Abstract
Neurofibromatosis type 1 (NF1) has regularly been associated with cognitive, social, and behavioral problems. The fact that many different cognitive and behavioral impairments have been observed in NF1 suggests that networks of brain regions are involved rather than specific brain regions. Here, we examined whether functional connectivity was different in NF1 and, if so, whether associations were present with cognitive, social, and behavioral outcomes. Fourteen NF1 patients (8 male, age: M=12.49, SD=2.65) and 30 healthy controls (HC; 23 male, age: M=12.30, SD=2.94; p=0.835) were included. Functional connectivity was assessed using functional resting-state scanning. We analyzed brain regions that have been associated with cognitive and social functions: the bilateral ventral anterior cingulate cortex (vACC), the bilateral amygdala, the bilateral orbitofrontal cortex (OFC), and the posterior cingulate cortex (PCC). For NF1 patients, connection strengths between brain regions showing HC-NF1 differences were correlated with parent reports of cognitive, social, and behavioral functioning. Compared to HC, patients showed differences in functional connectivity between the left vACC and the frontal cortex, insula, and subcortical areas (caudate, putamen), between the left amygdala and the frontal cortex, insula, supramarginal gyrus, and PCC/precuneus, and between the left OFC and frontal and subcortical areas (caudate, pallidum). In patients, indications were found for associations between increased frontofrontal and temporofrontal functional connectivity with cognitive, social, and behavioral deficits (r-range=0.536–0.851). NF1 patients showed differences in functional connectivity between areas associated with cognitive and social functioning when compared to controls. This, plus the fact that connectivity strengths in these networks were associated with worse cognitive, social, and behavioral outcomes, suggests a neuropathological basis for the widespread deficits observed in NF1.
Key words: : behavioral problems, executive dysfunction, functional connectivity, neurofibromatosis type 1, resting-state social problems
Introduction
Neurofibromatosis Type 1 (NF1) is an autosomal genetic disorder, affecting one in 3500 newborns (National Institutes of Health, 1988), characterized by many different symptoms such as glial neoplasms, café-au-lait spots, skin fold freckling, optic nerve glioma, osseous (bone) abnormalities, iris Lisch nodules, and cutaneous and plexiform neurofibromas (Payne et al., 2010). Brain manifestations include tumors, megalencephaly, and anomalies of the corpus callosum (Payne et al., 2010).
Core behavioral consequences include cognitive impairment, social malfunction, and behavioral abnormalities. Cognitive abnormalities have been expressed in lower intelligence quotient (IQ), learning disabilities, visuospatial processing impairments, language problems, and executive deficits (Billingsley et al., 2004; Clements-Stephens et al., 2008; Coudé et al., 2006; Cutting et al., 2002; Hyman et al., 2005, 2006; North et al., 1994; Payne et al., 2011; Rowbotham et al., 2009; Roy et al., 2010). Social and behavioral problems include difficulties with social skills and communication, problems with forming friendships, rejection by peers, and social isolation. Although social problems have been associated with cognitive difficulties, low academic achievement, and attention deficit hyperactivity disorder (Barton and North, 2004; Gresham et al., 1996; Huijbregts et al., 2010), it was shown that social malfunction remained, although attenuated, after controlling for cognitive status (Huijbregts et al., 2010).
Previous functional magnetic resonance imaging (fMRI) studies in other diseases, examining alterations in networks of brain regions associated with cognitive and social functioning (Salmi et al., 2013; Wheaton et al., 2014), highlighted several pivotal areas being associated with measured malfunction, including the amygdala, orbitofrontal cortex (OFC), and anterior (ACC) and posterior cingulate cortex (PCC) (Gasquoine, 2013; Jackowski et al., 2012; Leech and Sharp, 2014; Murray et al., 2014; Salmi et al., 2013). The amygdala has been associated with determining the emotional significance of visual, auditory, and olfactory signals, and is involved in coping with the social environment and making social judgments (Adolphs et al., 1998), especially when evaluating facial expressions. Lesion studies revealed that amygdala damage results in deficits in retrieving socially relevant knowledge on the basis of facial appearance or an impairment in the ability to recognize fear from facial expressions (Adolphs, 1994, 1998). Deficits in face recognition, identification of facial emotions, especially fearful expressions, and in matching facial emotions have already been reported in NF1 (Huijbregts et al., 2010). The amygdala shares extensive reciprocal connections with the OFC, damage to which has also been associated with social deficits (Beer et al., 2006). The OFC is involved in social and antisocial behavior (Passamonti et al., 2010), self-monitoring (Beer et al., 2006), and social adaptation (Beyer et al., 2014). However, imaging studies of the OFC in NF1 are sparse. Violante et al. (2013) reported on local structural orbitofrontal differences between NF1 patients and control subjects and suggested these might underlie the executive impairments in NF1. The ACC and the PCC, both part of the default mode network (DMN, an assembly of synchronized spatially remote areas shown to be deactivated during goal-directed behavior), have been reported to be fundamentally involved in both social and cognitive processes (Greicius et al., 2003; Mars et al., 2012). The ACC comprises a ventral section (vACC), associated specifically with emotional aspects, especially the assessment of salience of emotional and motivational information and the regulation of emotional responses, and a dorsal section (dACC), which has been associated with modulation of attention and executive function (Bush et al., 2000).
