Abstract
Simonson, Tatum S. Altitude adaptation: A glimpse through various lenses. High Alt Med Biol 16:125–137, 2015.—Recent availability of genome-wide data from highland populations has enabled the identification of adaptive genomic signals. Some of the genomic signals reported thus far among Tibetan, Andean, and Ethiopian are the same, while others appear unique to each population. These genomic findings parallel observations conveyed by decades of physiological research: different continental populations, resident at high altitude for hundreds of generations, exhibit a distinct composite of traits at altitude. The most commonly reported signatures of selection emanate from genomic segments containing hypoxia-inducible factor (HIF) pathway genes. Corroborative evidence for adaptive significance stems from associations between putatively adaptive gene copies and sea-level ranges of hemoglobin concentration in Tibetan and Amhara Ethiopians, birth weights and metabolic factors in Andeans and Tibetans, maternal uterine artery diameter in Andeans, and protection from chronic mountain sickness in Andean males at altitude. While limited reports provide mechanistic insights thus far, efforts to identify and link precise genetic variants to molecular, physiological, and developmental functions are underway, and progress on the genomics front continues to provide unprecedented movement towards these goals. This combination of multiple perspectives is necessary to maximize our understanding of orchestrated biological and evolutionary processes in native highland populations, which will advance our understanding of both adaptive and non-adaptive responses to hypoxia.
Key Words: : adaptation, altitude, Andean, Ethiopian, genetics, physiology, Tibetan
Three Continental Populations Have Inhabited High-Altitude Areas for Hundreds of Generations
High-altitude hypoxia poses strong environmental pressures for permanent human habitation. Despite this, populations in the Qinghai-Tibetan Plateau, Andean Altiplano, and Semien Plateau of Ethiopia (Fig. 1) have persisted at high altitudes (3500–4500 m above sea level) for hundreds of generations (Beall, 2006) and are therefore uniquely suited for studies of hypoxia adaptation (Moore, 2001). Due to complex population histories, it is not possible to generalize a single continuous duration of human occupation across each continental region, although records estimate initial Tibetan, Andean, and Ethiopian habitations occurred ∼25,000, ∼12,000, and ∼5000 to possibly ∼70,000 years ago, respectively (Hassen, 1990; Aldenderfer, 1993; 2011; Zhao et al., 2009; Rademaker et al., 2014).
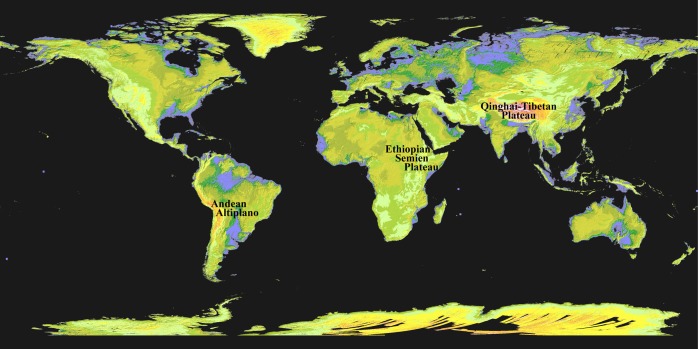
FIG. 1.
Map with three locations where high-altitude adapted populations have lived for hundreds of generations. (Image modified from http://www.nasa.gov/topics/earth/features/20090629.html; low elevations are purple, medium elevations are greens and yellows, and high elevations are orange, red and white.)
The largest high-altitude region continuously inhabited by human populations is the Qinghai-Tibetan Plateau, which includes the Tibet Autonomous Region and Qinghai Province in western China, as well as areas of Jammu and Kashmir in northern India. While some regions of the Plateau have been inhabited for up to ∼25,000 years, genetic relationships between ancestors of present-day Tibetan and Sherpa date to ∼30,000 years before present (Jeong et al., 2014). As discussed in the genetics section below, one region of adaptive gene sequence in these populations is more similar to that of an ancient human lineage (Denisovan) than any other human population, suggesting adaptive introgression of archaic genetic material into the gene pool of Tibetan ancestors ∼40,000 years ago, prior to habitation at altitude (Huerta-Sanchez et al., 2014). In addition to ancient genomic remnants, genetic data suggest migrations into the Plateau occurred before and after the last glacial maximum (Qin et al., 2010; Qi et al., 2013), and archaeological findings indicate areas of more recent occupation in the northeast region of the Plateau only ∼3000 to ∼5000 years ago (Chen et al., 2015). Considering this vast geographic area spans nearly 100,000 square miles, it is not surprising that various geographically distinct groups, such as the Amdo, Kham, and Ü-Tsang Tibetans, exhibit distinct population structure and vary in reports of adaptive signals (Simonson et al., 2012a; Wuren et al., 2014).
The Andean Altiplano in South America, the second largest high plateau, spans parts of Peru, Argentina, Ecuador, and Bolivia. Archaeological findings indicate that humans established residence in the Andean highlands shortly after inhabiting South America, ∼12,000 years before present, with subsequent migrations to and from neighboring coastal environments (Rademaker et al., 2014). European admixture in 17th and 20th centuries contributed to present-day linguistically distinct populations in this region, including the Aymara and Quechua. This mixture may underlie, at least in part, physiological differences (Brutsaert et al., 2003; 2005) and influence the detection of adaptive genetic signals (Eichstaedt et al., 2014) among Andean populations. The degree of admixture is variable, and is absent or limited in some studies of these groups (Rupert and Hochachka, 2001; Julian et al., 2009).
The demographic history of various highland Ethiopian groups proves complex. The Oromo and Amhara have resided at high altitudes more than 2500 m above sea level for ∼500 years and ∼5000 up to ∼70,000 years, respectively, in contrast to the Tigray, who inhabit intermediate to high altitudes (∼2000 m above sea level) (Hassen, 1990; Alkorta-Aranburu et al., 2012; Scheinfeldt et al., 2012; Huerta-Sanchez et al., 2013). A substantial amount of gene flow from northern regions of Africa, the Middle East, and sub-Saharan Africa is noted among Ethiopians (Semino et al. 2002), and non-African components comprise ∼50% of the ancestry in Ethiopian groups. This mixture, in addition to that between highland and lowland Ethiopian groups, has been accounted for in recent genomic analyses (Huerta-Sanchez et al., 2013). Subpopulations presently located in the intermediate and highland regions of Ethiopia exhibit a range of physiological traits, including variation in hemoglobin concentration ([Hb]). This variation likely reflects distinct subpopulation histories, i.e. genetic background and degree of gene flow, level of altitude residence and number of generations at altitude.
Physiological Lens: Distinct Composite of Traits Exhibited by Each Continental Highland Population
More than 100 studies regarding physiological adaptations in Tibetan and Sherpa highlanders have been published (Gilbert-Kawai et al., 2014), with an extensive amount of similar research reported in Andeans but less in Ethiopian highlanders (Petousi et al., 2014). Various studies provide different conclusions even within continental regions, likely reflecting outcomes of limited sample sizes, inconsistent methodologies, and/or within region subpopulation variation. Despite these limitations, the consensus suggests specific components of oxygen transport play unique roles in different continental highlanders' adaptation ( Moore, 2001; Beall, 2007; Petousi and Robbins, 2014), and the precise genetic factors underlying this variation are on the forefront of discovery. While a comprehensive review of physiological traits in native highlanders is essential for bridging physiological and genomic findings, only a brief overview of this topic is provided here. Thorough summaries of distinct characteristics in these populations are provided elsewhere (Moore et al., 1992; 1998; Beall, 2000; 2006; 2007; Moore, 2001; Bigham and Lee, 2014; Gilbert-Kawai et al., 2014; Petousi and Robbins, 2014).
Developmental differences among native highlanders: Views from in utero and early life stage studies
Reproductive success and infant survival are crucial components of trans-generational adaptation in highlanders. In native Andeans and Tibetans, increased utero-placental oxygen delivery at altitude is attributed to increased common iliac blood flow into uterine arteries, resulting in less intrauterine growth restriction compared to newcomer high-altitude populations and, in the case of Tibetans, less pre- and post-natal mortality (Zamudio et al., 1993; Moore et al., 1998; 2001; Moore, 2001; Tripathy and Gupta, 2005). Furthermore, compared to lowland groups, birth weights in native Tibetan and Andean highlanders are higher (Moore et al., 1998).
Single nucleotide polymorphisms (SNPs) in genes related to oxygen sensing and vascular control, PRKAA1 and EDNRA, both identified as adaptive candidates in Andeans (Bigham et al., 2009), and in the latter case Tibetans (Simonson et al., 2010), are associated with birth weight in Andeans (Bigham et al., 2014). Maternal adaptive gene copies of PRKAA1 are also associated with uterine artery diameter and metabolic homeostasis in Andeans (Bigham et al., 2014). Sustained SaO2 in Tibetan neonates compared to lower values observed in Andean or Han Chinese counterparts may underlie early lifecycle differences in highlanders, possibly due to a decrease in sleep-disordered breathing (Julian et al., 2013), differences in ventilatory patterns (Moore et al., 1998), or other unexplored but possibly developmental and/or genetically regulated phenomenon (Simonson et al., 2014)). SaO2 is also higher in Tibetan neonates compared to Andeans, who exhibit SaO2 and pulmonary arterial pressures similar to European infants (Niermeyer et al., 2015). It remains to be seen if and how the ability to tolerate hypoxia during early developmental stages relates to distinct physiology in highland adults.
