Abstract
Vyas, Kaetan J., David Danz, Robert H. Gilman, Robert A. Wise, Fabiola León-Velarde, J. Jaime Miranda, and William Checkley. Noninvasive assessment of excessive erythrocytosis as a screening method for chronic mountain sickness at high altitude. High Alt Med Biol 16:162–168, 2015.—Globally, over 140 million people are at risk of developing chronic mountain sickness, a common maladaptation to life at high altitude (>2500 meters above sea level). The diagnosis is contingent upon the identification of excessive erythrocytosis (EE). Current best practices to identify EE require a venous blood draw, which is cumbersome for large-scale surveillance. We evaluated two point-of-care biomarkers to screen for EE: noninvasive spot-check tests of total hemoglobin and oxyhemoglobin saturation (Pronto-7, Masimo Corporation). We conducted paired evaluations of total serum hemoglobin from a venous blood draw and noninvasive, spot-check testing of total hemoglobin and oxyhemoglobin saturation with the Pronto-7 in 382 adults aged ≥35 years living in Puno, Peru (3825 meters above sea level). We used the Bland-Altman method to measure agreement between the noninvasive hemoglobin assessment and the gold standard lab hemoglobin analyzer. Mean age was 58.8 years and 47% were male. The Pronto-7 test was unsuccessful in 21 (5%) participants. Limits of agreement between total hemoglobin measured via venous blood draw and the noninvasive, spot-check test ranged from −2.8 g/dL (95% CI −3.0 to −2.5) to 2.5 g/dL (95% CI 2.2 to 2.7), with a bias of −0.2 g/dL (95% CI −0.3 to −0.02) for the difference between total hemoglobin and noninvasive hemoglobin concentrations. Overall, the noninvasive spot-check test of total hemoglobin had a better area under the receiver operating characteristic curve compared to oxyhemoglobin saturation for the identification of EE as measured by a gold standard laboratory hemoglobin analyzer (0.96 vs. 0.82; p<0.001). Best cut-off values to screen for EE with the Pronto 7 were ≥19.9 g/dL in males and ≥17.5 g/dL in females. At these cut-points, sensitivity and specificity were both 92% and 89% for males and females, respectively. A noninvasive, spot-check test of total hemoglobin had low bias and high discrimination for the detection of EE in high altitude Peru, and may be a useful point-of-care tool for large-scale surveillance in high-altitude settings.
Key Words: : chronic mountain sickness, excessive erythrocytosis, noninvasive methods, screening
Introduction
Chronic mountain sickness is a common maladaptation to life at high altitude. It is characterized by excessive erythrocytosis (EE), severe hypoxemia, alveolar hypoventilation, sometimes leading to pulmonary hypertension cor pulmonale and congestive heart failure (León-Velarde et al., 2010). According to a 2001 estimate, globally over 150 million people are at risk of developing chronic mountain sickness, 30 million of whom are located in South America alone (Moore, 2001). Anywhere between 5% and 10% of high altitude dwellers (>2500 meters above sea level) may be affected by chronic mountain sickness (León-Velarde et al., 2005). The prevalence of chronic mountain sickness varies both geographically with the extent of elevation (Groepenhoff et al., 2012) and genetic make-up. For example, Tibetan populations living at high altitudes have been shown to have lower levels of chronic mountain sickness than Andean populations at comparable altitudes (Beall, 2006).
The diagnosis of chronic mountain sickness is contingent upon the identification of EE. EE is defined as a hemoglobin concentration ≥21 g/dL for men and ≥19 g/dL for women (León-Velarde et al., 2005). In Cerro de Pasco (4340 m above sea level), Peruvian investigators found the average prevalence of EE to be 16% (Monge-C et al., 1992). Currently, the diagnosis of EE requires a venous blood draw. The requirement of a blood draw may complicate the diagnosis of EE in resource-limited settings, as laboratory measurements are needed to measure hemoglobin. While the use of capillary blood samples using the HemoCue (Hemocue Inc., Brea, CA, USA) is an accurate alternative to a blood draw at sea level, photometric assessment of hemoglobin concentration has been found to have wide limits of agreement with laboratory-derived hemoglobin measurements in high altitude populations (Zhou et al., 2009) and is still considered invasive as it requires a finger prick.
