Abstract
Between April 2009 and July 2011, the NASA Haughton-Mars Project (HMP) led the Northwest Passage Drive Expedition (NWPDX), a multi-staged long-distance crewed rover traverse along the Northwest Passage in the Arctic. In April 2009, the HMP Okarian rover was driven 496 km over sea ice along the Northwest Passage, from Kugluktuk to Cambridge Bay, Nunavut, Canada. During the traverse, crew members collected samples from within the rover and from undisturbed snow-covered surfaces around the rover at three locations. The rover samples and snow samples were stored at subzero conditions (−20°C to −1°C) until processed for microbial diversity in labs at the NASA Kennedy Space Center, Florida. The objective was to determine the extent of microbial dispersal away from the rover and onto undisturbed snow. Interior surfaces of the rover were found to be associated with a wide range of bacteria (69 unique taxa) and fungi (16 unique taxa). In contrast, snow samples from the upwind, downwind, uptrack, and downtrack sample sites exterior to the rover were negative for both bacteria and fungi except for two colony-forming units (cfus) recovered from one downwind (1 cfu; site A4) and one uptrack (1 cfu; site B6) sample location. The fungus, Aspergillus fumigatus (GenBank JX517279), and closely related bacteria in the genus Brevibacillus were recovered from both snow (B. agri, GenBank JX517278) and interior rover surfaces. However, it is unknown whether the microorganisms were deposited onto snow surfaces at the time of sample collection (i.e., from the clothing or skin of the human operator) or via airborne dispersal from the rover during the 12–18 h layovers at the sites prior to collection. Results support the conclusion that a crewed rover traveling over previously undisturbed terrain may not significantly contaminate the local terrain via airborne dispersal of propagules from the vehicle. Key Words: Planetary protection—Contamination—Habitability—Haughton Crater—Mars. Astrobiology 15, 478–491.
1. Introduction
In preparation for robotic and human exploration missions to the Moon and Mars, terrestrial analog sites are used as proxies to evaluate a wide range of exploration technologies and strategies, including rovers, life-support systems, habitat design, research equipment, and operational protocols. Terrestrial analogues have been proposed for both the Moon and Mars and in general consist of cold and desiccated locations with low extant macroscopic flora and fauna, but none are devoid of microbial communities (see Horneck and Baumstark-Khan, 2002; Gilmour et al., 2003). At many analog sites, scientists are concerned with forward microbial contamination from human activity that may impact indigenous communities, reducing the scientific integrity of the sites, for instance in caves (e.g., Hunter et al., 2004; Fernandez-Cortes et al., 2011) and in the Antarctic Dry Valleys (e.g., Harris, 1998; Tow and Cowan, 2005). Furthermore, a key objective of the robotic and human exploration of Mars is the discovery of an extant martian microbiota, if present. Thus, forward contamination of pristine extremophile sites on Earth and Mars is of significant concern, and international efforts are underway to mitigate the dispersal of human-associated microorganisms into such sites (National Research Council, 2002, 2006).
In 1997, the NASA Haughton-Mars Project (HMP) was established on Devon Island in the High Arctic as a field research project dedicated to comparative planetary sciences and studies related to preparing for the human exploration of Mars (Lee, 1997, 2002). Since 2003, the HMP has been conducting simulated pressurized rover traverses using the Mars-1 Humvee rover, a modified ambulance version of the military High Mobility Multipurpose Wheeled Vehicle (e.g., Lee et al., 2011). To carry out studies with two identical rovers working in tandem (i.e., a dual-pressurized rover strategy), the HMP secured a second vehicle, the HMP Okarian (Fig. 1A; also known as the Moon-1 Humvee rover; Lee, 2010), and began developing a plan to deliver the new vehicle to Devon Island by driving it across Arctic sea ice along the length of the Northwest Passage. Thus emerged the Northwest Passage Drive Expedition (NWPDX) (Lee, 2010; Lee et al., 2015), a multi-staged long-distance crewed rover traverse that was eventually implemented over the course of three field seasons, between April 2009 and July 2011 (Lee, 2010; Lee et al., 2011, 2015). The present study relates to measurements during the first leg of the expedition: NWPDX-2009. The Okarian rover is a highly capable nonpressurized all-terrain rover simulating some of the basic attributes of a pressurized planetary rover, including the ability to traverse unprepared terrain and offer shelter to a crew of five.
FIG. 1.
Northwest Passage Drive Expedition (NWPDX) 2009 traverse images. (A) Deployment of the Okarian rover by a C-130 Hercules on snow in Kugluktuk, Nunavut, Canada. (B) Traverse team traveled with one scout snowmobile blazing the traverse track, the Okarian following behind, and one safety snowmobile bringing up the rear. (C) Equipment was deployed each night when stopped for evening meals and sleep (see text); image is from sample site B (see Table 1). (D) Dinner was cooked with traditional LP gas stoves placed on coolers offloaded to the rear of the Okarian rover for each stop; image of site C (see Table 1). (E) Microbial sampling by author P. Lee was conducted in the morning prior to restarting the NWPDX traverse and after 12–18 h of science and residence activities near the rover (see text); image of site B sampling. Inuk guide Joe Amarualik can be seen in (B) and (D). Color images are available at www.liebertonline.com/ast.
The NWPDX-2009 field team comprised five men and consisted of three vehicles, the Okarian rover and two accompanying snowmobiles to provide scouting and logistics support. Three crewmembers rode in the rover, while the snowmobiles were each driven by one person. In addition to cargo transported inside the rover, each vehicle had a qamutiq sled in tow to transport additional equipment and supplies, including food, fuel, spare parts, and emergency gear. At night, the rover would be emptied out to make room for the crew of five to sleep. During early spring, from 10 to 17 April 2009, the Okarian was successfully driven 496 km over sea ice from Kugluktuk to Cambridge Bay, Nunavut, Canada. The vehicle reached the southwest coast of Devon Island in May 2010 during a traverse from Resolute Bay, Cornwallis Island, to Devon Island, and completed the traverse to the HMP Research Station in 2011 (Lee et al., 2015). While the primary goal of the NWPDX was to safely transfer the Okarian rover from the North American continental mainland to Devon Island by way of the Northwest Passage, a secondary and opportunistic goal was to conduct a series of research experiments in Arctic and planetary science, astrobiology, and human exploration operations during the traverse (Lee et al., 2015).
The present paper presents the results of the NWPDX-2009 astrobiology investigation, which focused on planetary protection. The NWPDX traverse offered a unique opportunity to evaluate the forward contamination of an undisturbed snow-covered region by a crewed vehicle as a proxy for gauging potential forward contamination of pristine terrains on Mars. Microbial contamination of caves (Hunter et al., 2004; Lavoie and Northup, 2006; Fernandez-Cortes et al., 2011) and of the Antarctic Dry Valleys (e.g., Tow and Cowan 2005) has been noted following extensive human visits. However, the published literature in general does not examine the initial stages of exploration but instead assesses contamination from human activities after numerous sortie missions to specific sites. The primary objective of the present study was to determine if, and to what extent, aerial dispersal of human-associated microorganisms occurred during a rover traverse over one of the most pristine terrains occurring naturally on Earth, that is, fresh snow covering seasonal sea-ice in the High Arctic tens to hundreds of kilometers away from human settlements.
2. Materials and Methods
2.1. The Okarian Humvee rover
Background on the Okarian vehicle and its pre-expedition journeys is provided in order to establish the initial history of exposure of the Okarian rover to microbial contaminants prior to the NWPDX-2009 mission, The Okarian rover is a diesel-powered model M997A maxi-ambulance military Humvee manufactured by the AM General Corporation of Mishawaka, Indiana, USA. The vehicle never served in the military but instead was assigned upon factory rollout in 1987 to Hollywood, where it served 20 years as a film industry prop, featured in numerous productions (Lee et al., 2015). The Humvee was eventually recalled by AM General in 2008, reassigned to the HMP that same year, and received the designation of HMP Okarian. Between September 2008 and February 2009, the vehicle was refurbished, and a number of modifications were made, including the strengthening of transmission links to accommodate tracks instead of wheels and the addition of a third cockpit seat. The vehicle was repainted from 10 to 20 November 2008, including the cockpit and adjoining rear cabin.
To reach Kugluktuk, the starting point of the NWPDX-2009 mission, the rover was first transported on a flatbed truck between 19 and 23 February 2009 from Mishawaka to the Mars Institute at the NASA Research Park at NASA Ames Research Center, Moffett Field, California, USA. From 12 to 13 March 2009, the vehicle was driven from Moffett Field to Vancouver, British Columbia, Canada, via San Francisco, California; Portland, Oregon; and Seattle, Washington. On 12 March, the Okarian rover stopped at the Sir Francis Drake High School in San Anselmo, California, where it was toured by several dozen students. From 1 to 2 April 2009, the rover was transported by flatbed truck from Vancouver to Edmonton, Alberta, where it was transferred to another flatbed truck and transported from 3 to 6 April to Yellowknife, Northwest Territories. On 7 April, the rover wheels were replaced by snow tracks in a hangar at the Yellowknife airport. On 8 April, the rover was flown on board a commercial C-130 Hercules transport plane from Yellowknife to Kugluktuk. On 9 April, the rover visited the Kugluktuk high school where it was again toured by several dozen students. On 10 April 2009, the rover departed Kugluktuk and began its NWPDX-2009 sea-ice journey. The total elapsed time between leaving the Mishawaka factory and departure from Kugluktuk was 50 days.
