Abstract
Thioredoxin (Trx) and glutaredoxin (Grx1) are hydrogen donors for ribonucleotide reductase, the key enzyme for deoxyribonucleotide biosynthesis. The viability of a double mutant lacking both Trx and Grx1 implies the presence of a third, unknown hydrogen donor. This paper reports the purification and characterization of two proteins with glutaredoxin activity (using hydroxyethyl disulfide as a substrate) from an Escherichia coli mutant lacking Trx and Grx1 (delta trxA, grx::kan). Affinity chromatography was used to bind glutaredoxin on a glutathione-containing thiol-Sepharose column. The molecular weight of Grx2, 27,000, was atypical for glutaredoxins, whereas Grx3 had a molecular weight of 10,000. Amino acid sequence analysis revealed novel structures with putative active sites typical of glutaredoxins: Cys-Pro-Tyr-Cys. The proteins are therefore referred to as Grx2 and Grx3. The low hydrogen donor activity for ribonucleotide reductase in the crude extract was recovered in the purification of Grx3, whereas Grx2 was inactive. As a hydrogen donor for E. coli ribonucleotide reductase, Grx3 showed approximately the same Km value (0.35 microM) as Grx1, whereas its Vmax value was only 5% that of Grx1. The combination of the Grx3 hydrogen donor activity and a 25-fold induction of ribonucleotide reductase activity in a delta trxA, grx double mutant provides an explanation for its viability and deoxyribonucleotide biosynthesis. The physiological functions of Grx2 and Grx3 remain to be determined.
Full text
PDF

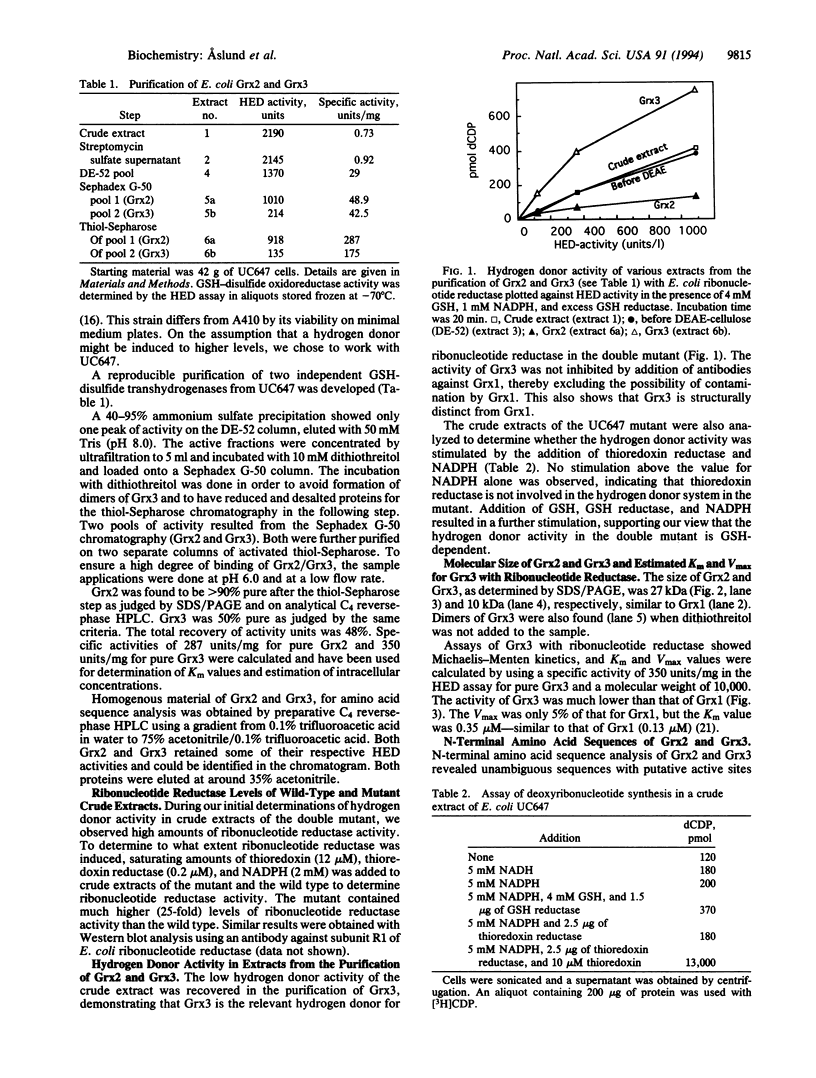
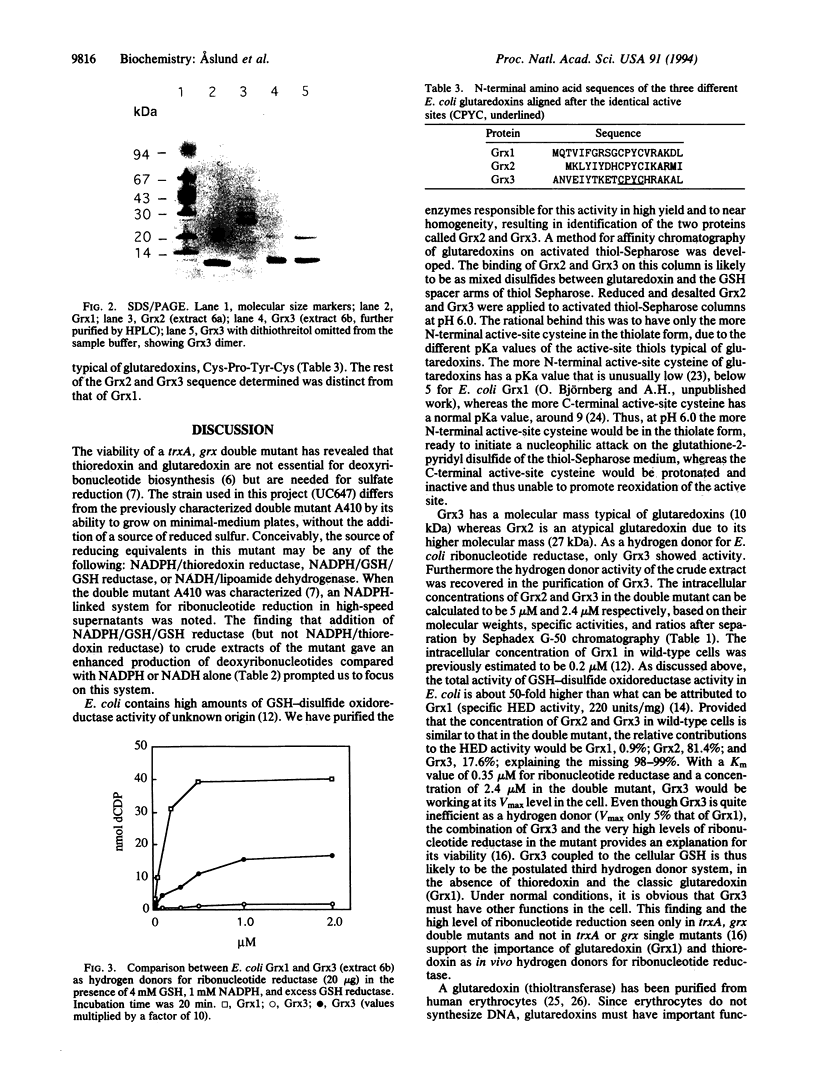

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abril N., Pueyo C. Mutagenesis in Escherichia coli lacking catalase. Environ Mol Mutagen. 1990;15(4):184–189. doi: 10.1002/em.2850150403. [DOI] [PubMed] [Google Scholar]
- Björnberg O., Holmgren A. Characterization of homogeneous recombinant glutaredoxin from Escherichia coli: purification from an inducible lambda PL expression system and properties of a novel elongated form. Protein Expr Purif. 1991 Aug;2(4):287–295. doi: 10.1016/1046-5928(91)90085-w. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Eklund H., Cambillau C., Sjöberg B. M., Holmgren A., Jörnvall H., Hög J. O., Brändén C. I. Conformational and functional similarities between glutaredoxin and thioredoxins. EMBO J. 1984 Jul;3(7):1443–1449. doi: 10.1002/j.1460-2075.1984.tb01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson H. A., Rozell B., Stemme S., Engström Y., Thelander L., Holmgren A. Different cellular distribution of thioredoxin and subunit M1 of ribonucleotide reductase in rat tissues. Exp Cell Res. 1986 Apr;163(2):363–369. doi: 10.1016/0014-4827(86)90067-4. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979 May 10;254(9):3672–3678. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Purification and characterization of glutaredoxin from Escherichia coli. J Biol Chem. 1979 May 10;254(9):3664–3671. [PubMed] [Google Scholar]
- Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Ohlsson I., Grankvist M. L. Thiroedoxin from Escherichia coli. Radioimmunological and enzymatic determinations in wild type cells and mutants defective in phage T7 DNA replication. J Biol Chem. 1978 Jan 25;253(2):430–436. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Hopper S., Iurlano D. Properties of a thioredoxin purified from rabbit bone marrow which fails to serve as a hydrogen donor for the homologous ribonucleotide reductase. J Biol Chem. 1983 Nov 25;258(22):13453–13457. [PubMed] [Google Scholar]
- Hopper S., Johnson R. S., Vath J. E., Biemann K. Glutaredoxin from rabbit bone marrow. Purification, characterization, and amino acid sequence determined by tandem mass spectrometry. J Biol Chem. 1989 Dec 5;264(34):20438–20447. [PubMed] [Google Scholar]
- Hög J. O., Jörnvall H., Holmgren A., Carlquist M., Persson M. The primary structure of Escherichia coli glutaredoxin. Distant homology with thioredoxins in a superfamily of small proteins with a redox-active cystine disulfide/cysteine dithiol. Eur J Biochem. 1983 Oct 17;136(1):223–232. doi: 10.1111/j.1432-1033.1983.tb07730.x. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., MOORE E. C., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. IV. ISOLATION AND CHARACTERIZATION OF THIOREDOXIN, THE HYDROGEN DONOR FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3436–3444. [PubMed] [Google Scholar]