In NF1, despite the disease-related impact on the brain [e.g., unidentified bright objects (Ferraz-Filho et al., 2012), macrocephaly (Steen et al., 2001), microstructural alterations (Ferraz-Filho et al., 2011)] and the established behavioral, cognitive, and social deficits, fMRI studies are scarce. A number of task-based functional imaging studies showed abnormal activation of brain regions associated with phonological and visual processing, as well as the effect of medication on the DMN (Billingsley et al., 2004; Chabernaud et al., 2012; Clements-Stephens et al., 2008; Violante et al., 2012), all reporting on task-related functional network changes.
Resting-state functional imaging has emerged as a valuable method of assessing integrity of brain functional connectivity networks in patient cohorts in the past decade, as data acquisition is less dependent on patient compliance, which appears to be particularly important in patient cohorts suffering from attention deficits, problems with task comprehension, or lying still during task execution. During resting-state fMRI, spontaneous fluctuations of the BOLD signal within remote brain regions are used to infer temporal coherencies (Biswal et al., 1995; Greicius et al., 2003). The temporal synchronization between remote brain regions is commonly interpreted as functional connectivity, which in turn is suggested to mirror brain functions (Smith et al., 2009).
Given the executive, social, and behavioral impairments in NF1, we hypothesized that these deficits would be associated with abnormal functional brain connectivity within brain networks related to these specific domains. Furthermore, we investigated whether any IQ differences were also associated with the functional connectivity differences of interest (and, if so, intended to correct for them). A seed-based connectivity approach, using seven seeds related to NF1 pathophysiology and sociocognitive problems (bilateral amygdala, bilateral OFC, bilateral vACC, and PCC), was used to provide a comprehensive overview of functional connectivity abnormalities in NF1.
Materials and Methods
Participants
In this cross-sectional study, 14 NF1 patients (8 male, age: M=12.49, SD=2.65) and 30 healthy controls (HCs, 23 male, age: M=12.30, SD=2.94; p=0.835, sex: χ2=1.748, df=1, p=0.186) underwent structural and functional MRI. All NF1 subjects fulfilled the diagnostic criteria specified by the National Institutes of Health (1988). Written informed consent was obtained.
The experiment was approved by the Ethics Committee of the Leiden University Medical Center (Leiden, The Netherlands) and the Department of Education and Child Studies, Leiden University (Leiden, The Netherlands).
Imaging protocol
All subjects underwent scanning at the Leiden University Medical Center. Imaging was performed on a Philips 3 Tesla Achieva MRI scanner using an 8-channel SENSE receiver head coil (Philips Healthcare, Best, The Netherlands). In each subject, a three-dimensional (3D) T1-weighted anatomical scan was acquired with the following scan parameters for registration purposes: 3D T1 TFE sequence, 140 axial slices, TR 9.8 msec, TE 4.6 msec, flip angle 8°, 1.16×0.92 mm, 1.2 mm slice thickness, and no slice gap. The scanning parameters of the resting-state fMRI scan were as follows: GE-EPI sequence, 160 volumes, 38 axial slices, TR 2.2 sec, TE 30 msec, flip angle 80°, 2.75 mm isotropic voxels, and 0.25 mm slice gap (duration=5.8 min). During the resting-state scan, participants were instructed to lie still with their eyes closed and not to fall asleep. All anatomical scans were reviewed by a neuroradiologist.
Preprocessing resting-state data
FMRI data preprocessing was performed using FMRIBs Software Library (FSL 5.0.4, Oxford, United Kingdom; www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004). To automatically denoise the resting-state data, FIX (v1.06 beta, FMRIBs ICA-based X-noiseifier; additionally requiring MATLAB [Statistics and Signal Processing Toolbox] and R) was conducted (Griffanti et al., 2014; Salimi-Khorshidi et al., 2014). Given a set of independent components, FIX classifies components as “signal” or “noise” (effects of motion, non-neuronal physiology, scanner artifacts, and other nuisance sources), the latter then being removed from the data (Salimi-Khorshidi et al., 2014). FIX denoising reduced absolute motion by 0.52 mm in controls (predenoising motion: M=0.547 mm, SD=0.760) and by 0.91 mm in NF1 patients (predenoising motion: M=0.933 mm, SD=0.816, in motion differences between groups before correction: p=0.036), resulting in nonsignificant motion differences between groups after correction (p=0.514). The following preprocessing steps were subsequently performed in the resting-state data: motion correction (Jenkinson et al., 2002), brain extraction (Smith, 2002), spatial smoothing using a Gaussian kernel of full width at a half-maximum of 5 mm and a high-pass temporal filtering of 100 sec (cutoff 0.01 Hz). After preprocessing, the functional images were boundary based registered to the corresponding high-resolution T1-weighted images, which afterward were registered to the MNI152 standard space image using nonlinear registration with a warp resolution of 10 mm (Greve and Fischl, 2010).
