Abstract
This study aimed to investigate the potential effect of interleukin 33 (IL-33) on humoral responses to hepatitis B virus (HBV) and the possible mechanisms underlying the action of IL-33 in regulating follicular helper T (TFH) cells. The impact of IL-33 treatment on the levels of serum HBV DNA, HBsAg, HBeAg, HBsAb, and HBeAb, as well as the frequencies of CD4+CXCR5+ TFH cells in wild-type HBV transgenic (HBV-Tg) mice and in a transwell coculture of HepG2.2.15 with IL-33-treated peripheral blood mononuclear cells (PBMCs) were determined. Furthermore, the gene transcription profiles in IL-33-treated TFH cells were determined by microarrays. IL-33 treatment significantly reduced the levels of serum HBV DNA, HBsAg, and HBeAg, but increased the levels of HBsAb and HBeAb in HBV-Tg mice, accompanied by increased frequency of splenic infiltrating CD4+CXCR5+ TFH cells in HBV-Tg. Similarly, coculture of HepG2.2.15 cells with IL-33-treated PBMCs reduced the levels of HBV DNA, HBsAg, and HBeAg, but increased the levels of HBsAb and HBeAb. Microarray analyses indicated that IL-33 significantly modulated the transcription of many genes involved in regulating TFH activation and differentiation. Our findings suggest that IL-33 may activate TFH cells, promoting humoral responses to HBV during the pathogenic process.
Introduction
Hepatitis B virus (HBV) is a major cause of acute and chronic hepatitis in humans. About 350 million people are chronically infected with HBV and are at a high risk of developing liver cirrhosis and hepatocellular carcinoma in the world (Yin and others 2011). The natural course of chronic HBV (CHB) infection is characterized by a period of immune tolerance, at which stage the virus coexists with the host without apparent injury to the host. As a result, CHB patients at the immune tolerance stage display high levels of HBsAg, HBeAg, and HBV DNA, but not HBeAb, accompanied by abnormal levels of serum alanine aminotransferase. More importantly, patients with CHB also show deficient cytotoxic T lymphocyte responses to HBsAg and HBcAg (Kang and others 2006; Morrey and others 2011). Thus, immune tolerance is a major factor underlying the maintenance of CHB status, and understanding the pathogenic process of CHB is of significance in the management of patients with CHB.
Engagement of T cell receptor (TCR) on naïve CD4+ T cells by the antigen determinant presented by antigen-presenting cells can activate CD4+ T cells, which can differentiate into different subsets of CD4+ helper T cells, such as Th1, Th2, Th17, follicular helper T (TFH), and others, depending on the expression of the lineage-specific transcription factor and cytokine environment (Ramiscal and Vinuesa 2013). TFH cells express a unique combination of surface markers and effector molecules, including chemokine receptor CXCR5 and inducible costimulator (ICOS), program death-1 (PD-1), and interleukin 21 (IL-21), which are critical for their development and function (Ramiscal and Vinuesa 2013). TFH cells positively regulate the germinal center formation, B cell differentiation, and humoral responses (Laurent and others 2010). Previous studies have shown that viral persistence and prolonged TCR stimulation progressively favor CD4+ T cell differentiation toward TFH cells during the pathogenic process of CHB (Forster and others 1996; Fahey and others 2011). Our previous studies have shown a high frequency of ICOS- and PD-1-expressing CD4+CXCR5+ TFH cells in CHB patients, particularly in CHB patients at immune active (IA), and the percentages of TFH cells were positively associated with the concentrations of serum aspartate aminotransferase (AST) in IA patients (Feng and others 2011). However, little is known about which cytokine can regulate TFH cell development during the pathogenesis of CHB.
IL-33 is a member of the IL-1 family and secreted by many types of cells, including endothelial, epithelial, dendritic, and mast cells, as well as macrophages. Interaction of IL-33 with its receptor ST2 promotes basophils, mast, macrophages, and Th2 cells to produce Th2-related cytokines, such as IL-5 and IL-13 (Xu and others 1998; Milovanovic and others 2012). Interestingly, a recent study has shown that IL-33 can induce B1 cell proliferation, type II cytokine synthesis, and IgM production in vitro and in vivo (Komai-Koma and others 2011). Several lines of evidence have demonstrated that IL-33 acts as an alarm to recruit immunocompetent cells, leading to hepatocytotoxicity and liver tissue injury (Arshad and others 2012). Indeed, significantly higher concentrations of serum IL-33 and sST2 are detected in patients with CHB and chronic HCV (CHC) infection related to that in healthy controls (Cacopardo and others 2012; Wang and others 2012a, 2012b), and treatment with antiviral therapies significantly reduces the levels of serum IL-33 in both CHB and CHC patients (Cacopardo and others 2012; Wang and others 2012a, 2012b). These findings suggest that IL-33 may be a pathogenic factor contributing to virus-related liver injury (Cacopardo and others 2012; Wang and others 2012a, 2012b). However, the precise role of IL-33 in the HBV replication and HBV-related humoral responses has not been clarified.
In this study, we tested the hypothesis that IL-33, through enhancing TFH activation, regulates humoral responses to HBV during the pathogenesis of CHB. We first tested the effect of treatment with IL-33 on HBV loads and HBV-related humoral responses, as well as the frequency of CD4+CXCR5+ TFH cells in HBV-Tg mice. Furthermore, we tested the impact of IL-33 on the HBV-induced humoral responses in vitro in a transwell coculture of HepG2.2.15 cells with peripheral blood mononuclear cells (PBMCs) from healthy controls. Finally, we employed microarrays to characterize the gene transcription profiles in TFH cells. Our findings indicated that IL-33 activated humoral immunity against HBV in vivo and in vitro by activating CD4+CXCR5+ TFH cells.
