrobiology of ore deposits. Econ Geol 100:1067–1084 [Google Scholar]
- Sparks W.B., Hough J., Germer T.A., Chen F., DasSarma S., DasSarma P., Robb F.T., Manset N., Kolokolova L., Reid N., Macchetto F.D., and Martin W. (2009a) Detection of circular polarization in light scattered from photosynthetic microbes. Proc Natl Acad Sci USA 106:7816–7821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks W.B., Hough J.H., Kolokolova L., Germer T.A., Chen F., DasSarma S., DasSarma R., Robb F.T., Manset N., Reid I.N., Macchetto F.D., and Martin W. (2009b) Circular polarization in scattered light as a possible biomarker. J Quant Spectrosc Radiat Transf 110:1771–1779 [Google Scholar]
- Squyres S.W., Arvidson R.E., Bell J.F., Bruckner J., Cabrol N.A., Calvin W., Carr M.H., Christensen P.R., Clark B.C., Crumpter L., Des Marais D.J., d'Uston C., Economou T., Farmer J., Farrand W., Folkner W., Golombek M., Gorevan S., Grant J.A., Greeley R., Grotzinger J., Haskin L., Herkenhoff K.E., Hviid S., Johnson J., Klingelhöfer G., Knoll A.H., Landis G., Lemmon M., Li R., Madsen M.B., Malin M.C., McLennan S.M., McSween H.Y., Ming D.W., Moersch J., Morris R.V., Parker T., Rice J.W., Jr., Richter L., Rieder R., Sims M., Smith M., Smith P., Soderblom L.A., Sullivan R., Wänke H., Wdowiak T., Wolff M., and Yen A. (2004a) The Opportunity Rover's Athena science investigation at Meridiani Planum, Mars. Science 306:1698–1703 [DOI] [PubMed] [Google Scholar]
- Squyres S.W., Grotzinger J.P., Arvidson R.E., Bell J.F., Calvin W., Christensen P.R., Clark B.C., Crisp J.A., Farrand W.H., Herkenhoff K.E., Johnson J.R., Klingelhöfer G., Knoll A.H., McLennan S.M., McSween H.Y., Jr., Morris R.V., Rice J.W., Jr., Rieder R., and Soderblom L.A. (2004b) In situ evidence for an ancient aqueous environment at Meridiani Planum, Mars. Science 306:1709–1714 [DOI] [PubMed] [Google Scholar]
- Stalport F., Glavin D.P., Eigenbrode J.L., Bish D., Blake D., Coll P., Szopa C., Buch A., McAdam A., Dworkin J.P., and Mahaffy P.R. (2012) The influence of mineralogy on recovering organic acids from Mars analogue materials using the “one-pot” derivatization experiment on the Sample Analysis at Mars (SAM) instrument suite. Planet Space Sci 67:1–13 [Google Scholar]
- Summons R.E., Albrecht P., McDonald G., and Moldowan J.M. (2008) Molecular biosignatures. Space Sci Rev 135:133–159 [Google Scholar]
- ten Kate I.L., Garry J.R.C., Peeters Z., Foing B., and Ehrenfreund P. (2006) The effects of martian near surface conditions on the photochemistry of amino acids. Planet Space Sci 54:296–302 [Google Scholar]
- Thomas M.M., Clouse J.A., and Longo J.M. (1993) Adsorption of organic-compounds on carbonate minerals. 1. Model compounds and their influence on mineral wettability. Chem Geol 109:201–213 [Google Scholar]
- Tung H.C., Bramall N.E., and Price P.B. (2005) Microbial origin of excess methane in glacial ice and implications for life on Mars. Proc Natl Acad Sci USA 102:18292–18296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaniman D.T., Bish D.L., Ming D.W., Bristow T.F., Morris R.V., Blake D.F., Chipera S.J., Morrison S.M., Treiman A.H., Rampe E.B., Rice M., Achilles C.N., Grotzinger J.P., McLennan S.M., Williams J., Bell J.F., III, Newsom H.E., Downs R.T., Maurice S., Sarrazin P., Yen A.S., Morookian J.M., Farmer J.D., Stack K., Milliken R.E., Ehlmann B.L., Sumner D.Y., Berger G., Crisp J.A., Hurowitz J.A., Anderson R., Des Marais D.J., Stolper E.M., Edgett K.S., Gupta S., Spanovich N., and the MSL science team.(2014) Mineralogy of a mudstone at Yellowknife Bay, Gale Crater, Mars. Science 343, doi: 10.1126/science.1243480 [DOI] [PubMed] [Google Scholar]
- Vargas M., Kashefi K., Blunt-Harris E.L., and Lovley D.R. (1998) Microbiological evidence for Fe(III) reduction on early Earth. Nature 395:65–67 [DOI] [PubMed] [Google Scholar]
