Abstract
Kiyamu, Melisa, Fabiola León-Velarde, María Rivera-Chira, Gianpietro Elías, and Tom D. Brutsaert. Developmental effects determine submaximal arterial oxygen saturation in Peruvian Quechua. High Alt Med Biol 16, 138–146, 2015.—Andean high altitude natives show higher arterial oxygen saturation (Sao2) during exercise in hypoxia, compared to acclimatized sojourners. In order to evaluate the effects of life-long exposure to high altitude on Sao2, we studied two groups of well-matched, self-identified Peruvian Quechua natives who differed in their developmental exposure to hypoxia before and after a 2-month training period. Male and female volunteers (18–35 years) were recruited in Lima, Peru (150 m). The two groups were: a) Individuals who were born and raised at sea-level (BSL, n=34) and b) Individuals who were born and raised at high altitude (BHA, n=32), but who migrated to sea-level as adults (>16 years old). Exercise testing was conducted using a submaximal exercise protocol in normobaric hypoxia in Lima (BP=750 mmHg, Fio2=0.12), in order to measure Sao2 (%), ventilation (VE L/min) and oxygen consumption (Vo2, L/min). Repeated-measures ANOVA, controlling for VE/VO2 (L/min) and sex during the submaximal protocol showed that BHA maintained higher Sao2 (%) compared to BSL at all workloads before (p=0.005) and after training (p=0.017). As expected, both groups showed a decrease in Sao2 (%) (p<0.001), as workload increased. Resting Sao2 levels were not found to be different between groups. The results suggest that developmental exposure to altitude contributes to the maintenance of higher Sao2 levels during submaximal exercise at hypoxia.
Key Words: : adaptation, development, hypoxia, saturation
Introduction
The capacity to maintain adequate oxygenation in a hypoxic environment is pivotal for the thousands of individuals residing in the highlands. The majority of studies that have measured Sao2 during submaximal and maximal exercise at hypoxia indicate that Quechua and Tibetan high altitude natives have higher exercise Sao2 compared to lowlanders (Sun et al., 1990; Ge et al., 1995; Zhuang et al., 1996; Chen et al., 1997; Brutsaert et al., 2000; Marconi et al., 2004). In particular, during exercise, the maintenance of higher arterial oxygen saturation (Sao2) is an important factor in the overall response to work, given the physiological demands imposed on the body at high altitude.
The examination of genetic and/or developmental origins of higher Sao2 levels in highlanders contributes to the broader question of adaptation to high altitude (Hochachka et al., 1999; Beall, 2000; Moore, 2001). Early studies conducted by Beall and colleagues (1994; 1998) in Tibetans reported that Sao2 showed significant heritability. Similarly, quantitative genetic studies indicate the presence of a higher resting Sao2 allele in pregnant Tibetan women that was associated with a higher offspring survival during the first year of life (Beall et al., 1997; 2004). In Andeans, the maintenance of arterial saturation during hypoxic exercise has been mainly associated to genetic factors as well (Frisancho et al., 1995; Brutsaert et al., 2000).
An alternative explanation for the genetic basis of the high Sao2 phenotype suggests that the observed difference in adults is determined through life-long exposure to hypoxia. The “Developmental Adaptation Hypothesis” is based on the plasticity of structural and functional systems in the individual at sensitive periods in their life (Frisancho, 2009). It is well-known that larger pulmonary volumes are strongly determined by developmental exposure to hypoxia in Andean populations (Greksa, 1990; Frisancho et al., 1997; Brutsaert et al., 2004; Kiyamu et al., 2012) in Tibetans (Droma et al., 1991), and in non-native populations who were born and raised at the highlands (Greksa, 1990). Moreover, there are a handful of studies suggesting the role of lifelong exposure to hypoxia on a more efficient gas exchange system (Dempsey et al., 1971; Zhuang et al., 1996; Brutsaert et al. 2000; Wagner et al., 2002). Nevertheless, the role of developmental processes in Sao2 has not been extensively studied in high altitude native populations, especially during exercise in hypoxia, where the presumed differences in arterial saturation would be most evident.
A partition of developmental and genetic factors is a challenging task, given the interaction of both factors to determine a complex phenotype like arterial oxygen saturation. Migrant study designs, where different groups are compared, are used to address these questions (Harrison, 1966). However, a common confounding factor is that groups differ in genetic, socioeconomic, and/or lifestyle factors. For instance, comparisons between European and Andean subjects show that Europeans are heavier, taller, and of higher socio-economic status than Quechua participants (Greksa, 1990; Frisancho et al., 1997; Brutsaert et al., 1999). In order to test developmental effects, a better approach is to use study groups that are well-matched, especially in their population ancestry.
In the present study, we aimed to test the hypothesis that developmental exposure to hypoxia confers higher submaximal arterial saturation, and lower VE/Vo2 in normobaric hypoxia at sea-level, 150 m (Fio2=0.12; BP=750 mmHg), in a group of Quechua males and females, using a partial migrant approach. In order to test this hypothesis, we compared two well-matched groups, who differed in their developmental exposure to hypoxia. The first group (BSL) consisted of individuals who were born and raised at sea-level (150 m) and the second group (BHA) consisted of individuals who were born and raised at high altitude, >2500 m, but who migrated to sea-level as adults (older than 16 years). Individuals were measured in normobaric hypoxia for submaximal Sao2, VE/Vo2 and Ventilation (VE), before and after a 2-month-training period. The training period was included in the study design to properly evaluate differences in aerobic capacity, explained elsewhere (Kiyamu et al., 2015).
Materials and Methods
Study population and subjects
Healthy, non-smoker volunteers (n=66, age 18–35) were recruited from the city of Lima (150 m), Peru. A qualified physician conducted medical screenings for all volunteers, including a medical interview, an electrocardiogram, and a spirometry test. The criteria of exclusion was: a) cardiovascular, renal disease and/or any other health impediment (recent injury or operation) that would prevent subjects from doing exercise; b) obstructive or restrictive diseases, following the medical guidelines for expected FEV1/FVC(%) healthy individuals; b) present or past history of smoking, c) anemic individuals: females who had Hb concentrations <11 g/dL and males who had Hb <13 g/dL were not accepted; d) pregnant or lactating women; and e) women who were taking any hormonal contraceptive.