Materials and Methods
Preparation for IL-33
Murine rIL-33 proteins were produced and kindly provided by Professor Damo Xu (Division of Immunology, Infection and Inflammation, Faculty of Medicine, Glasgow Biomedical Research Centre, University of Glasgow, Glasgow, the United Kingdom), as described previously (Xu and others 2008; Komai-Koma and others 2011). The levels of endotoxin in the prepared recombinant proteins were determined by the Limulus amebocyte lysate QCL-1000 pyrogen assay, according to the manufacturers' instruction (Lonza, Basel, Switzerland). The prepared rIL-33 containing <0.1 EU/μg protein was used for the following experiments.
Mice
Wild-type (WT) C57BL/6 and HBV transgenic mice C57BL/6J-TgN (AlblHBV) 44Bri (namely HBV-Tg mice; the Jackson Laboratory, Bar Harbor, ME) were purchased from Vital River Laboratories (Beijing, China). The HBV-Tg mice mimic the pathogenesis of CHB infection in humans and display high levels of serum HBV DNA and all forms of HBsAg particles. All mice were housed in a specific pathogen-free facility at the Jilin University and were allowed free access to food and water. The experimental protocols for animal studies were established in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health. The experimental protocols were approved by the Animal Research and Protection Committee of Jilin University (Changchun, China).
To investigate the role of IL-33 in vivo, HBV-Tg mice were randomly treated with vehicle phosphate buffer saline (PBS) or intraperitoneally (i.p.) with IL-33 (2 μg/mouse) twice per week for 4 weeks, respectively (n=6 per group). Three days after the last treatment, the mice were sacrificed and their blood samples were collected for characterizing the levels of HBV DNA and HBV-related antigens and antibodies. Their spleens were collected. After stopping the bleeding, the mice were subjected to perfusion with 25 mL of Hank's balanced salt solution within 10 min. Subsequently, their livers were dissected out and the hepatic mononuclear cells (HMNCs) were prepared by Percoll gradient centrifugation, as described previously (Feng and others 2011). Briefly, the liver tissue samples were meshed through a 200-gauge stainless steel filter; after being washed, the cells passing through the filter were centrifuged and resuspended in 40% Percoll (Pharmacia, Uppsala, Switzerland). The cell suspension was overlaid gently on the top of 70% Percoll and centrifuged at 1,500 g for 30 min at room temperature. HMNCs were obtained from the interphase and washed twice with PBS. The containing erythrocytes were removed using lysis solution (BD Biosciences, San Jose, CA).
Immunohistochemistry analysis of HBV-Tg mice
The liver tissue samples were fixed with formalin and paraffin embedded. The liver tissue sections (4 μm) were stained with antibodies against HBsAg, HBeAg, or anti-mouse IgG for detecting HBeAb (Shanghai Caiyou, China). The bound antibodies were detected by HRP-conjugated second antibodies or by using a Histostain-Plus Kit (Beijing 4A Biotech, Beijing, China) and visualized by 3,3-diaminobenzidine (Sigma-Aldrich, St. Louis, MO), followed by counterstaining with Mayer's hematoxylin or methyl green.
Flow cytometry
The frequency of CD4+CXCR5+ TFH cells in the mouse spleen and HMNCs was determined by flow cytometry, as previously described (Yin and others 2011). Briefly, splenic or HMNCs at 106/tube were stained in duplicate with PerCP Cy5.5-anti-CD3 (BD Pharmingen, San Diego, CA), FITC-anti-CD4 (eBioscience, San Diego, CA), and PE-anti-CXCR5 (R&D Systems, Minneapolis, MN) at room temperature for 30 min, respectively. After being washed with PBS, the cells were gated on the forward scatter of living cells and then centered on CD3+ T cells. The frequency of CD4+CXCR5+ TFH cells was determined by flow cytometric analysis on the FACSCalibur (BD Biosciences, San Jose, CA) using FlowJo software analysis (v5.7.2; Tree Star, Ashland, OR). At least 50,000 events per sample were analyzed.
Recombinant IL-33 treatment of PBMCs
Venous blood samples were obtained from 3 healthy subjects who had no history of and current diabetes mellitus, hypertension, hyperlipidemia, cardiovascular diseases, chronic systemic disorders, or infectious disease, but had received regular hepatitis B vaccines. Their PBMCs were isolated by Ficoll-Hypaque gradient centrifugation for 30 min. The PBMCs at 1.0×106 cells/mL were treated in duplicate with 1 or 100 ng/mL of rIL-33 in the RPMI 1640 medium containing 5% heat-inactivated fetal bovine serum (FBS) in 24-well round bottom plates at 37°C 5% CO2 for 72 h.
RNA isolation and microarray hybridization
To analyze the gene expression profiling of TFH cells following IL-33 stimulation in vitro, CD4+CXCR5+ TFH cells were purified by FACS on an Aria cell sorting system. Briefly, after treatment with rIL-33 for 72 h, the suspended cells were harvested and stained with FITC-anti-CD4 and PE-anti-CXCR5 on ice. The CD4+CXCR5+ TFH cells were purified by FACS. Subsequently, total RNA was extracted from the purified THF cells using TRIzol (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions (Wakashin and others 2009).