Resting-state data analyses
The functional connectivity analysis was performed using a dual regression method (part of FSL) (Filippini et al., 2009). We chose 4 mm spherical regions of interest in the vACC left (Montreal Neurologic Institute [MNI] coordinates: −2, 38, 10) and right (4, 36, 12), the amygdala left (−22, −6, −16) and right (28, −2, −20), the OFC left (−16, 12, −20) and right (20, 24, −20), and the PCC (0, −40, 30). The center coordinate of each seed was extracted from the Harvard-Oxford Cortical or Subcortical Atlases. Each seed was paired with two confound variables (white matter and cerebrospinal fluid) and used in the spatial and temporal dual regression technique [as described in Filippini et al. (2009)] to perform voxelwise between group comparisons. In the dual regression analysis, for each patient, each seed (and white matter and cerebrospinal fluid) was used as a spatial regressor in a general linear model to identify individual temporal dynamics (regression 1). The time courses were then used as a set of temporal regressors in a second general linear model (regression 2), resulting in beta-weights for each voxel. These were used in the subsequent higher level analysis. Group-level inferences were carried out using voxelwise nonparametric permutation testing (5000 permutations, Randomise v2.9, Nichols and Holmes, 2002). Results were corrected for multiple comparisons across space using cluster-based correction (threshold z=2.3, FDR-corrected p=0.05). Group comparisons were performed using two-tailed independent samples t-tests. To identify the direction of functional connectivity changes, we extracted the mean beta-values for each individual within clusters of significant difference of group comparisons.
Questionnaires
The neuropsychological assessment involved estimation of IQ based on the Wechsler Intelligence Scale for Children (WISC, Wechsler et al., 2011). The following subtests were obtained: verbal comprehension (vocabulary, comprehension), visual-spatial abilities (block design), fluid reasoning (picture completion), and processing speed (symbol search, coding). Verbal IQ was estimated based on the subscales vocabulary and comprehension and performance IQ was estimated on the basis of all other subtests. For other aspects of cognitive and social functioning, several questionnaires were administered. For social skills, the parent version of the Social Skills Rating System (SSRS) (Gresham and Elliott, 1990) was used. The four SSRS-dimensions (cooperation, assertion, responsibility, self-control) as well as the total SSRS score were used as indicators of social skills. The Social Responsiveness Scale (SRS) (Constantino, 2005) is a standard measure of autism spectrum. It includes five interrelated dimensions: social awareness, social cognition, reciprocal social communication, social motivation, and autistic mannerisms. The Child Behavior Checklist (CBCL) (Achenbach, 1992) was used to determine behavioral problems. Executive functioning was assessed using the parent-rated Behavior Rating Inventory of Executive Function (BRIEF) (Gioia et al., 2000) and the Dysexecutive Questionnaire (DEX, Wilson et al., 1996). The DEX is part of the Behavioral Assessment of the Dysexecutive Syndrome (Wilson et al., 1996) and is designed to assess a range of cognitive, behavioral, and emotional problems associated with executive dysfunction.
The BRIEF total score and the DEX cognition were used as indicators of executive function. Due to a high correlation between scores on the two questionnaires (r=0.836, p<0.001), principal component analyses for dimensionality reduction were used to extract one fixed component. To account for the reported influence of cognition on social functioning, this component was used as a covariate in the group comparison of social psychometrics to control for the effect. Test scores were available for all patients and nine control children.
Statistical analyses
SPSS 21 for Windows (SPSS, Chicago, IL) was used for statistical analyses. Whenever appropriate, tested with the Kolmogorov–Smirnov test for normal distribution of data, parametric tests were used. If data were not normally distributed, nonparametric tests were applied. For the NF1 patients, Pearson correlations were performed (uncorrected for multiple comparisons) between the beta-scores of areas of functional connectivity differences and IQ, executive, social, and behavioral outcome measures.
Results
IQ, cognitive and social profile
When compared to controls, NF1 patients showed a significantly lower performance IQ (MHC=11.36, SDHC=2.05; MNF1=8.91, SDNF1=2.29; p=0.016) and total IQ scores (MHC=11.17, SDHC=1.88; MNF1=8.92, SDNF1=1.77; p=0.009). Only a trend was found for verbal IQ (MHC=10.78, SDHC=2.43; MNF1=8.93, SDNF1=2.23; p=0.074). Also, NF1 patients showed poorer cognitive and social scores in all but one examined domains (Table 1). As social functioning was reported to be influenced by cognitive control deficits (Huijbregts et al., 2010), subscales of social questionnaires were corrected for executive function. The SRS total score, including the subscale social motivation and autistic mannerisms, as well as SSRS assertion and CBCL social problems, remained significantly different after correction (Table 1).
Table 1.