Noninvasive measurements that could identify EE would be an ideal solution for large-scale surveillance, especially in sparsely populated high altitude settings in resource-poor countries. Specifically, a point-of-care test would be able to reach and screen a larger population in a shorter amount of time, leading to better health outcomes. One possible biomarker that could be used for screening for EE is a noninvasive, spot-check test of total hemoglobin. An additional possible biomarker is blood oxyhemoglobin saturation as measured by pulse oximetry. The rationale behind using oxyhemoglobin saturation comes from a recent study conducted in Puno, Peru (3825 m above sea level) by our group, in which we found lower oxyhemoglobin saturation values in those with versus without EE (De Ferrari et al., 2014). Others have reported similar findings.
The noninvasive tests in this study use the Pronto-7 device (Masimo Corporation, Irvine, CA), which employs infrared light absorbance technology (Masimo, 2012). This is a new method of health monitoring with the potential for application in critical care environments and resource poor settings alike due to the rapidity and ease of testing. The objective of this study was to assess the viability of noninvasive biomarkers obtained using the Pronto-7 device vs. a standard venous blood draw and laboratory hemoglobin analyzer to screen for EE.
Methods
Study setting
The study population consisted of adults aged ≥35 years living in Puno, Peru. Puno is an Andean city located at 3825 meters above sea level and with a population of approximately 150,000 inhabitants. Average temperature was 16°C in Puno. On average, barometric pressure was 1014 mbar. The study protocol was approved by the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health in Baltimore, USA and A.B. PRISMA in Lima, Peru. All participants provided verbal informed consent after our research team read the entire informed consent document to them and any questions were answered.
Study design
This is an ancillary study of a larger ongoing cohort study in three Peruvian cities, one of which is Puno. In preparation for study activities, we first conducted a door-to-door household census of the study areas from which an age-, sex-, and site-stratified population-based cohort of approximately 500 participants were enrolled in both urban and rural settings in Puno (Miranda et al., 2012). At baseline, participants responded to a face-to-face questionnaire regarding sociodemographics and medical history. Field workers measured anthropometrics, blood pressure, and spirometry before and after bronchodilators, and obtained a fasting blood sample for analysis. Hemoglobin was determined using the Sysmex XE-2100 (Sysmex Corporation, Kobe, Japan), which utilized an automated sodium lauryl sulfate method for the detection of methemoglobin (our gold standard approach). All tests were processed in a centralized testing facility. Four years after initial recruitment and baseline measurements, participants were asked to repeat the above procedures. During this round of measurements, we conducted paired simultaneous evaluations of a blood draw for total hemoglobin assessment and a noninvasive spot-check test for total hemoglobin and oxyhemoglobin saturation with the Pronto-7 as outlined below.
Noninvasive assessment of hemoglobin
The Pronto-7 (SW 2.3.16 and 2.3.17) test was performed on study participants by trained field workers fewer than 15 minutes before venous blood draw. A Pronto-7 Rainbow 4D sensor (Rev. F) was placed on the participant's nondominant ring finger. Manufacturer best practices were followed when conducting the measurement. Participants were instructed not to move their hands or speak while spot-check testing took place, which on average lasted for one minute. We used two methods to increase the probability of success for tests registered as incomplete by the Pronto-7 device. We first warmed participant hands with a blanket or by having the participant hold a container filled with warm water. If that did not work, we conducted the test inside a box painted black to eliminate interference from ambient light or ultraviolet rays. We did not take note of which participants needed additional attempts or measures to acquire the data.
Biostatistical methods
One of our primary objectives was to conduct a comparative assessment of total hemoglobin levels estimated with the Pronto-7 versus that measured with a laboratory hematology analyzer from a blood draw. We used the traditional Bland Altman method to determine agreement between both approaches (Bland et al., 1986) by comparing the differences between the two methods to their averages. We used t-tests to compare continuous values and chi-square tests or Fisher exact tests to compare categorical values, as appropriate. Another primary objective was to compare the area under the receiver operating characteristic curves (AUC) between the Pronto-7 noninvasive hemoglobin concentration and Pronto-7 oxyhemoglobin saturation to screen for EE as measured by a laboratory hemoglobin analyzer in a model adjusted for differences in sex. A secondary objective was to determine the noninvasive total hemoglobin concentration that would serve as a cut-off to screen individuals for EE by identifying the value at which sensitivity and specificity were equal (Hosmer et al., 2004). We conducted statistical analyses with STATA Version 12 (StataCorp, College Station, Texas, USA) and R (www.r-project.org). We used the package OptimalCutpoints in R to calculate threshold values.