The same investigator interviewed all volunteers at the beginning of the study using a version of the Minnesota Leisure Time physical activity questionnaire (Kriska, 1997) to document levels of activity over the past year. None of the volunteers were athletes or had participated in elite competitions. Most individuals reported to be sedentary or moderately active at the start of the study.
Quechua ancestry was based on the three following criteria: 1) presence of one or more Quechua surnames (we registered the surnames from both parental lineages up to two generations—grandparents—for a total of eight surnames); 2) volunteers identified themselves as having ancestors from the highlands; and 3) knowledge of Quechua either by themselves or one of their parents or grandparents, given that Quechua is not taught at school and only transmitted in the family environment.
This research project was approved by the Institutional Review Board of the University at Albany, State University of New York and the Universidad Peruana Cayetano Heredia. All volunteers gave their written informed consent to participate in the study.
Study design
A version of the migrant approach proposed by Harrison (1966) was used. Selection of individuals was based on their different exposures to hypoxia during growth and development (full exposure versus no exposure), and their common Quechua ancestry. The study groups were: a) BSL (Born at sea level, n=34, 17 males, 17 females). Quechua individuals who were born and raised at sea-level (<150 m) with no significant exposure to hypoxia (>3000 m) during growth. Similar to BHA, parents and grandparents from the paternal and maternal side of BSL individuals were born and raised in the highlands; and b) BHA (Born at high altitude, n=32, 17 males, 15 females). Quechua individuals who were born and raised at altitudes higher than 3000 m and who migrated to sea-level as adults (older than 16 years old), and whose parents and grandparents from the paternal and maternal side were from high altitude. All individuals in the BHA had migrated to the lowlands at least a year before entering the study and no BHA subject had any significant exposure to normoxia during growth.
Baseline measurements were made (anthropometry, pulmonary volumes, and exercise tests), both in normoxia and normobaric hypoxia, followed by a 2-month training period under supervision. The inclusion of a training period was part of the study design to effectively test exercise phenotypes controlling for differing levels of initial fitness among volunteers (Kiyamu et al., 2015). Post-training measurements included anthropometric and exercise phenotypes.
Anthropometry and pulmonary volumes
Standard anthropometry (weight, height, and skinfolds) was performed by the same investigator. Skinfold measurements were taken three times at three sites (subscapular, triceps, and biceps), at the beginning of the study and after the training period. Body density was calculated according to age and sex specific equations given by Durnin and Womersley (1974), and percent body fat was calculated from the Siri equation (Siri, 1956). FFM (fat free mass) was calculated based on total body weight. In order to measure pulmonary volumes, each subject was instructed to perform a maximal expiration after a maximal inspiration. Pulmonary volumes were measured three times, using a Hillmed spirometer (HM Specialist 1), and the best attempt was recorded. Pulmonary volumes were corrected for BTPS (body temperature, pressure saturated). Hemoglobin (Hb) was documented from capillary blood obtained by finger prick. Blood samples were measured using a hemoglobin analyzer (Hemocue, Angelholm, Sweden).
Endurance training program
Volunteers trained for a period of 2 months at sea-level (150 m), 3 days per week for 40 min on a stationary bicycle. In the first phase (first 4 weeks) volunteers followed intervals at 60%, 70%, and 80% of individual maximal heart rate, based on their initial Vo2max test, so that all volunteers were training at the same relative workload. Training was designed so that approximately 50% of the time, volunteers would work at an intensity of 60%–70% of their maximal heart rate, 27% of the time, at an intensity of 70%–80% of their maximal heart rate and 23% of the time at 80%–90% of their maximal heart rate. The second phase (last 4 weeks) had the same intensity distribution as the first phase, with the addition of two separate 1-min intervals, where volunteers were asked to achieve near maximal heart rate, followed by 1-min period of recovery. Subjects wore heart rate monitors during each session (Polar Electric, Sweden) and heart rates were recorded to ensure compliance with the prescribed intensity levels. All training sessions were supervised to ensure compliance with intensity goals.
Submaximal Sao2 measurements
Measurements of arterial oxygen saturation at rest and during exercise were conducted using pulse oxymetry, with a fingertip sensor (Ohmeda oximeter, 5740). All measurements were conducted as part of Vo2max protocols, prior to the training sessions and after the 2-month training. Subjects received a verbal explanation of the test prior to the measurements. Each test started with a 5-min period of resting measurements of oxygen consumption (Vo2 L/min) and resting Sao2 values. The first workload consisted of 1 kg for both men and women. Subjects were instructed to pedal at 60 RPM on a graded cycle ergometer (Monark 818E) with a 0.5 kg weight increment every 3 min. Submaximal workloads consisted of 60 w and 90 w to ensure submaximal workloads, in both males and females. Arterial oxygen saturation was recorded every 30 sec and data are presented as the average for each 3-min interval. Tests were conducted twice: 1) under conditions of normobaric hypoxia, and 2) during normoxia with a period of 60–90 min to rest between each test. Cadence was closely monitored by one of the investigators, and all individuals were encouraged to keep the desired pedaling frequency during the test.
The order of each test (normoxia and normobaric hypoxia) was selected at random and the same order was followed for all volunteers before and after training. Normobaric hypoxia was simulated by delivering a gas mixture with low Fio2, constantly mixed in a reservoir until the gas reading was at the desired oxygen concentration (Fio2=0.12). The final oxygen concentration inside the balloon was measured with Ametek gas analyzers (Pittsburgh, PA) used to conduct all tests. Volunteers were connected to a low resistance breathing valve (Hans-Rudolph) and the concentrations of oxygen and carbon dioxide in expired air, as well as respiratory volume, measured by a pneumotacograh (VacuMed, Inc), were directed to a Dell laptop via an A:D interface (VacuMed, Inc) to calculate oxygen consumption (Vo2), CO2 production (Vco2), respiratory exchange ratio (RER), and minute ventilation in 30-sec intervals. Heart rate (HR) was also recorded continuously via telemetry (Polar Electric, Sweden). Gas analyzer calibration was conducted twice a day, and the pneumotach was calibrated using a 3-liter syringe.
Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics software (Version 20, IBM). Anthropometric differences and exercise variables were analyzed using independent t-tests. Pulmonary volumes were analyzed using ANCOVA, where height was introduced as a covariate. For the submaximal Sao2 analysis between groups, ANCOVA for repeated measures was assessed to determine a time effect (Rest, 60 w, and 90 w) and group (BHA vs. BSL) effect, controlling for sex differences, and VE/Vo2. The Bonferroni method was used as post hoc test for the repeated measures analysis. Additionally, for each individual workload (rest, 60 w, and 90 w), submaximal Sao2 comparisons between groups were analyzed using ANCOVA, introducing sex and VE/VO2 as covariates. In all tables and figures, values are presented as means±SEM. For all tests, statistical significance was indicated at p≥0.05.
Results
Subject characteristics
Volunteers were measured at baseline values (pre-training) and after 2 months of supervised training in stationary bicycles (post-training). The anthropometric characteristics of the BSL (n=34) and BHA (n=32) groups are given as unadjusted means presented by sex, both before and after the training period in Table 1. All p-values are expressed for between-group differences. BSL were taller (p=0.013) and heavier (p=0.017) than BHA. The weight difference remained in the post-training measurement, with BSL being heavier than their highland counterparts (p=0.026). BHA had lower body fat after training (p=0.03), but not before training (p=0.132). Fat free mass (FFM, kg) was higher in BSL than BHA, but this difference did not reach statistical significance before (p=0.081) or after training (p=0.088). No differences were detected for age and hemoglobin (Hb) levels (p=0.083) between groups. Adjusted FVC-BTPS means for height effects were significantly higher in BHA than the BSL group (p<0.001), as expected. The evaluations of physical activity from standard activity questionnaires (Kriska, 1997) conducted before training revealed no differences in total physical activity averaged over the past year in MET-hours per week between BHA and BSL (data not shown).
Table 1.
Anthropometric Characteristics (Means±SE) of Males and Females Born and Raised at High Altitude (BHA), or Born and Raised at Sea Level (BSL)
| BHA (n=32) | BSL (n=34) | ||||
|---|---|---|---|---|---|
| Females | Males | Females | Males | ||
| N | 15 | 17 | 17 | 17 | P value1 |
| Pre-training | |||||
| Age (years) | 23±1 | 27±1 | 27±1 | 24±1 | 0.71 |
| Height (m)* | 1.53±0.01 | 1.62±0.01 | 1.55±0.01 | 1.67±0.01 | 0.013 |
| Weight (kg)* | 53±2 | 66±2 | 60±2 | 70±2 | 0.017 |
| Body fat (%) | 32.7±1.1 | 25.4±1.1 | 35.7±1.0 | 25.7±1.1 | 0.132 |
| FFM (kg) | 36±1 | 50±1 | 38±1 | 52±1 | 0.081 |
| FVC-BTPS(l)*2 | 4.41±0.14 | 4.89±0.12 | 3.91±0.13 | 4.43±0.15 | <0.001 |
| % predicted FVC | 116±3 | 113±2 | 103±3 | 103±3 | <0.001 |
| Hb (g/dl) | 12.9±0.2 | 15.1±0.2 | 12.3±0.2 | 15.0±0.2 | 0.083 |
| Post-training | |||||
| Weight (kg)* | 53±3 | 66±2 | 59±3 | 70±3 | 0.026 |
| Body fat (%)* | 32.2±0.9 | 24.7±0.9 | 35.6±0.9 | 25.4±0.9 | 0.03 |
| FFM (kg) | 36±2 | 49±1 | 38±2 | 52±2 | 0.088 |
| Hb (g/dl) | 12.7±0.3 | 15.5±0.3 | 12.5±0.3 | 14.8±0.3 | 0.089 |
p values refer to differences between BHA and BSL from ANCOVA results (adjusted for gender).
FVC is corrected for BTPS and adjusted for height.
Significant difference between BHA and BSL (p<0.05).
Submaximal exercise
Submaximal work levels were given at a fixed external resistance for all individuals. Only the first two workloads were considered, at 1 kg and 1.5 kg of resistance, with the same pedal frequency (60 RPM) for all volunteers. Data for pedaling frequency were used in order to calculate the work output at each work level (watts) and results are shown in Table 2. There is no significant difference in work output between BHA and BSL before or after training.
Table 2.
Submaximal Exercise Traits (Means±SE) of Males and Females Born and Raised at High Altitude (BHA), and Born and Raised at Sea Level (BSL), Before and After Training
| BSL (Males) (n=17) | BHA (Males) (n=17) | |||||
|---|---|---|---|---|---|---|
| Pre-training | ||||||
| Rest | W1 | W2 | Rest | W1 | W2 | |
| Watts1 | 0 | 59.2±0.4 | 88.9±0.6 | 0 | 58.9±0.5 | 88.9±0.6 |
| SaO2(%)1 | 90.9±0.6 | 85.4±0.89* | 82.9±1.6* | 92.0±0.7 | 87.4±0.9 | 85.7±1.7 |
| SaO2(%)2 | 90.8±0.6 | 85.6±0.83* | 84.0±1.7* | 92.2±0.63 | 87.8±0.9 | 87.2±1.8 |
| VE-BTPS (L/min) | 12.1±0.6† | 25.8±1.0 | 39.2±1.5† | 12.9±0.6† | 25.4±1.0 | 38.3±1.6† |