During the vehicle's entire history, including the sequence of refurbishment and transportation events leading up to its departure from Kugluktuk, no special precautions were taken to mitigate the amount or diversity of microbial contamination on or in the rover. In particular, no special processing was undertaken to sanitize the rover prior to initiating the sea-ice traverse on 10 April 2009 (Fig. 2). Not sanitizing the inside of the Okarian vehicle was done on purpose to ensure that the inside and outside of the rover would be as naturally contaminated by microorganisms as possible prior to the start of the NWPDX-2009 mission. Thus, the NWPDX traverse would represent a plausible worst-case scenario for microbial dispersal from a crewed vehicle during a terrestrial traverse over a pristine snow-covered terrain.
FIG. 2.
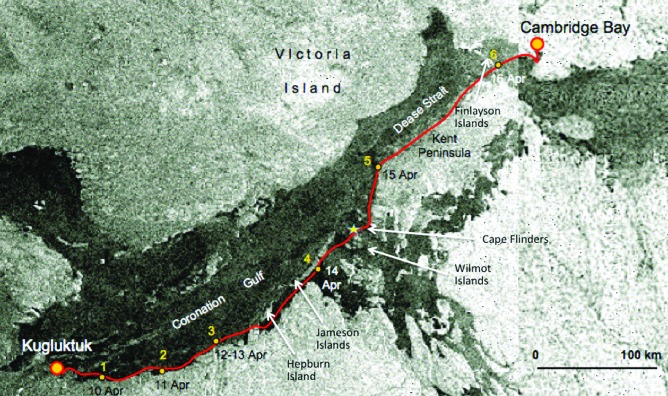
Map of the Okarian rover traverse route during the April 2009 campaign of the NWPDX, Nunavut, Canada. The expedition departed from Kugluktuk at 10:15 a.m. (local) on 10 April 2009. Overnight locations are designated by numbers. Snow sample site A was located approximately 10 km outside Kugluktuk on day one of the traverse. Snow sample sites B and C occurred at overnight locations 2 and 6, respectively. A total of 496 km was traversed by the Okarian rover expedition, arriving at Cambridge Bay at 2:00 p.m. (local) on 17 April 2009. (Adapted from Lee et al., 2015.) Color images are available at www.liebertonline.com/ast.
2.2. The NWPXD-2009 traverse
The Okarian rover and snowmobile escorts departed Kugluktuk on 10 April 2009. The rover remained on sea ice at all times until it reached Cambridge Bay on 17 April. The expedition rested overnight at a total of six camp locations between the two end points of the traverse, including two nights at Camp 3 due to bad weather (Fig. 2). Upon arriving at each overnight camp site, the rover rear cab double doors were opened, and the vehicle's contents (i.e., movable items such as coolers containing food and cooking gear stowed in its central aisle and on its sleeping bunks) were extracted and laid out on the snow or sea ice immediately behind the rover, within 3 m of the vehicle (Figs. 1C, 1D, and 3). Food preparation was done within a 3 m perimeter, either outside the rover or inside, depending on weather conditions (Figs. 1D, 3). Following dinner and in preparation for crew sleep, the rear doors of the rover were shut for the night. All five team members slept inside the Okarian rover, four on bunks and one on a floor mat in the center aisle. The rover's diesel engine was left running all night to avoid problematic cold engine restarts in the morning. The rear doors of the rover were reopened in the morning for breakfast preparations and restowing of all off-loaded gear. At each Camp, the front cockpit doors of the rover were frequently opened and closed for crew access. Urination and defecation at each Camp was carried out on the snow or sea-ice ∼20 m away from the Okarian, usually in the uptrack direction away from the rover (i.e., in the direction from which the traverse originated) and cross-wind. Human waste was not retrieved. Trash, however, was collected in plastic bags and stowed inside the rover for disposal in Cambridge Bay.
FIG. 3.
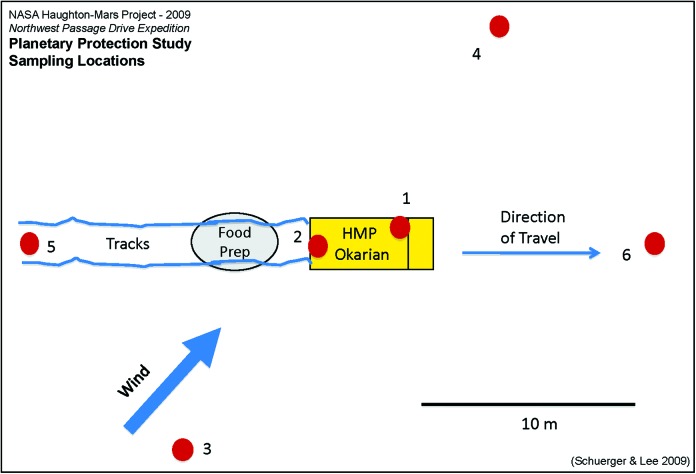
Map showing the six rover and snow sample locations in relation to the Okarian rover parked for field work at a sampling site (see text). (Adapted from Schuerger and Lee, 2010.) Color images are available at www.liebertonline.com/ast.
2.3. Collection of Okarian and snow samples
To characterize and quantify the amount of dispersed human-associated microorganisms during the traverse, snow samples were collected at several different sites along the traverse (sample sites), kept frozen, and shipped frozen at the end of the traverse from Cambridge Bay to NASA labs, Kennedy Space Center (KSC), Florida, USA. A total of three sites were sampled along the 496 km traverse. At each site, a series of six samples were collected from the same locations relative to the rover (Fig. 3), as follows: (1) inside the rover on the cockpit floor on the driver's side; (2) inside the rover in the drainage gutter on the rear cab steps; (3) 10 m outside and upwind; (4) 10 m outside and downwind; (5) 10 m outside and uptrack (behind the rover relative to the direction of travel); and (6) 10 m outside and downtrack (ahead of the rover relative to the direction of travel). At each sample site, the outside temperature and weather conditions were recorded (Table 1).
Table 1.
Sample Sites, Dates, GPS Coordinates, and Weather for the Locations Involved in the NWPDX-2009 Study
| Site | Date and Time | Coordinates | Temp. | Wind | Weather |
|---|---|---|---|---|---|
| Traverse start: Kugluktuk | 10 April 2009; 10:15 a.m. local | 67°49.32′N; 115°05.42′W | |||
| Sample site A | 10 April 2009; 11:20 a.m. local | 67°49.97′N; 115°01.19′W | −2.2°C | SW; 20 kt | Overnight; snow |
| Sample site B | 11 April 2009; 11:00 a.m. local | 67°44.12′N; 113°54.33′W | −6.8°C | SW; calm | Overnight; SW winds |
| Sample site C | 16 April 2009; 9:00 a.m. local | 69°01.02′N; 105°50.06′W | −5°C | SW; 20 kt | Overnight; snow |
| Traverse end: Cambridge Bay | 17 April 2009; 2:00 p.m. local | 69°07.02′N; 105°03.11′W |
The Okarian samples were collected with sterile spatulas and 50 cc polystyrene centrifuge tubes. The sterile spatulas were used to shovel snow and debris on the floor boards into the 50 cc tubes. The Okarian samples were collected from areas of ∼0.2 m2 during each sampling event at each site. In all cases, the sampling personnel had donned sterile surgical gloves prior to sampling but did not use sterile overcoats or sleeves due to the bulk of winter survival gear being worn (see Fig. 1).
The first site, site A, was a brief science stop. It was reached an hour after departing Kugluktuk. Site A was located ∼10 km east of Kugluktuk and was not an overnight Camp location. Site B was the first overnight site, Camp 1, and was reached after driving 56 km east from Kugluktuk. Site C was the last overnight site, Camp 6. It was located 45 km southwest of Cambridge Bay (Fig. 2). Table 1 gives dates and times, GPS coordinates, and general weather conditions at the five key locations considered in the study. Snow samples at sites B and C were collected in the morning immediately prior to departure and thus were collected following 12 and 18 h of human activity around the rover.
The snow sampling was designed to measure the aerial dispersal of microbial cells and spores falling upon undisturbed snow within ∼10 m of the rover and not in its immediate vicinity. Snow and ice surfaces upon which the crew walked, prepared food, conducted science experiments, eliminated wastes, and worked on vehicle maintenance were not directly sampled. Thus, the sample design was specifically constrained to measure aerial dispersal from human activities around a contaminated rover within a 10 m radius of the crewed vehicle but not the direct footsteps of the crew.
Samples of surface snow were collected in sterile 50 cc plastic tubes with sterile spatulas to shovel snow into the tubes. The snow samples were collected with the researcher kneeled and downwind of the sample locations, clothed in arctic polar gear, hands in sterile latex gloves, and samples collected from ∼0.2 m2 (Fig. 1E). Snow samples were kept frozen (−25°C to −5°C) during the traverse (HOBO data recorder, ONSET, Inc., Bourne, MA, USA). Once at Cambridge Bay, all samples were maintained at −20°C until shipped on ice to microbiological labs at NASA KSC, Florida, on 27 April 2009 for processing. Samples were received at KSC on 28 April 2009. Two HOBO data loggers were placed within the shipping container to KSC and confirmed that the samples were transported at temperatures below −1°C (data not shown).