Group Differences Between NF1 Patients and Healthy Controls on Cognitive/Executive Function and Social Questionnaires
| NF1 | Controls | p | p-corr | Partial η2 | |
|---|---|---|---|---|---|
| DEX | |||||
| Behavior | 1.830±0.923 | 0.666±0.946 | 0.0162 | na | na |
| Cognition | 1.628±0.772 | 0.622±0.738 | 0.005 | na | na |
| Emotion | 1.714±0.959 | 0.815±0.669 | 0.023 | na | na |
| Total | 34.9±16.1 | 15.0±14.0 | 0.002 | na | na |
| BRIEF | |||||
| Total | 158.15±31.14 | 96.78±21.75 | <0.001 | na | na |
| SRS | |||||
| Social awareness | 18.857±3.880 | 13.222±2.333 | 0.001 | 0.733 | 0.006 |
| Social cognition | 25.786±5.423 | 16.667±4.664 | <0.001 | 0.456 | 0.030 |
| Reciprocal social communication | 47.071±9.723 | 29.889±7.061 | <0.001 | 0.183 | 0.091 |
| Social motivation | 24.143±3.840 | 16.667±2.958 | <0.001 | 0.025 | 0.238 |
| Autistic mannerisms | 26.429±4.86 | 13.889±2.088 | <0.001 | 0.000 | 0.507 |
| Total | 142.286±23.695 | 90.333±16.651 | <0.001 | 0.013 | 0.284 |
| SSRSa | |||||
| Cooperation | 9.857±5.934 | 16.444±3.087 | 0.002 | 0.628 | 0.013 |
| Assertion | 12.143±3.325 | 17.889±2.421 | <0.001 | 0.022 | 0.245 |
| Responsibility | 12.214±3.468 | 17.000±2.236 | 0.001 | 0.773 | 0.004 |
| Self-control | 9.429±4.586 | 16.111±3.140 | 0.001 | 0.845 | 0.002 |
| Total | 43.643±13.921 | 67.444±7.748 | <0.001 | 0.198 | 0.086 |
| CBCL | |||||
| Anxious/depressed | 4.286±3.124 | 2.778±2.108 | 0.218 | na | na |
| Withdrawn/depressed | 5.214±3.068 | 1.000±1.225 | <0.001 | na | na |
| Somatic complaints | 3.857±2.214 | 1.556±1.810 | 0.023b | na | na |
| Social problems | 8.857±4.786 | 1.000±1.118 | <0.001b | 0.050 | 0.187 |
| Thought problems | 5.214±4.264 | 0.778±1.093 | <0.001b | na | na |
| Attention problems | 10.643±4.050 | 2.444±4.126 | <0.001 | na | na |
| Rule-breaking behavior | 3.071±1.940 | 1.333±1.658 | 0.038 | na | na |
| Aggressive behavior | 9.643±6.271 | 3.111±5.947 | 0.016b | na | na |
As executive functions can impact on social skills, p-corr shows group differences, in social scales only, after correction for cognition/executive function (uncorrected for multiple comparisons).
Higher scores indicate better performance.
Mann–Whitney U-Test, otherwise t-test.
DEX, Dysexecutive Questionnaire; BRIEF, Behavior Rating Inventory of Executive Functioning; SRS, Social Responsiveness Scale; SSRS, Social Skills Rating System; CBCL, Child Behavior Checklist; uncorrected for multiple comparisons; NF1, neurofibromatosis type 1.
Seed-based functional connectivity
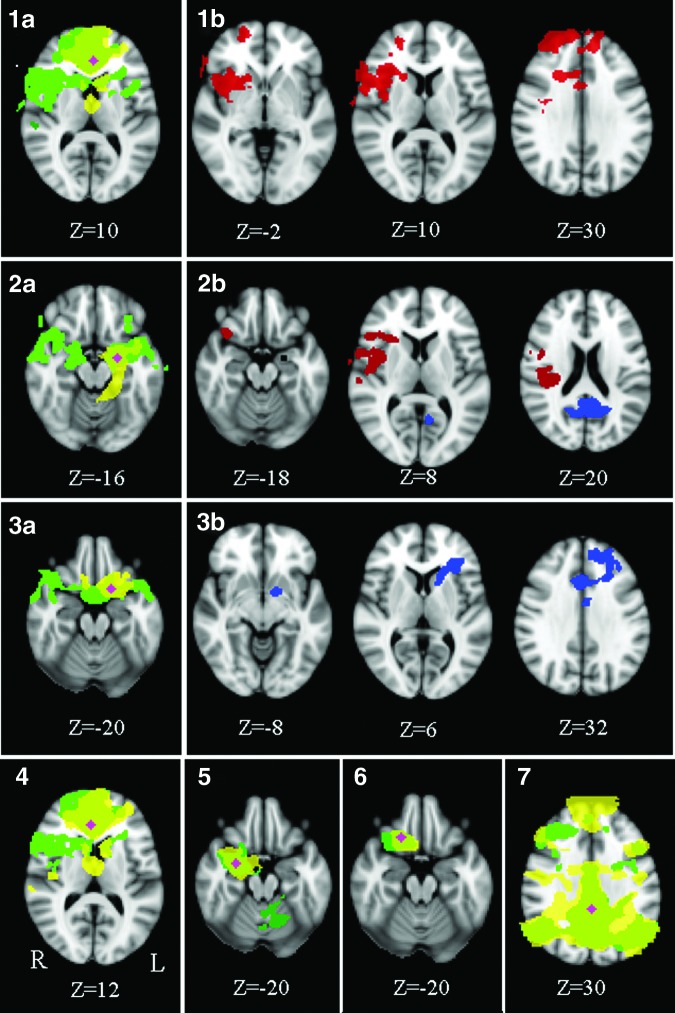
Mean functional connectivity maps for the seed regions and their associated areas can be seen in Figure 1 (1a, 2a, 3a, 4–7). For a precise group-specific network description, see Supplementary Data; Supplementary Data are available online at www.liebertpub.com/brain. Group differences in functional connectivity were found between the left vACC and the insular cortex, dACC, frontal pole, right caudate nucleus, putamen, and inferior frontal gyrus (Fig. 1: 1b), between the left amygdala and the central and frontal opercular cortex, insula, frontal pole, right supramarginal gyrus, OFC, and the PCC/precuneus (Fig. 1: 2b), and between the left OFC and the dACC, right frontal pole, middle frontal gyrus, left caudate, and left pallidum (Fig. 1: 3b).
