Abstract
Background and Purpose: Etiology of orchialgia or testicular pain after laparoscopic donor nephrectomy (LDN) has been found to be related to injury of the spermatic plexus during gonadal (testicular) vein (GV) or ureteral ligation. This study aimed to evaluate and validate the impact of the level of ligation of GV and ureter in relation to the crossing of iliac vessels (CIV) on incidence of orchialgia.
Patients and Methods: A prospective study was conducted on 70 males who underwent left LDN from January 2008 to December 2010 (group A) to determine the correlation between orchialgia and level of ligation of the GV and ureter with respect to CIV; this revealed that the ligation of the GV and/or ureter above the level of the CIV (level 1, n=40) is less likely to cause orchialgia than ligating them at or below (level 2, n=30). Subsequently, in 45 male patients (group B) for left LDN from January 2011 to June 2013, we ensured that clipping of the ureter and GV be performed above the CIV to validate the above findings. Patients with a history of scrotal pathology or surgical procedure were excluded. One-sided Z-test with pooled variance was used to calculate the sample size.
Results: In group A, orchialgia was seen in 10 (14.3%) patients. The clipping of the ureter and GV at level 2 (orchialgia, n=9) was associated with a significantly higher incidence of orchialgia than clipping them at level 1 (orchialgia, n=1) (P=0.001,95% confidence interval=0.0707 to 0.2471). In group B, 43 patients were finally analyzed, and none had orchialgia.
Conclusion: The level of ligation of the GV and ureter has significant impact on the incidence of orchialgia. Ipsilateral testicular pain in patients with left-sided LDN is preventable, if the ureter and GV are ligated or clipped above the level of iliac vessels bifurcation.
Introduction
The first nephrectomy using the laparoscopic approach was reported by Clayman and associates1 in 1991. Fabrizio and colleagues2 were the first to perform laparoscopic donor nephrectomy (LDN) in 1995. Since then, evolution in techniques and increased experience with minimally invasive surgery has established LDN as the standard approach for kidney harvesting. The major and minor complication rate after laparoscopic renal procedures and LDN has been reported in the range of 1% to 6% and 10% to 30%, respectively.3–5 Testicular pain after laparoscopic renal procedures has been infrequently cited in the literature. The etiopathogenesis of orchialgia has not been clearly defined, but clipping of the gonadal/testicular vein (GV) and ureter has been considered as the main culprit.6–8
The pelvic plexus gives rise to the inferior spermatic plexus that innervates the pelvic ureter. Injury to these delicate neural structures during ligation or clipping of the GV or ureter has been accepted as the principle etiology of ipsilateral testicular pain.6–10 Further, we found some anatomic evidence that the distribution of neural fibers of the spermatic plexus is much higher around the GV and ureter in the pelvis below the division of the common iliac vessels.6, 9–12 Hence, we hypothesized that the possibility of these nerve fibers being damaged should be higher if their ligation at a lower level is attempted.
Between January 2008 and December 2010, we prospectively evaluated the risk factors including impact of level of ligation of the GV and ureter for orchialgia in patients undergoing laparoscopic renal surgery, including 75 cases of LDN. We observed that if the GV and ureter were clipped above the crossing or bifurcation of the iliac vessels (CIV), the incidence of testicular pain was reduced significantly rather than clipping them at or below this level.13 In view of above findings, we ensured that the ureter and GV be ligated above the CIV in all cases of left LDN from January 2011 onward, and we prospectively evaluated them to confirm whether this modification can prevent or reduce the incidence of orchialgia.
Patients and Methods
Study design
Group A—A total of 75 male donors who underwent left-sided LDN from January 2008 to December 2010 (prospective observational study); group B—a total of 45 male donors who underwent left-sided LDN from January 2011 to January 2013. On the basis of outcome analysis of group A, we made the modification related to the level of clipping of the GV and ureter (prospective observational study). Exclusion criteria: Patients with a history of scrotal pathology, testicular pain or surgical procedure were excluded.
Surgical technique
LDN were performed by a technique almost similar to the standard.4 Clipping of the ureter and GV above the CIV was designated as level 1 and as level 2 when clipped below the CIV. In level 1, we routinely clipped the GV and ureter ∼1.5 cm (measured by jaw of standard Maryland grasper) above the CIV. We carefully preserved the periureteral tissue while performing the ureteral dissection down to the CIV to prevent ureteral ischemic injury.
