Abstract
Background
Measurement of prostate-specific antigen (PSA) advanced the diagnostic and prognostic potential for prostate cancer (PCa). However, due to PSA’s lack of specificity, novel biomarkers are needed to improve risk assessment and ensure optimal personalized therapy. A set of protein molecules as potential biomarkers was therefore evaluated in serum of PCa patients.
Methods
Serum samples from patients undergoing radical prostatectomy (RPE) for biopsy-proven PCa without neoadjuvant treatment were compared to serum samples from healthy subjects. Preliminary screening of 119 proteins in 10 PCa patients and 10 controls was carried out by the Proteome Profiler Antibody Array. Those markers showing distinct differences between patients and controls were then further evaluated by ELISA in the serum of 165 PCa patients and 19 controls. Uni- and multivariate as well as correlation analysis were performed to test the capability of these molecules to detect disease and predict pathological outcome.
Results
Screening showed that soluble (s)E-cadherin, E-selectin, MMP2, MMP9, TIMP1, TIMP2, Galectin and Clusterin warranted further evaluation. sE-Cadherin, TIMP1, Galectin and Clusterin were significantly over- and MMP9 under-expressed in PCa compared to controls. The concentration of sE-cadherin, MMP2 and Clusterin correlated negatively and that of MMP9 and TIMP1 positively with the Gleason Sum at prostatectomy. Only sE-cadherin significantly correlated with the highest Gleason pattern. Compared to serum PSA, sE-cadherin provided an independent and better matching predictive ability for discriminating PCas with an upgrade at RPE and aggressive tumors with a Gleason Sum ≥7.
Conclusions
sE-cadherin performed most favorably from a large panel of serum proteins in terms of diagnostic and predictive potential in curatively treatable PCa. sE-cadherin merits further investigation as a biomarker for PCa.
Keywords: Prostate cancer, Diagnosis, Prediction, Biomarker, sE-cadherin
Background
Prostate cancer (PCa) is currently the most prevalent male neoplasia in industrialized countries, accounting for almost 12 % of cancer cases annually [1]. Besides incumbent morbidity, the disease is associated with a significant economic burden. Declining mortality is expected to cause increases in healthcare costs as a result of increased diagnosis, diagnosis at an earlier stage and increased survival [2].
Due to the biological heterogeneity of PCa and rapidly expanding treatment options, individualized risk-adapted therapy incorporating patient- and tumor-specific characteristics is required to optimize outcome and avoid over-treatment with unnecessary adverse effects [3, 4]. Since not only radical prostatectomy (RPE) or radiation, but also active surveillance possibly postponing definite therapy, currently represent state-of-the-art care for patients with a localized PCa and a long life expectancy [5], identifying cancer types with high progression risk is indispensable in determining a treatment course. Nomograms, neural networks and predictive tables have all been developed in the past years to tailor decision-making at different stages of PCa diagnosis and treatment [6]. Being limited in their clinical application by problematic generalization and population-specific issues [7], they provide an average accuracy of 70 % [6]. To improve their performance, identification of appropriate molecular biomarkers and possibly their incorporation into predictive models is crucial [7].
Until now, prostate specific antigen (PSA) remains the only clinically relevant diagnostic and follow-up biomarker [8]. Recent research has been aimed at finding markers to overcome the limits of PSA, not only to diagnose PCa but also to distinguish between indolent and clinically significant disease [9]. Despite initial promising reports, these marker-candidates have fallen short following extensive validation and have not proven to be of routine value.
Marker detection in serum is attractive since sampling is simple and ideal for screening and prognostic evaluation of PCa to facilitate treatment choices for patients with local disease [10]. A panel of serum candidate markers known to be involved in inflammatory processes and tumorigenesis of different cancer types was evaluated for diagnostic and predictive potential in a cohort of patients with PCa.
Methods
Patients and controls
Study approval was granted by the local medical ethics committee (project number SUG_01-2014). Patients (n = 165) undergoing curative radical prostatectomy for biopsy-proven PCa without any neoadjuvant treatment between October 2010 and June 2014 in the Department of Urology, Goethe-University, Frankfurt am Main, Germany, were included in the study after having signed informed consent to utilize biomaterial for scientific assessment. The clinical tumor stage was classified according to the 7th edition of AJCC [11] and the pathological tumor stage was determined according to the 6th edition of the TNM classification [12]. Tumors were graded with the Gleason Sum (GS) [13]. Clinical and histological characteristics were collected from patient charts. Risk classification was determined according to D`Amico et al. [14]. Imaging was carried out in the high-risk group according to the guidelines of the European Association of Urology [15]. Epstein criteria [16] were used to assess the clinical significance of the tumor. Controls (n = 19) were healthy, age-matched, male volunteers.