Results
Participant characteristics
A total of 382 paired evaluations were conducted. Average age among these participants was 58.8 years and 47% were male. Average hemoglobin concentrations determined using the gold standard were 17.8 g/dL in men and 15.8 g/dL in women, and the overall prevalence of EE was 5%. The Pronto-7 spot-check reading was unsuccessful in 21 (5%) participants; however, we did not find differences in any of the measured variables between participants in whom the Pronto-7 test was successfully administered vs. those in whom it was unsuccessful (Table 1).
Table 1.
Differences in Participant Characteristics Between the Pronto-7 Successful and Failed Measurements
| Participant characteristics | Successful (n=361) | Failed (n=21) | P |
|---|---|---|---|
| Demographics | |||
| % male (n) | 47% (171) | 48% (10) | 1.00 |
| Age in years, mean (SD) | 58.7 (12.2) | 61.1 (14.3) | 0.38 |
| Anthropometrics | |||
| Height in cm, mean (SD) | 156.1 (8.7) | 155.7 (6.9) | 0.87 |
| Weight in kg, mean (SD) | 65.6 (11.9) | 63.7 (10.4) | 0.49 |
| Body mass index in kg/m2, mean (SD) | 26.9 (4.1) | 26.2 (3.8) | 0.49 |
| Laboratory values | |||
| Hemoglobin in g/dL, mean (SD) | 16.8 (2.0) | 16.3 (1.4) | 0.25 |
| Glucose in mg/dL, mean (SD) | 103 (42.4) | 90.6 (12.4) | 0.18 |
| Creatinine in mg/dL, mean (SD) | 0.8 (0.3) | 0.7 (0.1) | 0.22 |
| High sensitivity CRP in mg/L, mean (SD) | 2.2 (3.5) | 2.2 (2.6) | 0.98 |
| Cholesterol in mg/dL, mean (SD) | 188.8 (40.4) | 174.4 (32.3) | 0.11 |
| HDL in mg/dL, mean (SD) | 44.9 (11.4) | 46.7 (13.9) | 0.49 |
| Triglycerides in mg/dL, mean (SD) | 152.3 (84.3) | 153.8 (93.2) | 0.94 |
| Hemoglobin A1c in %, mean (SD) | 5.8 (1.0) | 5.7 (0.4) | 0.45 |
Agreement between measured and noninvasive hemoglobin concentrations
