Abstract
Fatty liver disease is a problem of growing clinical importance due to its association with the increasingly prevalent conditions of obesity and diabetes. While steatosis represents a reversible state of excess intrahepatic lipid, it is also associated with increased susceptibility to oxidative and cytokine stresses and progression to irreversible hepatic injury characterized by steatohepatitis, cirrhosis, and malignancy. Currently, the molecular mechanisms underlying progression of this dynamic disease remain poorly understood, particularly at the level of transcriptional regulation. We recently constructed a library of stable monoclonal green fluorescent protein (GFP) reporter cells that enable transcriptional regulation to be studied dynamically in living cells. Here, we adapt the reporter cells to create a model of steatosis that will allow investigation of transcriptional dynamics associated with the development of steatosis and the response to subsequent “second hit” stresses. The reporter model recapitulates many cellular features of the human disease, including fatty acid uptake, intracellular triglyceride accumulation, increased reactive oxygen species accumulation, decreased mitochondrial membrane potential, increased susceptibility to apoptotic cytokine stresses, and decreased proliferation. Finally, to demonstrate the utility of the reporter cells for studying transcriptional regulation, we compared the transcriptional dynamics of nuclear factor κB (NFκB), heat shock response element (HSE), and glucocorticoid response element (GRE) in response to their classical inducers under lean and fatty conditions and found that intracellular lipid accumulation was associated with dose-dependent impairment of NFκB and HSE but not GRE activation. Thus, steatotic reporter cells represent an efficient model for studying transcriptional responses and have the potential to provide important insights into the progression of fatty liver disease.
Keywords: steatosis, reporter cells, in vitro disease model
INTRODUCTION
Hepatic steatosis, or “fatty liver”, refers to the accumulation of triglycerides in hepatocytes. Originally, steatosis was attributed to excessive alcohol consumption, known as Alcoholic Fatty Liver Disease (AFLD). However, there is growing appreciation for fatty liver disease induced by a broad range of stimuli, including drugs, auto-immune diseases, diabetes, and obesity, referred to as Nonalcoholic Fatty Liver Disease (NAFLD) and nonalcoholic steatohepatitis (NASH). The prevalence of NAFLD has been estimated to be as high as 39% in some populations, and this is likely to grow due to the increasing number of obese individuals (Yu et al., 2002). Prior to progression to steatohepatitis, steatosis represents a reversible state of metabolic dysfunction. However, under certain circumstances that are not well-defined, steatosis progresses to inflammatory steatohepatitis, irreversible liver damage, fibrosis, cirrhosis, and even hepatocellular carcinoma (Angulo, 2002; Farrell and Larter, 2006; Reddy and Rao, 2006). As a result, the transition from steatosis to steatohepatitis is thought to be a central point of regulation in the development of progressive steatotic liver disease and its unfortunate clinical sequelae.
The most commonly accepted model explaining the development of fatty liver disease is the “two hit” hypothesis (Day and James, 1998). In this model, steatosis represents the “first hit,” and sensitizes cells to subsequent stresses, or “second hits,” which can take many forms including drugs, hypoxia, or cytokines, eventually leading to inflammation, or steatohepatitis. Animal models and human clinical studies have consistently demonstrated that the import and intracellular accumulation of extracellular free fatty acid (FFA) as lipid droplets is associated with cell signaling changes that increase susceptibility to cytokines, promote apoptotic or necrotic cell death, and impair compensatory proliferation (Bécuwe et al., 2003; Bianchi at al., 2002; Bianchi at al., 2004; Boden and Shulman, 2002; Cury-Boaventura and Curi, 2005; Finstad et al., 1994; Gómez-Lechón et al., 2007; Hennig at al., 1996; Malhi et al., 2006; Toborek et al., 1996; Toborek et al., 1997; Wang et al., 2006; Wu and Cederbaum, 2003). Nevertheless, the molecular details underlying these changes and their influence on stress response pathways remain unclear. Investigation of the links between intracellular lipid accumulation and stress response pathways are therefore critical for establishing a molecular description of the proposed “two hit” model and providing mechanistic insight into this complex dynamic disease.