The mRNA transcription profiles were determined by cRNA-based microarray by the Shanghai Biotechnology (Shanghai, China). Briefly, the RNA samples (10 μg/each) were sent to examine the RNA expression profiles of the TFH cell with Illumina microarray analysis (Illumina, San Diego, CA). The RNA samples (10 μg/each) were purified using the RNeasy kit (Qiagen, Valencia, CA) and used for the generation of biotinylated cRNA using the Illumina RNA Amplification Kit (Ambion, Austin, TX), according to the manufacturer's instructions. Individual biotinylated cRNA samples (∼100 ng) were hybridized to human whole-genome expression BeadChip human-6 V3 Plus 2.0 GeneChip microarrays (Illumina, San Diego, CA) and scanned, according to the Illumina BeadStation 500× manual. The quality and reproducibility of the microarray were controlled by the high levels of bead-type redundancy (up to an average of 30 beads per probe) on each array. The labeled cRNA samples were detected by hybridization to 50-mer probes on the Beadchip. After being washed and stained, the BeadChips were scanned on a HiScan™SQ, IScan, or BeadArray™ Reader. The Illumina data were extracted using software provided by the manufacturer (Wilcoxon statistic, 2-fold ratios, 0% false discovery rate). The potential functions of differentially expressing genes were analyzed using Illumina's GenomeStudio Gene Expression Module.
Bioinformatic analysis
The gene expression profiles were compared among TFH cells treated with the mock control (PBS) and rIL-33. The changes in the levels of mRNA transcripts were expressed as the fold change in values between treated cells and controls. The IL-33-induced differentially expressing genes were identified if a relative level of mRNA transcripts in the treated cells to the control was >1.5 (upregulated) or <0.75 (downregulated). All differentially expressing genes were analyzed by Gene Ontology to annotate genes and to classify their function, biological process, and cellular components.
Culture of HepG2.2.15 cells
HepG2.2.15 cells were from the China Center for Type Culture Collection (CCTCC, Wuhan, China) and maintained in 10% FBS RPMI-1640 medium containing 200 μg/mL of G418 at 37°C in a humidified atmosphere of 5% CO2. The cells were passaged every 3 days until HBV DNA was stably detected in the supernatants. HepG2.2.15 cells (2×104 cells/well) at the bottom chambers of 24-well transwell plates (BD Biosciences, Franklin Lakes, NJ) were cocultured in duplicate with PBMCs (2×105/well) from healthy subjects at the top chambers in the presence or absence of vehicle PBS, 1 or 100 ng/mL of rIL-33, and anti-IL-21 (5 μg/mL) for 1, 3, and 6 days. Subsequently, their supernatants were harvested for determining the concentrations of HBV DNA, HBV-related antigens, and antibodies.
Detection of HBV DNA and HBV-related antigens and antibodies
The concentrations of HBV DNA were examined in the serum samples from HBV-Tg mice, and the supernatants of HepG2.2.15 cells cocultured with human PBMCs were quantified by luciferase assays using the luciferase quantization detection kit (Roche Amplicor, Basel, Switzerland), according to the manufacturers' instruction (Jiang and others 2010). The limitation of detection for HBV DNA is 300 copies/mL. The levels of HBsAg, HBeAg, HBsAb, and HBeAb were determined by a chemiluminescent microparticle immunoassay (CMIA) using an Abbott I 2000 automated chemiluminescence immunoassay analyzer (Abbott Laboratories, Abbott Park, IL), according to a previous report (Feng and others 2011).
Enzyme-linked immunosorbent assay
The concentrations of anti-HBs and anti-HBe IgG and IgM in the supernatants of cocultured HepG2.2.1.5 and PBMCs were determined by enzyme-linked immunosorbent assay (ELISA) using specific kits (Cygnus Technologies, Southport, NC), according to the manufacturers' instruction.
Statistical analysis
Data are expressed as median and range unless specified. The difference between the 2 groups was analyzed by the Mann–Whitney U nonparametric test, using the SPSS version 18.0 software. The relationship between the 2 variables was evaluated using the Pearson rank correlation test. A 2-sided P value of <0.05 was considered statistically significant.
Results
IL-33 enhances HBsAb and HBeAb responses in HBV-Tg mice
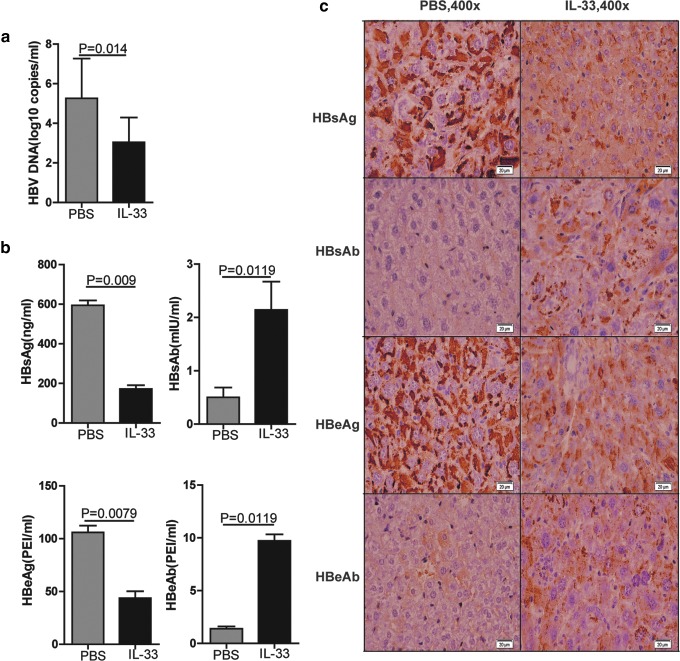
To elucidate the potential role of IL-33 in regulating HBV-specific humoral responses during the pathogenic process of CHB in vivo, HBV-Tg mice were treated i.p. with IL-33 or PBS twice a week for 4 weeks. The levels of serum HBV DNA and HBV-related antigens and antibodies were measured. As shown in Fig. 1, the levels of serum HBV DNA, HBsAg, and HBeAg in the IL-33-treated mice were significantly lower than that in the PBS-injected mice (P=0.014, P=0.009, P=0.0079, respectively, Fig. 1a, b). In contrast, the levels of serum HBsAb and HBeAb in the IL-33-treated mice were significantly higher than that in the PBS-injected mice (P=0.0119). In parallel, similar patterns of HBsAg, HBeAg, HBsAb, and HbeAb were detected in the liver of the IL-33-treated and PBS-injected mice (Fig. 1c). These data clearly demonstrated that treatment with IL-33 significantly reduced HBV virus loads and HBV-related antigens, but enhanced anti-HBV humoral responses in HBV-Tg mice. Given that HBeAg-specific B cells are tolerant in HBV-Tg mice (Akbar and Onji 1998), the elevated levels of HBeAb by IL-33 treatment suggest that IL-33 treatment may break immune tolerance and activate B cell responses in HBV-Tg mice.