Blood samples
10 ml peripheral blood was drawn from patients several days before surgery and from controls. Blood samples were allowed to coagulate and then centrifuged at 3000 rpm at +4 °C for 10 min. The serum supernatant was stored at -80 °C.
Proteome profiler antibody array
Serum from 10 PCa patients and from 10 controls were applied to a proteome profiler array to screen 119 markers (Human soluble Receptor Array Kit, Non Hematopoietic Panel, R&D systems, MN, USA) (Table 1) according to the manufacturer’s instructions. Briefly, membranes spotted with antibodies were blocked with Array buffer for 1 h at room temperature on a shaker followed by incubation with 1 ml serum mixed with 3 ml of array buffer overnight at 4 °C. On the next day membranes were washed 3x with wash buffer and further incubated with a detection antibody cocktail for 2 h at room temperature on a shaker. Membranes were again washed 3x with wash buffer and incubated with Streptavidin-HRP for 30 min at room temperature on a shaker. Signals were visualized using the Chemi reagent mix on a FUSION FX-7 imaging device (Vilber Lourmat, Torcy, France).
Table 1.
Markers screened with Proteome Profiling Antibody
| A: Non-Hematopoietic Array | ||||
| ADAM15 | βIG-H3 | BMPR-IB/ALK-6 | Cadherin-4/R-Cadherin | Cadherin-11 |
| Cadherin-13 | E-Cadherin | N-Cadherin | P-Cadherin | VE-Cadherin |
| Cathepsin-D | CD40/TNFRSF5 | CEACAM-5/CD66e | CHL-1/L1CAM-2 | Clusterin |
| Coagulation-Factor II/Thrombin | COMP/Thrombospondin | CRELD2 | Desmoglein 2 | ECM-1 |
| EGF R/ErbB1 | Endoglycan | EpCAM/TROP-1 | ErbB2/HER2 | ErbB3/HER3 |
| ErbB4/HER4 | ESAM | Galectin-2 | HPRG | Integrin α3/CD49c |
| Integrin α5/CD49e | Integrin α6/CD49f | Integrin α9 | Integrin αV/CD51 | Jagged 1 |
| JAM-B/VE-JAM | JAM-C | LRP-6 | MCAM/CD146 | MEPE |
| MUCDHL | Nectin-2/CD112 | Nectin-4 | Neurotrimin | Notch-1 |
| NrCAM | Periostin/OSF-2 | Podocalyxin | E-Selectin/CD62e | Semaphorin-3A |
| SREC-I/SR-F1 | SREC-II | Stanniocalcin 1 | Syndecan-1/CD138 | Syndecan-4 |
| Thrombospondin-2 | TIMP-4 | TROP-2 | VAP-1/AOC3 | VCAM-1 |
| VEGF R1/Flt-1 | VEGF R2/KDR/Flk-1 | |||
| B: Common analytes array | ||||
| ACE | ADAM8 | ADAM9 | ADAM10 | ALCAM/CD166 |
| Amphiregulin | APP (pan) | BACE-1 | BCAM | C1qR1/CD93 |
| CD9 | CD23/Fc ε RII | CD31/PECAM-1 | CD36/SR-B3 | CD40 Ligand/TNFSF5 |
| CD44H | CD58/LFA-3 | CD90/Thy1 | CD99 | CD155/PVR |
| CEACAM-1/CD66a | CX3CL1/Fractalkine | CXCL8/IL-8 | EMMPRINN/CD147 | Endoglin/CD105 |
| Epiregulin | Galectin-1 | Galectin-3 | Galectin-3BP/MAC-2BP | HB-EGF |
| ICAM-2/CD102 | IL-1 RII | IL-15 Rα | Integrin β1/CD29 | Integrin β2/CD18 |
| Integrin β3/CD61 | Integrin β4/CD104 | Integrin β5 | Integrin β6 | JAM-A |
| Lipoclain-2/NGAL | LOX-1/SR-E1 | MD-1 | MMP-2 (total) | NCAM-1/CD56 |
| NCAM-L1 | Osteopontin | PAR1 | Pref-1/DLK-1/FA1 | RECK |
| Stabilin-1 | TACE/ADAM17 | Thrombospondin | TIMP-1 | TIMP-2 |
| TIMP-3 | TNF RII/TNFRSF1B | |||
Elisa
E-selectin, soluble (s)E-Cadherin, MMP2, MMP9, TIMP1, TIMP2, Galectin and Clusterin, were identified as distinctly differently expressed in the serum of PCa patients and controls in the Proteome Profiler Antibody Array screening. The concentration of these markers was determined in the serum of 165 PCa patients and 19 controls using commercially available ELISA Kits (R&DSystems, MN, USA) according to the manufacturer’s instructions. Briefly, serum was diluted, pipetted into wells and incubated at 37 °C or RT for 1-2 h according to kit instructions. After incubation plates were washed 4x, followed by incubation with detection antibodies for 30 min or 1 h at RT and wells were subsequently washed 4x. TMB substrate was added to each well for 20 min at RT, followed by the addition of stop solution. The optical density was measured spectrophotometrically with an ELISA reader (Infinite M200 series, Tecan, Crailsheim, Germany). All assays were done in duplicate and the concentration was calculated from a standard curve using a 4-parameter curve fit (Magellan software, Tecan).