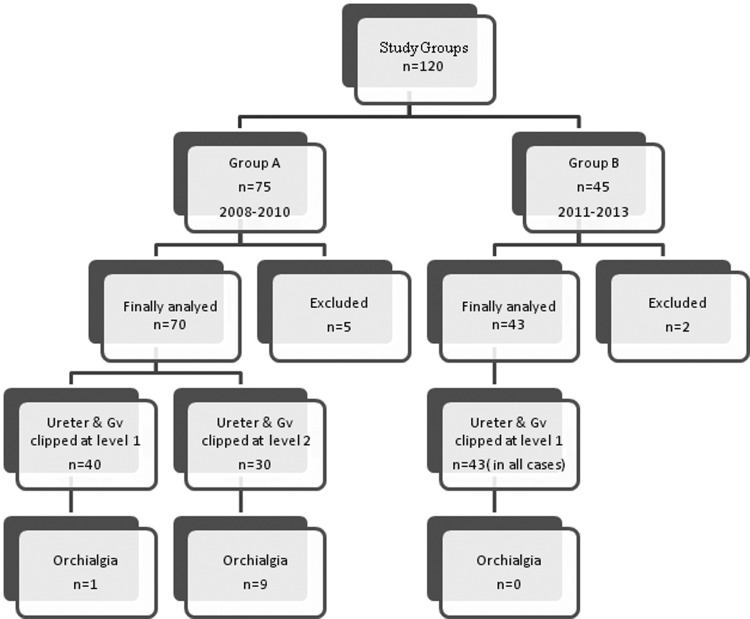
In group A, GV and/or ureter was clipped at level 1 and level 2 in 40 and 30 patients, respectively (Fig. 1). After analysis of data of group A, we found the incidence of ipsilateral orchialgia was 14.4% and the level of the ureter and/or GV clipping in relation to the CIV was an independent predictor of orchialgia.13 In view of the above findings, we ensured that the ligation of the ureter and GV will be carefully performed above the CIV in all patients of group B.
FIG. 1.
Study groups. GV=gonadal vein.
Pain assessment
The standard 10-point numeric rating scale was used for assessment of postoperative pain as documented by the National Institutes of Health. Scrotal ultrasonography (US) was performed in patients with a moderate degree of pain.
Statistical analysis
Statistical analyses were performed by using the SPSS® 17 software. Using the independent sample t tests, the variables of group A and group B with and without orchialgia were compared in terms of mean age (years), blood loss (mL), surgical duration (minutes), and the duration of hospital stay (days). To determine the incidence and association between orchialgia and the level of GV and ureteral clipping in relation to CIV, the chi-square test was applied. In all analyses, we have used two-sided hypothesis testing and probability values. P<0.05 were considered significant.
Sample size
A one sided Z-test with pooled variance was applied to compute the sample size. With existing incidence of orchialgia of 15% and an estimate reduction to 0% gave a sample of 44 patients to achieve 80% power of study at the significance level of 0.05.
Results
Seventy patients of group A and 43 patients of group B were evaluated for final analysis after satisfying the exclusion criteria. Age, duration of surgery, blood loss, body mass index, and mean follow-up of patients were comparable in both A and B (Table 1). A total of 10 (14.4%) patients in group A and none in group B had ipsilateral orchialgia (P=0.001) (Tables 1 and 2).
Table 1.
Demographic and Clinical Characteristics of Both the Groups
| Variables | Group A | Group B | P value |
|---|---|---|---|
| Number (N) | 70 | 43 | |
| Age (mean±SD) | 41±8.5 years | 39.6±10.2 | 0.10 |
| BMI (mean±SD) | 24.6±2.3 kg/m2 | 23.8±2.7 kg/m2 | 0.25 |
| Duration (mean±SD) | 165±30.2 minutes | 158±3 7.5 minutes | 0.09 |
| Mean blood loss (mL) | 90±45.5 mL | 95.5±40 mL | 0.30 |
| Orchialgia | 10 | 0 | <0.001 |
| Hospital stay (days) | 4.8±1.2 | 3.5±1.2 | 0.04 |
| Follow-up | 4.2±2.6 months | 3.8±1.8 | 0.09 |
SD=standard deviation; BMI=body mass index.
Table 2.
Level of Ligation of Gonadal Vein and Ureter and Incidence of Orchialgia in Group A
| Level of ligation of GV and/or ureter | Number (N) | Orchialgia (n) | P value |
|---|---|---|---|
| Level 1 | 40 | 1 | 0.001 |
| Level 2 | 30 | 9 |
GV=gonadal vein.