Transcription factor networks represent a critical point of regulation and cellular decision-making. However, at present, there is no consensus on the transcriptional profiles that characterize steatosis and their response to “second hits.” This is, in part due, to the complex nature of the stresses, which are not only multifactorial but dynamic. For example, systemic inflammation is characterized by early transient release of TNF-α and IL-1 followed by delayed-onset sustained release of HMGB1 (Ulloa and Tracey, 2005). Numerous studies have demonstrated that lipid accumulation and cytokine stimulation have independent effects on NFκB activation (Cao et al., 1996; Gilmore and Herscovitch, 2006; Lo et al., 1999; Michiels et al., 2002; Novak et al., 2003; Ross et al., 1999; Song et al.; 2002; Zhao et al., 2004) yet the combined effects of these stimuli remain unclear. Other transcriptional regulators such as heat shock factors and ligand-bound glucocorticoid receptors remain relatively uncharacterized in the context of steatohepatitis. Understanding the interrelationships between metabolic state (intracellular triglyceride accumulation), environmental stress (cytokines and oxidative stress), transcriptional regulation, and state-dependent stress responses (survival, necrosis or apoptosis, and compensatory proliferation) represent important steps in understanding the progression from steatosis to steatohepatitis.
Several cell culture models of steatosis have been developed previously (Donato et al., 2006; Feldstein et al., 2003a; Feldstein et al., 2003b; Gómez-Lechón et al., 2007; Malhi et al., 2006). Studies by Malhi et al. with human and rat hepatoma cell lines, as well as mouse primary hepatocytes, clearly demonstrated that saturated FFAs were more cytotoxic than monounsaturated FFAs and provided a mechanistic insight into the cellular mechanisms of hepatocyte lipoapoptosis (Malhi et al., 2006). Gómez-Lechón et al. used the human hepatocyte-derived cell line HepG2 to study the differential effect of saturated versus unsaturated FFA (Gómez-Lechón et al., 2007). Donato et al. incubated primary human hepatocytes with long-chain FFA to investigate the potential effects of steatosis on drug-metabolizing capacity of hepatocytes. Their results indicated a down-regulation of several P450 enzymes involved in drug metabolism (Donato et al., 2006). However, while many studies have explored the regulation and effects of fat accumulation on metabolism (Bécuwe et al., 2003; Bianchi at al., 2002; Bianchi at al., 2004; Boden and Shulman, 2002; Cury-Boaventura and Curi, 2005; Hennig at al., 1996; Malhi et al., 2006; Toborek et al., 1996; Toborek et al., 1997; Wu and Cederbaum, 2003), comparatively little is known about how fat accumulation impacts stress responses, particularly at the level transcriptional regulation. Furthermore, most of these models use laborious, expensive, and destructive single-time-point measurement techniques and do not allow efficient or dynamic measurement of transcriptional activation.
We recently developed a microfluidic living cell array to non-destructively study gene expression in living cells. As part of this effort, we constructed GFP reporter cells that continuously report on levels of transcriptional activation and can be measured by microscopy or flow cytometry (King et al., 2007; King et al., 2008; Thomson et al., 2004; Weider et al., 2005). In this work, we adapt the reporter cells to create a model of hepatic steatosis to enable non-destructive transcriptional profiling of cytokine stress responses under lean and steatotic conditions, and to understand how the degree and composition of steatosis impact susceptibility to cytokine stress. In particular, we describe the responses of reporter cells for NFκB, HSE, and GRE to their classical inducers under lean and fatty conditions. Further, we used these reporter cell models to demonstrate the effect of increased intracellular triglyceride accumulation on the increased susceptibility to cytokine stresses, specifically, to tumor necrosis factor-α (TNF-α).