Hemoglobin concentration among highland groups at altitude
Elevated [Hb] in sojourners at high altitude has long been noted as a hallmark response to high-altitude hypoxia. Consistently reported among many Tibetan and Amhara Ethiopian populations, however, is that while [Hb] increases with altitude, it is to a much lower extent than that exhibited by lowlanders (Beall et al., 2002; 2006; Wu et al., 2005; Scheinfeldt et al., 2012). Therefore, many reports indicate these groups exhibit average [Hb] that is within the sea-level range despite residence above 3500 m. This is unique not only in comparison to acclimatized lowlanders but Andean counterparts at comparable altitudes, who exhibit an average [Hb] up to a few g/dl higher than Tibetans (Beall et al., 1998) with further excessive erythrocytotic subgroups (Villafuerte et al., 2014). Putatively adaptive copies of EPAS1 (Beall et al., 2010; Yi et al., 2010), EGLN1 and PPARA (Simonson et al., 2010) loci are associated with relatively lower [Hb] in Tibetans. [Hb] in Amhara Ethiopians is associated with THRB gene copies and marginally associated with EPAS1 and PPARA putatively adaptive loci (Scheinfeldt et al., 2012), yet the precise genetic variants involved and physiological significance for these gene regions are unknown.
Oxygen saturation, binding affinities in highland groups
One study of Tibetan mothers at altitude indicates a high-SaO2 genotype affords less infant mortality compared to those without (Beall et al., 2004). Whether SaO2 is highest among Amhara Ethiopians, followed by Andeans, but lower in Tibetans (Beall et al., 1999; 2002; 2006) or comparable in Tibetans and lowlanders at altitude (Keyl et al., 2000) varies among studies, although comprehensive analyses support the former (Moore, 2001; Wu et al., 2005; Beall, 2007; Weitz and Garruto, 2007). Reports of Hb-O2 binding affinity in each of the highland populations also vary among studies (Morpurgo et al., 1976; Samaja et al., 1979; Moore et al., 1992; Balaban et al., 2003; Simonson et al., 2014; Tashi et al., 2014), with recent report of an even lower P50 in Tibetans (Simonson et al., 2014) compared to Andean residents who also exhibit a greater O2-binding affinity (Balaban et al., 2003) at high altitude.
Variation in the hypoxic ventilatory response and pulmonary physiology
While the hypoxic ventilatory response (HVR) varies widely among individuals, a body of evidence now supports that many Tibetans exhibit an elevated HVR (Zhuang et al., 1993) whereas Andeans exhibit a blunted response to hypoxia (Curran et al., 1997; Beall 2007). Andeans also exhibit lower resting minute ventilation than Tibetans (Beall et al., 1997), and it is suspected that differences in control of breathing underlie these patterns (Moore et al., 2000; Brutsaert et al., 2005). Himalayan highlanders also demonstrate decreased central and absent peripheral sensitivities to CO2 compared to lowlanders (Duffin et al., 2010).
In terms of lung physiology, few reports suggest Tibetans, compared to Han Chinese lowlanders, have greater lung volume, total lung and vital capacities, and residual and tidal volumes, in addition to greater diffusing capacity (Sun et al., 1990; Droma et al., 1991; Kapoor and Kapoor, 2005). Hypoxic pulmonary vasoconstriction (HPV), a response observed among some lowlanders at altitude (Penaloza and Arias-Stella, 2007), is rare among Tibetans during rest or exercise at altitude (Groves et al., 1993) or after examination post-hypoxia exposure following extended residence at sea level (Petousi et al., 2014). This is in contrast to histological findings in Andeans whose pulmonary artery structure is indicative of pulmonary hypertension (Arias-Stella and Saldana, 1963; Heath et al., 1981). In Ethiopians, the pulmonary vascular response to hypoxia includes a rise in pulmonary pressure but not resistance, which may be attributed to increased blood flow (Hoit et al., 2011).
Cardiac and metabolic differences observed in highlanders
Tibetan and Sherpa exhibit elevated heart rates compared to lowlanders at altitude (Pugh, 1962; 1964; Sun et al., 1990; Wu, 1990) and, compared to Han Chinese, greater stroke volume, cardiac output (Wu, 1990; Ge, 1995; Chen et al., 1997), and less right heart hypertrophy (Halperin et al., 1998). Sherpa at altitude also exhibit components of mechanical reserve typically observed only among lowlanders at low altitude as well as a relatively smaller left ventricle (Stembridge et al., 2014). Cardiac metabolism studies in Sherpa indicate a shift from fatty acids, typically utilized by cardiac muscle in the fasting resting state in lowlanders, to increased uptake of glucose for up to 3 weeks of de-acclimatization (Holden et al., 1995), yielding more ATP per oxygen molecule but limited energy reserve over time.
The ratio of myocardial phosphocreatine (which releases high-energy phosphates) to ATP, typically decreased in lowlanders only upon return from high altitude (Holloway et al., 2011), is approximately half that of lowlanders in Sherpa at low altitude (Hochachka et al., 1996a). Recent genetic studies indicate elevated serum lactate and free fatty acid levels in Tibetans are associated with adaptive copies of EPAS1 and PPARA, respectively (Ge et al., 2012). The physiological significance remains to be confirmed in a fasting state, but these findings are in line with a metabolic shift to anaerobic glucose metabolism. Involvement of the HIF pathway in metabolism (Majmundar et al., 2010) is also illustrated in studies of Chuvash polycythemia, whereby limited degradation of HIF is associated with higher lactate compared to standard HIF activity in non-Chuvash subjects during exercise (Formenti et al., 2010).
Increased capillarity but decreased muscle fiber per cross-sectional area, greater maximal oxygen consumption despite low mitochondrial density, and a metabolic shift to carbohydrate oxidation in skeletal muscle in five Sherpa compared to unacclimatized lowlanders suggest adaptive changes involve muscle structure/function (Kayser et al., 1991; Kayser et al., 1996). Higher mean maximal O2 consumption (VO2 max) and enhanced pulmonary gas exchange during exercise in Tibetan and Andean highlanders, greater endurance capacity among Tibetans (Brutsaert, 2008), and greater protection against decreased VO2 max at altitude based on Andean ancestry (Brutsaert et al., 2003), suggest genetic or developmental factors underlie such differences. While it is unknown whether selection candidate genes are associated specifically with these traits, various genes in regions exhibiting an adaptive signal are involved in cardiac and skeletal development and function.
Cerebral adaptations to hypoxia
It has been hypothesized that superior autoregulation or increased oxygen delivery to the brain would be beneficial at altitude. Less cerebral alterations have been reported in Sherpa, including psycho-neurological symptoms to extreme altitude (Garrido et al., 1996). Compared to lowlanders, Tibetans and Sherpa have greater internal carotid artery (ICA) blood flow velocity (Huang et al., 1992), which may result in increased oxygen delivery. Positron emission tomography (PET) scans of glucose metabolism in Sherpa and lowlanders are comparable, suggesting a reduction in cerebral metabolism does not occur in this highland group (Hochachka et al., 1996b).
Relationships among physiological traits exhibited in highland populations
Clearly many traits, shared and or unique to continental highland populations, are involved as primary components or secondary effects of evolutionary adaptation to high-altitude hypoxia that has occurred over many generations. A sea-level range of [Hb] at altitude, for example, while a hallmark of Tibetan and Amhara Ethiopian adaptation and a recent focus in the high-altitude literature, provides only a glimpse into the complexity of high-altitude adaptation. Whether [Hb] is secondary to other physiological changes at one or more steps of the O2 transport cascade or rather a direct target of adaptation (e.g., [Hb] is specifically targeted for reduction to ameliorate negative effects associated with high blood viscosity or mitigate unfavorable outcomes, as in utero/early development) is unknown (Simonson et al., 2010; 2012a; Storz et al., 2010). The genetic adaptations reported thus far may be linked to [Hb] and additionally, as well as independently, associated with variation in the assortment of traits described here. Recent advancements in genomics provide the opportunity to explore these potential connections and address long-standing questions in the field of high-altitude research.
Genomics Lens: Evidence for Genetic Adaptation in Highland Populations
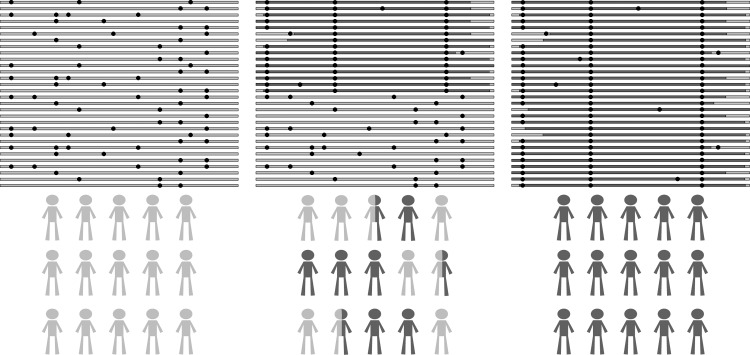
Native highlanders have survived for many generations at altitude despite physiological challenges associated with hypoxia. This is, at least in part, because ancestors of modern day highlanders had beneficial genetic variants that afforded the ability to survive and reproduce ( Bigham et al., 2009; 2010; Beall et al., 2010; Simonson et al., 2010; Yi et al., 2010a). Therefore, by means of Darwinian selection, adaptive copies of genetic variants (and linked DNA sequence in that region called haplotypes) have been continuously passed down over generations, leaving a pattern of decreased variation distinct from neutrally inherited loci in the genome. Due to the rapid nature of this event, the region subjected to selection has not experienced substantial shuffling of the genome nor accumulated many novel mutations. This striking pattern of haplotype homozygosity (referred to as a selective sweep, as it includes adaptive variants and “hitchhiking” neighboring sequence; Fig. 2) may be detected in genomic data from as few as 30 individuals in a uniquely adapted population (Pickrell et al., 2007).