| VO2 (Ll/min) | 0.31±0.02† | 0.97±0.04 | 1.48±0.04† | 0.35±0.02† | 0.96±0.05 | 1.46±0.04† |
| HR (bmp) | 88±5 | 120±3† | 140±4† | 90±5 | 117±3† | 137±4† |
| VE/VO2 | 40.4±2.8 | 28.2±1.5 | 26.8±1.2 | 37.8±2.9 | 28.1±1.5 | 26.2±1.2 |
| Post-training | ||||||
| Rest | W1 | W2 | Rest | W1 | W2 | |
| Watts | 0 | 59±0.27 | 88.9±0.3 | 0 | 58.6±0.3 | 89±0.4 |
| SaO2(%)1 | 90.3±0.7 | 85±1* | 82.4±1.1 | 92.1±0.7 | 87.5±1.0 | 84±1.1 |
| SaO2(%)2 | 90.5±0.6 | 85.7±0.9* | 83.4±0.9* | 92.1±0.7 | 87.7±0.9 | 85.3±0.9 |
| VE-BTPS (l/min) | 11.7±0.6† | 25.1±0.8 | 36.5±1.1† | 13±0.6† | 25.4±0.8 | 36.4±1.1† |
| VO2(l/min) | 0.36±0.23† | 1.02±0.03† | 1.48±0.03† | 0.37±0.23† | 1.01±0.03† | 1.51±0.03† |
| HR (bmp) | 83±3 | 114±3† | 130±4 † | 80±3 | 108±3 † | 122±4† |
| VE/VO2 | 33±1.9 | 25.3±1 | 24.8±0.8 | 35.7±1.9 | 26.3±1 | 24.3±0.9 |
| BSL (Females) (n=17) | BHA (Females) (n=15) | |||||
|---|---|---|---|---|---|---|
| Pre-training | ||||||
| Rest | W1 | W2 | Rest | W1 | W2 | |
| Watts | 0 | 58.7±0.4 | 87.5±0.6 | 0 | 58.9±0.5 | 87±0.7 |
| SaO2(%)1 | 90.7±0.7 | 83.7±0.9* | 80.3±1.7 | 91.2±0.7 | 86.8±0.9 | 86.5±1.8 |
| SaO2(%)2 | 90.6±0.6 | 82.9±0.9* | 78.1±2* | 91.3±0.6 | 87±0.9 | 86±2 |
| VE-BTPS (L/min) | 10.1±0.6 | 26.6±1.0 | 47.1±1.5 | 9.2±0.6 | 24.3±1 | 43±2 |
| VO2 (L/min) | 0.29±0.02 | 0.89±0.04 | 1.29±0.04 | 0.26±0.02 | 0.9±0.05 | 1.37±0.05 |
| HR (bmp) | 91±5 | 139±3 | 169±4 | 95±5 | 133±3 | 161±4 |
| VE/VO2 | 38.8±2.8 | 31.7±1.5 | 37.4±1.2* | 36.6±2.9 | 27.7±1.5 | 31.4±1.2 |
| Post-training | ||||||
| Rest | W1 | W2 | Rest | W1 | W2 | |
| Watts | 0 | 59.5±0.27 | 89.2±0.34 | 0 | 59.7±0.3 | 89.1±0.4 |
| SaO2(%)1 | 90.5±0.7 | 83.1±1* | 81.8±1.1* | 91.9±0.7 | 87.5±1.0 | 84.4±1.1 |
| SaO2(%)2 | 90.46±0.63 | 82.6±0.9* | 81.3±1* | 91.2±0.7 | 87.0±0.9 | 83.7±0.8 |
| VE-BTPS (L/min) | 9.9±0.6 | 26±0.8 | 41.2±1.1 | 10.3±0.6 | 23.9±0.8 | 37.7±1.1 |
| VO2 (L/min) | 0.3±0.2 | 0.96±0.03 | 1.38±0.03 | 0.27±0.23 | 0.89±0.03 | 1.33±0.03 |
| HR (bmp) | 89±3 | 131±3 | 152±4 | 88±3 | 128±3 | 151±4 |
| VE/VO2 | 35.2±1.9 | 27.7±1 | 29.9±0.8 | 38.3±1.9 | 27.5±1 | 28.1±0.8 |
Significantly different from BHA group (p<0.05).
Significantly different between males and females (p<0.05).
Watts, work output calculated for each resistance load during submaximal exercise.
Unadjusted SaO2.
Adjusted for VE/VO2.
At the beginning of the study, before training, the first workload represented about ∼55% of Vo2max in women, and ∼40% of Vo2max in men. The second workload was equivalent to ∼82% of Vo2max in women, and ∼61% of Vo2max in men. After training, the first workload represented about ∼55% of Vo2max in women, and ∼37% of Vo2max in men, whereas workload 2 represented about ∼70% of Vo2max in women and ∼54% of Vo2max in men. Because the absolute workloads established in the study for all individuals do not represent the same relative work in men and women, results showing submaximal exercise are presented by gender in Table 2. At each workload, Vo2 values were not significantly different between BHA and BSL. Heart rate (HR) was not different between BHA and BSL at rest or at any workload. However, males had significantly lower HR during workload 1 and workload 2 (p<0.001) compared to women, both before and after training.
Measurements of maximal aerobic capacity have been reported elsewhere (Kiyamu et al, 2015), but are included here to better inform the analysis of submaximal SaO2 values. BHA had significantly higher Vo2peak (40.31±1.0 mL/min/kg) compared to BSL (35.78±0.96 mL/min/kg, p=0.001), adjusting for sex, under conditions of normobaric hypoxia. Similarly, when FFM (kg) was introduced as a covariate for Vo2peak comparisons between groups, the BHA group had higher Vo2peak values than the BSL group (2.43±0.04 L/min vs. 2.26±0.04 L/min, respectively, p=0.01).
Arterial saturation
At rest, Sao2 values did not differ significantly between groups before or after training. However, submaximal Sao2 (%) differed between the groups at each workload. Because VE/Vo2 was significantly associated with Sao2 at every workload, we introduced VE/Vo2 as a covariate in the analysis. Table 2 shows unadjusted means for Sao2 (%) and adjusted Sao2 (%) means, controlling for VE/Vo2. Results are presented by group and sex. We found that BHA had consistently higher Sao2 (%) across all workloads, compared to the BSL group, without adjusting for VE/Vo2. Both the unadjusted and the adjusted Sao2 means were similar during rest and across workloads, before and after training.