Statistics
Univariate analysis was performed by the Wilcoxon-Man-Whitney-test for comparison between two groups and the Kruskal-Wallis-test with the Iman-Conover-method (Bonferroni-Holm-corrected) for more than two groups. Bi- and multivariate analysis was carried out by logistic regression for two target values and multiple regression for more than two target values. Correlation between two parameters was evaluated through Spearman’s coefficient. The statistical program applied was BiASfuer Windows (Version 9.11, Dr. rer. nat. Hanns Ackermann, epsilon-publishers, Frankfurt, Germany). The null hypothesis was rejected if p-values were less than 0.05. Continuous data were presented as median (range) or number (%), as applicable.
Results
Clinical and pathologic demographics of 165 patients are shown in Table 2. The median age at tumor diagnosis was 65 years and the median serum PSA was 8.1 ng/ml. Nearly all PCas submitted to surgery were clinically significant. All patients were clinically free of visceral or bone disease. None of the patients had evident clinical signs of infection or acute or chronic inflammation at surgery. Histologically, all tumors were conventional acinar adenocarcinomas.
Table 2.
Clinical and histopathological demographics of 165 PCa patients
| n = 165 (100 %) | |
|---|---|
| Age, yrs | 65 (range: 40-88) |
| pT-stage | |
| <3 | 113 (68.5) |
| ≥3 | 52 (31.5) |
| Extracapsular infiltration | 34 (20.6) |
| Infiltration of seminal vesicles | 18 (11.0) |
| cT-stage | |
| <3 | 158 (95.8) |
| ≥3 | 7 (4.2) |
| Serum-PSA, ng/ml | 8.1 (1.8-136) |
| PSAD, ng/ml/ml | 0.2 (0.1-1.8) |
| Abnormal DRE | 65 (39.4) |
| Prostate volume, ml | 33 (10-120) |
| Gleason Sum (Biopsy) | |
| ≤6 | 86 (52.1) |
| 7 | 55 (33.3) |
| ≥8 | 24 (14.5) |
| Highest Gleason Pattern (Biopsy) | |
| 3 | 70 (42.4) |
| 4 | 62 (37.6) |
| 5 | 14 (8.5) |
| Gleason Sum (RPE) | |
| 6 | 27 (16.4) |
| 7 | 99 (60.0) |
| ≥8 | 39 (23.6) |
| Highest Gleason Pattern (RPE) | |
| 3 | 28 (17.0) |
| 4 | 104 (63.0) |
| 5 | 33 (20.0) |
| Gleason Sum change | |
| upgrade | 85 (51.5) |
| downgrade | 15 (9.0) |
| Clinically significant PCa (Epstein) | 158 (95.8) |
| D’Amico Classification | |
| Low risk | 55 (33.3) |
| Intermediate risk | 61 (37.0) |
| High risk | 49 (29.7) |
| N+ | 22 (13.3) |
| R+ | 36 (21.8) |
| L+ | 29 (17.6) |
| V+ | 2 (1.2) |
| Pn+ | 106 (64.2) |
Values expressed as median with range or number (%). RPE, radical prostatectomy; DRE, digital rectal examination, PSAD, PSA density
The screening analysis by Proteome Profiler showed distinctly altered expression of sE-cadherin, E-selectin, MMP2, MMP9, TIMP1, TIMP2, Galectin and Clusterin in the serum of tumor patients compared to controls. Further ELISA investigations were based on these markers.