- Vreeland R.H., Rosenzweig W.D., and Powers D.W. (2000) Isolation of a 250 million-year-old halotolerant bacterium from a primary salt. Nature 407:897–900 [DOI] [PubMed] [Google Scholar]
- Wagner I. and Musso H. (1983) New naturally occurring amino acids. Angew Chem Int Ed Engl 22:816–828 [Google Scholar]
- Walker S.L., Redman J.A., and Elimelech M. (2004) Role of cell surface lipopolysaccharides in Escherichia coli K12 adhesion and transport. Langmuir 20:7736–7746 [DOI] [PubMed] [Google Scholar]
- Walter M.R. and Des Marais D.J. (1993) Preservation of biological information in thermal-spring deposits—developing a strategy for the search for fossil life on Mars. Icarus 101:129–143 [DOI] [PubMed] [Google Scholar]
- Weber K.A., Achenbach L.A., and Coates J.D. (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4:752–764 [DOI] [PubMed] [Google Scholar]
- Webster C.R., Mahaffy P.R., Atreya S.K., Flesch G.J., and Farley K.A. (2013) Low upper limit to methane abundance on Mars. Science 342:355–357 [DOI] [PubMed] [Google Scholar]
- Webster C.R., Mahaffy P.R., Atreya S.K., Flesch G.J., Mischna M.A., Meslin P.Y., Farley K.A., Conrad P.G., Christensen L.E., Pavlov A.A., Martín-Torres J., Zorzano M.P., McConnochie T.H., Owen T., Eigenbrode J.L., Glavin D.P., Steele A., Malespin C.A., Archer P.D., Jr., Sutter B., Coll P., Freissinet C., McKay C.P., Moores J.E., Schwenzer S.P., Bridges J.C., Navarro-Gonzalez R., Gellert R., Lemmon M.T., and the MSL science team. (2015) Mars methane detection and variability at Gale Crater. Science 347:415–417 [DOI] [PubMed] [Google Scholar]
- Westall F. (2009) Life on an anaerobic planet. Science 323:471–472 [DOI] [PubMed] [Google Scholar]
- Wey J.K., Scherwass A., Norf H., Arndt H., and Weitere M. (2008) Effects of protozoan grazing within river biofilms under semi-natural conditions. Aquat Microb Ecol 52:283–296 [Google Scholar]
- Whitman W.B., Coleman D.C., and Wiebe W.J. (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci USA 95:6578–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.B., Metge D.W., Eberl D.D., Harvey R.W., Turner A.G., Prapaipong P., and Poret-Peterson A.T. (2011) What makes a natural clay antibacterial? Environ Sci Technol 45:3768–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn-Williams D.D. (2000) Cyanobacteria in deserts—life at the limit? In The Ecology of Cyanobacteria, edited by Whitton B.A. and Potts M., Kluwer Academic Publishers, Dordrecht, the Netherlands, pp 341–366 [Google Scholar]
- Xiong J. and Bauer C.E. (2002) Complex evolution of photosynthesis. Annu Rev Plant Biol 53:503–521 [DOI] [PubMed] [Google Scholar]
- Yan B., Stoner D.L., Kotler J.M., Hinman N.W., and Scott J.R. (2007) Detection of biosignatures by geomatrix-assisted laser desorption/ionization (GALDI) mass spectrometry. Geomicrobiol J 24:379–385 [Google Scholar]
- Yen A.S., Kim S.S., Hecht M.H., Frant M.S., and Murray B. (2000) Evidence that the reactivity of the martian soil is due to superoxide ions. Science 289:1909–1912 [DOI] [PubMed] [Google Scholar]
- Zaia D.A.M. (2004) A review of adsorption of amino acids on minerals: was it important for origin of life? Amino Acids 27:113–118 [DOI] [PubMed] [Google Scholar]
- Zettler L.A.A., Gomez F., Zettler E., Keenan B.G., Amils R., and Sogin M.L. (2002) Eukaryotic diversity in Spain's River of Fire. Nature 417:137. [DOI] [PubMed] [Google Scholar]
- Zhang M., Kan Y.H., Zang Q.J., Su Z.M., and Wang R.S. (2003) Why silicon nanotubes stably exist in armchair structure? Chem Phys Lett 379:81–86 [Google Scholar]
- Zullig J.J. and Morse J.W. (1988) Interaction of organic-acids with carbonate mineral surfaces in seawater and related solutions. 1. Fatty-acid adsorption. Geochim Cosmochim Acta 52:1667–1678 [Google Scholar]