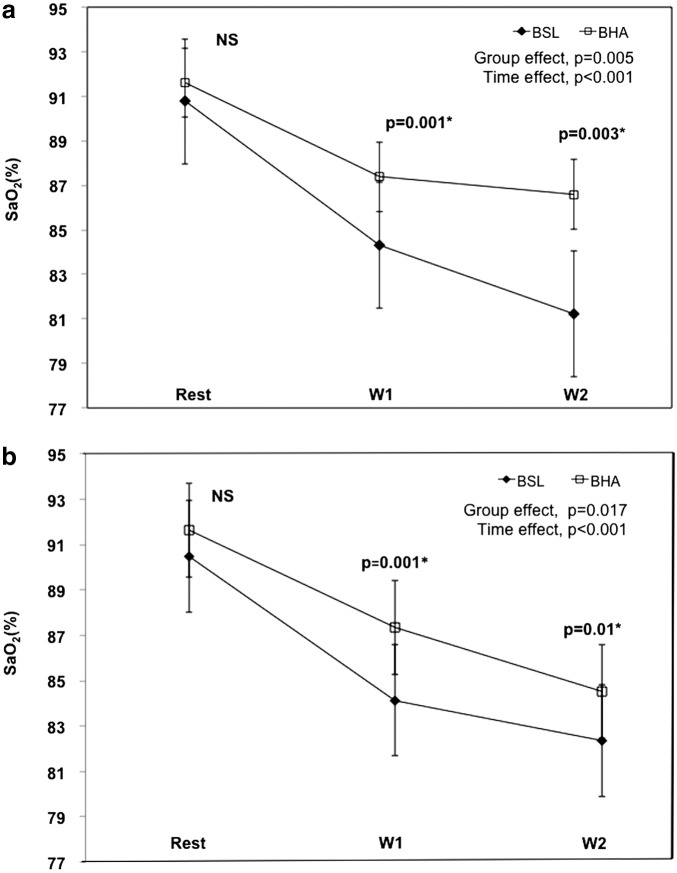
Figure 1 shows SaO2 with increasing submaximal work in BHA and BSL at normobaric hypoxia before training (Fig. 1a) and after training (Fig. 1b). Figure 1a shows a significant time effect (p<0.001), with a trend towards lower Sao2 as workload increases in both groups, as expected. More importantly, there is significant group effect (BHA vs. BSL) across workloads (p=0.005). When analyzed per workload, submaximal Sao2 values were significantly higher in BHA compared to the BSL at workload 1 (84.1±0.7 vs. 87.8±0.7; p=0.001) and workload 2 (80.5±1.5 vs. 87.1±1.5; p=0.003) before training. Similarly, when Sao2 was measured after training, there was a significant group effect (p=0.017), where the BHA group showed consistently higher Sao2 than the BSL group. Figure 1b illustrates the group effect and the time effect (p<0.001), with the expected trend towards lower Sao2 as workload increases. Differences at each workload are also shown for the post-training state. BSL had significantly lower Sao2 (%) than BHA, at workload 1 (84.1±0.6 vs. 87.4±0.7, p=0.001) and workload 2 (82.4±0.6 vs. 84.5±0.6; p=0.013), respectively.
FIG. 1.
(a) Sao2 (%) at rest and during submaximal exercise levels for BSL and BHA before training. (b) Sao2 (%) at rest and during submaximal exercise levels for BSL and BHA after training.*p-values are shown for each workload (Rest; W1=workload 1 ∼60 w; W2=workload 2 ∼90 w). Group effects and time effects were analyzed controlling for gender effects.
Ventilation (VE-BTPS) and ventilatory equivalent (VE/Vo2)
Ventilation levels (VE-BTPS, L/min) and VE/Vo2 are also shown in Table 2. No differences were found between BHA and BSL at rest, or during any of the submaximal levels, in VE-BTPS or VE/Vo2. Differences in ventilation between groups were analyzed controlling for Vo2 at each corresponding workload. Females had significantly higher ventilatory values (VE-BTPS, L/min) than males during workload 2 (90 w) both before and after training (p<0.05), as well as higher VE/Vo2 (p<0.05)
Discussion
In the present study, it was found that Quechua individuals who have a full developmental exposure to hypoxia maintain a higher arterial oxygen saturation (Sao2) during submaximal exercise, before and after a training period, compared to Quechua individuals who were born and raised at sea-level. However, submaximal ventilation (VE-BTPS) and ventilatory equivalent (VE/Vo2) were not different at any workload between the two groups.
Ventilation and ventilatory equivalent (VE/Vo2)
There were no differences in VE-BTPS (L/min) during submaximal exercise between BHA and BSL. Similarly, our results did not show any difference in VE/Vo2 between groups. A lower VE/Vo2 has been suggested to be more efficient during hypoxic exercise for the ability to extract the same volume of oxygen with lower ventilation. However, unlike many studies in the literature that support lower ventilation during exercise (Schoene et al., 1990; Brutsaert et al., 2000; Wagner et al., 2002) in Andean natives, as well as lower VE/Vo2 in Andeans compared to acclimatized lowlanders (Brutsaert et al., 2000), we did not find any blunted ventilation during rest or submaximal exercise in the BHA group.
A direct comparison with the aforementioned studies might not be straightforward though, considering that previous studies compared populations with differing ancestries (e.g., populations with foreign ancestry and/or acclimatized sojourners and Quechua natives living at high altitude). The present study compares individuals with Quechua ancestry, but who differed in their developmental exposure to hypoxia. Moreover, the BHA group is composed of adult migrants to sea-level, where all hypoxic tests were conducted under simulated conditions. Because ventilation (VE-BTPS, L/min) was not different between groups, we also tested the ΔVE (difference in VE at normoxia and hypoxia) at rest and for each workload, controlling for the Vo2 level during exercise (data not shown). Submaximal ΔVE was not significantly different between groups, and resting ventilatory values did not differ between BHA and BSL either.
Our results are consistent with a report on male high altitude migrants to sea-level, during submaximal exercise (60 w). Gamboa and colleagues (2001) found that ventilation was not blunted in high altitude natives residing at sea-level compared to lowland natives, which suggests that the low ventilation during exercise is dependent on the continuous exposure to a hypoxic stimulus in Andean natives. Moreover, it is important to note that the low ventilation in Andeans was established based on a comparison with fully acclimatized Europeans at altitude (Brutsaert et al., 2000; Wagner et al., 2002). Because we only compared individuals with Quechua ancestry, the lack of difference in ventilation during exercise found in the present study could only indicate that developmental factors are not playing an important role on this trait. The majority of the evidence indicates that ventilatory traits, such as lower submaximal ventilation and acute ventilatory response to hypoxia (HVR) at rest are mainly explained by genetic factors (Brutsaert et al., 2005).
Resting Sao2
No differences were observed between the two study groups in resting Sao2, before or after a period of training. The results in the present study are consistent with other reports in Andeans that did not find any difference in resting Sao2 among groups with different developmental exposure to hypoxia (Brutsaert et al., 2000) and in Tibetans (Chen et al., 1997), suggesting that developmental factors are not an important contributor to resting Sao2 values in high altitude populations.