Univariate analysis of the ELISA investigation demonstrated that serum sE-Cadherin, TIMP1, Galectin and Clusterin were significantly over- and MMP9 under-expressed in PCa patients compared to healthy controls (all p < 0.05, Fig. 1). The concentration of sE-Cadherin, MMP2 and Clusterin correlated negatively and that of MMP9 and TIMP1 positively with GS at prostatectomy (all p < 0.05, Table 3), but only sE-cadherin significantly correlated with the highest Gleason pattern (p < 0.05, Fig. 2a). In the group of patients with a GS upgrade from prostate biopsy to prostatectomy specimen, a decreased concentration of sE-cadherin was observed, compared to patients without upgrade (p < 0.05, Fig. 2b).
Fig. 1.
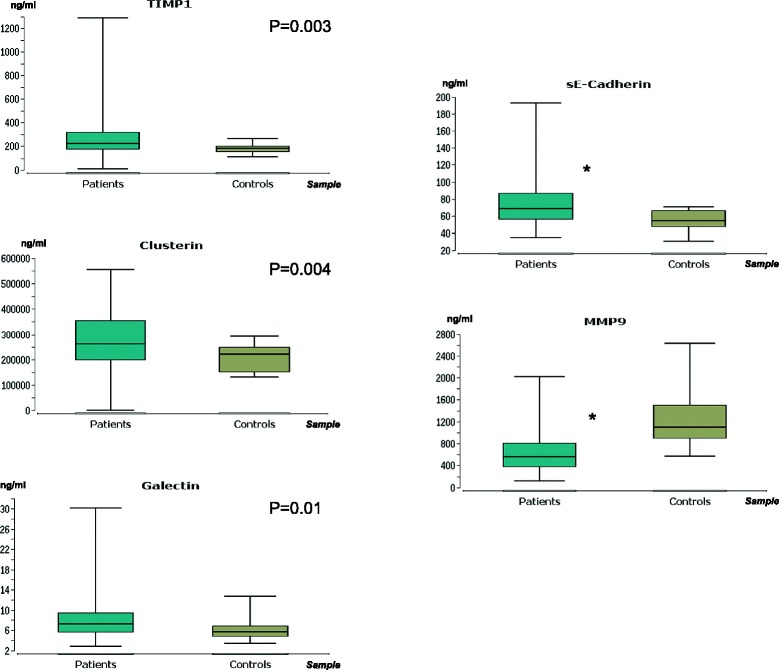
Marker concentrations in serum from tumor patients and controls. Only candidate molecules with significant differences are presented. Y-axis - concentration in ng/ml; X-axis - serum samples of tumor patients and controls. Box: lower line - quartile Q1 (25 %-quantile); middle line - median; upper line - quartile Q3 (75 %-quantile); aerials - extreme values. * - p < 0.001 (Wilcoxon-Mann–Whitney-test). 0.05 > p ≥ 0.01 are given in exact numbers
Table 3.
Correlation of markers with prostatectomy Gleason Sum
| sE-Cadherin | MMP2 | MMP9 | TIMP1 | Clusterin | |
|---|---|---|---|---|---|
| p-value | 0.004599 | 0.020268 | 0.048087 | 0.027881 | 0.031574 |
| Coefficient (rho) | −0.219991 | −0.180763 | 0.154655 | 0.171867 | −0.168062 |
Fig. 2.