FIG. 1.
IL-33 treatment inhibits HBV replication and promotes HBV antibody production in HBV-Tg mice. HBV-Tg mice were injected intraperitoneally with PBS (100 μL/mouse) and IL-33 (2 μg/mouse) twice per week for 4 weeks, respectively. The viral loads, expression of HBV antigens, and production of antibodies were examined. (a) The viral loads of serum HBV DNA examined by qPCR in HBV-Tg mice. (b) The concentrations of serum HBeAg, HBeAb, HBsAg, and HBsAb in HBV-Tg mice. (c) Representative images of HBeAg, HBeAb, HBsAg, and HBsAb staining detected by histological study in liver sections from HBV-Tg mice. The statistical significance of the data was determined using the Mann–Whitney test; P value is shown in each test. HBV, hepatitis B virus; IL-33, interleukin 33; PBS, phosphate buffer saline; qPCR, real-time quantitative PCR detecting system.
IL-33 increases the frequency of splenic infiltrating CD4+CXCR5+ TFH cells in HBV-tg mice
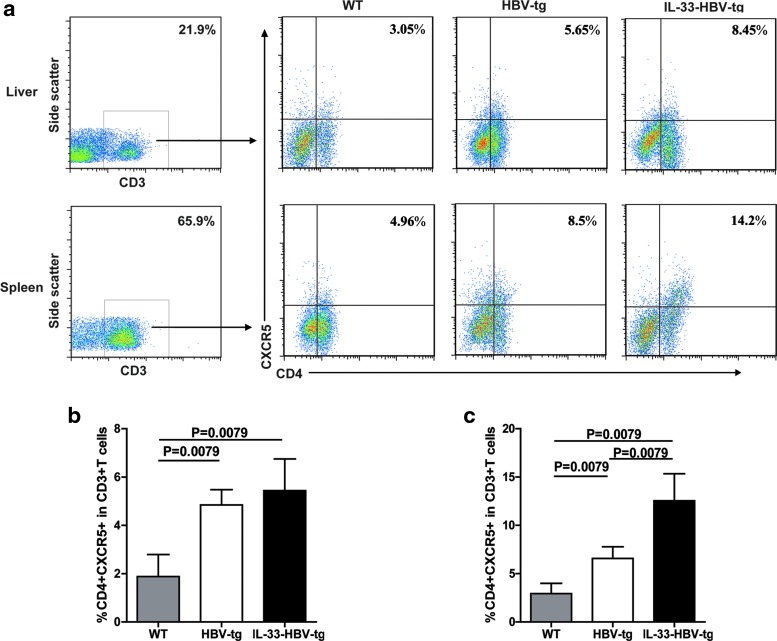
TFH cells are crucial for regulating humoral responses. To understand the effect of IL-33 on enhancing anti-HBV responses in HBV-Tg mice, we tested the impact of IL-33 treatment on the frequency of circulating CD4+CXCR5+ TFH cells in vivo. HBV-Tg mice were randomly treated i.p. with vehicle PBS or IL-33 (2 μg/mouse) twice per week for 4 weeks, respectively. Three days after the last treatment, the frequency of CD4+CXCR5+ TFH cells in CD3+ T cells in the spleens and hepatic infiltrates was determined by flow cytometry analysis (Fig. 2a). As shown in Fig. 2b and c, the numbers of splenic and liver CD4+CXCR5+ TFH cells in PBS-treated HBV-tg mice were significantly greater than WT mice (P=0.0079). The numbers of splenic CD4+CXCR5+ TFH cells in the IL-33-treated HBV-Tg were significantly higher than that in the PBS-treated HBV-Tg (P=0.0079, Fig. 2c), but there was no significant difference in the numbers of liver CD4+CXCR5+ TFH infiltrates between IL-33-treated and PBS-treated HBV-Tg. Therefore, IL-33 treatment increased the frequency of splenic TFH cells in HBV-Tg.
FIG. 2.
FACS analysis of TFH cells in HBV-Tg mice treated with PBS and IL-33, respectively. (a) Splenic and hepatic mononuclear cells from the WT and HBV-Tg mice with different treatments were stained in duplicate with anti-CD3, anti-CD4, and anti-CXCR5, respectively. The cells were gated initially on lymphocytes, then on CD3+ T cells (left), and then on CD4+CXCR5+ T cells (right). Subsequently, the frequency of CD4+CXCR5+ T cells was analyzed in the liver (b) and spleen (c) of the 2 groups by flow cytometry. At least 50,000 events were analyzed for each sample and data are representatives of different groups of samples from at least 2 independent experiments. The statistical significance of the data was determined using the Mann–Whitney test; P value is shown in each test. TFH, follicular helper T; WT, wild-type.