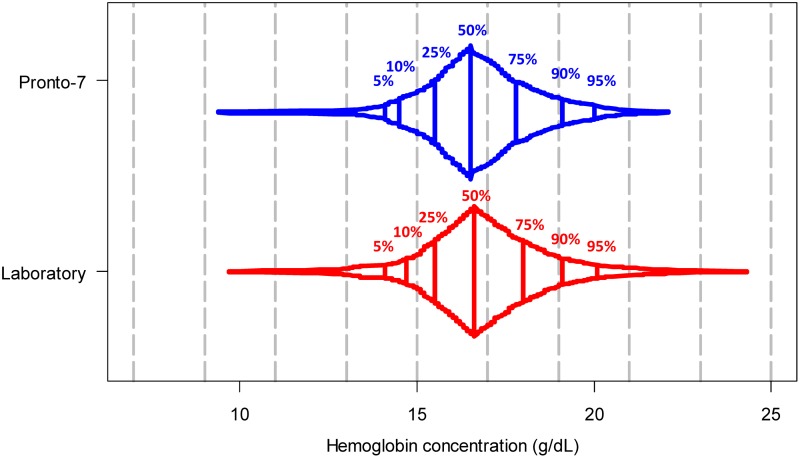
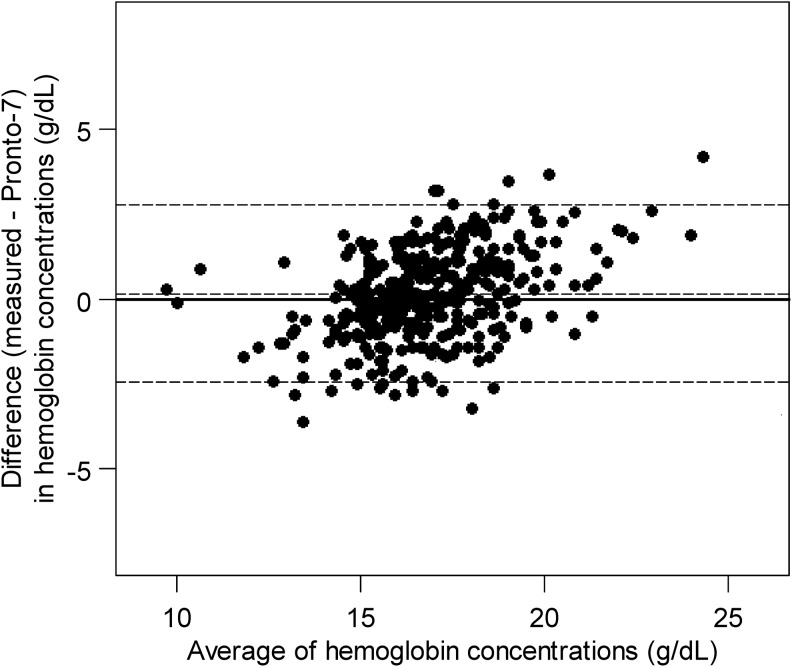
Among the 361 participants with a successful Pronto-7 reading, average spot-check hemoglobin concentrations were 17.5 g/dL in men and 15.9 g/dL in women. The distributions of values between both methods of hemoglobin assessment appeared relatively similar (Fig. 1). We display the level of agreement between hemoglobin readings obtained from the Pronto-7 and the laboratory hemoglobin analyzer in Figure 2. The limits of agreement between methods ranged from −2.8 g/dL (95% CI, −3.0 to −2.5) to 2.5 g/dL (95% CI, 2.2 to 2.7), with an bias of −0.2 g/dL (95% CI, −0.3 to −0.02) for the difference between total hemoglobin and noninvasive hemoglobin concentrations (i.e., favoring higher values in the laboratory hemoglobin analyzer). On visual inspection of the data outcomes, the Pronto-7 showed a trend bias wherein it overestimated hemoglobin values in the lower concentrations and underestimated values in the higher concentrations.
FIG. 1.
Box percentile plot of hemoglobin concentrations using the Pronto 7 noninvasive hemoglobin assessment and the laboratory hemoglobin analyzer.
FIG. 2.
Bland-Altman agreement scatterplot for noninvasive hemoglobin concentration (g/dL) by using Pronto 7 versus that measured by laboratory hemoglobin analyzer (g/dL) in a blood sample as the gold standard.
Correlation between oxyhemoglobin saturation and measured hemoglobin concentrations
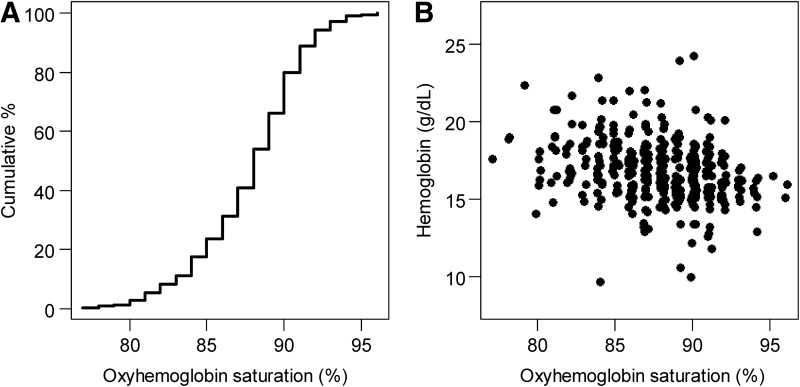
Average oxyhemoglobin saturation was 88% (SD=3.4%), with values ranging between 77% and 96% (Fig. 3). The relationship between oxyhemoglobin saturation and measured hemoglobin concentrations was approximately linear (Fig. 3) and negatively associated (r=−0.28, 95% CI −0.37 to −0.18; p<0.001).
FIG. 3.