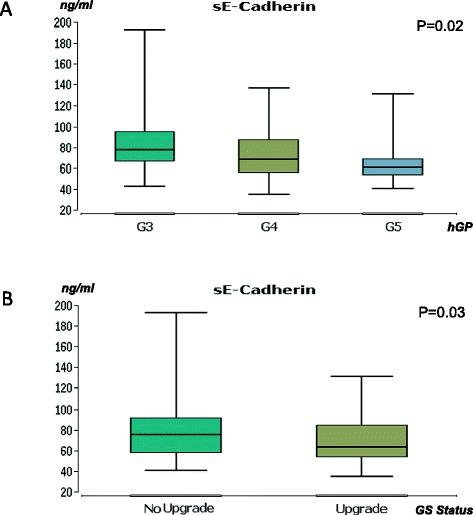
Associations between sE-cadherin and histopathological parameters. a: Association between sE-cadherin expression and highest Gleason pattern. Y-axis - concentration in ng/ml; X-axis - serum samples of tumor patients distributed by the highest Gleason pattern (hGP). G3-G5 - Gleason pattern 3-5. P-value (Kruskal-Wallis-test). b: Association between sE-cadherin expression and Gleason sum upgrade. Y-axis - concentration in ng/ml; X-axis - serum samples of tumor patients distributed by upgrade status of the Gleason sum in prostatectomy specimen. P-value (Wilcoxon-Mann–Whitney-test). Box: lower line - quartile Q1 (25 %-quantile); middle line - median; upper line - quartile Q3 (75 %-quantile); aerials - extreme values
Multivariate analysis with logistic regression for all 8 candidate markers as well as serum PSA was unsuitable because, taking into account the cohort size and ‘the rule of ten’ [17], sufficient stability of the model could not be ensured. Therefore, a bivariate logistic regression analysis was performed, incorporating each of the candidate molecules and serum PSA to assess their ancillary predictive potential over PSA for clinical and pathological T-stage, L-, N-, R-, Pn-status, biopsy (b) and prostatectomy (p)GS after dichotomization of the study cohort into GS < 7 and ≥ 7, as well as Gleason upgrade. Only sE-cadherin yielded favorable discriminative ability over PSA for more aggressive tumors with a pGS ≥ 7 (p = 0.01). Moreover, sE-cadherin better detected PCas with a Gleason upgrade at RPE (p = 0.01) than did serum PSA. Neither sE-cadherin, TIMP1, Galectin, Clusterin nor MMP9 correlated significantly with serum PSA.
Discussion
Estimating PCa malignancy in an era of personalized healthcare requires novel diagnostic and prognostic biomarkers to develop individual treatment strategies. Therefore, the potential of a panel of serum proteins to detect PCa and predict histopathological outcome in curatively treated disease was evaluated.
Out of 119 candidate molecules, sE-cadherin yielded the most promising potential to serve as a biomarker in the current study cohort with almost 96 % clinically significant PCas according to the Epstein criteria [16]. E-cadherin, a type-I-cadherin, belongs to the cadherin family of transmembrane or membrane-associated glycoproteins located in the adherens junction and basolateral membrane in epithelial cells. It consists of a large extracellular domain, a transmembrane segment and a conserved cytoplasmic domain [18]. E-cadherin is indispensable in promoting cell-cell adhesion in a Ca2+-dependant manner and steers tissue morphogenesis [19]. During cancer progression, transcriptional E-cadherin reprogramming induces decreased adhesion and enhanced migration and invasion during the epithelial-to-mesenchymal transition of epithelial cells [20, 21]. sE-cadherin is an 80-kDa ectodomain of the full-length E-cadherin, which is proteolytically shed into the extracellular space through cleavage by secretases and caspases [18]. Concurrent to the disruption of adhesion junctions, EGFR and Wnt/β-catenin pathway signaling, often involved in tumorigenesis, are activated [22]. Since E-cadherin cleavage has been linked to neoplastic adenoma-cancer progression, serum levels of sE-cadherin have been shown to be augmented in patients with breast, gastric and colorectal cancer [23]. This is in line with our findings demonstrating that the serum concentration of sE-cadherin was elevated in patients with PCa, more than other investigated candidates. Thanks to its stability in blood, sE-cadherin may be a suitable indicator for early PCa detection, due to accumulation during tumor-associated proteolysis [23].
So far, little has been reported about sE-cadherin in connection with PCa. Ahmed et al. [24] investigated its potential as a biomarker in a group of 71 Egyptian males with PCa. In agreement with our findings, significant over-expression of sE-cadherin in the serum of tumor patients, compared to healthy individuals, was observed. No correlation with the clinical tumor stage was apparent. In contrast to our findings, the authors reported high expression of sE-cadherin, correlating with a poor Gleason grade and serum PSA. It is difficult to interpret their data in the context of our findings due to the limited sample size of that cohort, median PSA of 70.7 ng/ml for tumor patients, most cancers classified as stage D and details about their staging procedure absent. These study characteristics harbor significant probability of undetected metastasis and therefore deviate from the present study population, which was clinically free from visceral- and bone-disease. Furthermore, alterations in Gleason grading procedures over time have resulted in significant tumor up-grading [25], making a study published in 1999 [24] difficult to compare with the present study carried out more than a decade later, due to bias stemming from the Will Rogers phenomenon. Later, Kuefer et al. [26] observed no difference between the expression of serum sE-cadherin in 61 men with localized PCa compared to controls. On the contrary, a more contemporary series also involving 61 patients with clinically localized PCa, reported by Iacopino et al. [27], demonstrated serum sE-cadherin over-expression in PCa patients compared to that of healthy individuals, which is similar to our observation. However, no correlation of the marker with PSA, T-stage, GS, grade or R- and Pn-status was observed by those investigators. To best knowledge, the current assessment of sE-cadherin has taken place in the largest cohort of clinically non-metastatic males with PCa. sE-cadherin correlated with the highest Gleason pattern, which is an important prognosis factor [28] and was also associated with a Gleason upgrade and pGS, which is more accurate than bGS at predicting biochemical recurrence after RPE [29]. Most importantly, for identifying more aggressive tumors with GS ≥ 7 and tumors with a Gleason upgrade, sE-cadherin outmatched serum PSA without correlating with this marker.