Nevertheless, there are a handful of studies that suggest similar resting Sao2 in Tibetans and Han Chinese who migrated to the highlands as children (Zhuang, 1993, Chen, 1997). A more recent study comparing Han Chinese with full developmental exposure to hypoxia with Tibetans across a wide age range did not find any difference between those two groups (Weitz and Garruto, 2007), suggesting that developmental exposure to hypoxia might play a role as important as ancestral exposure to hypoxia.
The majority of the evidence in the literature has indicated a stronger case for genetic effects on resting Sao2, especially in Tibetans, where genetic effects have been established through a series of heritability studies (Beall et al., 1997; 2004).
Submaximal Sao2
The results of our study suggests that developmental exposure to hypoxia plays a role in determining submaximal Sao2 in a group of Peruvian Quechua. Unlike measurements of aerobic capacity, differences in physical fitness are not likely to be a significant factor in determining higher Sao2, especially at submaximal levels. In fact, if any difference were to be expected, it would be the opposite. A review of the literature shows that highly trained (elite) individuals are more likely to show lower levels of Sao2, compared to untrained controls (Nielsen, 2003), and even at altitudes as low as 580 m endurance-trained athletes have been found to have increased desaturations at maximal exercise (Gore et al., 1996).
When Sao2 values were analyzed between groups without including covariates (unadjusted), results showed consistently higher Sao2 in BHA during workload 1 and workload 2. However, because Sao2 is largely determined by ventilation and differing levels of relative intensity, we controlled for VE/ Vo2. After adjusting for VE/ Vo2, submaximal Sao2 values were still higher in the BHA group compared to BSL at each workload, as it is shown in Table 2.
The contribution of developmental factors in the maintenance of higher Sao2 levels during exercise has not been extensively studied, and the literature shows conflicting results. Prior to the present report, Brutsaert and colleagues (1999) showed no developmental effects on submaximal Sao2, by comparing European populations with full life-long exposure to hypoxia with Europeans who were recently acclimatized in La Paz. In fact, when comparing Europeans and Aymaras born and raised at altitude, Brutsaert and colleagues (2000) found higher Sao2 in Aymaras, suggesting a genetic effect.
The only study that has supported a developmental effect on submaximal Sao2 was reported by the same research group (Brutsaert et al., 2004). They reported a ∼5% contribution of development in determining higher Sao2 during exercise in Quechua natives born and raised in the highlands compared to lowland-born individuals. In the present study, all individuals were exposed to acute normobaric hypoxia, and were unacclimatized to hypoxia at the time of the tests. Moreover, the repeated-measures analysis in both pre-training and post-training phases showed that BHA had consistently higher submaximal Sao2 than their lowland counterparts.
The evidence for a genetic basis explaining higher Sao2 levels during exercise in Andeans is scarce. Ancestry informative markers (AIMs) have been used to give a measure of Quechua ancestry by some studies. However, ancestry was not associated to exercise Sao2 in Andeans (Brutsaert et al., 2004; 2005). Additionally, the I-allele of the Insertion/Deletion (I/D) polymorphism of the ACE genotype has been associated with higher Sao2 in Andeans (Bigham et al., 2008). While this might appear to suggest a case for selection of the I-allele in Quechua individuals, the frequency of the I-allele is not different from other non-altitude native populations in the Americas (Rupert et al., 1999).
Arterial saturation is a multifactorial phenotype that could be explained by a combination of cardiovascular, hematological factors, and pulmonary factors. In our results, VE/Vo2 and Hb were not different between groups, implying there must be other mechanisms that account for the higher submaximal Sao2 levels in BHA. Oxygen affinity to hemoglobin is another factor that determines Sao2. Winslow (2007) summarized the P50 limitations in humans at altitude, emphasizing the absence of differences in oxygen affinity to hemoglobin in Andeans compared to sea level values. Similarly, Simonson et al. (2014) reported no benefit of P50 during exercise in Tibetans. Therefore, the contributions of Hb-O2 affinity to the differences observed in Sao2 do not appear to be significant.
Another factor that could account for the differences observed in Sao2 is a more efficient pulmonary gas exchange system in hypoxia. Enlarged pulmonary volumes could be associated with higher diffusion capacities by increasing the total surface area of the alveoli. Given that FVC values were higher in the BHA group, the enhanced pulmonary gas exchange constitutes a likely factor. Indeed, Schoene et al. (1990) measured steady state diffusion capacity (DLCO) in Andeans and found that it was higher compared to acclimatized lowlanders, hence suggesting that a better diffusion capacity is the main factor driving the higher Sao2 in Andeans during exercise. Other studies measuring pulmonary diffusion are also consistent with the higher pulmonary efficiency in Andeans at rest (Dempsey et al., 1971) and during exercise (Wagner et al., 2002; Lundby et al., 2004; Faoro et al, 2012; Groepenhoff et al., 2012). In sum, considering the literature showing an enhanced pulmonary diffusion capacity in highlanders, pulmonary gas exchange traits could play an important role determining higher Sao2 during exercise.
Limitations of the study
A limitation of this study is the unknown proportion of Quechua versus Spanish ancestry in the samples. Based on the colonial history of Peru, the current population has differing levels of admixture, especially with European (i.e., Spanish) populations, and to a much lesser extent with African populations. However, there is no a priori reason to consider differing levels of Quechua ancestry between BHA and BSL, based on the criteria for selection mentioned in earlier sections: both sets of maternal and parental lineages from the highlands, surnames, self-identification, and knowledge of Quechua language. Moreover, by using the same selection criteria of the present study, previous reports sampling individuals in Lima, as well as migrants to sea-level, showed a posteriori assessments of ancestry that ranged between 80%–95% using ancestry-informative markers (AIMS) ( Brutsaert et al. 2003; 2005; Bigham et al. 2008).
Finally, a possible limitation of the study is that pulmonary function in our BSL group could have been negatively affected by air pollution during growth, since BSL subjects were born and raised in Lima, which is an urban environment. This, in turn, might have an effect on oxygenation during exercise. However, we consider that possible exposure to air pollution did not confound the results of the present study significantly. A recent report showed higher FVC values in Lima subjects, compared to individuals from lowland rural towns, even if these rural towns had lower levels of pollution than Lima (Robinson et al., 2011). Moreover, even if air pollution tends to be higher in urban settings, highlanders could also be exposed to household air pollution, given the common practice of burning biomass fuel to cook in Andean towns (Pollard et al., 2014).