MATERIALS AND METHODS
Construction of Reporter Cell Lines
The Reuber H35 rat hepatoma is a well-differentiated rat hepatoma cell line that has been routinely used for in vitro cell culture (Pitot et al., 1964). Their responses to inflammatory cytokines have been extensively characterized (Itoh et al., 1989; Richards et al., 1996). These cells have been used to study stressor-specific activation of the heat shock genes in response to a variety of stimuli such as heat, arsenite ions, and cadmium ions (Ovelgonne et al., 1995). Reporter cells were constructed by transfecting H35 hepatoma cells with plasmid DNA encoding a fluoresecent GFP reporter protein under the transcriptional control of a single transcription factor response element. Details of the construction were described previously (King et al., 2007; Thomson et al., 2004; Weider et al., 2005). Briefly, response elements for each transcription factor of interest were selected and cloned upstream of a CMV minimal promoter and a destabilized enhanced green fluorescent protein (d2EGFP) to create each reporter plasmid. The reporter plasmids were also designed to express a drug resistance gene for drug selection. In this work, we focus on three transcription factors, NFκB, HSF1 (referred to as HSE), and GR (referred to as GRE) with κB, HSE, and GRE consensus sequences GGGAMTNYCC, CNNGAANNTTCNN, and AGAACANNNTGTTCT, which were used to create reporter response elements with sequences GGGAATTTCC, CTAGAATGTTCTAG, and AGAACAAAATGTTGT, respectively (King et al., 2007). Reporter plasmids were then introduced into H35 cells by electroporation, and cells with stable DNA integration were selected by G418. Stable transfectants were sorted for minimal background fluorescence and maximal responsiveness to classic inducers (TNF-α for NFκB, 2 hours at 42°C for HSE, and 1 mmol/l dexamethasone for GRE) using flow cytometry. Finally, sorted cells were then subcloned to obtain the stable monoclonal reporter cell lines. Non-transfected (NT) cells were used to measure cellular auto-fluorescence as well as viability and proliferation experiments.
Cell Culture
H35 cells were maintained in phenol red free DMEM (Gibco) supplemented with 10% fetal bovine serum and 100 units/mL penicillin - 100 µg/µL streptomycin in a humidified 5% CO2 incubator at 37°C. Experimental base medium was prepared by supplementing the maintenance medium with 2% bovine serum albumin (Sigma), and conjugating with 0 – 4 mmol/l of one of the two selected fatty acids, oleic acid (OA) or linoleic acid (LA) (Sigma). Oleic acid (an ω-9 FFA) and linoleic acid (an ω-6 FFA) were chosen because they represent different classes of fatty acids that are converted to a unique set of derivative molecules as described previously (Das, 2006). In particular, linoleic acid is known for its ability to be converted into arachadonic acid, a potentant modulator of inflammation. Meanwhile, oleic acid serves as a non-arachadonicacid-forming comparison (Das, 2006). After adding the fatty acids, the medium was sonicated at 40°C for 45 min and cooled to 4°C. Finally, insulin (0.5 U/ml) and glucagon (7 ng/ml) were added, and the medium was sterilized by passage through a 0.2 µm filter.
Cells were plated in 24-well plates (Costar), at a seeding density of approximately 1×105 cells per well in maintenance medium. After 24 h, the medium was changed to the experimental medium for up to 72 h. To investigate the effect of TNF-α, a prototypic inflammatory cytokine, on fat-laden cells, the cells were exposed to 10 ng/ml TNF-α (R&D Systems) for 18 h before the viability and flow cytometry studies.
Oil-Red-O Staining
The NT H35 cells were treated with varying doses of OA or LA for 72 h and fixed by incubating in 4% paraformaldehyde at room temperature for 10 min. After extensive washing with PBS, the intracellular triglyceride deposits were stained with Oil-Red-O dye for 12 h at room temperature. Cells were imaged using a Zeiss Axiovert 200 optical microscope under 200× magnification. Images were captured using an AxiocamMR color digital camera with Axiovision 4.5 image acquisition software.