FIG. 2.
Selective sweep in human genomes across evolutionary time. Each panel represents a collection of the same chromosomal region sampled at successive generations over time. A beneficial genetic variant will increase in frequency over many generations, leaving a pattern of decreased genetic variation (extended haplotype homozygosity). The first panel represents a neutrally evolving region; the second and third panels represent signals detected from an incomplete and complete selective sweep, respectively, that occurred over many generations of selective pressure. In the latter case, the region harboring the adaptive genetic variant(s) is fixed (or nearly fixed) in the population.
Differences in analytical methods, including the examination of allele frequencies in highland and non-highland groups and genome-wide analyses of extended patterns of homozygosity have been used to identify adaptive signatures. As regions exhibiting these patterns are expected to harbor functional variants favored by selection, they are likely associated with sizable developmental/survival effects and are therefore amenable to detecting genotype–phenotype associations. Therefore, populations that have adapted over many generations are exceptional for identifying such regions and assessing genetic contributions to important traits such as those involved in hypoxia adaptation and tolerance.
Genes that exhibit both an adaptive signal and are involved in oxygen sensing and response have been prioritized as top candidate genes in highland populations (Scheinfeldt and Tishkoff, 2010; Simonson et al., 2012a; Petousi and Robbins, 2014) (Table 1). Many are involved in the hypoxia-inducible factor (HIF) pathway, which regulates expression of hundreds of genes throughout various developmental stages and in several different tissues under acute, chronic, and intermittent hypoxia (Semenza, 1996; Manalo et al., 2005; Greer et al., 2012). Additional non-HIF and few non a priori candidates are also highlighted as top genomic candidates.
Table 1.
Signals of Altitude Adaptation Reported in More Than One Study of Human Highlanders and Exhibits Phenotype Association or is Reported as Significant in Non-Human Species
| Candidate gene region | Tibetan/Sherpa, Andean, Ethiopian Populations | Other intermediate/highland populations | Phenotype association in human highland population | Adaptive significance in non-human species |
|---|---|---|---|---|
| EPAS1 | Tibetan1,2,3,5,6,7,9; Sherpa10 | Deedu Mongolian15 | Hemoglobin concentration in (Tibetan2,3; Amhara Ethiopian23); lactate, free fatty acids (Tibetan27) | Dog7,18 |
| EGLN1 | Tibetan1,3,5,6,7,8,9,13; Andean 4,11; Sherpa10 | Daghestani16 | Hemoglobin concentration (Tibetan1); Gain29 and loss30 of function in exon 1 Asp4Glu/Cys127Ser | |
| PPARA | Tibetan1, Amhara and Omotic Ethiopian23 | Hemoglobin concentration (Tibetan1, Amhara and Omotic Ethiopian23); lactate, free fatty acids (Tibetan27) | ||
| HMOX2/NMRAL1 | Tibetan1,5,9 | |||
| β globin gene region | Tibetan1,3; Andean4 | Deer mice19,20, hummingbird21, dog17,18 | ||
| PKLR | Tibetan1,3 | Deedu Mongolian15 | ||
| CYP17A1 | Tibetan1,9 | |||
| HFE | Tibetan1,3,9 | |||
| EDNRA | Tibetan1; Andean4 | Birth weight (Andeans26) | ||
| CYP2E1 | Tibetan1 | Deedu Mongolian15 | ||
| PPARG | Tibetan1 | Deedu Mongolian15 | ||
| HYOU1/HMBS | Sherpa10 | Hemoglobin concentration (Sherpa10) | ||
| SENP1/ANP32D | Andean14, Andean28 | Drosophila14 | ||
| ADAM17 | Tibetan1 | Yak22 | ||
| ARNT2, CBARA1, THRB, VAV3 | Amhara Ethiopian23 | THRB and hemoglobin concentration (Amhara Ethiopian23) | ||
| SNP rs10803083 (chromosome 1) | Amhara Ethiopian24 | Hemoglobin concentration in Amhara Ethiopian24 | ||
| BHLHE41 | Amhara, Oromo, and Tigray Ethiopian25 | |||
| PRKAA1 | Andean11 | Birth weight (Andeans26); Maternal genotypes associated with uterine artery diameter and metabolic homeostasis (Andeans26) | ||
| EDNRB | Andean4, Amhara Ethiopian27 | |||
| CIC, LIPE, PAFAH1B3 | Amhara/Oromos Ethiopian27 | Involved in hypoxia tolerance in Drosophila27 |
1. Simonson et al. (2010) Science; 2. Beall et al. (2010) PNAS; 3. Yi et al. (2010) Science; 4. Bigham et al. (2010) PLoS Genetics; 5. Peng et al. (2010) Molecular Biology and Evolution; 6. (Xu and others 2011) Molecular Biology and Evolution; 7. (Wang and others 2011) PLoS One; 8. Ge et al. (2012) Molecular Genetics and Metabolism; 9. Wuren et al. (2014) PLoS One; 10. Jeong et al. (2014) Nature Communications;11. Bigham et al. (2009) Human Genomics; 12. Aggarwal et al. (2010) PNAS; 13. (Xiang and others 2013) Molecular Biology and Evolution;14. Zhou et al. (2013) AJHG; 15. Xing et al. (2013) PLoS Genetics; 16. Pagani et al. (2012) Human Genetics; 17. (Li and others 2014) Molecular Biology and Evolution; 18. (Gou and others 2014) Genome Research; 19. Storz et al. (2009) PNAS; 20. Natarajan et al. (2013) Science; 21. Projecto-Garcia et al. (2013) PNAS; 22. (Qiu and others 2012) Nature; 23. Schienfeldt et al. (2012) Genome Biology; 24. Alkorta-Aranburu et al. (2012) PLoS Genetics; 25. Huerta-Sanchez et al. (2012) Molecular Biology and Evolution; 26. Bigham et al. (2014) Physiological Genomics; 27. Udpa et al. (2014) Genome Biology; 28. Cole et al. (2014) High Altitude Medicine and Biology; 29. Lorenzo et al. (2014) Nature Genetics; 30. Song et al. (2014) Journal of Biological Chemistry.
Efforts to account for these latter sets of candidates, the population structure reported within continental groups, and different analytical approaches may further reveal regions of functional relevance in highlanders' genomes. Whole-genome sequence, epigenetic, and molecular investigations will continue to aid in the understanding of how evolutionary processes shape distinct patterns of adaptation in native highlanders.
Genetic candidates prioritized in studies of altitude adaptation
The majority of genomic altitude adaptation studies published to date focus on Tibetan highlanders and/or HIF-pathway candidate genes. Despite differences in geographic sampling across the Qinghai-Tibetan Plateau and analytical methods employed, EPAS1 and EGLN1 candidate genes, both key modulators of the HIF pathway, have been identified as top selection candidates in more than a dozen studies of Tibetan adaptation (Scheinfeldt and Tishkoff, 2010; Simonson et al., 2012a; Petousi et al., 2014). HIFs are heterodimeric transcription factors comprising a constitutively expressed β subunit and one of three α subunits, which, in combination with the β subunit, initiate gene transcription under conditions of hypoxia (Semenza, 1996; Manalo et al., 2005; Greer et al., 2012). The EPAS1 gene encodes the HIF-2α subunit, and EGLN1 encodes the oxygen-sensing prolyl hydroxylase PHD2, which targets HIFs for destruction under normoxic conditions. The HIF pathway is involved in regulating various responses to hypoxia, including angiogenesis, erythropoiesis, iron regulation, metabolism, as well as immunity and developmental biology (Greer et al., 2012), and plays various pleiotropic roles in physiology and medicine (Semenza, 2012).
The genotype–phenotype relationship examined the most among highlanders thus far is that between putatively adaptive HIF candidate loci and [Hb]. As mentioned above, SNPs representing the EPAS1 (Beall et al., 2010; Yi et al., 2010a), and EGLN1 and PPARA (Simonson et al., 2010) adaptive regions are associated with low (within sea-level range rather than elevated) [Hb] in Tibetans at altitude, suggesting a beneficial evolutionary process, primary or otherwise, is associated with this trait. Sherpa, who share adaptive signals at EPAS1 and EGLN1, also exhibit an association between [Hb] and EPAS1, and exhibit an adaptive signal at a genomic segment associated with high-altitude ancestry ∼1 kb upstream of the gene HYOU1, hypoxia upregulated protein 1, with [Hb]. This region is also a cis-expression quantitative trait locus for a neighboring candidate gene HMBS, hydroxymethylbilane, and SNPs in this gene region are associated with Sherpa [Hb] (Jeong et al. 2014). Putatively adaptive copies of THRB in addition to PPARA and EPAS1, the latter two also identified in Tibetans (Simonson et al., 2010), show relationships with [Hb] in Amhara Ethiopians (Scheinfeldt et al., 2012). BHLHE41, although not associated with [Hb], is a key HIF pathway gene and top selection candidate in Amhara, Oromo, and Tigray Ethiopians (Huerta-Sanchez et al., 2013).