2.4. Microbial procedures
All six internal rover samples arrived intact at KSC. However, only 11 of 12 snow samples survived intact during shipment to the KSC microbiological labs for processing. The uptrack sample C5 (Table 1; Fig. 3) was lost during shipment due to a cracked sidewall on the 50 cc tube. Surviving snow samples were thawed at 4°C for 48 h, vortexed for 2 min, and 100 μL aliquots plated on individual R2A agar plates at undiluted rates. All melted rover and snow samples (∼5 mL per sample) were plated on R2A agar (Difco, Fisher Scientific, Pittsburgh, PA, USA); thus there are no archived solutions. Melted snow samples were then incubated on R2A media at 4°C, 25°C, or 37°C and read after 28 days, 7 days, or 48 h, respectively. Samples incubated at 37°C were assayed for presumptive clinical isolates, at 25°C for total microbial counts, and at 4°C for psychrophiles. Unique colonial morphotypes were recovered and streak-purified on R2A agar (Fig. 4); 203 individual isolates of bacteria and 34 isolates of fungi were collected and archived.
FIG. 4.
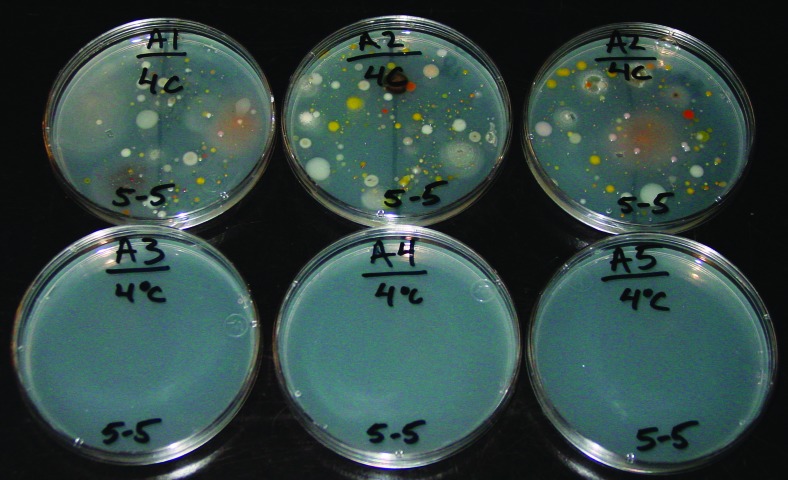
Snow samples were melted and then incubated on R2A media at 4°C, 25°C, or 37°C and read after 28 days, 7 days, or 48 h, respectively. Cultures depicted here were from 28-day cultures incubated at 4°C from sample site A. Top row of R2A plates were from within the Okarian rover from sites A1 and A2. Bottom row of R2A plates were from snow samples from sites A3, A4, and A5 (see text and Fig. 3 for sampling details). Color images are available at www.liebertonline.com/ast.
All samples were plated onto the single medium, R2A, based on two criteria: (1) our team had previous experience successfully using R2A for isolating diverse culturable bacteria and fungi from oligotrophic environments; (2) because of the small number of samples available, and the expected low numbers of dispersed cells, R2A was selected to optimize the recovery of culturable bacteria and fungi. It was undesirable to split the limited number of samples into multiple media assays and diverse incubation methods to capture what may have been extremely low to zero numbers of other taxa. Thus, the current study should be viewed as a first-order approximation of microbial transfer rates away from a crewed rover and onto pristine terrains in extreme environments.
All recovered isolates of bacteria and fungi were processed for 16S and 18S sequencing, respectively. Universal bacterial 16S primers B27F (5′-GAGTTTGATC MTGGCTCAG-3′) and B1512R (5′-AAGGAGGTGATCCANCCRCA-3′) were used as described previously (Lueders et al., 2004). Universal eukaryotic 18S primers EU18sF (5′-GGAGGGCAAGTCTGGT-3′) and EU18sR (5′-ACGGGCGGTGTGTRC-3′) were used as described by Diez et al. (2001) and Lane et al. (1985). Deoxyribonucleic acid extracts from microbial isolates were amplified by polymerase chain reaction (Benardini et al., 2003), and the 16S and 18S sequences were then characterized at the Interdisciplinary Center for Biotechnology Research (ICBR) at the University of Florida (Gainesville, FL, USA).
Taxonomic affinities were based on BLAST analysis at the National Center for Biotechnology Information (NCBI) Web site (www.ncbi.nlm.nih.gov). Isolates were identified to species based on affinities ≥97.5% (Stackebrandt et al., 2002). All isolates were archived at −70°C in 25% sterile aqueous glycerol solutions, and all 16S and 18S sequences were uploaded to GenBank [www.ncbi.nlm.nih.gov/genbank; National Center for Biotechnology Information (US), 2011]. All data on the sources and geographical locations of closest-match strains in Tables 2, 3, and 4 were derived from GenBank entries or published literature.
Table 2.
Taxonomy of Fungal Diversity within the Okarian Rover Samples from Sites A, B, and C (see Fig. 2) during the NWPDX-2009 Traverse from Kugluktuk to Cambridge Bay, Canada
| Taxonomy with 18S contigs in current studya | Strain No. current studyb | GenBank accession No. current study | Source location for NCBI closest match strainc | NCBI closest match accession No. | NCBI closest match | Number of isolates (sites) | R2A recovery temp. (°C) |
|---|---|---|---|---|---|---|---|
| Alternaria botrytis | A2F-4c-8 | JX470350 | aerosols, Sweden | AF548106 | 0.995 | 1(A) | 4 |
| Alternaria cheiranthi | B2F-25c-3 | JX456605 | plant debris, CA, USA | AF229508 | 0.997 | 1(B) | 25 |
| Aspergillus fumigatus | B1F-25c-2 | JX456606 | China | FJ560718 | 0.991 | 1(B) | 25 |
| Aspergillus oryzae | B1F-25c-2c | JX470334 | China | HM064501 | 0.985 | 1(B) | 25 |
| Chaetomium globosum | C2F-4c-1 | JX470335 | China | JN639019 | 0.997 | 1(C) | 4 |
| Cladosporium cladosporioides | A2F-4c-1 | JX470336 | South Africa; China | AY251093 | 0.998 | 1(A), 5(B) | 4, 25 |
| Cochliobolus lunatus | B1F-25c-6 | JX470339 | Echinochloa pathogen, China | DQ337381 | 0.996 | 1(B) | 25 |
| Geomyces destructans | A2F-4c-9 | JX470340 | bat pathogen, France | GQ489025 | 0.999 | 3(A), 1(C) | 4 |
| Geomyces pannorum | A2F-4c-10 | JX470341 | soil, Antarctica | AY129548 | 0.998 | 1(A) | 4 |
| Neophaeosphaeria filamentosa | C2F-25c-2 | JX470342 | USA | AF250825 | 0.999 | 2(C) | 25 |
| Penicillium expansum | B2F-4c-4 | JX470343 | human food, China | GU561988 | 0.999 | 2(B) | 4, 25 |
| Penicillium freii | A2F-25c-1 | JX470344 | Denmark | AJ005446 | 0.999 | 2(A), 3(B) | 4, 25 |
| Phoma macrostoma | A2F-25c-3 | JX470346 | plant leaf, Japan | AB454217 | 0.995 | 1(A) | 25 |
| Pleospora herbarum | B1F-25c-6b | JX470347 | Oregon, USA | DQ767648 | 0.993 | 2(B) | 25 |
| Tetracladium maxilliforme | A2F-4c-5 | JX470348 | lake water, Canada | EU883429 | 0.992 | 1(A) | 4 |
| Thelebolus microsporus | A2F-4c-11 | JX470349 | soil, Antarctica | AY942191 | 0.998 | 1(A) | 4 |
Sixteen unique fungal taxa were identified from 31 isolates recovered from interior samples in the Okarian rover.
Taxonomy was based on BLAST analysis with full contigs.
Isolate names represent samples from sites A, B, or C; sample location at each site (1–6; see Fig. 3); F=fungi; temperature for lab incubation (4°C, 25°C, or 37°C); and strain number in sequence from 1. When multiple strains were identified for a specific taxon, the highest affinity with the closest match accession number was given in the table.
CA, California; USA, United States of America.
Table 3.