The negative serum sE-cadherin correlation with more aggressive PCa in this investigation requires further research since serum sE-cadherin has been reported to rise with more aggressive or advanced stages in many other cancer types [23]. However, Juhasz et al. has provided evidence for significantly decreased expression of serum sE-cadherin in individuals with advanced and metastasized diffuse-type gastric cancer in contrast to intestinal cancer [30]. Careful analysis of cancer type as well as stage and grade specific molecular machinery pertaining to E-cadherin turnover is therefore warranted. Ongoing studies on sE-cadherin with respect to PCa should also take into consideration that proinflammatory cytokines [31], growth factors [32], as well as chemically induced oxidative stress [33] may contribute to its generation and possibly falsify results based on its expression.
Although sE-cadherin seems to be a promising biomarker, it may not be alone. Catenins, as well as CD44, are associated with E-cadherin in regulating prostate cancer cell adhesion [34]. In particular, CD44v8–10 have been shown to be negatively correlated with c-Myc expression, both in vitro and in vivo. The negative correlation may partly be attributable to redox stress-enhanced Wnt/beta-catenin signaling [35].
The reason no distinct difference was apparent between the CD44 serum level in healthy subjects and tumor patients may have been because CD44 variants such as CD44v5, CD44v6 and CD44v10, possibly involved in prostate cancer progression, were not individually evaluated [36,37]. EpCAM differences between tumor patients and controls were also not apparent in the present investigation, which is in line with other reports [38]. EpCAM has been reported to be responsible for persistence of minimal residual disease and latent relapse of prostate cancer, presumably by affecting the susceptibility to the EGF ligand and regulating the AMPK signaling pathway [39]. Nevertheless, a prognostic/diagnostic role of EpCAM in EGFR activated prostate cancer cells should not be ruled out.
The same ambivalence might apply to MMP9. Although this protein has been suggested to correlate with prostate cancer progression, the correlation has not always been substantiated. Recent experiments documented that MMP9 might even exert tumor-suppressive properties. In fact, elevated MMP9 secretion has been shown to result in decreased prostate cancer cell proliferation, coupled to increased sE-cadherin shedding [40]. The combined effect might explain the inverse correlation between sE-cadherin and MMP9 found in the present investigation, whereby reduced MMP9 and elevated sE-cadherin may drive tumor proliferation forward. Still, this is hypothetical and requires further evaluation.
The present investigation is limited by its single-center design and lack of external validation. To account for regional and racial disparity regarding the biological characteristics of PCa [41], validation studies with patient cohorts from other centers would be desirable. The potential of sE-cadherin as a biomarker could thereby be more exactly appraised.
Conclusions
To sum up, sE-cadherin performed best out of a large panel of serum proteins in terms of diagnostic and predictive potential in 165 patients with clinically localized PCa. Therefore, this molecule merits further investigation as a biomarker for PCa.
Acknowledgments
This work was supported by the Dr. Illing Stiftung, Hans und Wolfgang Schleussner-Stiftung and Banss Stiftung.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
IT: study conception, data analysis, statistics and manuscript writing. KT: investigations, statistics. EJ: investigations. KMG: data interpretation, manuscript revision. HB: data interpretation, manuscript revision. RM: data interpretation, statistics. GB: study conception, data analysis. EO: investigations. HA: statistics. KN: manuscript revision, data analysis. AH: study conception, data analysis, statistics and manuscript revision. RAB: study conception, data analysis, statistics and manuscript revision. All authors read and approved the final manuscript.
Authors’ information
Axel Haferkamp and Roman A Blaheta contributed equally as senior authors.
Contributor Information
Igor Tsaur, Phone: 0049-69-6301-7671, Email: igor.tsaur@kgu.de.
Kristina Thurn, Email: kristina.thurn@kgu.de.
Eva Juengel, Email: eva.juengel@kgu.de.
Kilian M. Gust, Email: kilian.gust@kgu.de
Hendrik Borgmann, Email: hendrik.borgmann@kgu.de.