FIG. 1.
Mean functional connectivity maps of the seed regions in the vACC (1a), amygdala left (2a), OFC left (3a), vACC right (4), amygdala right (5), orbitofrontal cortex right (6), and the PCC (7) (green, NF1 patients; yellow, controls; pink, the seed). Group differences are shown in (1b, 2b, and 3b). In NF1 patients, areas show positive coupling in functional connectivity with the vACC left (1b) and the amygdala left (2b, red), and negative coupling with the amygdala left (2b, blue) and the orbitofrontal cortex left (3b, blue) when compared to controls. No differences in functional connectivity were found for right hemispheric seeds or for the PCC (corrected p=0.05, z=2.3). NF1, neurofibromatosis type 1; vACC, ventral anterior cingulate cortex; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex.
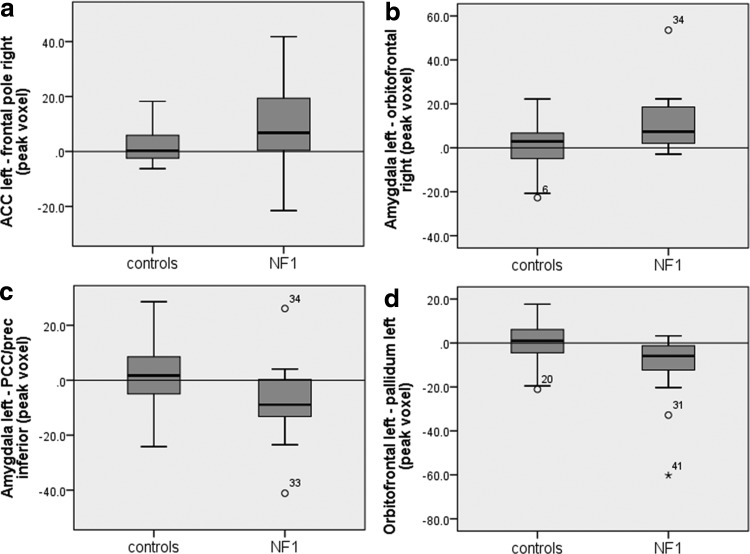
To determine whether functional connectivity is higher or lower in NF1 patients when compared to controls between the respective areas (e.g., between the vACC left and the frontal pole right), beta-scores served to indicate on directionality (Figs. 1 and 2). Functional connectivity in the abovementioned areas of group differences was not significantly different from zero in controls, highlighting that within the patient group, positive or negative connectivity exists between areas where no significant connectivity on average in controls is observed. Therefore, all regions with a difference show an increased connection (either positive or negative interaction) in NF1.
FIG. 2.
Mean beta-values of NF1 children and controls in areas of connectivity difference (peak voxel) seen in Figure 1. NF1 patients show positive coupling between the vACC left and the frontal pole (a) and between the left amygdala and OFC (b) compared to controls, who do not show significant functional connectivity between those areas (values toward 0). In contrast, NF1 patients show negative coupling between the amygdala left and the PCC/precuneus (c) and between the OFC left and the pallidum left (d), when compared to controls, who again do not show significant functional connectivity. Circles and asterisks constitute outliers. FC, functional connectivity; ACC, anterior cingulate cortex; prec, precuneus.
To examine the association between differences in functional connectivity and executive, social, and behavioral outcomes, as well as IQ, correlations between betas of areas of significant differences (i.e., frontal pole right, OFC right, PCC/precuneus, and the pallidum left, Fig. 2) and questionnaire scores were conducted (Table 2). These revealed that higher functional connectivity between (1) the left vACC and the right frontal pole correlated positively with the DEX, BRIEF, and SRS scores, and subscores of the CBCL and (2) a higher functional connectivity between the left amygdala and the right OFC correlated with subscores of the CBCL. These results indicate that the higher the functional connectivity between the stated areas, the worse the executive, social, and behavioral performance of NF1 patients. No correlation was found between areas of lower functional connectivity (PCC/precunus, pallidum left) and questionnaire outcome measures. Also, no correlation was found with IQ.
Table 2.
Significant Correlations Between Areas of Changed Functional Connectivity and Social/Executive Measures in NF1 Patients Only (Uncorrected for Multiple Comparisons)
| Correlated variables | r | p | |
|---|---|---|---|
| lvACC-rFP | DEX total | 0.582 | 0.029 |
| lvACC-rFP | DEX behavior | 0.633 | 0.015 |
| lvACC-rFP | DEX emotion | 0.540 | 0.046 |
| lvACC-rFP | SRS total | 0.564 | 0.036 |
| lvACC-rFP | BRIEF | 0.554 | 0.049 |
| lvACC-rFP | CBCL social problems | 0.536 | 0.048 |
| lvACC-rFP | CBCL thought problems | 0.635 | 0.015 |
| lvACC-rFP | CBCL withdrawn/depression | 0.586 | 0.028 |
| lAMG-rOFC | CBCL attention problems | 0.637 | 0.014 |
| lAMG-rOFC | CBCL thought problems | 0.851a | 0.000 |
| lAMG-rOFC | CBCL withdrawn/depression | 0.623 | 0.017 |
Significant after correction for multiple comparisons (FDR, q=0.05).