Taxonomy of Bacteria Recovered from within the Okarian Rover from Sample Sites A, B, and C (see Fig. 2) during the NWPDX-2009 Traverse from Kugluktuk to Cambridge Bay, Canada
| Taxonomy with 16S contigs in current studya | Strain No. current studyb | GenBank accession No. current study | Source locations for NCBI strains closest matchc | NCBI accession No. closest match | NCBI closest match | Number of isolates (sites) | R2A recovery temp. (°C) |
|---|---|---|---|---|---|---|---|
| Aeromicrobium tamlense | C1-37c-2 | JX517204 | dried seaweed, South Korea | DQ411541 | 0.983 | 1(C) | 37 |
| Arthrobacter agilis | A2-4c-5 | JX517205 | groundwater, South Korea | EU730943 | 0.987 | 1(A) | 4 |
| Arthrobacter flavus | A2-4c-14 | JX517206 | pond water, Antarctica | FR691390 | 0.991 | 1(A) | 4 |
| Arthrobacter sp. | A2-25c-5 | JX517207 | ice/snow, Antarctica; permafrost, Norway | DQ341415 | 0.986 | 2(A), 1(B) | 4, 25 |
| Arthrobacter sulfonivorans | A2-4c-15 | JX517208 | glacial sediments, Svalbard | FM955888 | 0.995 | 3(A) | 4 |
| Arthrobacter tumbae | C1-4c-3 | JX517209 | polar ices, location unknown | EU090712 | 0.999 | 1(A) | 4 |
| Bacillus amyloliquefaciens | B1-25c-5 | JX517210 | potato rhizosphere, Peru; soil, Brazil; soil, China; melon fruit, China; potassium mine, China; water, Hungary | JX036499 | 0.999 | 5(A), 6(B) | 25, 37 |
| Bacillus cereus | B2-37c-7 | JX517211 | marine sediment, Costa Rica | EU741083 | 1.000 | 2(B), 1(C) | 25, 37 |
| Bacillus circulans | C2-37c-4 | JX517212 | mosquito midgut, India | JN644554 | 0.999 | 1(A), 2(C) | 25, 37 |
| Bacillus clausii (B1512R) | A1-37c-4 | JX517213 | Belgium | AF329475 | 0.994 | 1(A) | 37 |
| Bacillus drentensis | A1-25c-6 | JX517214 | airline cabin, CA, USA; desert granite, AZ, USA | EU379279 | 0.996 | 2(A) | 25 |
| Bacillus firmus | A2-37c-11 | JX517215 | salt lake, China | GQ903397 | 0.999 | 1(A), 2(B) | 37 |
| Bacillus horikoshii | B2-37c-8 | JX517216 | lake water, India | GU001902 | 0.999 | 1(B) | 37 |
| Bacillus korlensis | A2-37c-4 | JX517217 | desert soil, China | EU603328 | 0.996 | 2(A) | 37 |
| Bacillus licheniformis (B1512R) | A1-25c-5 | JX517218 | fermented fish, China, India, and South Korea | JX025165 | 0.999 | 2(A), 3(B) | 25 |
| Bacillus megaterium | A1-25c-21 | JX517219 | JPL clean room, CA, USA; rhizosphere, China; desert granite, AZ, USA | AY030338 | 0.999 | 2(A), 2(B) | 25, 37 |
| Bacillus nealsonii | A2-37c-15 | JX517220 | plant debris, China | FJ544393 | 0.999 | 1(A), 1(B) | 37 |
| Bacillus niacini (B1512R) | A2-37c-23 | JX517221 | soil, India | GU339292 | 0.991 | 1(A) | 37 |
| Bacillus pumilus | A1-25c-19 | JX517223 | potassium mine, China; JPL and KSC clean rooms, USA | GU332600 | 0.999 | 2(A), 2(B), 2(C) | 25, 37 |
| Bacillus selenatarsenatis | A1-37c-12 | JX517224 | mushrooms, China | EU239470 | 0.995 | 2(A) | 25, 37 |
| Bacillus simplex | A1-25c-12 | JX517225 | plant leaves, roots, alpine grass, China; desert granite, AZ, USA; Arctic Ocean | FJ999940 | 0.999 | 4(A), 2(B) | 25, 37 |
| Bacillus sp. | A2-37c-20 | JX517226 | JPL clean room, CA, USA; ocean water, Japan; desert granite, AZ, USA; uranium mine, Germany | AY030333 | 0.998 | 4(A), 4(B), 3(C) | 25, 37 |
| Bacillus thuringiensis | A1-25c-14 | JX517227 | Jatropha endophyte, Singapore | JQ659733 | 1.000 | 3(A) | 25, 37 |
| Bacillus weihenstephanensis | A2-25c-6b | JX517228 | forest soil, France | CP000903 | 1.000 | 1(A) | 25 |
| Brevibacillus brevis | B1-37c-14 | JX517229 | plant compost, Spain | EF079071 | 0.998 | 1(B) | 37 |
| Brevibacillus borstelensis | A1-37c-13 | JX517230 | fermented soybean sauce, South Korea | FJ982663 | 0.999 | 2(A) | 37 |
| Brevibacterium sp. (B1512R) | A2-37c-14 | JX517231 | oil-contaminated soil, India | GQ865646 | 1.000 | 1(A) | 37 |
| Brevundimonas mediterranea | C2-25c-16 | JX517232 | Mediterranean Sea; airline cabin, USA | AJ244706 | 0.998 | 4(C) | 4, 25 |
| Brevundimonas bullata | B2-37c-16 | JX517233 | KSC spacecraft facility, FL, USA | EU977700 | 0.997 | 3(B), 1(C) | 25, 37 |
| Brevundimonas sp. | B2-25c-4 | JX517234 | swine effluent, USA | DQ337577 | 1.000 | 3(B) | 25 |
| Cellulomonas sp. | C1-25c-1 | JX517235 | soil, Taiwan | EU303275 | 0.990 | 1(C) | 25 |
| Citricoccus sp. | C2-25c-5 | JX517236 | seawater, Japan | AB594473 | 0.999 | 1(C) | 25 |
| Cryobacterium sp. | A2-4c-18 | JX517237 | lake sediment, Antarctica | GU244362 | 1.000 | 3(A), 2(B) | 4 |
| Dietzia maris | C2-4c-4 | JX517238 | Phoenix spacecraft, KSC, FL, USA | EU977748 | 0.992 | 7(C) | 4, 25 |
| Frigoribacterium sp. | B1-25c-3 | JX517239 | alpine plants, China; soil, Antarctica | DQ339618 | 0.997 | 1(A), 1(B) | 4, 25 |
| Georgenia soli | C1-25c-3 | JX517240 | iron-ore soil, India | FN356976 | 0.987 | 1(C) | 25 |
| Knoellia sinensis | A2-25c-9 | JX517241 | JPL clean room, CA, USA | AY167851 | 0.994 | 1(A) | 25 |
| Kocuria rosea | C2-37c-6 | JX517242 | KSC clean room, FL, USA; soil, Himalayas; cucumber leaves, China | JN084149 | 0.985 | 3(C) | 25, 37 |
| Leifsonia sp. | A2-25c-1 | JX517243 | oil shale semi-coke, Estonia | EF540446 | 0.993 | 1(A) | 25 |
| Lysinibacillus sphaericus | C2-37c-8 | JX517244 | cattle manure, Sweden; beach sand, Costa Rica | EU869258 | 0.999 | 3(A), 1(B), 1(C) | 25, 37 |
| Microbacterium oxydans | A2-4c-16 | JX517246 | barnyard soil, Finland | AM237353 | 0.987 | 3(A) | 4 |
| Microbacterium sp. | C2-25c-10 | JX517247 | glacial soil, China; raw milk, India; clinical specimen, Germany; environmental, China; oil shale semi-coke, Estonia; mountain snow, China; glacial meltwater, Mt. Everest | JQ946048 | 0.999 | 5(A), 7(C) | 25, 37 |
| Mycetocola reblochoni | C2-4c-9 | JX517249 | cheese, United Kingdom | DQ062100 | 0.976 | 1(C) | 4 |
| Mycetocola sp. | A2-25c-11 | JX517250 | location unknown | GU217690 | 0.982 | 1(A) | 25 |
| Nesterenkonia sp. | C1-25c-2 | JX517251 | soda lake sediments, China | GQ404473 | 0.989 | 1(C) | 25 |
| Paenibacillus amylolyticus | C2-37c-12 | JX517252 | forest soil, China; ryegrass rhizosphere, Switzerland | AM921628 | 0.980 | 1(B), 2(C) | 25, 37 |
| Paenibacillus contaminans | A1-25c-9 | JX517254 | lab bench, Taiwan | NR044325 | 0.988 | 1(A) | 25 |
| Paenibacillus glebae | C2-37c-1 | JX517255 | soil, Netherlands | AM745264 | 0.995 | 1(C) | 37 |
| Paenibacillus illinoisensis | A1-37c-14 | JX517256 | KSC clean room, FL, USA; garden soil, Russia | DQ870759 | 0.989 | 2(A) | 37 |
| Paenibacillus lautus | A1-25c-1 | JX517257 | Etruscan tomb and soil, Italy | EU249586 | 0.999 | 1(A), 2(B) | 25, 37 |
| Paenibacillus sp. | C2-4c-13 | JX517258 | plant debris, China; environmental sample, India | JN411476 | 0.997 | 1(A), 1(C) | 4, 37 |
| Paenibacillus polymyxa | A1-25c-13 | JX517259 | lake sediment, Italy | EU362611 | 1.000 | 1(A) | 25 |
| Paenibacillus ruminocola (B27F) | B2-37c-10 | JX517260 | cattle rumen, China | DQ085279 | 0.950 | 1(B) | 37 |
| Paenibacillus sp. | A2-37c-10 | JX517261 | JPL clean room, CA, USA; plants and soils in South Korea, China, and France; sawdust, Japan | EF690425 | 1.000 | 2(A), 2(B), 2(C) | 25, 37 |
| Paenibacillus terrigena | B2-37c-9 | JX517262 | cinnamon leaves, China | FJ174658 | 0.999 | 1(B) | 37 |
| Planococcus sp. | C2-4c-3 | JX517265 | snow, Tibet | HQ327129 | 0.999 | 3(C) | 4 |
| Pseudoclavibacter helvolus | B2-25c-12 | JX517266 | snow crab, Korea | HM584267 | 0.998 | 1(B) | 25 |
| Psychrobacillus psychrodurans | B1-25c-9 | JX517222 | marine brown alga, South Korea | EF101552 | 0.985 | 1(A), 1(B) | 25, 37 |
| Rhodococcus cercidiphylli | A2-25c-2 | JX517267 | plant leaves, China | EU325542 | 0.999 | 1(A) | 25 |
| Rhodococcus sp. | A2-25c-13 | JX517268 | deep ocean sediment, China | AY188941 | 0.993 | 1(A) | 25 |
| Rhodococcus globerulus | A2-4c-12 | JX517269 | potassium mine, China | GU332596 | 0.979 | 1(A) | 4 |
| Rummeliibacillus stabekisii | B1-37c-21 | JX517270 | KSC spacecraft facility, FL, USA | DQ870754 | 0.999 | 3(B) | 37 |
| Sanguibacter antarcticus | A2-25c-3 | JX517271 | beach sand, Antarctica | EF211071 | 0.993 | 1(A) | 25 |
| Solibacillus silvestris (B27F) | C2-37c-15 | JX517272 | plant leaves, India | JQ313581 | 1.000 | 1(C) | 37 |
| Sporosarcina aquimarina | A1-37c-1 | JX517273 | JPL clean room, CA, USA; seawater, South Korea | AY167819 | 0.988 | 2(A), 3(B), 1(C) | 25, 37 |
| Sporosarcina ginsengisoli | B1-37c-26 | JX517274 | ginseng soil, South Korea | AB245381 | 0.992 | 3(B) | 37 |
| Sporosarcina sp. | A2-4c-7 | JX517264 | alpine permafrost, China | JF778686 | 0.999 | 2(A) | 4 |
| Staphylococcus pasteuri | A2-37c-19 | JX517276 | soil, Iran | FR839669 | 0.999 | 1(A) | 37 |
| Terrabacter terrae | C2-4c-14 | JX517277 | soil, Spain | AY944176 | 0.945 | 1(C) | 4 |
Sixty-nine unique bacterial taxa were identified from 185 isolates recovered from interior samples in the Okarian rover.