Quantification of Intracellular Triglyceride Concentration
The NT H35 cells, treated with varying doses of OA or LA for 72 h, were lysed on ice for six 30 sec cycles of mechanical sonication followed by a 30 sec recovery period in between using a Branson Sonifier. The cell lysate was used to quantify the triglyceride content, using a triglyceride assay kit (Sigma), following the manufacturer’s protocol.
Measurement of Intracellular Reactive Oxygen Species (ROS)
2',7'-dichlorodihydrofluorescein diacetate, H2DCFDA, (Invitrogen) was used as a cell-permeant indicator for reactive oxygen species. This dye is nonfluorescent until the removal of the acetate groups by intracellular esterases and subsequent oxidation. We used a Spectra MAX Gemini XPS fluorescence microplate reader (Molecular Devices) to measure the fluorescence of the dye at 520 nm as a quantitative indication of intracellular ROS. Typically, the NT H35 cells were treated with 10 µM of H2DCFDA by incubating in PBS for 30 min at room temperature. The cells were then washed twice with PBS and cultured in the maintenance medium for 2 h. Subsequently, the medium was changed to the experimental medium with varying doses of OA or LA and the fluorescence was measured for up to 72 h.
Measurement of Mitochondrial Membrane Potential (MMP)
MMP was measured using 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolcarbocyanine iodide (JC-1), a cationic lipophilic dye that exhibits membrane potential-dependent aggregation in negatively charged mitochondria. The dye emits at 590 nm in the aggregated form, but emits at 527 nm in its monomeric form. Aggregation of JC-1 monomers causes its fluorescence emission maximum to shift from 527 to 590 nm. The ratio of red (590 nm) to green (527 nm) fluorescence provides a measure of the magnitude of MMP. A detailed experimental procedure is summarized elsewhere (Berthiaume et al., 2003).
Measurement of Proliferation and Viability
The NT H35 cells were treated with varying doses of OA or LA for 72 h. Trypan blue exclusion, was used to establish the viability of the cells, and this showed that cells floating in the medium atop the cultured cells were consistently found to be dead while those attached to the plate surface were consistently alive (data not shown). The dead cells were counted, to provide an indication of the viability of the cells after the treatment and the cytotoxicity of the OA or LA doses. Additionally, the cells attached to the cell culture plate surface were trypsinized and counted to provide an indication of the cell proliferation during the treatments.
Flow Cytometry
The NFκB and HSE reporter cells, were incubated with varying doses of OA or LA for 72 h, stimulated with 10 ng/ml TNF-α for 18 h, and cellular fluorescence was measured by flow cytometry. Similarly, the GRE reporter cells were stimulated with 4 mmol/l dexamethasone. The cells were then trypsinized and resuspended in the maintenance medium. The cells were immediately assayed for d2EGFP expression on a Becton-Dickinson FACS Calibur flow cytometer. The flow cytometry profiles were gated for EGFP-expressing cells based on unstimulated control samples.
Statistical Analysis
Statistical evaluation of the cell culture studies was performed with one way ANOVA and multiple comparisons least-square difference (LSD) post hoc analysis procedures. Values with p ≤ 0.05 were deemed significantly different. All results were performed at least in triplicate as specified in the figure legends. Results are reported as mean ± 95% confidence intervals.