While the functional variants of most candidate genes have yet to be identified, variants within the first exon of EGLN1 (Asp4Glu; Cys127Ser), found at markedly high frequency in Tibetans, exhibit a lower Km value for oxygen, suggesting a gain of PHD2 function and increased HIF degradation under hypoxic conditions (Lorenzo et al., 2014). A disruption in erythroid progenitor proliferation associated with these variants provides a potential mechanism for decreased [Hb] in Tibetans at altitude (Lorenzo et al., 2014). In a separate study, these variants were shown to result in loss of PHD2 function, and increased HIF activation, via defective binding of co-chaperon p23 (Song et al., 2014). Neighboring variants, within the first intron of EGLN1, are also associated with its expression and high-altitude pulmonary edema (HAPE) in a population from India (Aggarwal et al., 2010).
Recent studies indicate that Tibetan sea-level residents exhibit lower than sea-level average [Hb], suggesting a hypo-responsiveness of HIF. This is further demonstrated by decreased lymphocyte expression of HIF-2α mRNA and select HIF-targeted genes and dampened pulmonary vascular responses compared to Han Chinese at sea level (Petousi et al., 2014). How these findings relate to each other, specifically under relevant tissue, developmental, and environmental conditions, and the resulting effects on the HIF pathway and other compensatory expression changes, remains to be determined (Simonson and Powell, 2014).
In addition to the replicated HIF candidate findings in Tibetans, HIF pathway genes have also been reported as adaptive candidates in other populations and continental highland groups (Table 1). Interestingly, the first report of an adaptive signal at the EGLN1 locus came from a study of Andeans, although it remains to be determined whether the adaptive variants at this locus are the same as those reported in Tibetan/Sherpa highlanders (Bigham 2009; Bigham et al., 2010). This is also the case for the EDNRA selection candidate, identified in both Andeans (Bigham, 2009) and highland Tibetan and Mongolian groups (Simonson et al., 2010; Xing et al., 2013) and EDNRB, reported as a selection candidate in Andeans and Amhara Ethiopians (Bigham et al., 2010; Udpa et al., 2014).
Additional and perhaps equally important non-HIF pathway candidate genes have emerged as top candidates in more than one study. The HMOX2 locus, involved in hypoxia response in the O2-sensing carotid body (Prabhakar, 2012), and neighboring NMRAL1, involved in synthesis of nitric oxide, hypothesized as a contributor to adaptation (Beall, 2006; Erzurum et al., 2007; Beall et al., 2012), is a top candidate gene region in three Tibetan studies (Simonson et al., 2010; 2012a; Peng et al., 2011; Wuren et al., 2014). Other selection candidate genes reported at least twice in Tibetans include CYP17A1, HBB/HBG2, HFE, and PKLR (Simonson et al., 2010; 2012k; Yi et al., 2010a), with HBE1 reported among top candidates in Andeans as well (Bigham et al., 2010). Additional HIF and non-HIF gene candidates reported at least once in the literature are summarized in (Bigham and Lee, 2014). In addition to these genes, the potential for non-coding regulatory regions involvement in adaptation has yet to be fully explored (Jeong et al., 2014; Udpa et al., 2014) but should be prioritized through examination of overlapping signals of non-coding selection candidate regions.
While too numerous to mention in this review, various genes have been reported as high-altitude candidates in a single study. As fewer Ethiopian studies have been published to date (and are based on different subpopulations in Ethiopia), many candidate genes have been reported only once in this population. Examples include THRB, ARNT2, CBARA1, and VAV3 (Scheinfeldt et al., 2012), a non-coding genomic region on chromosome 1 (Alkorta-Aranburu et al., 2012), and HIF-pathway gene BHLHE41 (Huerta-Sanchez et al., 2013). Whole-genome sequence analysis of Oromo and Simen Ethiopians revealed an adaptive signal on chromosome 19 that contains CIC, LIPE, and PAFAH1B3 genes, whose orthologs in Drosophila influence survival rate under hypoxia (Udpa et al., 2014). One report of mitochondrial DNA (mtDNA) variants in Tibetans revealed that 3394C, when present on a particular Tibetan haplotype background, is associated with increased complex 1 activity relative to the alternate variant or 3394C variant on another mtDNA background (Ji et al., 2012).
Replicated genetic signals of adaptation in other populations and species at altitude
While most focus has been placed on studies of three continental highland groups, other populations with more recent multi-generation history at intermediate or high altitudes exhibit genomic signals of selection at some of the same loci reported in Tibetans, Andeans, and/or Ethiopians. Highly differentiated intronic SNPs in EGLN1 are prevalent among a Daghestani population at intermediate altitude (Pagani et al., 2012a). Genome-wide selection analysis in a highland (Deedu) Mongolian population revealed adaptive signals at EPAS1, PKLR, CYP2E1, and PPARG, also reported as top signals in neighboring Tibetan populations, but not lowland Buryat Mongolians (Simonson et al., 2010; Xing et al., 2013; Wuren et al., 2014).
Some high-altitude adaptive candidate genes from human studies are also reported as targets of selection in other species. Candidate gene studies in high-altitude deer mice identified variants at alpha and beta hemoglobin loci that underlie greater Hb-O2 binding affinity at high altitude ( Storz et al., 2009; Storz et al., 2010; Natarajan et al., 2013) and pikas (Tufts et al., 2015). Variants at the same locus also prove adaptive in high-altitude hummingbirds in South America (Projecto-Garcia et al., 2013). Adaptive signals are noted at EPAS1 and HBB loci in domesticated dogs at altitude (Gou et al., 2014; Wang et al., 2014; Fan et al., 2015), and yak share an adaptive selection signal with Tibetans at the ADAM17 locus (Qiu et al., 2012).
In the Drosophila laboratory model, genes involved in the Notch pathway are top candidates for hypoxia adaption (Zhou et al., 2011; Zhou and Haddad, 2013), and orthologs of genes identified in highland Ethiopians influence Drosophila survival rate under hypoxia (Udpa et al., 2014). Decreased expression of two neighboring candidates genes, SENP1 and ANP32D, originally identified through comparative whole genome sequence analysis of Andeans with and without chronic mountain sickness (CMS), exhibit increased survival in Drosophila exposed to hypoxia (Zhou et al., 2013), and gene expression at this locus is lower in fibroblasts derived from non-CMS versus CMS cells (Zhou et al., 2013). Replication of the SENP1 finding supports the association identified between variants in this gene and CMS individuals of Quechua ancestry (Cole et al., 2014).
Considering geographic substructure and distinct analytical approaches in adaptation studies
The various approaches employed in genetic studies of adaptation across human populations and different species have been fruitful but somewhat limited in standardization and presentation of results. One issue of concern is that many studies of a single continental population identify none to few of the same genes as having been subject to selection. While such concerns are valid, it is important to consider that most high-altitude genomic studies are based upon data collected in subgeographic regions, or a dispersed sample of data collected throughout a vast area, which may influence the strength or detection of adaptive signals (Simonson et al., 2012a; Huerta-Sanchez et al., 2013). Efforts to identify and account for admixture and genetic ancestry in Andeans (Brutsaert et al., 2003; 2005), population structure among Tibetans and Sherpa (Xing et al., 2013; Jeong et al., 2014; Wuren et al., 2014), and subgroups in Ethiopia (Alkorta-Aranburu et al., 2012; Pagani et al., 2012b; Scheinfeldt et al., 2012; Huerta-Sanchez et al., 2013) attempt to address these concerns.
Discrepant signals may also be due to different approaches designed to detect incomplete (ongoing) versus complete (fixed or nearly fixed) adaptive events (Nielsen et al., 2007), “hard” or “soft” sweeps, novel or standing (pre-existing) genetic variation (Pritchard et al., 2010), or long-range haplotype versus allele-frequency based tests of selection (Sabeti et al., 2007). Tools developed to analyze complete genome sequence data, such as the Composite of Multiple Signals (CMS), which combines information from multiple tests of selection, improve the resolution of adaptive regions containing functional variants (Grossman et al., 2010). Sequence data will also provide greater insight into relevant genomic changes, rather than single nucleotide variant (SNV) or protein-coding (exome) data alone. These rich data sets, coupled with future efforts to characterize genome-wide expression, epigenomic, and/or proteonomic profiles will accelerate progress in signal detection and work towards defining molecular roles.
Physiological Genomics Lens: How Do Adaptive Genetic Factors Orchestrate a System of Traits at Altitude?
Considering multiple reported links between adaptive signals and [Hb], one unanswered questions is whether [Hb] was the direct target or secondary consequence of other adaptive changes in the oxygen transport system (Storz, 2010; Simonson et al., 2012a; 2014). Therefore, a starting point for integrative analysis revolves around the assessment of both oxygen transport components, which may provide insight to developmental changes, and genetic associations both related and unrelated to low [Hb], and the timing of, genetic background contribution to, adaptive events. An understanding of common and distinct characteristics and genetic catalogues in each continental population are essential to address this question.
Evolutionary steps through distinct adaptive landscapes
The combination of physiological and genomic analyses provide valuable insights into the timing and the mechanisms underlying genetic events that have led to the current physiological states of the Tibetan, Andean, Ethiopian, and other multi-generation populations at altitude.