Oxyhemoglobin saturation (%) and measured laboratory hemoglobin concentrations. (A) Empirical cumulative distribution function of oxyhemoglobin saturation values. (B) Scatterplot of oxyhemoglobin saturation values and measured hemoglobin concentrations by a laboratory analyzer.
Differences in discrimination between the two noninvasive methods
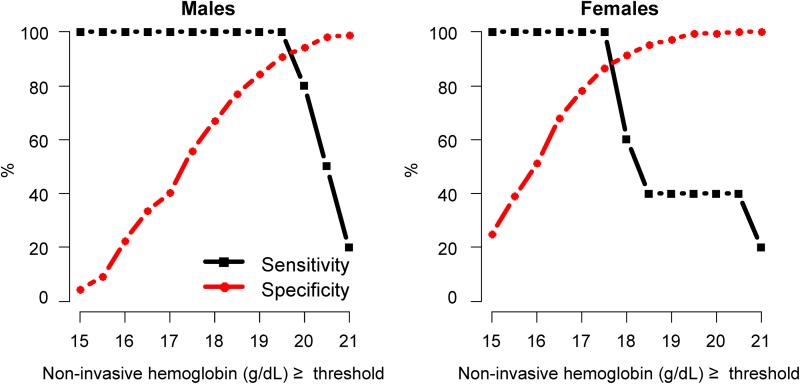
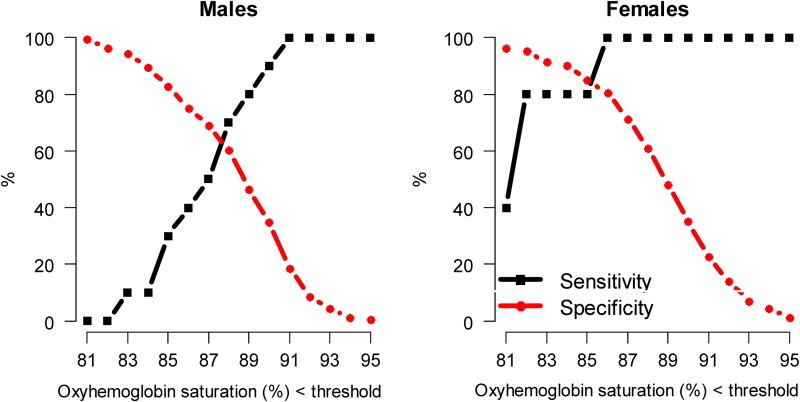
We constructed ROC curves by sex for the spot-check test of hemoglobin using the Pronto-7 to determine the best cut-off value to screen for EE (Fig. 3). Cut-off values were determined to be the hemoglobin concentration that would necessitate additional testing using a venous blood draw to identify EE by the equality of sensitivity and specificity. The best cut-off value for the noninvasive assessment of hemoglobin was ≥19.9 g/dL in men, for which both sensitivity and specificity were 92%; and ≥17.5 g/dL in women, for which both sensitivity and specificity were 89% (Fig. 4). We also constructed ROC curves by sex for oxyhemoglobin saturation to determine the best cut-off value to screen for EE (Fig. 5). The best cut-off value for oxyhemoglobin saturation was 88% in men, for which both sensitivity and specificity were 64%; and, 85% in women, for which both sensitivity and specificity were 82%. The use of noninvasive hemoglobin concentration (AUC=0.96, 95% CI 0.93 to 0.99) had better discrimination than did oxyhemoglobin saturation values (AUC=0.82, 95% CI 0.74 to 0.89) to screen for EE (p<0.001 for difference between AUCs).
FIG. 4.
Sensitivity and specificity by values of Pronto-7 noninvasive hemoglobin assessment to identify excessive erythrocytosis (assessed using a laboratory hemoglobin analyzer). Specifically, we calculated sensitivity and specificity of having excessive erythrocytosis (defined as a measured hemoglobin concentration ≥21.0 g/dL in males and ≥19.0 g/dL in females) based on having an equal or greater threshold value for a range of Pronto-7 hemoglobin concentrations.
FIG. 5.
Sensitivity and specificity by oxyhemoglobin saturation values to identify excessive erythrocytosis (as assessed using a laboratory hemoglobin analyzer). Specifically, we calculated sensitivity and specificity of having excessive erythrocytosis (defined as measured hemoglobin concentrations ≥21.0 g/dL in males and ≥19.0 g/dL in females) for lower threshold value for a range of oxyhemoglobin saturation values.