In group A, 10 patients had testicular pain that was continuous dull aching and heavy in nature and was unrelated to manipulation of the testes. Mean intensity of pain was 3.1 with a range of 2 to 5. Eight and two patients had mild and moderate intensity of pain, respectively, whereas none of them had severe pain. In all cases of orchialgia, pain started in the first week (range 2–7, mean 3.2±0.6 days), more so within the first 4 days. All of them had pain relief with conservative management by 13±4.2 days (range 4–30 days). Scrotal US was normal in two patients with a moderate degree of pain.
In Group A, we clipped the vein and/or ureter at level 2 in 30 patients, of whom 9 patients had pain (Fig. 1, Table 2). In 40 patients, both GV and ureter were ligated at level 1; only one patient had orchialgia (Fig.1). On statistical analysis, the clipping of the ureter and GV at level 2 (orchialgia, n=9) was associated with a significantly higher incidence of orchialgia than clipping them at level 1 (orchialgia, n=1) (P=0.001, 95% confidence interval=0.0707–0.2471).
In group B, both GV and ureter were clipped at level 1 in all patients, and none had orchialgia with mean follow-up of 3.8±1.8 months.
Discussion
In our initial part of the study (group A), we found that testicular pain after LDN related to the level of clipping of the GV and ureter in relation to CIV. Clipping them below the CIV considerably increased the risk of testicular pain compared with clipping above the CIV. On further study, we found that careful ligation of the GV and ureter above the CIV prevents testicular pain in left-sided LDN.
Overall incidence of orchialgia was 8.52% after various laparoscopic renal surgeries including LDN as reported by Srivastava and associates.13 They found that orchialgia was more frequent when the GV or ureter was ligated or clipped at or below the common iliac bifurcation. The later part of this study (group B) was based on the above findings, and ultimately we were able to find the possible way to prevent orchialgia in LDN.
Incidence of orchialgia was 1% in a series of 381 LDN by Su and colleagues7 and was related to GV ligation. Gjerston and coworkers8 reported a much higher incidence (21%) of testicular pain following laparoscopic renal procedures. The incidence of orchialgia was reported in a range of 6% to 10% after LDN.6,12 Gjerston and coworkers8 assessed the character of pain in their study, which was mild, self-limiting, and experienced within 4 to 7 days of the surgical procedure. In our study, too, in 80% of the patients, the pain was mild in intensity and started within first postoperative week. Pain lasted for a mean of 13±4.2 (range 4–30 days) days and improved with conservative management.
Two hypotheses related to the etiopathogenesis of orchialgia have been proposed in the literature,6,8,13 the first being venous congestion secondary to GV ligation as noticed by Gjerston and coworkers8 in their large series of various renal procedures. Second is the neural hypothesis, related to collateral damage of neural fibers of the spermatic plexus or cord during clipping or ligation of the GV and ureter.6,13 On the basis of neural hypothesis, Kim and colleagues 6 described that reflex sympathetic dystrophy after injury to these neural fibers led to the neuropathic pain in this group of patients. The anatomic basis of orchialgia in the study by Srivastava and associates13 was principally related to the injury of testicular nerve fibers.
The testicular neurophysiology is poorly understood. The neural fibers of the spermatic plexus (SP), which arises from superior, middle, and inferior SP, provide afferent innervation to the testis.9,10 Fibers from these plexuses supply the middle and pelvic part of the ureter and subsequently travel inferolaterally toward the deep inguinal ring to merge in the cord structures and innervate the testis. Both the pelvic ureter and testis are innervated by the pelvic plexus via the inferior SP. These delicate fibers are prone to injury, leading to orchialgia during clipping of the GV and ureter. It is not clear why only a small percentage of patients had orchialgia after clipping of the GV and ureter. We thought that during clipping of the GV and ureter, if the surrounding fatty tissue (which may contain the neural fibers) got entrapped, the possibility of orchialgia would be higher. This hypothesis, however, needs further scientific validation.
In group A, we found that the orchialgia was significantly associated with the level of clipping of the ureter and GV in relation to the bifurcation of the common iliac vessels. The probable reason may be related to the higher density of nerve fibers of the SP (mainly middle and inferior spermatic nerves) around the pelvic ureter and surrounding tissues. The anatomic location makes these neural fibers more vulnerable for disruption while clipping these structures beyond the CIV.9–12 The evidence given by Shirodkar and coworkers12 indirectly supports this hypothesis to some extent. They reported that none of their patients undergoing LDN had orchialgia when the GV was clipped near its insertion at the renal vein and spared it lower down.