Taxonomy was based on BLAST analysis with full contigs unless otherwise noted for situations in which only one primer sequence was used.
Isolate names represent bacteria from snow sites A, B, or C; sample locations at each site (1–6; see Fig. 3); temperature for lab incubation (4°C, 25°C, or 37°C); and strain number in sequence from 1.
AZ, Arizona; CA, California; FL, Florida; JPL, Jet Propulsion Laboratory; KSC, Kennedy Space Center; TX, Texas; USA, United States of America.
Table 4.
Taxonomy of One Bacterium and One Fungus Recovered from Snow Samples at Sample Sites A and B, Respectively, during the NWPDX-2009 Traverse from Kugluktuk to Cambridge Bay, Canada
| Taxonomy 16S or 18S with contigs in current studya | Strain No. current studyb | GenBank accession No. current study | Source location for NCBI closest match strain | NCBI closest match accession No. | NCBI closest match | Number of isolates | R2A recovery temp. (°C) |
|---|---|---|---|---|---|---|---|
| Aspergillus fumigatus | B6F-25C-1 | JX517279 | plant debris, China | FJ560718 | 0.991 | 1(B) | 25 |
| Brevibacillus agri | A4-25C-2 | JX517278 | brick wall, China | GQ927168 | 0.998 | 1(A) | 25 |
Only one colony of each species was recovered on R2A cultures for the snow samples.
Taxonomy based on BLAST analysis with full contigs.
Isolate names represent samples from sites A, B, or C; sample location at each site (1–6; see Fig. 3); F=fungi; temperature for lab incubation (4°C, 25°C, or 37°C); and strain number in sequence from 1.
3. Results
Weather conditions during snow sampling (Table 1) ranged between calm and clear conditions (sample site B) to light to moderate snow with winds ∼20 knots from the southwest (sample site C). The individuals (P. Lee and J. Weaver) who collected all samples were positioned downwind of the actual snow or rover sample sites. The temperature ranged between −2°C and −7°C at the time of collecting snow samples.
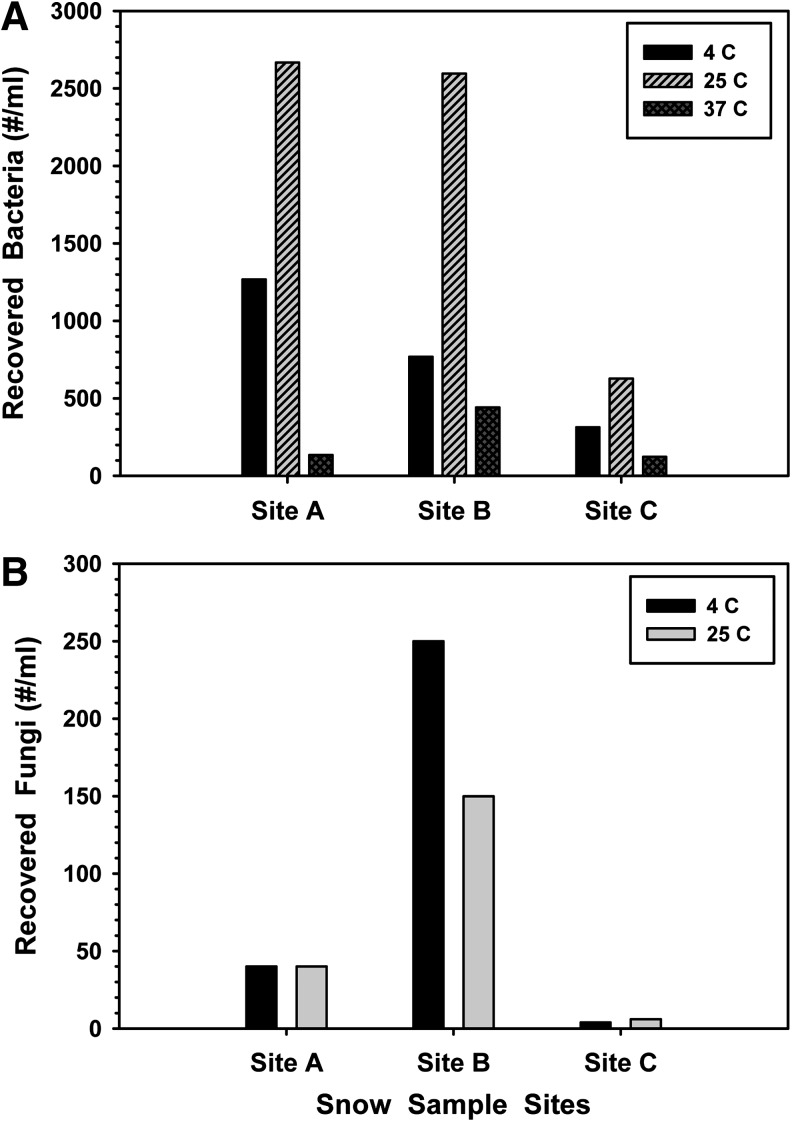
All samples collected from within the Okarian rover (from sites 1 and 2; Fig. 3) were heavily populated by both fungal and bacterial species (Tables 2 and 3). First, for cultures incubated at 37°C, 25°C, or 4°C, bacterial populations ranged between 1.25×102 and 4.50×102 cfu/mL, 6.5×102 and 2.7×103 cfu/mL, or 3.25×102 and 1.29×103 cfu/mL, respectively (Fig. 5A). In general, there was a decline in the total abundance and diversity of bacterial species (Fig. 5) recovered from samples over time, with the greatest losses observed between sample sites B (11 April 2009) and C (16 April 2009). Second, for cultures incubated at 25°C or 4°C, fungal populations ranged between <10 and 2.5×102 cfu/mL, respectively (Fig. 5B). In contrast, the population dynamics for fungi recovered from within the rover were slightly different when compared to the dynamics of recovered bacteria over time. For fungi, the populations were initially low (∼40–50 cfu/mL) at sample site A, spiked to closer to 250 cfu/mL at site B, and then plunged to <10 cfu/mL at sample site C (Fig. 5B). Third, when the numbers of bacterial isolates and unique 16S sequences were plotted for sample sites A, B, and C (Fig. 6), both the number of bacterial isolates recovered from the inside of the rover and the number of unique 16S sequences from the recovered isolates decreased linearly over time from site A to site C. Results suggest that a general decrease in microbial diversity over time occurred within the Okarian rover habitation area. And fourth, no archaea were recovered from either the snow or rover samples.
FIG. 5.
Changes in the interior Okarian microbial population densities over time between sample sites A (10 April 2009), B (11 April), and C (16 April). (A) Bacterial populations within the Okarian rover appeared to decline in a generally linear fashion over time. (B) In contrast, fungi appeared to bloom between sites A and B but then declined significantly over the 5 days between sites B and C.
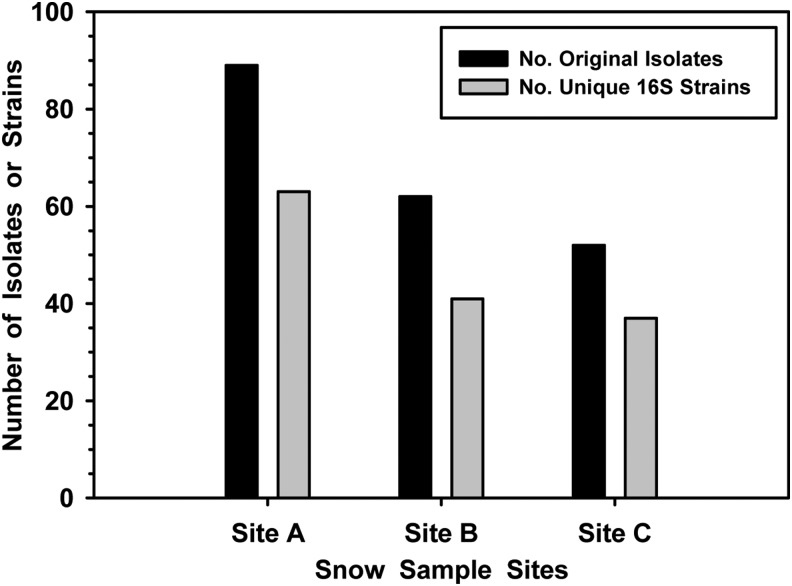
FIG. 6.