IL-33 inhibits HBV replication in HepG2.2.15 and promotes humoral response in vitro
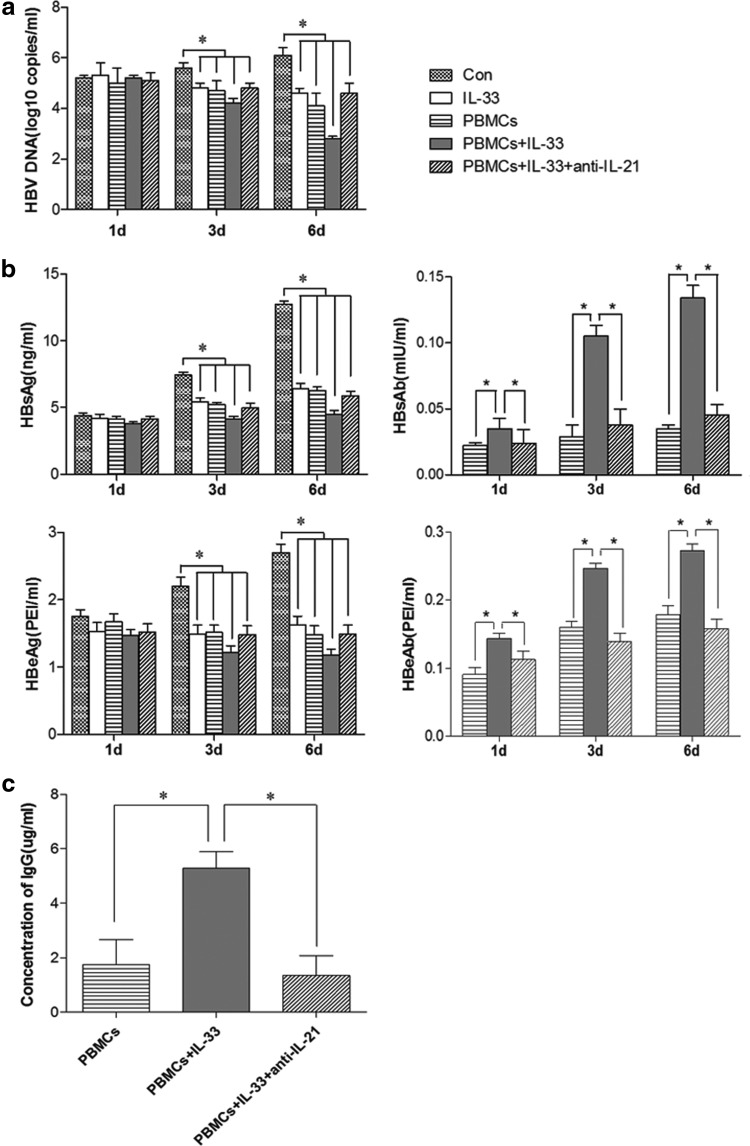
HBV constitutively replicates in HepG2.2.15 cells that secrete HBsAg and HBeAg. To further investigate the effect of IL-33 on the HBV replication and HBV-specific humoral responses, PBMCs were isolated from healthy subjects and treated with IL-33 in vitro, followed by coculture with HepG2.2.15 cells for 1, 3, and 6 days in a transwell culture system in the presence or absence of IL-33 and anti-IL-21. Additional HepG2.2.15 cells were treated with IL-33 alone in the absence of PBMCs to test the direct effect of IL-33 on the HBV replication in HepG2.2.15 cells. The levels of HBV DNA, HBsAg, HBeAg, HBsAb, and HBeAb in the supernatants were measured. As shown in Fig. 3, the levels of HBV DNA, HBsAg, and HBeAg in the supernatants were significantly reduced in the IL-33+PBMCs group compared with that in other groups. In contrast, the levels of anti-HBs IgG and anti-HBe IgG obviously increased in the IL-33+PBMCs group compared with that in other groups. However, the significantly decreased HBV replication and enhanced ant-HBV humoral responses by IL-33 and PBMCs were almost completely abrogated by treatment with anti-IL-21 (Fig. 3). The levels of HBsAb and HBeAb in the IL-33+PBMCs group were generally higher than that in the PBMCs group and anti-IL-21 group (Fig. 3a, b), indicating an effect of IL-33 on promoting B cell activation. Moreover, treatment with IL-33 alone significantly reduced the levels of HBV DNA, HBeAg, and HBeAg, which represented HBV replication in HepG2.2.1.5 cells. Collectively, these data suggested that IL-33 and PBMCs, like through activating TFH cells, synergistically inhibited HBV replication in HepG2.2.15 cells and enhanced humoral responses in vitro.
FIG. 3.
Viral loads, HBV antigens, and antibodies in coculture of HepG2.2.15 with IL-33-stimulated PBMCs. HepG2.2.15 cells were cocultured alone, treated with IL-33 (1 ng/mL), cocultured with PBMCs, IL-33, and PBMCs or IL-33/PBMCs/anti-IL-21, respectively. The levels of HBV DNA, HBsAg, HBeAg, HBsAb, and HBeAb, as well as anti-HBs and anti-HBe IgG and IgM were detected. (a) Viral loads of HBV DNA were determined in the medium using qPCR on days 1, 3, or 6 post coculture. (b) Concentrations of HBsAg, HBsAb, HBeAg, and HBeAb in the medium were detected using ELISA. (c) The concentration of IgG in cell culture supernatant. There was no detectable anti-HBsAb and anti-HBeAb as well as anti-HBs and anti-HBe IgG and IgM in the supernatants of cultured HepG2.2.15 cells and IL-33-treated HepG2.2.15 cells (data not shown). The statistical significance of the data was determined using the Mann–Whitney U nonparametric test. *p<0.05 vs. the Control. ELISA, enzyme-linked immunosorbent assay; PBMCs, peripheral blood mononuclear cells.