Rene Mager, Email: rene.mager@kgu.de.
Georg Bartsch, Email: georg.bartsch@kgu.de.
Elsie Oppermann, Email: oppermann@em.uni-frankfurt.de.
Hanns Ackermann, Email: ackermann@add.uni-frankfurt.de.
Karen Nelson, Email: kar_nelson@hotmail.com.
Axel Haferkamp, Email: axel.haferkamp@kgu.de.
Roman A. Blaheta, Email: roman.blaheta@kgu.de
References
- 1.Shafique K, Oliphant R, Morrison DS. The impact of socio-economic circumstances on overall and grade-specific prostate cancer incidence: a population-based study. Br J Cancer. 2012;107:575–82. doi: 10.1038/bjc.2012.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roehrborn CG, Black LK. The economic burden of prostate cancer. BJU Int. 2011;108:806–13. doi: 10.1111/j.1464-410X.2011.10365.x. [DOI] [PubMed] [Google Scholar]
- 3.Joniau S, Pfister D, de la Taille A, Gaboardi F, Thompson A, Ribal MJ. Controversies on individualized prostate cancer care: gaps in current practice. Ther Adv Urol. 2013;5:233–44. doi: 10.1177/1756287213490053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adami HO. The prostate cancer pseudo-epidemic. Acta Oncol. 2010;49:298–304. doi: 10.3109/02841860903584945. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Caras RJ, Sterbis JR. Prostate cancer nomograms: a review of their use in cancer detection and treatment. Curr Urol Rep. 2014;15:391. doi: 10.1007/s11934-013-0391-0. [DOI] [PubMed] [Google Scholar]
- 7.Freedland S, Mithal P. Editorial comment. Urology. 2013;81:1208. doi: 10.1016/j.urology.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 8.Tsaur I, Noack A, Makarevic J, Oppermann E, Waaga-Gasser AM, Gasser M et al. CCL2 Chemokine as a Potential Biomarker for Prostate Cancer: A Pilot Study. Cancer Res Treat. 2014; doi:10.4143/crt.2014.015. [DOI] [PMC free article] [PubMed]
- 9.Dijkstra S, Birker IL, Smit FP, Leyten GH, de Reijke TM, van Oort IM, et al. Prostate cancer biomarker profiles in urinary sediments and exosomes. J Urol. 2014;191:1132–8. doi: 10.1016/j.juro.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Amaro A, Esposito AI, Gallina A, Nees M, Angelini G, Albini A, et al. Validation of proposed prostate cancer biomarkers with gene expression data: a long road to travel. Cancer Metastasis Rev. 2014;33:657–71. doi: 10.1007/s10555-013-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edge SB. American joint committee on cancer., american cancer society., Teton data systems (firm). AJCC cancer staging handbook from the AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 12.Wittekind C. International union against cancer. TNM-klassifikation maligner tumoren. 6. Aufl., korrig. Nachdr. ed. Heidelberg: Springer Medizin; 2005. [Google Scholar]
- 13.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL, Committee IG. The 2005 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 14.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Leyten GH, Hessels D, Jannink SA, Smit FP, de Jong H, Cornel EB, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol. 2014;65:534–42. doi: 10.1016/j.eururo.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–8. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 18.David JM, Rajasekaran AK. Dishonorable discharge: the oncogenic roles of cleaved E-cadherin fragments. Cancer Res. 2012;72:2917–23. doi: 10.1158/0008-5472.CAN-11-3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 20.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–88. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci. 2013;116:317–36. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 22.De Freitas Silva BS, Yamamoto-Silva FP, Pontes HA, Pinto Junior Ddos S. E-cadherin downregulation and Twist overexpression since early stages of oral carcinogenesis. J Oral Pathol Med. 2014;43:125–31. doi: 10.1111/jop.12096. [DOI] [PubMed] [Google Scholar]
- 23.Repetto O, De Paoli P, De Re V, Canzonieri V, Cannizzaro R. Levels of soluble E-cadherin in breast, gastric, and colorectal cancers. Biomed Res Int. 2014;2014:408047. doi: 10.1155/2014/408047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed MI, Abd-Elmotelib F, Farag RM, Ziada NA, Khalifa A. Evaluation of some tissue and serum biomarkers in prostatic carcinoma among Egyptian males. Clin Biochem. 1999;32:439–45. doi: 10.1016/S0009-9120(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 25.Danneman D, Drevin L, Robinson D, Stattin P, Egevad L. Gleason inflation 1998-2011: a registry study of 97 168 men. BJU Int. 2015;115:248–55. doi: 10.1111/bju.12671. [DOI] [PubMed] [Google Scholar]