Discussion
In this study we tested two noninvasive approaches to the assessment of EE at high altitude. Specifically, we found that a spot-check hemoglobin assessment had a high diagnostic accuracy and discrimination to screen for EE in participants at high altitude. The device used in this study is advantageous over other assessment methods because it is noninvasive and provides a result in real time.
Previous studies have compared the Pronto-7 and other noninvasive hemoglobin measurement devices to capillary tube assessments and venous blood samples. Belardinelli et al. (2013) found that the Pronto-7 had a similar bias of −0.53 g/dL, SDD of 1.04 and 95% limits of agreement from −2.57 to 1.51 when compared to the Beckman Coulter Cell Counter at sea level. That study also showed the same trend bias that we observed in our study. In a study by Shah et al. (2013), the Pronto-7 performed with similar accuracy at sea level as compared to the HemoCue point of care device in screening patients at risk for anemia in outpatient settings. In our study, we found that Pronto-7 had an overall bias of −0.2 g/dL, when compared to a laboratory hemoglobin analyzer, which is well within the limits of acceptable difference of 1.0 g/dL (Gehring et al., 2002. Adam et al., 2012. Skelton et al., 2013).
Research studies focusing on evaluating the prevalence of chronic mountain sickness via diagnosis of EE most commonly measure methemoglobin, which requires a blood draw. Point-of-care testing using the HemoCue has been shown to be a reliably accurate alternative to the gold standard methemoglobin test at sea level,15 and has been used in prior high altitude studies in Peru (León-Velarde et al., 1994; Gonzales et al., 2009). In a recent study in Tibet (3700 m above sea level), however, Zhou et al. (2009) reported that the use of HemoCue device at high altitude for the estimation of hemoglobin had wide limits of agreement when compared with methemoglobin assessment of hemoglobin concentrations, questioning its reliability at high altitude. The Pronto-7 device may be an effective alternative to the HemoCue device. Although neither of the devices requires a laboratory to measure hemoglobin, the Pronto-7 has the added benefit of a completely noninvasive test. Moreover, the Pronto-7 is a point-of-care device that would make hemoglobin assessment easy to manage in difficult to reach villages at high altitude.
While total hemoglobin assessment by the Pronto-7 operates with a low bias in our high altitude setting, we also found that it tends to have problems at both the high and low ends of total hemoglobin concentrations. Specifically, the Pronto-7 noninvasive hemoglobin measurement overestimated hemoglobin at low values and underestimated it at high values when compared to our laboratory hemoglobin analyzer. This suggests that the Pronto-7 spot check for total hemoglobin cannot be used a diagnostic tool to identify EE. Nonetheless, the Pronto-7 could be used as a screening tool using alternate hemoglobin cutoffs to screen for individuals who are at risk of having EE. At high altitude, it is well recognized that oxyhemoglobin saturation values are associated with hemoglobin concentrations (Hurtado, 1942. León-Velarde et al., 1994, Peñaloza et al., 2007, Sahota et al., 2013).
Additionally, we have shown in a recent study that oxyhemoglobin saturation values were lower in participants with EE compared to those without EE (De Ferrari et al., 2014). Our findings prompted our group to test if oxyhemoglobin saturation could be used as a screening tool for EE. While pulse oximetry as a screening tool is both easier and less costly to measure than noninvasive hemoglobin, our analyses revealed that oxyhemoglobin saturation was inferior to the noninvasive hemoglobin to identify EE. An additional complication in using pulse oximetry as a screening tool is that the threshold of oxyhemoglobin saturation will likely vary with altitude, body mass index, pregnancy, and behavioral factors such as chronic alcohol drinking and tobacco smoking (Sahota et al., 2013, Peng et al., 2013).
Use of the noninvasive hemoglobin test to screen for EE has several advantages. It provides a result in real time; it increases participant safety and satisfaction; it can be used to screen repeatedly over time in those who are at high risk of developing EE; it is easy to use by a wide range of health professionals without the requisite or expertise in phlebotomy; it does not require any laboratory consumables; and, it reduces the risk of exposure to blood borne pathogens by avoiding needlesticks. Although our results show that the Pronto-7 noninvasive hemoglobin test cannot replace the gold standard for the assessment of total hemoglobin, using the device as a screening tool will enhance comfort for the majority of participants because unnecessary finger pricks and invasive venous blood samples could be avoided.