IL-33 treatment upregulates the transcription of genes associated with TFH activation in PBMCs
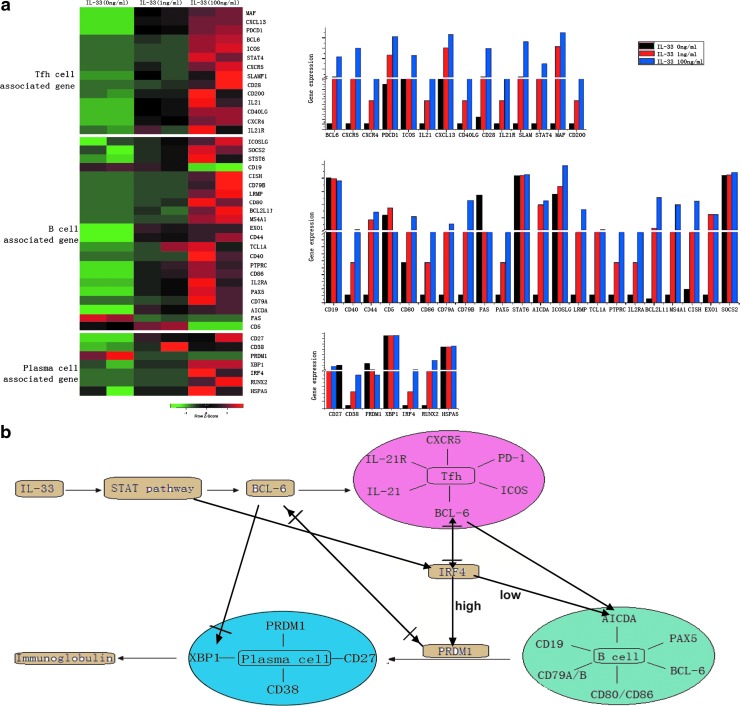
To elucidate the potential molecular mechanisms underlying the action of IL-33, PBMCs were isolated from individual healthy subjects and treated with, or without IL-33, for 72 h. The CD4+CXCR5+ TFH cells were purified by flow cytometric sorting, and the gene-expressing profiles of TFH cells were characterized by microarrays (Fig. 4a). Following stimulation with IL-33, there were 14 TFH-associated genes, including Bcl6, CXCR5, CXCR4, PDCD1, ICOS, IL-21, CXCL13, CD40LG, CD28, IL-21R, SLAM, STAT4, MAF, and CD200, which were upregulated in the IL-33-treated group compared with the IL-33 untreated controls, and the effects of IL-33 on upregulating the gene expression tended to be dose dependent (Fig. 4b).
FIG. 4.
RNA expression profiles of TFH cells in response to IL-33 stimulation. (a) RNA profiles were examined in TFH cells with PBS treatment and IL-33 stimulation, respectively. The TFH cell-associated genes were examined with Illumina microarray analysis. The differentially expressed genes in response to stimulation were determined with a SAM supervised analysis (Wilcoxon statistic, 2-fold ratio, 0% false discovery rate). The normalized expression level of each gene is represented by the color in the heat map: red represents high expression, while green represents low expression. Columns (right) represent normalized expression levels. (b) A postulated signal pathway of immune responses following IL-33 stimulation. SAM, significance analysis of microarrays.
Discussions
The IL-33/ST2 axis plays dual roles in immune responses. It can promote both Th1 and Th2 immune responses depending on the type of activated cell, microenvironment, and cytokine network in various diseases (Wakashin and others 2009; Alves-Filho and others 2010; Miller and others 2010; Mok and others 2010; Sammicheli and others 2011). The IL-33/ST2 axis has been show to play a protective role in Con A hepatitis, an acute liver injury, through prevention of hepatocytes from apoptosis and Th2 amplification (Volarevic and others 2012). In this study, we showed that IL-33 could effectively break immune tolerance and induce a specific adaptive immune response against HBV in HBV-Tg mice with low serum levels of HBeAg.
The expression of IL-33 was shown to be augmented in CHB and associated with virus-related liver injury (Wang and others 2012a). Therefore, we investigated the effects of IL-33 treatment on the HBV-Tg mice, which mimic CHB infection in humans. Treatment with IL-33 for 4 weeks significantly reduced HBV viral loads and the levels of serum HBeAg and HBsAg, but increased the levels of serum HBeAb and HBsAb in the HBV-Tg mice. The antiviral effects were confirmed with in situ staining, which showed a similar expression pattern of the relative antigens and antibodies in the liver tissue. Furthermore, when hepatic cells with stable HBV replication and protein expression were cocultured with PBMCs stimulated with IL-33, a dose- and time-dependent suppression of viral replication and seroconversion of relative antigens/antibodies were detected. As a combination of HBeAg seroconversion and complete suppression of HBV replication represents the favorable endpoint of clinical therapy for CHB (Wiegand and others 2010), our results suggested that IL-33 could be considered as a potential treatment for chronic HBV infection.