Thus far, different dates have been estimated for the EPAS1 selective event in Tibetans: ∼3000 years (Yi, 2010), ∼18,000 years (Peng, 2011), and introgression into the ancestral Tibetan gene pool more than ∼40,000 years ago (Heurta-Sanchez et al., 2014). A date of ∼8000 years has been reported for EGLN1 in two studies thus far (Peng et al., 2011; Lorenzo et al., 2014). Differences may reflect uncertainty or differences in demographic models, which likely vary across this vast geographic region. Efforts to collect and analyze whole-genome sequence data from individuals located throughout the Plateau will help clarify this issue.
Another remaining question is whether present-day physiological differences in geographically distinct highlanders reflect distinct evolutionary trajectories or rather different temporal snapshots of a consistent evolutionary process in each highland population. The former is the most parsimonious when viewed through a genetics lens: each population began with a different genetic background, providing a unique basis for evolutionary processes, with subsequent random, possibly compensatory, changes to the system; the chance of the same novel, beneficial mutations occurring along the same path in separate populations unlikely. The identification of different adaptive regulatory variants upstream of the LCT gene, which underlie lactase persistence, arose independently in European and African pastoralists populations, providing a direct example of convergent adaptation in humans (Tishkoff et al., 2007). Parallel mechanisms of adaptation are reported in other high-altitude species, such as hummingbird species, which exhibit adaptive genetic variants associated with increased Hb-O2 binding affinity at altitude (Projecto-Garcia et al., 2013). Again, the availability of whole-genome sequence data in all populations will provide a clearer view of the adaptive process.
Archaic insights into genetic determinants of adaptation
The recent linking of the Tibetan EPAS1 adaptive genomic region to the Denisovan lineage (Huerta-Sanchez et al., 2014) underscores an important point regarding the role of existing variation in a population. The adaptive EPAS1 signal has been identified in Tibetan, Sherpa, and Mongolian populations resident on the Plateau (Xing et al., 2013) thus far, indicating the adaptive signal is unique to populations in this region although a marginal association between EPAS1 and [Hb] is reported in Amhara Ethiopians (Scheinfeldt et al., 2012). That this genomic segment made its way into the ancestral Tibetan gene pool from Denisovans is attributed to the population history of this geographic area. The adaptive variant(s) existed as part of the population's genetic background and proved favorable in a high-altitude environment. Remarkably, this introgression provided the basis for what has turned out to be one of the strongest adaptive signals reported in highlanders, and the cascade of adaptive events that followed were likely shaped by this pre-existing evolutionary landscape.
Considering again the adaptive signal of EPAS1 is exclusive to Tibetans and neighboring highland populations, any physiological effect(s) are suspected to be unique (if not convergent) component(s) of their physiology. Indeed, EPAS1 is associated with the relatively low [Hb] in many Tibetans at altitude, distinct from most other populations with the exception of Amhara Ethiopians. Comparisons of sequence data from these putatively adaptive regions across populations and assessment of the same physiological variables in these groups will help clarify these important questions.
Broad views regarding genetics and physiology
As with physiological studies, genetic analyses are neither entirely conclusive nor exhaustive, but provide important steps towards understanding functional and evolutionary mechanisms of hypoxia adaptation. This review is limited to scans of the current genomics literature, which, in the absence of extensive molecular of physiological data, may be skewed towards only top empirical candidate genes with potential (a priori) functional relevance. Efforts to minimize this concern, through more comprehensive, unbiased, standardized, and integrative analyses are currently underway.
By developing a more complete view of genomic adaptations shared among or unique to particular groups, it will become increasingly clear how multi-gene and gene-by-environment factors work in concert and possibly as compensatory responses to a chain of adaptive events. Understanding the associations between genetic and physiological variation in highlanders has additional application for understanding maladaptive and general responses to hypoxia, which remain an important biomedical component of hypoxia research. This is also of clinical value when considering distinct and shared hypoxia-associated genetic variants and combinations thereof may contribute to physiological responses in residents and visitors to the environmental hypoxia at altitude as well as chronic (e.g., cardiopulmonary, developmental) or intermittent (e.g., sleep apnea) states of hypoxia.
Future efforts to complete additional whole-genome sequence analyses, supported by epigenetic, and other “omic” analyses (proteonomic, metabolic, etc) in native highlanders, and functional assessments in laboratory models are necessary to determine the relevance of the precise genomic variants underlying adaptive traits in these populations. Given the advancements in genomics technology, regulatory variants, underlying tissue and developmentally specific effects (ENCODE Consortium, 2011), will likely become increasingly evident and important contributors to the adaptive story in different highland groups. Integrative physiological and genomic efforts that aim to characterize functional relevance at a system level, extending beyond single associations, will provide insight into the evolutionary processes that continue to shape these unique populations and influence hypoxia response in all humans.
Acknowledgments
I thank P.W. Wagner, F.L. Powell, and M.J. MacInnis, and an anonymous reviewer for helpful comments.
References
- Aggarwal S, Negi S, Jha P, Singh PK, Stobdan T, Pasha MAQ, Ghosh S, Agrawal A, Indian Genome Variation Consortium, Prasher B, and Mukerji M. (2010). EGLN1 involvement in high-altitude adaptation revealed through genetic analysis of extreme constitution types defined in Ayurveda. Proc Natl Acad Sci USA 107:18961–18966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldenderfer M. (2011). Peopling the Tibetan plateau: Insights from archaeology. High Alt Med Biol 12:141–147 [DOI] [PubMed] [Google Scholar]
- Aldenderfer MS. (1993). Domestic Architecture, Ethnicity, and Complementarity in the South-Central Andes. University of Iowa Press, Iowa City [Google Scholar]
- Alkorta-Aranburu G, Beall CM, Witonsky DB, Gebremedhin A, Pritchard JK, and Di Rienzo A. (2012). The genetic architecture of adaptations to high altitude in Ethiopia. PLoS Genet 8:e1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Stella J, and Saldana M. (1963). The terminal portion of the pulmonary arterial tree in people native to high altitudes. Circulation 28:915–925 [DOI] [PubMed] [Google Scholar]
- Balaban DY, Duffin J, Preiss D, Mardimae A, Vesely A, Slessarev M, Zubieta-Calleja GR, Greene ER, Macleod DB, and Fisher JA. (2003). The in-vivo oxyhaemoglobin dissociation curve at sea level and high altitude. Respir Physiol Neurobiol 186:45–52 [DOI] [PubMed] [Google Scholar]
- Beall CM. (2000). Tibetan and Andean patterns of adaptation to high-altitude hypoxia. Hum Biol 72:201–228 [PubMed] [Google Scholar]
- Beall CM. (2006). Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol 46:18–24 [DOI] [PubMed] [Google Scholar]
- Beall CM. (2007). Two routes to functional adaptation: Tibetan and Andean high-altitude natives. Proc Natl Acad Sci USA 104:8655–8660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Almasy LA, Blangero J, Williams-Blangero S, Brittenham GM, Strohl KP, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, and Gonzalez C. (1999). Percent of oxygen saturation of arterial hemoglobin among Bolivian Aymara at 3,900–4,000 m. Am J Phys Anthropol 108:41–51 [DOI] [PubMed] [Google Scholar]
- Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, and Gonzalez C. (1998). Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol 106:385–400 [DOI] [PubMed] [Google Scholar]