L, left; r, right; vACC, ventral anterior cingulate cortex; FP frontal pole; AMG, amygdala; OFC, orbitofrontal cortex.
Discussion
Using resting-state fMRI to investigate functional connectivity alterations within networks associated with cognitive, social, and behavioral performance, we observed several significant functional alterations in the connectivity patterns of NF1 children when compared to controls. These differences revealed increases of interactions between certain brain regions in NF1 patients.
Positive coupling was found between left vACC and areas of the frontal cortex, insula cortex, and subcortical areas, and between the left amygdala and the frontal cortex, parietal cortex, and insula cortex. Negative coupling was found between the left amygdala and the PCC/precuneous, and between left OFC and frontal and subcortical areas. These changes were partly associated with behavioral outcome measures in NF1 patients. The positive coupling between the left vACC and the frontal pole and between the left amygdala and the right OFC was correlated with worse executive, social, and behavioral performances in NF1 patients. Importantly, equivalent correlations between functional connectivity and IQ have not been found, corroborating that deficits in executive functions, social skills, and behavior cannot be ascribed to global deficits only. We conclude that the increased functional connectivity between frontofrontal areas and temporofrontal areas is maladaptive rather than a failed attempt of the brain to compensate by functional reorganization.
In addition to the functional connectivity changes, we found in line with previous literature (Huijbregts and de Sonneville, 2011; Rowbotham et al., 2009), widespread impairments in executive, social, and behavioral function in the patients. The social deficits partly remained even after controlling for executive dysfunction (Huijbregts and de Sonneville, 2011; Huijbregts et al., 2010). Together, these findings further inform us on the neuropathological basis for the executive, social, and behavioral phenotype that has consistently been associated with NF1.
The seed-based approach revealed NF1-related connectivity deviations of the left vACC, left amygdala, and left OFC. The most prominent deviations were found in both the left vACC and left amygdala showing functional increases in connectivity with the insular cortex and frontoparietal operculum. The insular cortex has been associated with a variety of different functions, such as cognitive control (Cole and Schneider, 2007), individual emotions (Nieuwenhuys, 2012; Phan et al., 2002), and social emotion, including empathy and compassion, as well as interpersonal phenomena such as fairness and cooperation (Lamm and Singer, 2010). Also, the importance of the insular cortex has already been shown in several diseases associated with social-cognitive dysfunction such as mood disorders, post-stroke depression, panic disorders, post-traumatic stress disorders, obsessive compulsive disorder, and schizophrenia (Nagai et al., 2007). Hence, a shift in functional connectivity of the vACC and the amygdala with the insular cortex might be a strong indicator of underlying pathophysiological change in the context of social and cognitive status in NF1.
In addition, differences in functional connectivity were identified between the vACC and the dACC and the frontal pole. Previous literature highlighted the segregated functional role of the ventral and dorsal cingulate cortex, ascribing them an emotional and cognitive function, respectively (Bush et al., 2000). A reciprocal suppression between these two cingulate areas was reported, that is, deactivation of the vACC during cognitive tasks and deactivation of the dACC during emotional tasks (Bush et al., 2000). Furthermore, the dACC has strong connections to the frontal pole, which has been associated with social cognition, attention, episodic memory, and multitasking (Burgess et al., 2007; Gilbert et al., 2006, 2010). The imbalance between dorsal and ventral suppression within the anterior cingulate in adolescents with NF1, seen in their increased functional connectivity when compared to controls, together with the increased connectivity to the frontal pole, might explain the cognitive influence on social functioning in NF1 children, and hence, the attenuation of social deficits when controlling for cognitive dysfunction. Additionally, a divergence in the functional connectivity of the ACC, together with the medial prefrontal cortex and the retrosplenial cortex, all part of the DMN, has already been reported in a prior study examining visual processing of NF1 patients (Violante et al., 2012). The authors reported that patients failed to deactivate these areas when compared to controls. As the DMN has been associated with daydreaming and self-referential thought, they concluded that patients had a higher tendency toward task-irrelevant thoughts. Although we cannot corroborate this conclusion with our results, the repeated finding of functional differences between patients and controls associated with the ACC indicates its significant role in NF1-related brain pathology.
Finally, a negative coupling in functional connectivity between the amygdala and the PCC/precuneus was found. Both the PCC and precuneus are main hubs of the DMN (Damoiseaux et al., 2006). Previously, altered connectivity between regions of the DMN has been associated with a variety of mental and developmental disorders, including schizophrenia, autism, attention deficit, and anxiety disorders (Leech and Sharp, 2014). The fact that patients show more negative coupling between the amygdala and the PCC/precuneus could be indicative of a frontoparietal pathophysiological process. The network associated with the PCC seed did not reveal any group differences. Although this seems counterintuitive, considering the wide range of abnormalities in NF1 and the disease-related sensibility of the DMN, seen in other diseases (Leech and Sharp, 2014), outcomes of networks depend on the precise location of the seed. We therefore cannot exclude that various other seed coordinates within the PCC might reveal connectivity differences between groups. Further studies are needed to examine its potential role in NF1.