The induction of HBV antibody indicated that humoral immunity was activated by IL-33 stimulation. Our observations were consistent with the previous study showing that IL-33 activated B1 cell proliferation and enhanced cytokine production in vitro and in vivo in an ST2-dependent manner (Komai-Koma and others 2011). Since TFH cells play a crucial role in helping the B cells, supporting the GC formation and facilitating the generation of long-lived plasma cells and memory B cells (Bentebibel and others 2011), we sought to examine the frequency of TFH in HBV-Tg mice with IL-33 treatment. Using flow cytometry, the proportion of CD4+CXCR5+ TFH cells in the spleen and liver of HBV-Tg mice significantly increased compared with that in WT mice. In HBV-Tg mice, the frequency of splenic CD4+CXCR5+ TFH cells was significantly higher in the IL-33-treated group than the PBS-treated group, however, there was no significant difference in the frequency of TFH cells in the liver between these 2 groups. These observations suggested that the activation of humoral immunity by IL-33 could be mediated by TFH, which may take place in the spleen. Alternatively, the lack of statistical significance in the frequency of liver TFH cells between these 2 groups may reflect poor capacity of the TFH cell to migrate into the inflammatory areas. Actually, the frequency of liver TFH was higher in the IL-33-treated mice than that in the untreated mice (P=0.0632), although there was no statistical significant difference. The lack of statistical significance in the frequency of liver TFH cells between these 2 groups may reflect a small sample size. In this study, we showed for the first time that IL-33 activated CD4+CXCR5+ TFH cells in the CHB model.
The activation of TFH cells further induces the expression of downstream cytokines and cell surface molecules and facilitates the generation of the B cells and plasma cells (Crotty 2011). Therefore, transcript expression profiles were compared among TFH cells with mock treatment and IL-33 stimulation. All TFH-associated genes were expressed at low levels in HBV-Tg mice, indicating low activities of the TFH cells in CHB. As expected, all TFH cell-associated genes are upregulated by IL-33 stimulation in PBMCs, indicating activation of TFH cells by IL-33 stimulation. A unique combination of effector molecules has been elucidated to be critical for the development and function of TFH cells, including high levels of the surface receptors ICOS, CD40 ligand (CD40L), PD-1 and CD84, the cytokine IL-21, the cytoplasmic adaptor protein SLAM, and the transcription factors, Bcl-6 and c-MAF (Crotty 2011; Nakayamada and others 2011). These molecules play critical roles in promoting the activation, differentiation, and survival of B cells and plasma cells. We are interested in further investigating the molecular mechanisms underlying the action of IL-33 in regulating TFH responses.
Our previous study showed that the CD4+CXCR5+ TFH cells expended in CHB patients (Jiang and others 2010). In general, TFH cells provide the required signals to B cells for germinal center reactions that are necessary for long-lived antibody responses. Therefore, a protective role for TFH cells is expected in CHB patients. However, a positive correlation between the frequencies of TFH cells and the concentrations of serum AST was identified in CHB patients, which further suggested that TFH cells were associated with HBV-related damages in the liver (Jiang and others 2010). A similar paradox is observed in HIV infection. Even though an expansion of TFH cells is shown in HIV-infected individuals, the cells are unable to provide adequate B cell help (Cubas and others 2013). It is shown that the heightened levels of activation and antigen overload in HIV infection leads to excessive and persistent triggering of PD-1 on TFH cells, which affects their capacity to provide adequate B cell help. In CHB infection, the TFH pathway may be impaired by the same mechanism. In this study, IL-33 treatment might provide an external stimulation to overcome the impairment and lead to TFH cell-mediated recovery of humoral immune responses.
Accordingly, we speculate an IL-33-based regulation of TFH cell activation in Fig. 4. IL-33 binds to its receptors and induces the STAT4 (but not STAT6) activation, enhancing Bcl6 expression, and promoting TFH differentiation. Subsequently, TFH cells interact with B cells through the CD40/CD40L, ICOS/ICOS-L, and CD28/CD86 to promote B cell activation and plasma cell differentiation. The process is regulated by a feedback mechanism involving IRF4. When the IRF4 expression level is upregulated by TFH differentiation, it activates the expression of the gene, PRDM1 (BLIMP1), a master regulator of plasma cells; when IRF4 expression is suppressed by Bcl-6, it activates AICDA, which is upregulated in B cells (Pilbrow etaland others 2012). A balanced regulation of IRF4 expression upon IL-33 stimulation can activate B cells and plasma cells. In addition, IL-21 produced by TFH cells could contribute to the maintenance of TFH cells at various stages. Comprehensively, IL-33 stimulates the differentiation of B cells and plasma cells through TFH cell mediation.