We always intentionally avoided clipping of the left GV close to its insertion during left donor nephrectomy. During donor nephrectomy, one should try to avoid entering into the golden triangle (triangular area between the lower pole of the kidney and ureter containing adipose tissue) to preserve the blood supply of the allograft ureter. The GV, if preserved in its upper part, could serve as an excellent landmark for the golden triangle. It prevents inadvertent entry by the surgeon into the golden triangle area. Furthermore, while performing LDN, a surgeon generally tries to obtain as much length of ureter that can be easily accessible. For practical purposes, however, sufficient ureteral length can be obtained for a tension free ureteroneocystostomy even if the ureter is ligated at level 1.
It is pertinent to note that we have not encountered even a single case of orchialgia after open donor nephrectomy at our center, and this complication has not been reported even after open renal surgeries. The probable justification may be related to the fact that we tend to dissect the ureter and GV more distally (level 2 as per our design) in laparoscopic procedures, because of improved vision and ease of dissection lower down in the pelvis than open donor nephrectomy. Another likely explanation could be that the open procedures cause greater neuropathic pain at or near the long incision lines. Orchialgia, which is generally mild in nature, could probably have gone unnoticed by these patients.
A drawback of the study was that it did not include a control group of patients in whom neither the ureter nor GV was ligated. It is difficult to create such a model in the setting of live related donor nephrectomy.
Conclusion
Ipsilateral orchialgia is not unusual but a preventable complication in patients after left-sided LDN. It is likely to be related to disruption of neural fibers supplying the testis while dissecting or clipping the GV and/or ureter below the level of the common iliac bifurcation. Thus, we recommend that the GV and ureter should be clipped above the CIV iliac vessels crossing rather than below to prevent such complication.
Abbreviations Used
- CIV
crossing of iliac vessels
- GV
gonadal vein
- LDN
laparoscopic donor nephrectomy
- SP
spermatic plexus
- US
ultrasonography
Disclosure Statement
No competing financial interests exist.
References
- 1.Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy initial case report. J Urol 1991;146:278–282 [DOI] [PubMed] [Google Scholar]
- 2.Fabrizio MD, Ratner LE, Kavoussi LR. Laparoscopic live donor nephrectomy: Pro. Urology 1999; 53:665–667 [DOI] [PubMed] [Google Scholar]
- 3.Pareek G, Hedican SP, Gee JR, et al. Meta-analysis of the complications of laparoscopic renal surgery: Comparison of procedures and techniques. J Urol 2006;175:1208–1213 [DOI] [PubMed] [Google Scholar]
- 4.Kim FJ, Ratner LE, Kavoussi LR. Renal transplantation: Laparoscopic live donor nephrectomy. Urol Clin North Am 2000;27:777–785 [DOI] [PubMed] [Google Scholar]
- 5.Philosophe B, Kuo PC, Schweitzer EJ, et al. Laparoscopic versus open donor nephrectomy: Comparing ureteral complications in the recipients and improving the laparoscopic technique. Transplantation 1999;68:497–502 [DOI] [PubMed] [Google Scholar]
- 6.Kim FJ, Pinto P, Su LM, et al. Ipsilateral orchialgia after laparoscopic donor nephrectomy. J Endourol 2003;17:405–409 [DOI] [PubMed] [Google Scholar]
- 7.Su LM, Ratner LE, Montgomery RA, et al. Laproscopic live donor nephrectomy: Trends in donor and recipient morbidity following 381 cases. Ann Surg 2004;240:358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gjertson CK, Sundaram CP. Testicular pain following laparoscopic renal surgery. J Urol 2008;180:2037–2041 [DOI] [PubMed] [Google Scholar]
- 9.Rauchenwald M, Steers WD, Desjardins C. Efferent innervation of the rat testis. Biol Reprod 1995;52:1136–1143 [DOI] [PubMed] [Google Scholar]
- 10.Colby F. Embryology, anatomy and physiology of the testis and epididimis. In: Colby FH, ed. Essential Urology, 2nd ed. Baltimore: Williams & Wilkins, 1953, pp 101–103 [Google Scholar]
- 11.Starling JR, Harms BA. Diagnosis and treatment of genitofemoral and ilioinguinal neuralgia. World J Surg 1989;13:586–591 [DOI] [PubMed] [Google Scholar]
- 12.Shirodkar SP, Gorin MA, Sageshima J, et al. Technical modification for laparoscopic donor nephrectomy to minimize testicular pain: A complication with significant morbidity. Am J Transplant 2011;11:1031–1034 [DOI] [PubMed] [Google Scholar]
- 13.Srivastava A, Kapoor R, Srivastava A, et al. Orchialgia after laparoscopic renal surgery: A common problem with questionable etiology. Are there any predictors? World J Urol 2013;31:1153–1157 [DOI] [PubMed] [Google Scholar]