Conclusions
In a comparison of two groups of Quechua natives who differed by their developmental exposure to hypoxia, this study found that Sao2 was significantly higher during submaximal exercise in those individuals born and raised in the highlands, compared to those born and raised at sea-level. The results of the present study suggest that developmental factors are important to determine higher arterial oxygen saturation in Quechua natives at exercise; thus, consistent with the developmental adaptation hypothesis. Future research should focus on understanding: a) the physiological factors that determine the maintenance of higher Sao2 in Andeans, such as the enhanced pulmonary diffusion capacity associated with highlanders, and b) the genetic basis for the higher Sao2 during exercise in Andeans.
Acknowledgments
We are greatly indebted to all volunteers in our study, who contributed their time and energy to participate in this research. We also thank Cesar de Albertis, Margarita Posso, and Franco Portocarrero for their research assistance. This study was supported by a grant from NSF BCS-0824420 to T. Brutsaert.
Author Disclosure Statement
The authors of this article have no conflicts of interests or financial ties to disclose.
References
- Beall CM, Almasy LA, Blangero J, Williams-Blangero S, Brittenham GM, Strohl KP, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, and Gonzales C. (1999). Percent of oxygen saturation of arterial hemoglobin among Bolivian Aymara at 3,900-4,000 m. Am J Phys Anthropol 108:41–51 [DOI] [PubMed] [Google Scholar]
- Beall CM. (2000). Oxygen saturation increases during childhood and decreases during adulthood among high altitude native Tibetans residing at 3,800-4,200m. High Alt Med Biol 1:25–32 [DOI] [PubMed] [Google Scholar]
- Beall CM, Blanguero J, Williams-Blanguero S, and Goldstein M. (1994). Major gene for percent of oxygen saturation of arterial hemoglobin in Tibetan highlanders. Am J Phys Anthropol 95:271–276 [DOI] [PubMed] [Google Scholar]
- Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, and Gonzales C. (1998). Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol 106:385–400 [DOI] [PubMed] [Google Scholar]
- Beall CM, Song K, Elston RC, and Goldstein MC. (2004). Higher offspring survival among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Proc Natl Acad Sci USA 101:14300–14304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Strohl KP, Blangero J, Williams-Blangero S, Decker MJ, Brittenham GM, and Goldstein MC. (1997). Quantitative genetic analysis of arterial oxygen saturation in Tibetan highlanders. Hum Biol 69:597–604 [PubMed] [Google Scholar]
- Bigham AW, Kiyamu M, León-Velarde F, Parra EJ, Rivera-Ch M, Shriver MD, and Brutsaert TD. (2008). Angiotensin-converting enzyme genotype and arterial oxygen saturation at high altitude in Peruvian Quechua. High Alt Med Biol 9:167–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert TD, Araoz M, Soria R, Spielvogel H, and Haas JD. (2000). Higher arterial oxygen saturation during submaximal exercise in Bolivian Aymara compared to European sojourners and Europeans born and raised at high altitude. Am J Phys Anthropol 113:169–181 [DOI] [PubMed] [Google Scholar]
- Brutsaert TD, Haas JD, and Spielvogel H. (2004). Absence of work efficiency differences during cycle ergometry exercise in Bolivian Aymara. High Alt Med Biol 5:41–59 [DOI] [PubMed] [Google Scholar]
- Brutsaert T, Parra E, Shriver M, Gamboa A, Rivera-Ch M, and León-Velarde F. (2005). Ancestry explains the blunted ventilatory response to sustained hypoxia and lower exercise ventilation of Quechua altitude natives. Am J Physiol Regul Integr Comp Physiol 289:R225–234 [DOI] [PubMed] [Google Scholar]
- Chen QH, Ge RL, Wang XZ, Chen HX, Wu TY, Kobayashi T, and Yoshimura K. (1997). Exercise performance of Tibetan and Han adolescents at altitudes of 3,417 and 4,300 m. J Appl Physiol 83:661–667 [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Reddan WG, Birnbaum ML, Forster HV, Thoden JS, Grover RF, and Rankin J. (1971). Effects of acute through life-long hypoxic exposure on exercise pulmonary gas exchange. Respir Physiol 13:62–89 [DOI] [PubMed] [Google Scholar]
- Droma T, McCullough RG, McCullough RE, Zhuang JG, Cymerman A, Sun SF, Sutton JR, and Moore LG. (1991).Increased vital and total lung capacities in Tibetan compared to Han residents of Lhasa (3,658 m). Am J Phys Anthropol 86:341–351 [DOI] [PubMed] [Google Scholar]
- Durnin JV, and Womersley J. (1974). Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br J Nutr 32:77–97 [DOI] [PubMed] [Google Scholar]
- Faoro V, Huez S, Vanderpool R, Groepenhoff H, de Bisschop C, Martinot JB, Lamotte M, Pavelescu A, Guénard H, and Naeije R. (2014). Pulmonary circulation and gas exchange at exercise in Sherpas at high altitude. J Appl Physiol 116:919–926 [DOI] [PubMed] [Google Scholar]
- Frisancho A. (2009). Developmental adaptation: Where we go from here. Am J Hum Biol 21:694–703 [DOI] [PubMed] [Google Scholar]
- Frisancho A, Frisancho HG, Albalak R, Villain M, Vargas E, and Soria R. (1997). Developmental, genetic and environmental components of lung volumes at high altitude. Am J Hum Biol 9:191–203 [DOI] [PubMed] [Google Scholar]
- Frisancho AR, Frisancho HG, Milotich M, Brutsaert T, Albalak R, Spielvogel H, Villena M, Vargas E, and Soria R. (1995). Developmental, genetic, and environmental components of aerobic capacity at high altitude. Am J Phys Anthropol 96:431–442 [DOI] [PubMed] [Google Scholar]
- Gamboa A, León-Velarde F, Rivera-Ch M, Vargas M, Palacios JA, and Monge-C C. (2001). Ventilatory and cardiovascular responses to hypoxia and exercise in Andean natives living at sea level. High Alt Med Biol 2:341–347 [DOI] [PubMed] [Google Scholar]