Decline in bacterial biodiversity over time in Okarian rover samples. The numbers of original isolates representing unique colony morphotypes on R2A agar declined 62% over the course of the 6-day NWPDX-2009 traverse between sites A and C. The numbers of unique 16S bacterial sequences (Table 3) declined 59% over the same time period.
The dominant fungi recovered from within the rover (Table 2) included species from the genera Alternaria, Aspergillus, Cladosporium, Geomyces, Phoma, Penicillium, and Pleospora. The dominant bacterial species recovered from within the rover (Table 3) were Arthrobacter spp., Bacillus spp., B. amyloliquefaciens, B. circulans, B. licheniformis, B. megaterium, B. pumilus, B. simplex, Brevibacillus spp., Cryobacterium sp., Kocuria rosea, Lysinibacillus sphaericus, Microbacterium spp., Paenibacillus spp., and Sporosarcina aquimarina.
Most snow samples from sites exterior to the Okarian were negative for both bacteria and fungi except for one bacterial colony recovered at site A4 (downwind) and one fungal colony recovered from site B6 (downtrack) (Table 4). The bacterial isolate was identified as Brevibacillus agri (accession JX517278; closest match 0.998), and the fungal isolate was identified as Aspergillus fumigatus (accession JX517279; closest match 0.991). Thus, while the internal samples from the rover contained a wide range of bacterial and fungal colony morphotypes, only two individual colonies were recovered from snow samples collected from the undisturbed local terrain around the rover. Snow samples yielded ∼5 mL/tube of meltwater (equal to 50 cc of packed snow) that were completely dispensed onto multiple R2A plates during the assays. Thus, in most cases, 5 mL of meltwater was free of culturable microbial contamination.
Most bacteria and fungi exhibited close affinity to other cosmopolitan species from terrestrial ecosystems. However, a large percentage of the closest-match neighbors similar to the isolates recovered during the NWPDX mission were previously identified as environmental samples from Asia. For example, when comparing the 16S or 18S sequences of the Okarian rover assays to the NCBI closest matches, 7 of 16 (44%) unique fungal taxa and 34 of 69 (49%) unique bacterial taxa were similar to species described in Asia.
4. Discussion
A Special Region on Mars is defined as “a region within which terrestrial organisms are likely to propagate, or a region which is interpreted to have a high potential for the existence of extant martian life forms” (Beaty et al., 2006; Rummel et al., 2014). Special Regions are of keen scientific interest on Mars because of the possibility of finding a second lineage of life in the Solar System. Planetary protection protocols (National Research Council, 2002, 2006; COSPAR, 2005) have been established in order to mitigate forward contamination of other planetary bodies, with an exclusionary principle adopted for Special Regions. However, to accurately model the forward contamination of planetary bodies or Special Regions under diverse mission scenarios, dispersal of terrestrial microorganisms from rovers, equipment, and humans onto local terrains must be characterized and modeled. The current research explored the microbial contamination of undisturbed snow during a crewed rover traverse along the Northwest Passage in the Arctic. The NWPDX-2009 Okarian rover was not cleaned prior to the start of the traverse and thus was considered a possible worst-case scenario for characterizing dispersal of human commensals on to a pristine snowy terrain. In addition, no special arrangements were taken to mitigate microbial dispersal during housekeeping chores around the rover at sample sites, including processing of human wastes.
As expected, the internal microbial diversity of the rover was rich in both bacteria and fungi, with at least 69 and 16 unique culturable taxa, respectively, recovered from internal surfaces. The species diversity included both environmental taxa and human commensals. In contrast, most samples collected from undisturbed snow surfaces, ∼10 m from the Okarian vehicle, were free of microbial contamination. Culturable species were recovered from only 2 of 11 samples of undisturbed snow (Table 4) and were composed of just a single culturable bacterium (Brevibacillus agri) and a single culturable fungus (Aspergillus fumigatus). The presence of two other species of Brevibacillus and of A. fumigatus in samples from within the rover suggests that the isolates recovered from the snow were also present within the rover and therefore represent plausible isolates dispersed from the rover. However, if B. agri was present as an indigenous isolate from the Arctic snow (i.e., it was not recovered in the rover), the transfer rate from the rover to the snow would be reduced by 50%. In either case, very low levels of culturable bacteria and fungi were recovered in 11 snow samples from three waypoints during the Okarian traverse.
The low recovery of bacterial and fungal taxa away from the Okarian vehicle is consistent with at least four possibilities including (1) low shedding from the rover, equipment, and human clothing (Fig. 1); (2) dispersal of microbial species but with a concomitant significant dilution effect at the sampled locations; (3) ultralow or zero dispersal away from the rover, equipment, and human clothing with direct contamination of the snow at the moment of collecting the snow samples; or (4) lab contamination during the processing of the samples. The procedures described herein were not adequate to discriminate among these alternative hypotheses, but it seems most likely that the rates of shedding and dispersal were extremely low in the cold and harsh Arctic environment during the NWPDX-2009 traverse.
Low recoveries of human commensals away from habitats or field sites in extreme environments have been reported for both Antarctica (Upton et al., 1997; Hughes, 2003; Tow and Cowan, 2005) and caves (Somavilla et al., 1978; Lavoie and Northup, 2006). In contrast, other literature has documented long-term survival of nonindigenous species in polar regions (Sjöling and Cowan, 2000; Hughes and Nobbs, 2004) and caves (Hunter et al., 2004; Fernandez-Cortes et al., 2011) that were most likely introduced by human activities. Furthermore, it appears that the initial high rates of contamination noted in some studies decreased significantly over time once the sites were abandoned (Upton et al., 1997; Lavoie and Northup, 2006). Based on our results here, and the literature cited above, we propose that the dispersal of human-associated microorganisms is likely to be low during early sortie missions in pristine environments, is likely to increase slightly over time with continued exploration of a specific site, and will decrease over time once the exploration of a given site is halted. Further study is warranted for human exploration activities at temporary field sites on pristine terrains in order to characterize the temporal changes in dispersed bioloads and microbial diversity.
In addition, it may be possible to tolerate low to moderate levels of contamination at sites knowing that the harsh environmental conditions present will constrain long-term survival or colonization of nonindigenous species. For example, several studies (Beaty et al., 2006; Stoker et al., 2010; Schuerger et al., 2013) have listed on average ∼20 biocidal or inhibitory factors present at the surface of Mars, including (top five factors): solar UV irradiation, extreme desiccation, hypobaria, anoxia, and extremely low temperatures (global average on Mars is −61°C). Of all the biocidal factors listed in these studies, the solar UV flux at the surface of Mars appears to be the most aggressive factor for the short-term inactivation of terrestrial microorganisms on spacecraft hardware. Numerous studies have demonstrated extremely short survival times of viable terrestrial microbes under martian conditions and suggest that microbial contamination on external surfaces of spacesuits, equipment, rovers, and habitats will survive only a few hours to a few sols on Sun-exposed surfaces (e.g., Horneck et al., 1971; Mancinelli and Klovstad, 2000; Schuerger et al., 2003, 2006; Newcombe et al., 2005) and only a few weeks on partially shielded surfaces like the undersides of vehicles (Moores et al., 2007).
In Fig. 7, two alternative approaches are depicted for the human exploration of planetary bodies, including Mars. First, if the astronauts will be on foot or in unpressurized rovers (Fig. 7A), the solar UV irradiation is likely to sterilize the external surfaces of the rovers, equipment, and spacesuits within very short time periods (on the order of tens of minutes) during the initial portion of each extravehicular activity (EVA) sortie (Schuerger et al., 2003; Schuerger, 2004). In contrast, if long-range pressurized rovers are employed (Fig. 7B), the risk of contamination may rise slightly when the human occupants periodically exit the pressurized habitat for short-range EVAs, although the astronauts will still be bathed in the extremely biocidal solar UV flux present on most planetary surfaces likely to be explored. One solution for mitigating against the forward contamination risk involved in cycling pressurized rovers is to employ spacesuits that remain on the exterior surface of the rover and are entered through attachment airlocks on the backs of the suits (Fig. 7C; called suit ports). For example, such an approach was recently tested in 2008 by using the Mars-1 Humvee rover at the NASA HMP site on Devon Island (Lee et al., 2010) and the NASA Space Exploration Vehicle (SEV) at Black Point Lava Flow, Arizona, USA (Fig. 7) (National Aeronautics and Space Administration, 2011). Although dust settling onto vehicles or spacesuits may attenuate a portion of the UV irradiation incident on surfaces (Schuerger et al., 2003, 2012), the biocidal activity of UV photons on the viable contamination is likely to be significant and accumulative because the dust does not cover the entire surface (Groemer et al., 2011) and scattering of UV photons around dust particles is likely to occur (Schuerger et al., 2003).
FIG. 7.
Crewed extravehicular activity (EVA) vehicles for planetary explorations. (A) Apollo 17 astronaut Eugene Cernan during third EVA at Taurus-Littrow standing adjacent to the unpressurized Lunar Rover (NASA image AS17-134-20476). (B) NASA's Space Exploration Vehicle (SEV) configured here as a small pressurized rover during field testing at the Black Point Lava Flow site, Arizona. (C) EVA suits mounted on suit ports on the rear bulkhead of the SEV. Color images are available at www.liebertonline.com/ast.