In general, our findings indicate that IL-33 suppressed HBV replication and the secretion of HBeAg. In addition, it induced the production of HBeAb and HBsAb. IL-33 treatment induced active humoral immunity against HBV in a mouse model through the recovery of CD4+CXCR5+ TFH cell functions. In conclusion, IL-33 could be further explored as a potential therapeutic target in the treatment of chronic HBV infection. However, further detailed studies are needed to elucidate the underlying mechanisms.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (No. 30972610 and 81273240), the National High Technology Research and Development Program of China (863 Program, No. 2011AA02A106), the Jilin Province Science and Technology Agency (No. 20110716), the Health Department Research Projects in Jilin Province (2009Z054), the Norman Bethune Program of Jilin University (2012206), and the Special Research Foundation of Jilin University (No. B03). The authors thank Medjaden Bioscience Limited for assisting in the preparation of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- Akbar SK, Onji M. 1998. Hepatitis B virus (HBV)-transgenic mice as an investigative tool to study immunopathology during HBV infection. Int J Exp Pathol 79(5):279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Filho JC, Sonego F, Souto FO, Freitas A, Verri WA, Jr., Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. 2010. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med 16(6):708–712 [DOI] [PubMed] [Google Scholar]
- Arshad MI, Piquet-Pellorce C, Samson M. 2012. IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int 32(8):1200–1210 [DOI] [PubMed] [Google Scholar]
- Bentebibel SE, Schmitt N, Banchereau J, Ueno H. 2011. Human tonsil B-cell lymphoma 6 (BCL6)-expressing CD4+ T-cell subset specialized for B-cell help outside germinal centers. Proc Natl Acad Sci U S A 108(33):E488–E497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacopardo B, Rita Pinzone M, Palermo F, Nunnari G. 2012. Changes in serum Interleukin-33 concentration before and after treatment with pegylated interferon alfa-2a plus ribavirin in patients with chronic hepatitis C genotype 1b infection. Hepat Mon 12(12):e7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. 2011. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29:621–663 [DOI] [PubMed] [Google Scholar]
- Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr., Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. 2013. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19(4):494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey LM, Wilson EB, Elsaesser H, Fistonich CD, McGavern DB, Brooks DG. 2011. Viral persistence redirects CD4 T cell differentiation toward T follicular helper cells. J Exp Med 208(5):987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Lu L, Hua C, Qin L, Zhao P, Wang J, Wang Y, Li W, Shi X, Jiang Y. 2011. High frequency of CD4+ CXCR5+ TFH cells in patients with immune-active chronic hepatitis B. PLoS One 6(7):e21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. 1996. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87(6):1037–1047 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ma Z, Xin G, Yan H, Li W, Xu H, Hao C, Niu J, Zhao P. 2010. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediators Inflamm 2010:143026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EH, Kown TY, Oh GT, Park WF, Park SI, Park SK, Lee YI. 2006. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antiviral Res 72(2):100–106 [DOI] [PubMed] [Google Scholar]
- Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, Liew FY. 2011. IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol 186(4):2584–2591 [DOI] [PubMed] [Google Scholar]
- Laurent C, Fazilleau N, Brousset P. 2010. A novel subset of T-helper cells: follicular T-helper cells and their markers. Haematologica 95(3):356–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Asquith DL, Hueber AJ, Anderson LA, Holmes WM, McKenzie AN, Xu D, Sattar N, McInnes IB, Liew FY. 2010. Interleukin-33 induces protective effects in adipose tissue inflammation during obesity in mice. Circ Res 107(5):650–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, Lukic ML. 2012. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res 52(1–2):89–99 [DOI] [PubMed] [Google Scholar]
- Mok MY, Huang FP, Ip WK, Lo Y, Wong FY, Chan EY, Lam KF, Xu D. 2010. Serum levels of IL-33 and soluble ST2 and their association with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 49(3):520–527 [DOI] [PubMed] [Google Scholar]
- Morrey JD, Motter NE, Chang S, Fairman J. 2011. Breaking B and T cell tolerance using cationic lipid—DNA complexes (CLDC) as a vaccine adjuvant with hepatitis B virus (HBV) surface antigen in transgenic mice expressing HBV. Antiviral Res 90(3):227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayamada S, Kanno Y, Takahashi H, Jankovic D, Lu KT, Johnson TA, Sun HW, Vahedi G, Hakim O, Handon R, Schwartzberg PL, Hager GL, O'Shea JJ. 2011. Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35(6):919–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilbrow AP, Folkersen L, Pearson JF, Brown CM, McNoe L, Wang NM, Sweet WE, Tang WH, Black MA, Troughton RW, Richards AM, Franco-Cereceda A, Gabrielsen A, Eriksson P, Moravec CS, Cameron VA. 2012. The chromosome 9p21.3 coronary heart disease risk allele is associated with altered gene expression in normal heart and vascular tissues. PLoS One 7(6):e39574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiscal RR, Vinuesa CG. 2013. T-cell subsets in the germinal center. Immunol Rev 252(1):146–155 [DOI] [PubMed] [Google Scholar]
- Sammicheli S, Dang VP, Ruffin N, Pham HT, Lantto R, Vivar N, Chiodi F, Rethi B. 2011. IL-7 promotes CD95-induced apoptosis in B cells via the IFN-gamma/STAT1 pathway. PLoS One 6(12):e28629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volarevic V, Mitrovic M, Milovanovic M, Zelen I, Nikolic I, Mitrovic S, Pejnovic N, Arsenijevic N, Lukic ML. 2012. Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J Hepatol 56(1):26–33 [DOI] [PubMed] [Google Scholar]
- Wakashin H, Hirose K, Iwamoto I, Nakajima H. 2009. Role of IL-23-Th17 cell axis in allergic airway inflammation. Int Arch Allergy Immunol 149 Suppl 1:108–112 [DOI] [PubMed] [Google Scholar]
- Wang J, Cai Y, Ji H, Feng J, Ayana DA, Niu J, Jiang Y. 2012a. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis B. J Interferon Cytokine Res 32(6):248–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhao P, Guo H, Sun X, Jiang Z, Xu L, Feng J, Niu J, Jiang Y. 2012b. Serum IL-33 levels are associated with liver damage in patients with chronic hepatitis C. Mediators Inflamm 2012:819636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand J, van Bommel F, Berg T. 2010. Management of chronic hepatitis B: status and challenges beyond treatment guidelines. Semin Liver Dis 30(4):361–377 [DOI] [PubMed] [Google Scholar]
- Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. 1998. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med 187(5):787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. 2008. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A 105(31):10913–10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wu C, Song J, Wang J, Zhang E, Liu H, Yang D, Chen X, Lu M, Xu Y. 2011. DNA immunization with fusion of CTLA-4 to hepatitis B virus (HBV) core protein enhanced Th2 type responses and cleared HBV with an accelerated kinetic. PLoS One 6(7):e22524. [DOI] [PMC free article] [PubMed] [Google Scholar]