- Meng M., Hogenkamp H. P. Purification, characterization, and amino acid sequence of thioredoxin from Corynebacterium nephridii. J Biol Chem. 1981 Sep 10;256(17):9174–9182. [PubMed] [Google Scholar]
- Mieyal J. J., Starke D. W., Gravina S. A., Dothey C., Chung J. S. Thioltransferase in human red blood cells: purification and properties. Biochemistry. 1991 Jun 25;30(25):6088–6097. doi: 10.1021/bi00239a002. [DOI] [PubMed] [Google Scholar]
- Miranda-Vizuete A., Martinez-Galisteo E., Aslund F., Lopez-Barea J., Pueyo C., Holmgren A. Null thioredoxin and glutaredoxin Escherichia coli K-12 mutants have no enhanced sensitivity to mutagens due to a new GSH-dependent hydrogen donor and high increases in ribonucleotide reductase activity. J Biol Chem. 1994 Jun 17;269(24):16631–16637. [PubMed] [Google Scholar]
- Reichard P. From RNA to DNA, why so many ribonucleotide reductases? Science. 1993 Jun 18;260(5115):1773–1777. doi: 10.1126/science.8511586. [DOI] [PubMed] [Google Scholar]
- Russel M., Holmgren A. Construction and characterization of glutaredoxin-negative mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Feb;85(4):990–994. doi: 10.1073/pnas.85.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P., Holmgren A. Thioredoxin or glutaredoxin in Escherichia coli is essential for sulfate reduction but not for deoxyribonucleotide synthesis. J Bacteriol. 1990 Apr;172(4):1923–1929. doi: 10.1128/jb.172.4.1923-1929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T., Oshida T., Nishimura M., Maeda H., Hara T., Hosomi S., Mizoguchi T., Nishihara T. Study on human erythrocyte thioltransferase: comparative characterization with bovine enzyme and its physiological role under oxidative stress. J Biochem. 1992 May;111(5):688–692. doi: 10.1093/oxfordjournals.jbchem.a123819. [DOI] [PubMed] [Google Scholar]
- Thelander L., Sjöberg B. R., Eriksson S. Ribonucleoside diphosphate reductase (Escherichia coli). Methods Enzymol. 1978;51:227–237. doi: 10.1016/s0076-6879(78)51032-x. [DOI] [PubMed] [Google Scholar]
- Yang Y. F., Wells W. W. Identification and characterization of the functional amino acids at the active center of pig liver thioltransferase by site-directed mutagenesis. J Biol Chem. 1991 Jul 5;266(19):12759–12765. [PubMed] [Google Scholar]