For these reasons, noninvasive hemoglobin measurements are particularly applicable in critical care and resource-limited settings, and in populations like ours where cultural aversion against taking blood samples still exists. While the Pronto-7 is a new device, the noninvasive hemoglobin measurement is currently more cost-effective than a hemoglobin assessment at a Clinical Laboratory Improvement Amendments (CLIA) certified private laboratory in our setting (USD 2 per test including Pronto-7 equipment when 1000 tests are purchased vs USD 12 per test at a private laboratory, respectively). Moreover, we believe that this technology has the advantage of becoming even more cost-effective over time as it has for pulse oximetry. Additionally, the Pronto-7 device is rugged, robust, and requires little operator training. This makes the Pronto-7 an ideal health technology for resource-poor settings (McGuire et al., 2014). In contrast, taking blood samples in resource-poor settings will always require laboratory analysis for every sample taken, in addition to the costs of transporting and managing samples over long distances at high altitude.
Specific strengths of our study include a moderate to large sample size and the ability to concurrently measure hemoglobin both noninvasively and via blood draw within the same day. Additionally, this study was able to assess the viability of using oxyhemoglobin saturation values to screen for EE. Some limitations of this study include a low percentage of the studied population with EE, and that, while the bias was low for the Pronto-7 noninvasive hemoglobin measurement, the device appeared to overestimate low values and overestimate high values.
In summary, noninvasive assessment of total hemoglobin may aid in the surveillance of EE, and thus chronic mountain sickness in high altitude populations. We provide cutoffs to screen for EE, which will reduce the need for blood samples for the majority of participants. This is advantageous in low resource settings because obtaining and analyzing blood samples is cumbersome for both participants and health workers as it requires transport from hard to reach villages to laboratory settings. This device may be used frequently to monitor individuals at high risk for chronic mountain sickness.
Acknowledgments
The authors are indebted to all participants who kindly agreed to participate in the study and to the field team for their commitment and hard work. The authors would like to acknowledge Masimo Corporation for providing the equipment for the study.
WC and KV conceived study design and were responsible for study conduct, KV and WC performed the statistical analysis and wrote the first draft of the manuscript. DD, RHG, RAW, JJM and FLV participated in study design and conduct, and writing of manuscript. WC takes ultimate responsibility over study design and administration, analysis, and writing of the manuscript.
Funding: This work was supported in part by the Center for Global Health of Johns Hopkins University (Checkley), by the Foundation of the American Medical Association (Painschab), and by federal funds of the National Heart, Lung And Blood Institute, United States National Institutes of Health, Department of Health and Human Services under contract number HHSN268200900033C.
Author Disclosure Statement
The authors have no financial conflicts of interest to disclose.
References
- Adam I, Ahmed S, Mahmoud MH, and Yassin MI. (2012). Comparison of HemoCue® hemoglobin-meter and automated hematology analyzer in measurement of hemoglobin levels in pregnant women at Khartoum hospital, Sudan. Diag Pathol 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM. (2006). Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol 46:18–24 [DOI] [PubMed] [Google Scholar]
- Belardinelli A, Benni M, Tazzari PL, and Pagliaro P. (2013). Noninvasive methods for haemoglobin screening in prospective blood donors. Vox Sanguinis 105:116–120 [DOI] [PubMed] [Google Scholar]
- Bland JM, and Altman D. (1986). Statistical methods For assessing agreement between two methods of clinical measurement. Lancet 327:307–310 [PubMed] [Google Scholar]