- Beall CM, Cavalleri GL, Deng L, Elston RC, Gao Y, Knight J, Li C, Li JC, Liang Y, McCormack M, Montgomery HE, Pan H, Robbins PA, Shianna KV, Tam SC, Tsering N, Veeramah KR, Want W, Wangdui P, Weale ME, Xu Y, Xu Z, Yang L, Zaman MJ, Zeng C, Zhang L, Zhang X, Zhaxi P, and Zheng YT. (2010). Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci USA 107:11459–11464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, and Strohl KP. (2002). An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci USA 99:17215–17218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Laskowski D, and Erzurum SC. (2012). Nitric oxide in adaptation to altitude. Free Radic Biol Med 52:1123–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall CM, Song K, Elston RC, and Goldstein MC. (2004). Higher offspring survival among Tibetan women with high oxygen saturation genotypes residing at 4,000 m. Proc Natl Acad Sci USA 101:14300–14304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham A, Bauchet M, Pinto D, Mao X, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, Lopez Herraez D, Brutsaert T, Parra EJ, Moore LG, and Shriver MD. (2010). Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet 6:e1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Julian CG, Wilson MJ, Vargas E, Browne VA, Shriver MD, and Moore LG. (2014). Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol Genomics 46:687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, and Lee FS. (2014). Human high-altitude adaptation: Forward genetics meets the HIF pathway. Genes Dev 28:2189–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Mao X, Mei R, Brutsaert T, Wilson MJ, Julian CG, Parra EJ, Akey JM, Moore LG, and Shriver MD. (2009). Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Hum Genomics 4:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert TD. (2008). Do high-altitude natives have enhanced exercise performance at altitude? Appl Physiol Nutr Metab 33:582–592 [DOI] [PubMed] [Google Scholar]
- Brutsaert TD, Parra EJ, Shriver MD, Gamboa A, Palacios JA, Rivera M, Rodriguez I, and Leon-Velarde F. (2003). Spanish genetic admixture is associated with larger V(O2) max decrement from sea level to 4338 m in Peruvian Quechua. J Appl Physiol (1985) 95:519–528 [DOI] [PubMed] [Google Scholar]
- Brutsaert TD, Parra EJ, Shriver MD, Gamboa A, Rivera-Ch M, and Leon-Velarde F. (2005). Ancestry explains the blunted ventilatory response to sustained hypoxia and lower exercise ventilation of Quechua altitude natives. Am J Physiol Regul Integr Comp Physiol 289:R225–234 [DOI] [PubMed] [Google Scholar]
- Chen FH, Dong GH, Zhang DJ, Liu XY, Jia X, An CB, Ma MM, Xie YW, Barton L, Ren XY, Zhao ZJ, Wu XH, and Jones MK. (2015). Agriculture facilitated permanent human occupation of the Tibetan Plateau after 3600 B.P. Science 347:248–250 [DOI] [PubMed] [Google Scholar]
- Chen QH, Ge RL, Wang XZ, Chen HX, Wu TY, Kobayashi T, and Yoshimura K. (1997). Exercise performance of Tibetan and Han adolescents at altitudes of 3,417 and 4,300 m. J Appl Physiol (1985) 83:661–667 [DOI] [PubMed] [Google Scholar]
- Cole AM, Petousi N, Cavalleri GL, and Robbins PA. (2014). Genetic variation in SENP1 and ANP32D as predictors of chronic mountain sickness. High Alt Med Biol 15:497–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium EP. (2011). A user's guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol 9:e1001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran LS, Zhuang J, Sun SF, and Moore LG. (1997). Ventilation and hypoxic ventilatory responsiveness in Chinese-Tibetan residents at 3,658 m. J Appl Physiol (1985) 83:2098–2104 [DOI] [PubMed] [Google Scholar]
- Droma T, McCullough RG, McCullough RE, Zhuang JG, Cymerman A, Sun SF, Sutton JR, and Moore LG. (1991). Increased vital and total lung capacities in Tibetan compared to Han residents of Lhasa (3,658 m). Am J Phys Anthropol 86:341–351 [DOI] [PubMed] [Google Scholar]
- Eichstaedt CA, Antao T, Pagani L, Cardona A, Kivisild T, and Mormina M. (2014). The Andean adaptive toolkit to counteract high altitude maladaptation: Genome-wide and phenotypic analysis of the Collas. PLoS One 9:e93314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ, Feelisch M, and Beall CM. (2007). Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA 104:17593–17598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Liu F, Wu H, Wu S, Zhu C, Li Y, Wang G, and Zhang Y. (2015). A positive correlation between elevated altitude and frequency of mutant alleles at the EPAS1 and HBB loci in Chinese indigenous dogs. J Genetics Genomics 42:173–177 [DOI] [PubMed] [Google Scholar]
- Formenti F, Constantin-Teodosiu D, Emmanuel Y, Cheeseman J, Dorrington KL, Edwards LM, Humphreys SM, Lappin TR, McMullin MF, McNamara CJ, Mills W, Murphy JA, O'Connor DF, Percy MJ, Ratcliffe PH, Smith TG, Treacy M, Frayn KN, Greenhaff PL, Karpe F, Clarke K, and Robbins PA. (2010). Regulation of human metabolism by hypoxia-inducible factor. Proc Natl Acad Sci USA 107:12722–12727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido E, Segura R, Capdevila A, Pujol J, Javierre C, and Ventura JL. (1996). Are Himalayan Sherpas better protected against brain damage associated with extreme altitude climbs? Clin Sci (Lond) 90:81–85 [DOI] [PubMed] [Google Scholar]
- Ge RL, Simonson TS, Cooksey RC, Tanna U, Qin G, Huff CD, Witherspoon DJ, Xing J, Zhengzhong B, Prchal JT, Jorde LB, and McClain DA. (2012). Metabolic insight into mechanisms of high-altitude adaptation in Tibetans. Mol Genet Metab 106:244–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge RLH, Chen QH, Li HL, Gen D, Kubo K, Matsuzawa Y, Fujimoto K, Yoshimura K, Takeoka M, Kubo K, and Kobayashi T. (1995). Comparisons of oxygen transport between Tibetan and Han residents at moderate altitude. Wilderness Environ Med 6:391–400 [Google Scholar]
- Gilbert-Kawai ET, Milledge JS, Grocott MP, and Martin DS. (2014). King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology (Bethesda) 29:388–402 [DOI] [PubMed] [Google Scholar]
- Gou X, Wang Z, Li N, Qiu F, Xu Z, Yan D, Yang S, Jia J, Kong X, Wei Z, et al. (2014). Whole-genome sequencing of six dog breeds from continuous altitudes reveals adaptation to high-altitude hypoxia. Genome Res 24:1308–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer SN, Metcalf JL, Wang Y, and Ohh M. (2012). The updated biology of hypoxia-inducible factor. EMBO J 31:2448–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SR, Shlyakhter I, Karlsson EK, Byrne EH, Morales S, Frieden G, Hostetter E, Angelino E, Garber M, Zuk O, Lander ES, Schaffner SF, and Sabeti PC. (2010). A composite of multiple signals distinguishes causal variants in regions of positive selection. Science 327:883–886 [DOI] [PubMed] [Google Scholar]
- Groves BM, Droma T, Sutton JR, McCullough RG, McCullough RE, Zhuang J, Rapmund G, Sun S, Janes C, and Moore LG. (1993). Minimal hypoxic pulmonary hypertension in normal Tibetans at 3,658 m. J Appl Physiol (1985) 74:312–318 [DOI] [PubMed] [Google Scholar]
- Halperin BD, Sun S, Zhuang J, Droma T, and Moore LG. (1998). ECG observations in Tibetan and Han residents of Lhasa. J Electrocardiol 31:237–243 [PubMed] [Google Scholar]
- Hassen M. (1990). The Oromo of Ethiopia: A History, 1570–1860. Great Britain: Cambridge University Press [Google Scholar]
- Heath D, Smith P, Rios Dalenz J, Williams D, and Harris P. (1981). Small pulmonary arteries in some natives of La Paz, Bolivia. Thorax 36:599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Clark CM, Holden JE, Stanley C, Ugurbil K, and Menon RS. (1996a). 31P magnetic resonance spectroscopy of the Sherpa heart: A phosphocreatine/adenosine triphosphate signature of metabolic defense against hypobaric hypoxia. Proc Natl Acad Sci USA 93:1215–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Clark CM, Monge C, Stanley C, Brown WD, Stone CK, Nickles RJ, and Holden JE. (1996b). Sherpa brain glucose metabolism and defense adaptations against chronic hypoxia. J Appl Physiol (1985) 81:1355–1361 [DOI] [PubMed] [Google Scholar]
- Hoit BD, Dalton ND, Gebremedhin A, Janocha A, Zimmerman PA, Zimmerman AM, Strohl KP, Erzurum SC, and Beall CM. (2011). Elevated pulmonary artery pressure among Amhara highlanders in Ethiopia. Am J Hum Biol 23:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JE, Stone CK, Clark CM, Brown WD, Nickles RJ, Stanley C, and Hochachka PW. (1995). Enhanced cardiac metabolism of plasma glucose in high-altitude natives: Adaptation against chronic hypoxia. J Appl Physiol (1985) 79:222–228 [DOI] [PubMed] [Google Scholar]
- Holloway CJ, Montgomery HE, Murray AJ, Cochlin LE, Codreanu I, Hopwood N, Johnson AW, Rider OJ, Levett DZ, Tyler DJ, Francis JM, Neubauer S, Grocott MP, Clarke K, Caudwell Xtreme Everest Research Group. (2011). Cardiac response to hypobaric hypoxia: Persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J 25:792–796 [DOI] [PubMed] [Google Scholar]
- Huang SY, Sun S, Droma T, Zhuang J, Tao JX, McCullough RG, McCullough RE, Micco AJ, Reeves JT, and Moore LG. (1992). Internal carotid arterial flow velocity during exercise in Tibetan and Han residents of Lhasa (3,658 m). J Appl Physiol (1985) 73:2638–2642 [DOI] [PubMed] [Google Scholar]