RESULTS
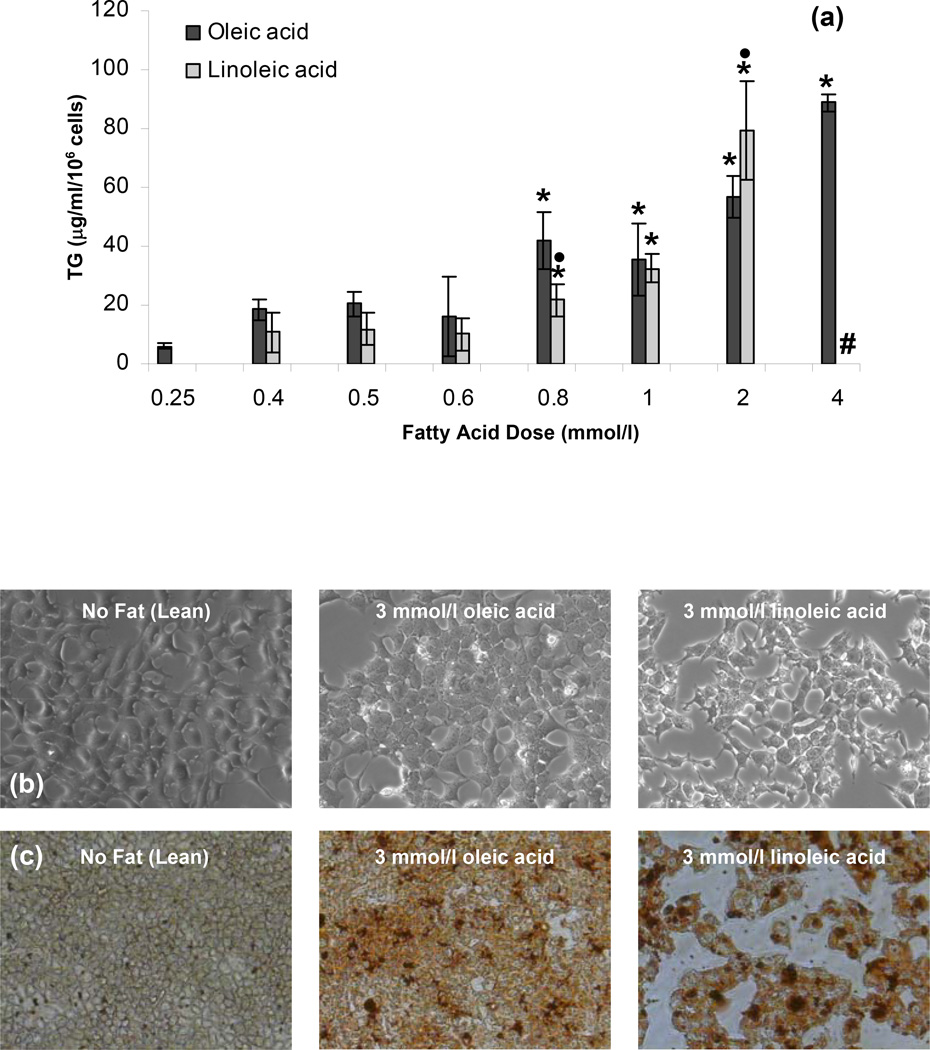
To create an in vitro model of steatosis, we cultured the NT H35 cells in medium with OA or LA. The degree of steatosis was evaluated by optical microscopy, Oil-Red-O staining, and intracellular triglyceride measurements. Figure 1a shows that the intracellular triglyceride content increased with increasing FFA concentration. We found this to be true for both OA as well as LA. These results demonstrated that the intracellular lipid content can be controlled by controlling the medium composition. Optical microscopy revealed that the lean NT H35 cells had a well-spread morphology with polygonal shapes. Compared to their lean counterparts, the H35 cells exposed to 3 mmol/l OA or LA showed partially spread morphology with evidence of intracellular lipid droplets (Fig. 1b). In order to obtain a qualitative representation of the induced steatosis, the NT H35 cells were stained with Oil-Red-O, a lipophilic dye. In general, Oil-Red-O staining increased with the amount of FFA supplemented in the medium and a similar level of staining was obtained for similar levels of the OA and LA. An example of the Oil-Red-O staining is shown in Fig. 1c, where 3 mmol/l of both LA and OA supplemented in the medium resulted in similar levels of Oil-Red-O staining.
Figure 1.
Intracellular TG accumulation in H35 cells as a function of fatty acid dose (a) measured by TG assay, (b) optical microscopy, and (c) Oil-Red-O staining. All measurements were performed after the H35 cells were exposed to different FFA doses for 72 h. Error bars represent 95% confidence intervals. * p ≤ 0.05 compared to values for 0.25 mmol/l fatty acid dose. • p ≤ 0.05 compared to values for same OA dose. # cells treated with 4 mmol/l LA did not survive over the 72 h culture period.