- 26.Kuefer R, Hofer MD, Gschwend JE, Pienta KJ, Sanda MG, Chinnaiyan AM, et al. The role of an 80 kDa fragment of E-cadherin in the metastatic progression of prostate cancer. Clin Cancer Res. 2003;9:6447–52. [PubMed] [Google Scholar]
- 27.Iacopino F, Pinto F, Bertaccini A, Calarco A, Proietti G, Totaro A, et al. Soluble E-cadherin and IL-6 serum levels in patients affected by prostate cancer before and after prostatectomy. Oncol Rep. 2012;28:370–4. doi: 10.3892/or.2012.1785. [DOI] [PubMed] [Google Scholar]
- 28.Epstein JI. An update of the Gleason grading system. J Urol. 2010;183:433–40. doi: 10.1016/j.juro.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 29.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Schnall M, Tomaszewski JE, et al. A multivariate analysis of clinical and pathological factors that predict for prostate specific antigen failure after radical prostatectomy for prostate cancer. J Urol. 1995;154:131–8. doi: 10.1016/S0022-5347(01)67248-3. [DOI] [PubMed] [Google Scholar]
- 30.Juhasz M, Ebert MP, Schulz HU, Rocken C, Molnar B, Tulassay Z, et al. Dual role of serum soluble E-cadherin as a biological marker of metastatic development in gastric cancer. Scand J Gastroenterol. 2003;38:850–5. doi: 10.1080/00365520310003985. [DOI] [PubMed] [Google Scholar]
- 31.Grabowska MM, Day ML. Soluble E-cadherin: more than a symptom of disease. Front Biosci (Landmark Ed) 2012;17:1948–64. doi: 10.2741/4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrish AR, Catania JM, Orozco J, Gandolfi AJ. Chemically induced oxidative stress disrupts the E-cadherin/catenin cell adhesion complex. Toxicol Sci. 1999;51:80–6. doi: 10.1093/toxsci/51.1.80. [DOI] [PubMed] [Google Scholar]
- 34.Kallakury BV, Sheehan CE, Ross JS. Co-downregulation of cell adhesion proteins alpha- and beta-catenins, p120CTN, E-cadherin, and CD44 in prostatic adenocarcinomas. Hum Pathol. 2001;32:849–55. doi: 10.1053/hupa.2001.26463. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida GJ, Saya H. Inversed relationship between CD44 variant and c-Myc due to oxidative stress-induced canonical Wnt activation. Biochem Biophys Res Commun. 2014;443:622–7. doi: 10.1016/j.bbrc.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Lein M, Jung K, Weiss S, Schnorr D, Loening SA. Soluble CD44 variants in the serum of patients with urological malignancies. Oncology. 1997;54:226–30. doi: 10.1159/000227693. [DOI] [PubMed] [Google Scholar]
- 37.Tei H, Miyake H, Harada K, Fujisawa M. Expression profile of CD44s, CD44v6, and CD44v10 in localized prostate cancer: effect on prognostic outcomes following radical prostatectomy. Urol Oncol. 2014;32:694–700. doi: 10.1016/j.urolonc.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Rupp AK, Rupp C, Keller S, Brase JC, Ehehalt R, Fogel M, et al. Loss of EpCAM expression in breast cancer derived serum exosomes: role of proteolytic cleavage. Gynecol Oncol. 2011;122:437–46. doi: 10.1016/j.ygyno.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida GJ, Saya H. EpCAM expression in the prostate cancer makes the difference in the response to growth factors. Biochem Biophys Res Commun. 2014;443:239–45. doi: 10.1016/j.bbrc.2013.11.093. [DOI] [PubMed] [Google Scholar]
- 40.Biswas MH, Du C, Zhang C, Straubhaar J, Languino LR, Balaji KC. Protein kinase D1 inhibits cell proliferation through matrix metalloproteinase-2 and matrix metalloproteinase-9 secretion in prostate cancer. Cancer Res. 2010;70:2095–104. doi: 10.1158/0008-5472.CAN-09-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Messing EM, Travis LB, Hyrien O, Chen R, Milano MT, et al. Age and Racial Differences among PSA-Detected (AJCC Stage T1cN0M0) Prostate Cancer in the U.S.: A Population-Based Study of 70,345 Men. Front. Oncol. 2013;3:312. doi: 10.3389/fonc.2013.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]