- Huerta-Sanchez E, Degiorgio M, Pagani L, Tarekegn A, Ekong R, Antao T, Cardona A, Montgomery HE, Cavalleri GL, Robbins PA, Weale ME, Bradman N, Bekele E, Kivisild T, Tyler-Smith C, and Nielsen R. (2013). Genetic signatures reveal high-altitude adaptation in a set of Ethiopian populations. Mol Biol Evol 30:1877–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta-Sanchez E, Jin X, Asan , Bianba Z, Peter BM, Vinckenbosch N, Liang Y, Yi X, He M, Somel M, Ni P, Wang B, Ou X, Huasang , Luosang J, Cuo ZX, Li K, Gao G, Yin Y, Wang W, Zhang X, Xu X, Yang H, Li Y, Wang J, and Nielsen R. (2014). Altitude adaptation in Tibetans caused by introgression of Denisovan-like DNA. Nature 512:194–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong C, Alkorta-Aranburu G, Basnyat B, Neupane M, Witonsky DB, Pritchard JK, Beall CM, and Di Rienzo A. (2014). Admixture facilitates genetic adaptations to high altitude in Tibet. Nat Commun 5:3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, Sharpley MS, Derbeneva O, Alves LS, Qian P, Wang Y, Chalkia D, Lvova M, Xu J, Yao W, Simon M, Platt J, Xu S, Angelin A, Davila A<. Huang T, Wang PH, Chuang LM, Moore LG, Qian G, and Wallace DC. (2012). Mitochondrial DNA variant associated with Leber hereditary optic neuropathy and high-altitude Tibetans. Proc Natl Acad Sci USA 109:7391–7396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Vargas E, Gonzales M, Davila RD, Ladenburger A, Reardon L, Schoo C, Powers RW, Lee-Chiong T, and Moore LG. (2013). Sleep-disordered breathing and oxidative stress in preclinical chronic mountain sickness (excessive erythrocytosis). Respir Physiol Neurobiol 186:188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, and Moore LG. (2009). Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol 296:R1564–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor S, and Kapoor AK. (2005). Body structure and respiratory efficiency among high altitude Himalayan populations. Coll Antropol 29:37–43 [PubMed] [Google Scholar]
- Kayser B, Hoppeler H, Claassen H, and Cerretelli P. (1991). Muscle structure and performance capacity of Himalayan Sherpas. J Appl Physiol (1985) 70:1938–1942 [DOI] [PubMed] [Google Scholar]
- Kayser B, Hoppeler H, Desplanches D, Marconi C, Broers B, and Cerretelli P. (1996). Muscle ultrastructure and biochemistry of lowland Tibetans. J Appl Physiol (1985) 81:419–425 [DOI] [PubMed] [Google Scholar]
- Keyl C, Schneider A, Greene RE, Passino C, Spadacini G, Bandinelli G, Bonfichi M, Arcaini L, Malcovati L, and Bernardi L. (2000). Effects of breathing control on cardiocirculatory modulation in Caucasian lowlanders and Himalayan Sherpas. Eur J Appl Physiol 83:481–486 [DOI] [PubMed] [Google Scholar]
- Li Y, Wu DD, Boyko AR, Wang GD, Wu SF, Irwin DM, and Zhang YP. (2014). Population variation revealed high-altitude adaptation of Tibetan mastiffs. Mol Biol Evol 31:1200–1205 [DOI] [PubMed] [Google Scholar]
- Lorenzo FR, Huff C, Myllymaki M, Olenchock B, Swierczek S, Tashi T, Gordeuk V, Wuren T, Ri-Li G, McClain DA, Khan TM, Koul PA, Guchhait P, Salama ME, Xing J, Semenza GL, Liberzon E, Wilson A, Simonson TS, Jorse LB, Kaelin WG, Koivunen P, and Prchal JT. (2014). A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet 46:951–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, and Simon MC. (2010). Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40:294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, and Semenza GL. (2005). Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105:659–669 [DOI] [PubMed] [Google Scholar]
- Moore LG. (2001). Human genetic adaptation to high altitude. High Alt Med Biol 2:257–279 [DOI] [PubMed] [Google Scholar]
- Moore LG, Curran-Everett L, Droma TS, Groves BM, McCullough RE, McCullough RG, Sun SF, Sutton JR, Zamudio S, and Zhuang JG. (1992). Are Tibetans better adapted? Int J Sports Med 13:S86–88 [DOI] [PubMed] [Google Scholar]
- Moore LG, Niermeyer S, and Zamudio S. (1998). Human adaptation to high altitude: Regional and life-cycle perspectives. Am J Phys Anthropol Suppl 27:25–64 [DOI] [PubMed] [Google Scholar]
- Moore LG, Young D, McCullough RE, Droma T, and Zamudio S. (2001). Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol 13:635–644 [DOI] [PubMed] [Google Scholar]
- Morpurgo G, Arese P, Bosia A, Pescarmona GP, Luzzana M, Modiano G, and Krishna ranjit S. (1976). Sherpas living permanently at high altitutde: A new pattern of adaptation. Proc Natl Acad Sci USA 73:747–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan C, Inoguchi N, Weber RE, Fago A, Moriyama H, and Storz JF. (2013). Epistasis among adaptive mutations in deer mouse hemoglobin. Science 340:1324–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen R, Hellmann I, Hubisz M, Bustamante C, and Clark AG. (2007). Recent and ongoing selection in the human genome. Nat Rev Genet 8:857–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niermeyer S, Andrade-M MP, Vargas E, and Moore LG. (2015). Neonatal oxygenation, pulmonary hypertension, and evolutionary adaptation to high altitude (2013 Grover Conference series). Pulm Circ 5:48–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani L, Ayub Q, MacArthur DG, Xue Y, Baillie JK, Chen Y, Kozarewa I, Turner DJ, Tofanelli S, Bulayeva K, Kidd K, Paoli G, and Tyler-Smith C. (2012a). High altitude adaptation in Daghestani populations from the Caucasus. Hum Genet 131:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani L, Kivisild T, Tarekegn A, Ekong R, Plaster C, Gallego Romero I, Ayub Q, Mehdi SQ, Thomas MG, Luiselli D, Bekele E, Bradman N, Balding DJ, and Tyler-Smith C. (2012b). Ethiopian genetic diversity reveals linguistic stratification and complex influences on the Ethiopian gene pool. Am J Hum Genet 91:83–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penaloza D, and Arias-Stella J. (2007). The heart and pulmonary circulation at high altitudes: Healthy highlanders and chronic mountain sickness. Circulation 115:1132–1146 [DOI] [PubMed] [Google Scholar]
- Peng Y, Yang Z, Zhang H, Cui C, Qi X, Luo X, Tao X, Wu T, Ouzhuluobu , Basang , Ciwangsangbu , Danzengduojie , Chen H, Shi H, and Su B. (2011). Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol 28:1075–1081 [DOI] [PubMed] [Google Scholar]
- Petousi N, Croft QP, Cavalleri GL, Cheng HY, Formenti F, Ishida K, Lunn D, McCormack M, Shianna KV, Talbot NP, ratcliffe PJ, and Robbins PA. (2014). Tibetans living at sea level have a hyporesponsive hypoxia-inducible factor system and blunted physiological responses to hypoxia. J Appl Physiol 116:893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petousi N, and Robbins PA. (2014). Human adaptation to the hypoxia of high altitude: The Tibetan paradigm from the pregenomic to the postgenomic era. J Appl Physiol (1985) 116:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR. (2012). Carbon monoxide (CO) and hydrogen sulfide (H(2)S) in hypoxic sensing by the carotid body. Respir Physiol Neurobiol 184:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard JK, Pickrell JK, and Coop G. (2010). The genetics of human adaptation: Hard sweeps, soft sweeps, and polygenic adaptation. Curr Biol 20:R208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projecto-Garcia J, Natarajan C, Moriyama H, Weber RE, Fago A, Cheviron ZA, Dudley R, McGuire JA, Witt CC, and Storz JF. (2013). Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc Natl Acad Sci USA 110:20669–20674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh LG. (1962). Physiological and medical aspects of the Himalayan scientific and mountaineering expedition, 1960–61. Br Med J 2:621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh LG. (1964). Man at high altitude: Studies carried out in the Himalaya. Sci Basis Med Annu Rev 32–54 [PubMed] [Google Scholar]
- Qi X, Cui C, Peng Y, Zhang X, Yang Z, Zhong H, Zhang H, Xiang K, Cao X, Wang Y, Ouzhuluobu , Basang , Ciwangsangbu , Bianba , Gonggalanzi , Wu T, Chen H, SHi H, and Su B. (2013). Genetic evidence of paleolithic colonization and neolithic expansion of modern humans on the tibetan plateau. Mol Biol Evol 30:1761–1778 [DOI] [PubMed] [Google Scholar]
- Qin Z, Yang Y, Kang L, Yan S, Cho K, Cai X, Lu Y, Zheng H, Zhu D, Fei D, Li S, Jin L, Li H, and Genographic Consortium. (2010). A mitochondrial revelation of early human migrations to the Tibetan Plateau before and after the last glacial maximum. Am J Phys Anthropol 143:555–569 [DOI] [PubMed] [Google Scholar]
- Qiu Q, Zhang G, Ma T, Qian W, Wang J, Ye Z, Cao C, Hu Q, Kim J, Larkin DM, Auvil L, Capitanu B, Ma J, Lewin HA, Qian X, Lang Y, Zhou R, Wang L, Wang K, Xia J, et al. (2012). The yak genome and adaptation to life at high altitude. Nat Genet 44:946–949 [DOI] [PubMed] [Google Scholar]
- Rademaker K, Hodgins G, Moore K, Zarrillo S, Miller C, Bromley GR, Leach P, Reid DA, Alvarez WY, and Sandweiss DH. (2014). Paleoindian settlement of the high-altitude Peruvian Andes. Science 346:466–469 [DOI] [PubMed] [Google Scholar]
- Rupert JL, and Hochachka PW. (2001). Genetic approaches to understanding human adaptation to altitude in the Andes. J Exp Biol 204:3151–3160 [DOI] [PubMed] [Google Scholar]
- Sabeti PC, Varilly P, Fry B, Lohmueller J, Hostetter E, Cotsapas C, Xie X, Byrne EH, McCarroll SA, Gaudet R, et al. (2007). Genome-wide detection and characterization of positive selection in human populations. Nature 449:913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaja M, Veicsteinas A, and Cerretelli P. (1979). Oxygen affinity of blood in altitude Sherpas. J Appl Physiol 47:337–341 [DOI] [PubMed] [Google Scholar]