- Ge RL, Lun GWH, Chen QH, Li HL, Gen D, Kubo K, Matsuzawa Y, Fujimoto K, Yoshimura K, Takeoka M, and Kobayashi T. (1995). Comparisons of oxygen transport between Tibetan and Han residents at moderate altitude. Wilderness Environ Med 6:391–400 [Google Scholar]
- Gore CJ, Hahn AG, Scroop GC, Watson DB, Norton KI, Wood RJ, Campbell DP, and Emonson DL. (1996). Increased arterial desaturation in trained cyclists during maximal exercise at 580 m altitude. J Appl Physiol 80:2204–2210 [DOI] [PubMed] [Google Scholar]
- Greksa LP. (1990). Developmental responses to high-altitude hypoxia in Bolivian children of European ancestry: A test of the developmental adaptation hypothesis. Am J Hum Biol 2:603–612 [DOI] [PubMed] [Google Scholar]
- Groepenhoff H, Overbeek MJ, Mulè M, van der Plas M, Argiento P, Villafuerte FC, Beloka S, Faoro V, Macarlupu JL, Guenard H, de Bisschop C, Martinot JB, Vanderpool R, Penaloza D, and Naeije R. (2012). Exercise pathophysiology in patients with chronic mountain sickness exercise in chronic mountain sickness. Chest 142:877–884 [DOI] [PubMed] [Google Scholar]
- Harrison G. (1966). Human adaptability with reference to the IBP proposals for high altitude research. In: The Biology of Human Adaptability. Baker PT, and Weiner JS, eds. Oxford: Clarendon Press; pp. 509–519 [Google Scholar]
- Hochachka PW, Rupert JL, and Monge C. (1999). Adaptation and conservation of physiological systems in the evolution of human hypoxia tolerance. Comp Biochem Physiol A Mol Integr Physiol 124:1–17 [DOI] [PubMed] [Google Scholar]
- Kiyamu M, Bigham A, Parra E, León-Velarde F, Rivera-Chira M, and Brutsaert TD. (2012). Developmental and genetic components explain enhanced pulmonary volumes of female Peruvian Quechua. Am J Phys Anthropol 148:534–542 [DOI] [PubMed] [Google Scholar]
- Kiyamu M, Elías G, León-Velarde F, Rivera M, and Brutsaert TD. (2015). Aerobic capacity of Peruvian Quechua: A test of the Developmental Adaptation Hypothesis. Am J Phys Anthropol 156:363–373 [DOI] [PubMed] [Google Scholar]
- Kriska A. (1997). Modifiable activity questionnaire. Med Sci Sports Exerc 29:S73–S78 [Google Scholar]
- Lundby C, Calbet JA, van Hall G, Saltin B, and Sander M. (2004). Pulmonary gas exchange at maximal exercise in Danish lowlanders during 8 wk of acclimatization to 4,100 m and in high-altitude Aymara natives. Am J Physiol Regul Integr Comp Physiol 287:R1202–1208 [DOI] [PubMed] [Google Scholar]
- Marconi C, Marzorati M, Grassi B, Basnyat B, Colombini A, Kayser B, and Cerretelli P. (2004). Second generation Tibetan lowlanders acclimatize to high altitude more quickly than Caucasians. J Physiol 556:661–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LG. (2001). Human genetic adaptation to high altitude. High Alt Med Biol 2:257–279 [DOI] [PubMed] [Google Scholar]
- Nielsen HB. (2003). Arterial desaturation during exercise in man: Implication for O2 uptake and work capacity. Scand J Med Sci Sports 13:339–358 [DOI] [PubMed] [Google Scholar]
- Pollard SL, Williams DL, Breysse PN, Baron PA, Grajeda LM, Gilman RH, Miranda JJ, and Checkley W; CRONICAS Cohort Study Group. (2014). A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ Health 13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CL, Baumann LM, Romero K, Combe JM, Gomez A, Gilman RH, Cabrera L, Gonzalvez G, Hansel NN, Wise RA, Barnes KC, Breysse PN, and Checkley W. (2011). Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax 66:1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupert JL, Devine DV, Monsalve MV, and Hochachka PW. (1999). Angiotensin-converting enzyme (ACE) alleles in the Quechua, a high altitude South American native population. Ann Hum Biol 26:375–380 [DOI] [PubMed] [Google Scholar]
- Schoene RB, Roach RC, Lahiri S, Peters RM, Jr., Hackett PH, and Santolaya R. (1990). Increased diffusion capacity maintains arterial saturation during exercise in the Quechua Indians of Chilean Altiplano. Am J Hum Biol 2:663–668 [DOI] [PubMed] [Google Scholar]
- Simonson TS, Wei G, Wagner HE, Wuren T, Bui A, Fine JM, Qin G, Beltrami FG, Yan M, Wagner PD, and Ge RL. (2014). Increased blood-oxygen binding affinity in Tibetan and Han Chinese residents at 4200 m. Exp Physiol 99:1624–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siri W. (1956). The gross composition of the body. Adv Biol Med Phys 4:239–280 [DOI] [PubMed] [Google Scholar]
- Sun SF, Droma TS, Zhang JG, Tao JX, Huang SY, McCullough RG, McCullough RE, Reeves CS, Reeves JT, and Moore LG. (1990). Greater maximal O2 uptakes and vital capacities in Tibetan than Han residents of Lhasa. Respir Physiol 79:151–161 [DOI] [PubMed] [Google Scholar]
- Wagner PD, Araoz M, Boushel R, Calbet JA, Jessen B, Rådegran G, Spielvogel H, Søndegaard H, Wagner H, and Saltin B. (2002). Pulmonary gas exchange and acid-base state at 5,260 m in high-altitude Bolivians and acclimatized lowlanders. J Appl Physiol 92:1393–1400 [DOI] [PubMed] [Google Scholar]
- Weitz CA, and Garruto RM. (2007). A comparative analysis of arterial oxygen saturation among Tibetans and Han born and raised at high altitude. High Alt Med Biol 8:13–26 [DOI] [PubMed] [Google Scholar]
- Winslow RM. (2007). The role of hemoglobin oxygen affinity in oxygen transport at high altitude. Respir Physiol Neurobiol 158:121–127 [DOI] [PubMed] [Google Scholar]
- Zhuang J, Droma T, Sutton JR, Groves BM, McCullough RE, McCullough RG, Sun S, and Moore LG. (1996). Smaller alveolar-arterial O2 gradients in Tibetan than Han residents of Lhasa (3658 m). Respir Physiol 103(1): 75–82 [DOI] [PubMed] [Google Scholar]