- De Ferrari A, Miranda JJ, Gilman RH, Dávila-Román VG, León-Velarde F, Rivera-Ch M, Huicho L, Bernabé-Ortiz A, Wise RA, and Checkley W. (2014). Prevalence, clinical profile, iron status, and subject-specific traits for excessive erythrocytosis in Andean adults living permanently at 3,825 meters above sea level. Chest 146:1327–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring H, Hornberger C, Dibbelt L, Rothsigkeit A, Gerlach K, Schumacher J, and Schmucker P. (2002). Accuracy of point-of-care-testing (POCT) for determining hemoglobin concentrations. Acta Anaesthesiol Scand 46:980–986 [DOI] [PubMed] [Google Scholar]
- Gonzales GF, Gasco M, Tapia V, and Gonzales-Castañeda C. (2009). High serum testosterone levels are associated with excessive erythrocytosis of chronic mountain sickness in men. Am J Physiol Endocrinol Metab 296:E1319–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groepenhoff H, Overbeek MJ, Mule M, van der Plas M, Argiento P, Villafuerte FC, Beloka S, Faoro V, Macarlupu JL, Guenard H, de Bisschop C, Martinot JB, Vanderpool R, Penaloza D, and Naeije R. (2012). Exercise pathophysiology in patients with chronic mountain sickness exercise in chronic mountain sickness. Chest 142:877–884 [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Jr, Lemeshow S, and Sturdivant R. (2004). Applied Logistic Regression. John Wiley & Sons, New York, USA [Google Scholar]
- Hurtado A. (1942). Chronic mountain sickness. JAMA 120:1278–1280 [Google Scholar]
- León-Velarde F, Arregui A, Vargas M, Huicho L, and Acosta R. (1994). Chronic mountain sickness and chronic lower respiratory tract disorders. Chest 106:151–155 [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge R, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, and Zubieta-Calleja G. (2005). Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol 6:147–157 [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Villafuerte F, and Richalet JP. (2010). Chronic mountain sickness and the Heart. Prog Cardiovasc Dis 52:540–549 [DOI] [PubMed] [Google Scholar]
- Masimo Corporation. (2012). Technical Bulletin: Accuracy of Noninvasive Total Hemoglobin (SpHb®) Measurement with Rainbow® Pulse CO-Oximetry. Irvine, CA, USA [Google Scholar]
- McGuire H, and Weigl B. (2014). Medical devices and diagnostics for cardiovascular diseases in low-resource settings. J Cardiovasc Transl Res 7:737–748 [DOI] [PubMed] [Google Scholar]
- Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, and Checkley W. (2012). Addressing geographical variation in the progression of non-communicable diseases in Peru: The CRONICAS cohort study protocol. BMJ Open 2:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge-C C, Arregui A, and León-Velarde F. (1992). Pathophysiology and epidemiology of chronic mountain sickness. Intl J Sports Med 13:S79–81 [DOI] [PubMed] [Google Scholar]
- Moore LG. (2001). Human genetic adaptation to high altitude. High Alt Med Biol 2:257–279 [DOI] [PubMed] [Google Scholar]
- Peñaloza D, and Arias-Stella J. (2007). The heart and pulmonary circulation at high altitudes: Healthy highlanders and chronic mountain sickness. Circulation 115:1132–1146 [DOI] [PubMed] [Google Scholar]
- Peng Q, Basang Z, Cui CY, Li L, Qian J, Gesang Q, Yang L, La Z, De Y, Dawa P, Qu N, Suo Q, Dan Z, Xiao D, Wang XF, and Jin L. (2013). Physiological responses and evaluation of effects of BMI, smoking and drinking in high altitude acclimatization: A cohort study in Chinese Han young males. PLoS One 8:e79346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahota IS, and Panwar NS. (2013). Prevalence of chronic mountain sickness in high altitude districts of Himachal Pradesh. Ind J Occupat Environ Med 17:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N, Osea EA, and Martinez GJ. (2013). Accuracy of noninvasive hemoglobin and invasive point-of-care hemoglobin testing compared with a laboratory analyzer. Intl J Labor Hematol 36:56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton VA, Wijayasinghe N, Sharafudeen S, Sange A, Parry NS, and Junghans C. (2013). Evaluation of point-of-care haemoglobin measuring devices: A comparison of Radical-7™ pulse co-oximetry, HemoCue(®) and laboratory haemoglobin measurements in obstetric patients. Anaesthesia 68:40–45 [DOI] [PubMed] [Google Scholar]
- Zhou X, Yan H, Xing Y, Dang S, Zhuoma B, and Wang D. (2009). Evaluation of a portable hemoglobin photometer in pregnant women in a high altitude area: A pilot study. BMC Public Health 9:228. [DOI] [PMC free article] [PubMed] [Google Scholar]