- Scheinfeldt LB, Soi S, Thompson S, Ranciaro A, Woldemeskel D, Beggs W, Lambert C, Jarvis JP, Abate D, Belay G, and Tishkoff SA. (2012). Genetic adaptation to high altitude in the Ethiopian highlands. Genome Biol 13:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinfeldt LB, and Tishkoff SA. (2010). Living the high life: High-altitude adaptation. Genome Biol 11:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. (1996). Transcriptional regulation by hypoxia-inducible factor 1 molecular mechanisms of oxygen homeostasis. Trends Cardiovasc Med 6:151–157 [DOI] [PubMed] [Google Scholar]
- Semino O, Santachiara-Benerecetti AS, Falaschi F, Cavalli-Sforza LL, and Underhill PA. (2002). Ethiopians and Khoisan share the deepest clades of the human Y-chromosome phylogeny. Am J Hum Genet 70:265–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, McClain DA, Jorde LB, and Prchal JT. (2012a). Genetic determinants of Tibetan high-altitude adaptation. Human Genetics 131:527–533 [DOI] [PubMed] [Google Scholar]
- Simonson TS, and Powell FL. (2014). Less is more: Blunted responses to hypoxia revealed in sea-level Tibetans. J Appl Physiol (1985) 116:711–712 [DOI] [PubMed] [Google Scholar]
- Simonson TS, Wei G, Wagner HE, Wuren T, Bui A, Fine JM, Qin G, Beltrami FG, Yan M, Wagner PD, and Ge RL. (2014). Increased blood-oxygen binding affinity in Tibetan and Han Chinese residents at 4200 m. Exp Physiol 99:1624–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, Wei G, Wagner HE, Wuren T, Qin G, Yan M, Wagner PD, Ge RL. (2015). Low hemoglobin concentration in Tibetan males is associated with greater high-altitude exercise capacity. J Physiol. doi: 10.1113/JP270518 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Bai Z, Lorenzo FR, Xing J, Jorde LB, Prchal JT, and Ge RL. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329:72–75 [DOI] [PubMed] [Google Scholar]
- Song D, Li LS, Arsenault PR, Tan Q, Bigham AW, Heaton-Johnson KJ, Master SR, and Lee FS. (2014). Defective Tibetan PHD2 binding to p23 links high altitude adaption to altered oxygen sensing. J Biol Chem 289:14656–14665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stembridge M, Ainslie PN, Hughes MG, Stohr EJ, Cotter JD, Nio AQ, and Shave R. (2014). Ventricular structure, function, and mechanics at high altitude: chronic remodeling in Sherpa vs. short-term lowlander adaptation. J Appl Physiol (1985) 117:334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF. (2010). Evolution. Genes for high altitudes. Science 329:40–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J, Runck AM, Sabatino SJ, Kelly JK, Ferrand N, Moriyama H, Weber RE, and Fago A. (2009). Evolutionary and functional insights into the mechanism underlying high altitude adaptation of deer mouse hemoglobin. Proc of the Natl Acad of Sci USA 106:14450–14455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz JF, Runck AM, Moriyama H, Weber RE, and Fago A. (2010). Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol 213:2565–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SF, Droma TS, Zhang JG, Tao JX, Huang SY, McCullough RG, McCullough RE, Reeves CS, Reeves JT, and Moore LG. (1990). Greater maximal O2 uptakes and vital capacities in Tibetan than Han residents of Lhasa. Respir Physiol 79:151–161 [DOI] [PubMed] [Google Scholar]
- Tashi T, Feng T, Koul P, Amaru R, Hussey D, Lorenzo FR, Rili G, and Prchal JT. (2014). High altitude genetic adaptation in Tibetans: No role of increased hemoglobin-oxygen affinity. Blood Cells Mol Dis 53:27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy V, and Gupta R. (2005). Birth weight among Tibetans at different altitudes in India: Are Tibetans better protected from IUGR? Am J Hum Biol 17:442–450 [DOI] [PubMed] [Google Scholar]
- Tufts DM, Natarajan C, Revsbech IG, Projecto-Garcia J, Hoffmann FG, Weber RE, Fago A, Moriyama H, and Storz JF. (2015). Epistasis constrains mutational pathways of hemoglobin adaptation in high-altitude pikas. Mol Bio Evol 32:287–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udpa N, Ronen R, Zhou D, Liang J, Stobdan T, Appenzeller O, Yin Y, Du Y, Guo L, Cao R, Guo G, Claydon VE, Hainsworth R, Gamboa JL, Zibeginus M, Zenebe G, Xue J, Liu S, Frazer KA, Li Y, Bafna V, and Haddad GG. (2014). Whole genome sequencing of Ethiopian highlanders reveals conserved hypoxia tolerance genes. Genome Biol 15:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte FC, Macarlupu JL, Anza-Ramirez C, Corrales-Melgar D, Vizcardo-Galindo G, Corante N, and Leon-Velarde F. (2014). Decreased plasma soluble erythropoietin receptor in high-altitude excessive erythrocytosis and chronic mountain sickness. J Appl Physiol (1985) 117:1356–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhang Y-B, Zhang F, Lin H, Wang X, Wan N, Ye Z, Weng H, Zhang L, Li X, Yan J, Wang P, Wu T, Cheng L, Wang J, Wang DM, Ma X, and Yu J. (2011). On the origin of Tibetans and their genetic basis in adapting high-altitude environments. PLoS One 6:e17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Fan RX, Zhai W, Liu F, Wang L, Zhong L, Wu H, Yang HC, Wu SF, Zhu CL, Li Y, Gao Y, Ge RL, Wu CI, and Zhang YP. (2014). Genetic convergence in the adaptation of dogs and humans to the high-altitude environment of the tibetan plateau. Genome Biol Evol 6:2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz CA, and Garruto RM. (2007). A comparative analysis of arterial oxygen saturation among Tibetans and Han born and raised at high altitude. High Alt Med Biol 8:13–26 [DOI] [PubMed] [Google Scholar]
- Wu T. (1990). [Changes in cardiac function at rest and during exercise in mountaineers at an extreme altitude]. Zhonghua Yi Xue Za Zhi 70:72–66, 6 [PubMed] [Google Scholar]
- Wu T, Wang X, Wei C, Cheng H, Wang X, Li Y, Ge D, Zhao H, Young P, Li G, and Wang Z. (2005). Hemoglobin levels in Qinghai-Tibet: Different effects of gender for Tibetans vs. Han. J Appl Physiol 98:598–604 [DOI] [PubMed] [Google Scholar]
- Wuren T, Simonson TS, Qin G, Xing J, Huff CD, Witherspoon DJ, Jorde LB, and Ge RL. (2014). Shared and unique signals of high-altitude adaptation in geographically distinct Tibetan populations. PLoS One 9: e88252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang K, Ouzhuluobu , Peng Y, Yang Z, Zhang X, Cui C, Zhang H, Li M, Zhang Y, Bianba , Gonggalanzi , Basang , Ciwangsangbu , Wu T, Chen H, Shi H, Qi X, and Su B. (2013). Identification of a Tibetan-specific mutation in the hypoxic gene EGLN1 and its contribution to high-altitude adaptation. Mol Biol Evol 30:1889–1898 [DOI] [PubMed] [Google Scholar]
- Xing J, Wuren T, Simonson TS, Watkins WS, Witherspoon DJ, Wu W, Qin G, Huff CD, Jorde LB, and Ge RL. (2013). Genomic analysis of natural selection and phenotypic variation in high-altitude mongolians. PLoS Genet 9:e1003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Li S, Yang Y, Tan J, Lou H, Jin W, Yang L, Pan X, Wang J, Shen Y, Wu B, Wang H, and Jin L. (2011). A genome-wide search for signals of high altitude adaptation in Tibetans. Mol Biol Evol 28:1003–1011 [DOI] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, et al. (2010a). Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329:75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Droma T, Norkyel KY, Acharya G, Zamudio JA, Niermeyer SN, and Moore LG. (1993). Protection from intrauterine growth retardation in Tibetans at high altitude. Am J Phys Anthropol 91:215–224 [DOI] [PubMed] [Google Scholar]
- Zhao M, Kong Q-P, Wang H-W, Peng M-S, Xie X-D, Wang W-Z, Jiayang , Duan J-G, Cai M-C, Zhao S-N, Cidanpingcuo , Tu YQ, Wu SF, Yao YG, Bandelt HJ, and Zhang YP. (2009). Mitochondrial genome evidence reveals successful Late Paleolithic settlement on the Tibetan Plateau. Proc Natl Acad Sci USA 106:21230–21235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, and Haddad GG. (2013). Genetic analysis of hypoxia tolerance and susceptibility in Drosophila and humans. Annu Rev Genomics Hum Genet 14:25–43 [DOI] [PubMed] [Google Scholar]
- Zhou D, Udpa N, Gersten M, Visk DW, Bashir A, Xue J, Frazer KA, Posakony JW, Subramaniam S, Bafna V, et al. (2011). Experimental selection of hypoxia-tolerant Drosophila melanogaster. Proc Natl Acad Sci USA 108:2349–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Udpa N, Ronen R, Stobdan T, Liang J, Appenzeller O, Zhao HW, Yin Y, Du Y, Guo L, Cao R, Wang Y, Jin X, Huang C, Jia W, Cao D, Guo G, Gamboa JL, Villafuerte F, Callacondo D, Xue J, Liu S, Frazer KA, Li Y, Bafna V, and Haddad GG. (2013). Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am J Hum Genet 93:452–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Droma T, Sun S, Janes C, McCullough RE, McCullough RG, Cymerman A, Huang SY, Reeves JT, and Moore LG. (1993). Hypoxic ventilatory responsiveness in Tibetan compared with Han residents of 3,658 m. J Appl Physiol (1985) 74:303–311 [DOI] [PubMed] [Google Scholar]