A limitation of the current study is the modest size of the patient group, although similar group sizes have been used in other fMRI studies in NF1 (Chabernaud et al., 2012; Violante et al., 2012). Behavioral data were available for only a subset of control subjects, although group differences were very much in line with those observed in earlier studies with larger control samples. Another limitation concerns the fact that results are uncorrected for multiple comparisons. Differences in functional connectivity strongly indicate on deviant brain organization in NF1 patients, but due to limited power, results become nonsignificant after correction. Also, the correlation results remained uncorrected. It therefore needs to be emphasized that the results have to be interpreted with care and need further verification in bigger samples.
Despite these limitations, this study provides comprehensive insights into the role of abnormal functional connectivity and its association to executive, social, and behavioral deficits observed in NF1.
Supplementary Material
Acknowledgments
This study was funded by the Austrian Science Fund (FWF: J3500 B23) and the Dutch Neurofibromatosis Association. S.A.R. is supported by a grant of the Netherlands Organization for Scientific Research (NWO).
Author Disclosure Statement
No competing financial interests exist.
References
- Achenbach T. 1992. Manual for the Child Behavior Checklist/2-3 and 1992 Profile. Burlington, VT: Department of Psychiatry, University of Vermont [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. 1998. The human amygdala in social judgment. Nature 393:470–474 [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. 1994. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372:669–672 [DOI] [PubMed] [Google Scholar]
- Barton B, North K. 2004. Social skills of children with neurofibromatosis type 1. Dev Med Child Neurol 46:553–563 [DOI] [PubMed] [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. 2006. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci 18:871–879 [DOI] [PubMed] [Google Scholar]
- Beyer F, Münte TF, Göttlich M, Krämer UM. 2014. Orbitofrontal cortex reactivity to angry facial expression in a social interaction correlates with aggressive behavior. Cereb Cortex pii: [DOI] [PubMed] [Google Scholar]
- Billingsley RL, Jackson EF, Slopis JM, Swank PR, Mahankali S. 2004. Functional MRI of visual–spatial processing in neurofibromatosis, type I. Neuropsychologia 42:395–404 [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. 2007. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends Cogn Sci 11:290–298 [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M. 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222 [DOI] [PubMed] [Google Scholar]
- Chabernaud C, Mennes M, Kardel PG, Gaillard WD, Kalbfleisch ML, Vanmeter JW, Packer RJ, Milham MP, Castellanos FX, Acosta MT. 2012. Lovastatin regulates brain spontaneous low-frequency brain activity in neurofibromatosis type 1. Neurosci Lett 515:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements-Stephens AM, Rimrodt SL, Gaur P, Cutting LE. 2008. Visuospatial processing in children with neurofibromatosis type 1. Neuropsychologia 46:690–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. 2007. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage 37:343–360 [DOI] [PubMed] [Google Scholar]
- Constantino J. 2005. Social Responsiveness Scale. Los Angeles: Western Psychological Service [Google Scholar]
- Coudé FX, Mignot C, Lyonnet S, Munnich A, 2006. Academic impairment is the most frequent complication of neurofibromatosis type-1 (NF1) in children. Behav Genet 36:660–664 [DOI] [PubMed] [Google Scholar]
- Cutting LE, Cooper KL, Koth CW, Mostofsky SH, Kates WR, Denckla MB, Kaufmann WE. 2002. Megalencephaly in NF1: predominantly white matter contribution and mitigation by ADHD. Neurology 59:1388–1394 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. 2006. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 103:13848–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz-Filho JRL, da Rocha AJ, Muniz MP, Souza AS, Goloni-Bertollo EM, Pavarino-Bertelli EC. 2011. Diffusion tensor MR imaging in neurofibromatosis type 1: expanding the knowledge of microstructural brain abnormalities. Pediatr Radiol 42:449–454 [DOI] [PubMed] [Google Scholar]
- Ferraz-Filho JRL, José da Rocha A, Muniz MP, Souza AS, Goloni-Bertollo EM, Pavarino-Bertelli EC. 2012. Unidentified bright objects in neurofibromatosis type 1: conventional MRI in the follow-up and correlation of microstructural lesions on diffusion tensor images. Eur J Paediatr Neurol 16:42–47 [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 106:7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasquoine PG. 2013. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev 37:340–348 [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Henson RNA, Simons JS. 2010. The scale of functional specialization within human prefrontal cortex. J Neurosci 30:1233–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, Burgess PW. 2006. Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci 18:932–948 [DOI] [PubMed] [Google Scholar]
- Gioia G, Isquith P, Guy S, Kenworthy L. 2000. Test review: behavior rating inventory of executive function. Child Neuropsychol 6:235–238 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresham FM, Elliott SN. 1990. Social Skills Rating System manual. Circle Pines, MN, American Guidance Service [Google Scholar]