The second significant finding of the NWPDX-2009 traverse astrobiology study was the observation that significant declines in bacterial and fungal diversity occurred within the Okarian rover between sample sites A and C. Both bacterial and fungal taxa declined over time, with the simplification of the bacterial community proceeding in a linear manner from site A to C (Figs. 5A and 6). The population dynamics of fungi were more complicated, with an initial surge in fungal populations observed over a 24 h period between samples taken at sites A and B, followed by a dramatic decline in species diversity between sites B and C. Similar simplifications of microbial diversity have been observed in the underwater Tektite I dive (Levine et al., 1970), closed ecological life support systems (see reviews by Schuerger, 1998, 2004), and spacecraft (Fox, 1971; Henney et al., 1978). What was surprising here was that the process of species simplification was observed for bacteria in only 24 h and for fungi in only 6 days, which is significantly faster than previous reports for isolated crewed habitats (Levine et al., 1970; Fox, 1971; Henney et al., 1978). Community simplification has been attributed to the isolation of microbial communities leading to decreased microbial exchange rates between ecosystems or niches and to the presence of the harsh environmental conditions themselves (see reviews by Horneck et al., 2010; Schuerger, 2004; Taylor, 1974). Thus, it is reasonable to expect that reductions in species diversity inside human habitats on planetary surfaces will in turn lead to decreased species diversity potentially dispersed onto planetary surfaces. The results may be lowered risks of forward contamination of pristine terrains on planetary surfaces or Special Regions on Mars.
And the third significant finding of the current work was that the microbial diversity of recovered taxa from within the rover was related to bacterial and fungal species from widely spaced ecosystems on Earth. Of special note is the recovery of a wide diversity of both bacteria and fungi in which the closest match strains in the NCBI database were from China and southeast Asia (Tables 2, 3, and 4). The routes of microbial contamination into the Okarian rover are not identified here but are likely to include airborne, soil-borne, and human routes of infestation. One intriguing possibility is that the significant abundance of Okarian taxa with 16S or 18S affiliations with related species from Asia may indicate that seasonal dust storms from Asia to the west coast of the United States (Smith et al., 2013) may constitute a source of potential contamination into spacecraft handling facilities in California, Oregon, and Washington.
The Okarian vehicle was not sanitized during any portion of its trip beginning at the refurbishment facility in Mishawaka, Indiana; followed by travel through California, Oregon, Washington, British Columbia, Alberta, and the Northwest Territories; and terminating in a C-130 flight to Kugluktuk, Canada. Two school visits and the NWPDX-2009 traverse preparations all contributed to the internal microbial bioloads of the Okarian rover. But given the wide range of cosmopolitan species that were recovered from within the rover, only one colony-forming unit each of A. fumigatus and B. agri were recovered from undisturbed snow samples during the NWPDX-2009 traverse. Thus, we propose that a combination of (1) low rates of dispersal from hardware or spacesuits in extreme environments, (2) simplification of microbial diversity in human habitats or on robotic rovers over time, and (3) biocidal and inhibitory factors on the surface of Mars suggest that the forward contamination of pristine martian terrains is unlikely to constitute a significant risk in the robotic or human exploration of Mars.
The current project should be viewed as a first-order approximation of the transfer rate for microbial taxa away from crewed vehicles and onto previously untraveled terrains. The information is particularly useful for modeling the forward contamination risks of human missions to Mars. However, the current study was limited by design (i.e., due to budget, time, and safety constraints) to the characterization of culturable bacteria and fungi away from the Okarian rover and did not examine the dispersal of archaea, viruses, or other nonculturable taxa. In addition, the Okarian rover was not pressurized, and pressure differentials between the interior of a pressurized rover and the external martian environment might exacerbate forward contamination risks on Mars. Additional research is warranted to better characterize dispersal rates, transfer distances and mechanisms, and the metagenomics of all microbial taxa transported away from crewed habitats and rovers in extreme environments.
Abbreviations Used
- cfus
colony-forming units
- EVA
extravehicular activity
- HMP
Haughton-Mars Project
- KSC
Kennedy Space Center
- NCBI
National Center for Biotechnology Information
- NWPDX
Northwest Passage Drive Expedition
Acknowledgments
The research was supported by two research grants (NNX12AJ84G and NNX08AQ81A) from NASA's Planetary Protection Office and through a Cooperative Agreement (NNX08AO59A) by NASA's Human Exploration and Operations Mission Directorate (HEOMD). The NASA Haughton-Mars Project and the Northwest Passage Drive Expedition (NWPDX) were sponsored by the Mars Institute, the SETI Institute, and the National Aeronautics and Space Administration (NASA). We would like to thank W.L. Nicholson for his suggestions on 16S and 18S sequencing protocols and the loan of specialized equipment for a portion of the lab work described herein. Thanks are also owed to the many other sponsors and supporters of the NWPDX expedition including the California Air National Guard; AM General Corporation; Cornell University; Simon Fraser University; University of Alberta; the National Space Biomedical Research Institute; the Canadian Space Agency, Bombardier, Inc.; First Air, Inc.; Hamilton Sundstrand Corporation; the Nunavut Research Institute; Aboriginal Affairs and Northern Development Canada; the Polar Continental Shelf Project of Natural Resources Canada; and the Nunavut communities of Kugluktuk, Cambridge Bay, Gjoa Haven, Resolute Bay, and Grise Fiord. Pascal Lee expresses special thanks to NWPDX-2009 field team members John W. Schutt, Joe Amarualik, Jesse T. Weaver, and Mark Carroll for the excellent support and enthusiasm during the NWPDX-2009 traverse. Kira Lorber and Stephen Braham are thanked for logistics and communications support, respectively.
Author Disclosure Statement
No competing financial interests exist for the authors, Andrew Schuerger and Pascal Lee.
References
- Beaty D.W., Buxbaum K., Meyer M., Boynton W.V., Clark B.C., Deming J.W., Doran P.T., Edgett K.S., Hecht M.H., Hipkin V., Kieft T., McDonald E., McKay C.P., Mellon M.T., Newsom H.E., Ori G., Paige D., Schuerger A.C., Sogin M., Spry A., Steele A., Tanaka K.L., and Voytek M.A. (2006) Findings of the Mars Special Regions Science Analysis Group. Astrobiology 6:677–732 [DOI] [PubMed] [Google Scholar]
- Benardini J.N., Sawyer J., Venkateswaran K., and Nicholson W.L. (2003) Spore UV and acceleration resistance of endolithic Bacillus pumilus and Bacillus subtilis isolates obtained from Sonoran Desert basalt: implications for lithopanspermia. Astrobiology 3:709–717 [DOI] [PubMed] [Google Scholar]
- COSPAR. (2005) COSPAR Planetary Protection Policy (20 October 2002; amended 24 March 2005), COSPAR, Paris: Available online at http://cosparhq.cnes.fr/Scistr/Pppolicy.htm [Google Scholar]
- Diez B., Pedros-Alio C., and Massana R. (2001) Study of genetic diversity of eukaryotic picoplankton in different oceanic regions by small-subunit rRNA gene cloning and sequencing. Appl Environ Microbiol 67:2932–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cortes A., Cuezva S., Sanchez-Moral S., Canaveras J.C., Porca E., Jurado V., Martin-Sanchez P.M., and Saiz-Jimenez C. (2011) Detection of human-induced environmental disturbances in a show cave. Environ Sci Pollut Res Int 18:1037–1045 [DOI] [PubMed] [Google Scholar]
- Fox L. (1971) The ecology of micro-organisms in a closed environment. Life Sci Space Res 9:69–74 [PubMed] [Google Scholar]
- Gilmour I., Sephton M.A., Conway A., Jones B.W., Rothery D.A., and Zarnecki J.C. (2003) An Introduction to Astrobiology, Cambridge University Press, New York [Google Scholar]
- Groemer G.E., Storrie-Lombardi M., Sattler B., Hauser O., Bickert K., Hauth E., Hauth S., Luger U., Schildhammer D., Foeger D., and Klauck J. (2011) Reducing biological contamination by a space suited astronaut: laboratory and field results from Aouda.X. Acta Astronaut 68:218–225 [Google Scholar]
- Harris C.M. (1998) Science and environmental management in the McMurdo Dry Valleys, Antarctica. In Ecosystem Processes in a Polar Desert: The McMurdo Dry Valleys, Antarctica, Antarctic Research Series Vol. 72, edited by Priscu J., American Geophysical Union, Washington, DC, pp 1–13 [Google Scholar]