- Gresham FM, MacMillan DL, Bocian KM. 1996. Learning disabilities, low achievement, and mild mental retardation: more alike than different? J Learn Disabil 29:570–581 [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. 2010. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM. 2014. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage 95:232–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbregts S, Jahja R, De Sonneville L, de Breij S, Swaab-Barneveld H. 2010. Social information processing in children and adolescents with neurofibromatosis type 1. Dev Med Child Neurol 52:620–625 [DOI] [PubMed] [Google Scholar]
- Huijbregts SCJ, de Sonneville LMJ. 2011. Does cognitive impairment explain behavioral and social problems of children with neurofibromatosis type 1? Behav Genet 41:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SL, Arthur Shores E, North KN. 2006. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Dev Med Child Neurol 48:973–977 [DOI] [PubMed] [Google Scholar]
- Hyman SL, Shores A, North KN. 2005. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 65:1037–1044 [DOI] [PubMed] [Google Scholar]
- Jackowski AP, de Araújo Filho GM, de Almeida AG, de Araújo CM, Reis M, Nery F, Batista IR, Silva I, Lacerda ALT. 2012. The involvement of the orbitofrontal cortex in psychiatric disorders: an update of neuroimaging findings. Rev Bras Psiquiatr 34:207–212 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841 [DOI] [PubMed] [Google Scholar]
- Lamm C, Singer T. 2010. The role of anterior insular cortex in social emotions. Brain Struct Funct 214:579–591 [DOI] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137(Pt 1):12–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, Rushworth MFS. 2012. On the relationship between the ‘default mode network’ and the ‘social brain’. Front Hum Neurosci 6:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Brosch T, Sander D. 2014. The functional profile of the human amygdala in affective processing: insights from intracranial recordings. Cortex 60:10–33 [DOI] [PubMed] [Google Scholar]
- Nagai M, Kishi K, Kato S. 2007. Insular cortex and neuropsychiatric disorders: a review of recent literature. Eur Psychiatry 22:387–394 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. 1988. Consensus development conference statement. Neurofibromatosis. Arch Neurol 45:575–578 [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R. 2012. The insular cortex: a review. Prog Brain Res 195:123–163 [DOI] [PubMed] [Google Scholar]
- North K, Joy P, Yuille D, Cocks N, Mobbs E, Hutchins P, McHugh K, de Silva M. 1994. Specific learning disability in children with neurofibromatosis type 1: significance of MRI abnormalities. Neurology 44:878–883 [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, Calder AJ. 2010. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Arch Gen Psychiatry 67:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JM, Hyman SL, Shores EA, North KN. 2011. Assessment of executive function and attention in children with neurofibromatosis type 1: relationships between cognitive measures and real-world behavior. Child Neuropsychol 17:313–329 [DOI] [PubMed] [Google Scholar]
- Payne JM, Moharir MD, Webster R, North KN. 2010. Brain structure and function in neurofibromatosis type 1: current concepts and future directions. J Neurol Neurosurg Psychiatry 81:304–309 [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. 2002. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16:331–348 [DOI] [PubMed] [Google Scholar]
- Rowbotham I, Pit-ten Cate IM, Sonuga-Barke EJS, Huijbregts SCJ. 2009. Cognitive control in adolescents with neurofibromatosis type 1. Neuropsychology 23:50–60 [DOI] [PubMed] [Google Scholar]
- Roy A, Roulin J-L, Charbonnier V, Allain P, Fasotti L, Barbarot S, Stalder J-F, Terrien A, Le Gall D. 2010. Executive dysfunction in children with neurofibromatosis type 1: a study of action planning. J Int Neuropsychol Soc 16:1056–1063 [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90:449–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi J, Roine U, Glerean E, Lahnakoski J, Nieminen-von Wendt T, Tani P, Leppämäki S, Nummenmaa L, Jääskeläinen IP, Carlson S, Rintahaka P, Sams M. 2013. The brains of high functioning autistic individuals do not synchronize with those of others. Neuroimage Clin 3:489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp 17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. 2009. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1)S208–S219 [DOI] [PubMed] [Google Scholar]
- Steen RG, Taylor JS, Langston JW, Glass JO, Brewer VR, Reddick WE, Mages R, Pivnick EK. 2001. Prospective evaluation of the brain in asymptomatic children with neurofibromatosis type 1: relationship of macrocephaly to T1 relaxation changes and structural brain abnormalities. AJNR Am J Neuroradiol 22:810–817 [PMC free article] [PubMed] [Google Scholar]
- Violante IR, Ribeiro MJ, Cunha G, Bernardino I, Duarte JV, Ramos F, Saraiva J, Silva E, Castelo-Branco M. 2012. Abnormal brain activation in neurofibromatosis type 1: a link between visual processing and the default mode network. PLoS One 7:e38785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violante IR, Ribeiro MJ, Silva ED, Castelo-Branco M. 2013. Gyrification, cortical and subcortical morphometry in neurofibromatosis type 1: an uneven profile of developmental abnormalities. J Neurodev Disord 5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, Petermann F, Petermann U. 2011. Wechsler Intelligence Scale for Children—Fourth Edition
- Wheaton MG, Fitzgerald DA, Phan KL, Klumpp H. 2014. Perceptual load modulates anterior cingulate cortex response to threat distractors in generalized social anxiety disorder. Biol Psychol 101C:13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Alderman N, Burgess P, Emslie H, Evans J. 1996. The Behavioural Assessment of the Dysexecutive Syndrome. Bury St Edmunds, UK: Thames Valley Company [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.