- Henney M.R., Raylor G.R., and Molina T.C. (1978) Mycological profile of crew during 56-day simulated orbital flight. Mycopathologia 63:131–144 [DOI] [PubMed] [Google Scholar]
- Horneck G. and Baumstark-Khan C. (2002) Astrobiology: The Quest for the Conditions of Life, Springer-Verlag, Köln, Germany [Google Scholar]
- Horneck G., Bucker H., and Wollenhaupt H. (1971) Survival of bacterial spores under some simulated lunar surface conditions. Life Sci Space Res 9:119–124 [PubMed] [Google Scholar]
- Horneck G., Klaus D.M., and Mancinelli R.L. (2010) Space microbiology. Microbiol Mol Biol Rev 74:121–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K.A. (2003) Influence of seasonal environmental variables on the distribution of presumptive fecal coliforms around an Antarctic research station. Appl Environ Microbiol 69:4884–4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K.A. and Nobbs S.J. (2004) Long-term survival of human faecal microorganisms on the Antarctic Peninsula. Antarct Sci 16:293–297 [Google Scholar]
- Hunter A.J., Northup D.E., Dahm C.N., and Boston P.J. (2004) Persistent coliform contamination in Lechugilla Cave pools. J Caves Karst Stud 66:102–110 [Google Scholar]
- Lane D.J., Pace B., Olsen G.J., Stahl D.A., Sogin M.L, and Pace N.R. (1985) Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci USA 82:6955–6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie K.H. and Northup D.E. (2006) Bacteria as indicators of human impact in caves. In Proceedings of the 2005 National Cave and Karst Management Symposium: Albany, New York, October31 to November4, 2005, the NCKMS Steering Committee, Huntsville, AL, pp 40–47 [Google Scholar]
- Lee P. (1997) A unique Mars/Early Mars analog on Earth: the Haughton impact structure, Devon Island, Canadian Arctic [abstract 3059]. In Conference on Early Mars: Geologic and Hydrologic Evolution, Physical and Chemical Environments and the Implications for Life, LPI Contribution Number 916, Lunar and Planetary Institute, Houston [Google Scholar]
- Lee P. (2002) Mars on Earth: the NASA Haughton-Mars Project. Ad Astra May-Jun 2002, pp 12–17, continued on pp 51–53 [Google Scholar]
- Lee P. (2010) Northwest Passage Drive: Preparing for Mars. Above & Beyond, Canada's Arctic Journal. Sep-Oct 2010, pp 35–39 [Google Scholar]
- Lee P., Braham S., Fong T., Helper M.A., Hodges K., Hurtado J.M., Jr., McKay C.P., and Schutt J.W. (2010) Planetary field geology: right and wrong lessons from terrestrial analogs [abstract 181719]. GSA Abstracts with Programs 42:67 [Google Scholar]
- Lee P., Braham S., Deans D., Fong T., Heggy E., Helper M., Hodgson E., Hoffman S.J., and Schutt J.W. (2011) Pressurized rover-based IVA field science: lessons learned from Moon and Mars analog studies at the Haughton-Mars Project, Devon Island, High Arctic [abstract 2656]. In 42nd Lunar and Planetary Science Conference Abstracts, Lunar and Planetary Institute, Houston [Google Scholar]
- Lee P., Schutt J.W., Amarualik J., Weaver J.T., Carroll J.T., Carroll M., Jeauffre J.-C., Mueller R., Shapiro J., Boucher M., Braham S., Lorber K., Fong T., Haas C., Hoffman S.J., Leonard M., Schuerger A.C., Thomas P.C., Thompson W., Bonadies T., Camblin J., Jr., and Godbout D. (2015) The Northwest Passage Drive Expedition: Lessons for the Human Exploration of the Moon, Mars, and Near-Earth Objects, Using Pressurized Vehicles, Mars Institute Technical Publication 2015-001, Mars Institute, Moffett Field, CA, in press [Google Scholar]
- Levine H.B., Cobb J.M., and Cobet A.B. (1970) The Tektite-I drive: mycological aspects. Arch Environ Health 20:500–505 [DOI] [PubMed] [Google Scholar]
- Lueders T., Manefield M., and Friedrich M.W. (2004) Enhanced sensitivity of DNA- and rRNA-based stable isotope probing by fractionation and quantitative analysis of isopycnic centrifugation gradients. Environ Microbiol 6:73–78 [DOI] [PubMed] [Google Scholar]
- Mancinelli R.L. and Klovstad M. (2000) Martian soil and UV radiation: microbial viability assessment on spacecraft surfaces. Planet Space Sci 48:1093–1097 [Google Scholar]
- Moores J.E., Smith P.H., Tanner R., Schuerger A.C., and Venkateswaran K. (2007) The shielding effect of small-scale martian surface geometry on ultraviolet flux. Icarus 192:417–433 [Google Scholar]
- National Aeronautics and Space Administration. (2011) NASA's Analog Missions: Paving the Way for Space Exploration, NP-2011-06-395-LaRC, NASA Langley Research Center, Hampton, VA [Google Scholar]
- National Center for Biotechnology Information (US). (2011) The GenBank Submissions Handbook, NCBI, Bethesda, MD [Google Scholar]
- National Research Council. (2002) Safe on Mars: Precursor Measurements Necessary to Support Human Operations on the Martian Surface, edited by Hauck F.H., National Academy Press, Washington, DC [Google Scholar]
- National Research Council. (2006) Preventing the Forward Contamination of Mars, edited by Chyba C.C., National Academies Press, Washington, DC [Google Scholar]
- Newcombe D.A., Schuerger A.C., Benardini J.N., Dickinson D., Tanner R., and Venkateswaran K. (2005) Survival of spacecraft-associated microorganisms under simulated martian UV irradiation. Appl Environ Microbiol 71:8147–8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel J.D., Beaty D.W., Jones M.A., Bakermans C., Barlow N.G., Boston P.J., Chevrier V.F., Clark B.C., de Vera J.-P., Gough R.V., Hallsworth J.E., Head J.W., Hipkin V.J., Kieft T.L., McEwen A.S., Mellon M.T., Mikucki J.A., Nicholoson W.L., Omelon C.R., Peterson R., Roden E.E., Lollar B.S., Tanaka K.L., Viola D., and Wray J.J. (2014) A new analysis of Mars “Special Regions”: findings of the second MEPAG Special Regions Science Analysis Group (SR-SAG2). Astrobiology 14:887–968 [DOI] [PubMed] [Google Scholar]
- Schuerger A.C. (1998) Microbial contamination of advanced life support (ALS) systems poses a moderate threat to the long-term stability of space-based bioregenerative systems. Life Support Biosph Sci 5:325–337 [PubMed] [Google Scholar]
- Schuerger A.C. (2004) Microbial ecology of the surface exploration of Mars with human-operated vehicles. In Martian Expedition Planning, edited by Cockell C.S., Univelt Publishers, Escondido, CA, pp 363–386 [Google Scholar]
- Schuerger A.C. and Lee P. (2010) Extremely low levels of dispersal observed for human-associated microbes onto a local pristine terrain during a simulated Moon traverse on sea ice along the Northwest Passage in the Arctic [abstract 5377]. In Astrobiology Science Conference 2010: Evolution and Life: Surviving Catastrophes and Extremes on Earth and Beyond, Lunar and Planetary Institute, Houston [Google Scholar]
- Schuerger A.C., Mancinelli R.L., Kern R.G., Rothschild L.J., and McKay C.P. (2003) Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated martian environments: implications for the forward contamination of Mars. Icarus 165:253–276 [DOI] [PubMed] [Google Scholar]
- Schuerger A.C., Richards J.T., Newcombe D.A., and Venkateswaran K. (2006) Rapid inactivation of seven Bacillus spp. under simulated Mars UV irradiation. Icarus 181:52–62 [Google Scholar]
- Schuerger A.C., Golden D.C., and Ming D.W. (2012) Biotoxicity of Mars soils: 1. Dry deposition of analog soils on microbial colonies and survival under martian conditions. Planet Space Sci 72:91–101 [Google Scholar]
- Schuerger A.C., Ulrich R., Berry B.J., and Nicholson W.L. (2013) Growth of Serratia liquefaciens under 7 mbar, 0 °C, and CO2-enriched anoxic atmospheres. Astrobiology 13:115–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöling S. and Cowan D.A. (2000) Detecting human bacterial contamination in Antarctic soils. Polar Biol 23:644–650 [Google Scholar]
- Smith D.J., Timonen H.J., Jaffe D.A., Griffin D.W., Birmle M.N., Perry K.D., Ward P.D., and Roberts M.S. (2013) Intercontinental dispersal of bacteria and archaea by transpacific winds. Appl Environ Microbiol 79:1134–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somavilla J.F., Khayyat N., and Arroyo V. (1978) A comparative study of the microorganisms present in the Altamira and La Pasiega caves. International Biodeterioration Bulletin 14:103–109 [Google Scholar]
- Stackebrandt E., Fredrickson J.K., Garrity G.M., Grimont P.A.D., Kämpfer P., Maiden C.J., Nesme X., Roselló-Mora R., Swings J., Trüper H.G., Vauterin L., Ward A.C., and Whitman W.B. (2002) Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int J Syst Evol Microbiol 52:1043–1047 [DOI] [PubMed] [Google Scholar]
- Stoker C.R., Zent A.P., Catling D.C., Douglas S., Marshall J.R., Archer D., Jr., Clark B.C., Kounaves S.P., Lemmon M.T., Quinn R., Renno N.O., Smith P.H., and Young S.M.M. (2010) Habitability of the Phoenix landing site. J Geophys Res Planets 115, doi: 10.1029/2009JE003421 [DOI] [Google Scholar]
- Taylor G.R. (1974) Space microbiology. Annu Rev Microbiol 28:121–137 [DOI] [PubMed] [Google Scholar]
- Tow L.A. and Cowan D.A. (2005) Dissemination and survival of non-indigenous bacterial genomes in pristine Antarctic environments. Extremophiles 9:385–389 [DOI] [PubMed] [Google Scholar]
- Upton M., Pennington T.H., Haston W., and Forbes K.J. (1997) Detection of human commensals in the area around an Antarctic research station. Antarct Sci 9:156–161 [Google Scholar]