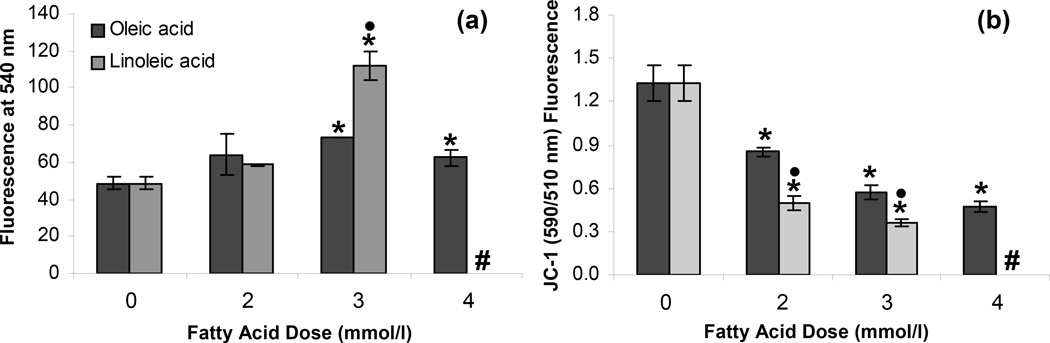
We next asked if our cell culture model of steatosis demonstrated the characteristic intracellular features of hepatic steatosis. Excess intracellular lipid accumulation is known to lead to the generation of intracellular reactive oxygen species (ROS) (Cury-Boaventura and Curi, 2005; Malhi et al., 2006). The oxidation of fatty acids represents an important source of ROS in vivo (Browning and Horton, 2004; Garcia-Ruiz et al., 1995; Hensley et al., 2000; Lieber, 2004; Mannaerts et al., 2000). Consequences of excessive intracellular ROS include depletion of nicotinaminde dinucleotide and ATP, DNA damage, alteration in protein stability, destruction of membranes via lipid peroxidation, and release of proinflammatory cytokines (Bergamini et al., 2004; Helling, 2006). These intracellular effects of ROS have been hypothesized to play an important role in the overall pathophysiology of NAFLD (Browning and Horton, 2004). Therefore, we examined the generation of ROS in the steatotic H35 cells. We used 2',7'-dichlorodihydrofluorescein diacetate to quantify intracellular ROS. Our results demonstrated that the amount of intracellular ROS increased with an increase in the amount of FFA supplemented in the medium (Fig. 2a). This increase was especially significant for FFA doses of greater than 2 mmol/l (p < 0.001). It was also noted that supplementation of 3 mmol/l LA led to a greater increase in the generation of ROS compared to an equivalent dose of OA (p < 0.001).
Figure 2.
Effect of steatosis on (a) generation of intracellular reactive oxygen species and (b) mitochondrial membrane potential. All measurements were performed after the H35 cells were exposed to different FFA doses for 72 h. Error bars represent 95% confidence intervals. * p ≤ 0.05 compared to values for 0 mmol/l FFA dose. • p ≤ 0.05 compared to values for same OA dose. # Cells treated with 4 mmol/l LA did not survive over the 72 h culture period.
Intracellular ROS and lipid peroxidation-induced oxidative stress have been shown to reduce the mitochondrial membrane potential (MMP). A reduced MMP could result in the release of caspsases and activation of apoptotic signaling pathways, and ultimately lead to cell death (Wu and Cederbaum, 2003). Figure 2b demonstrates that the MMP of NT H35 cells decreased when treated with varying doses of OA or LA for 72 h. Supplementation with LA led to a greater decrease in MMP compared to an equivalent dose of OA (p < 0.001), which may be attributed to a greater generation of ROS in the case of LA supplementation (Fig. 2a).
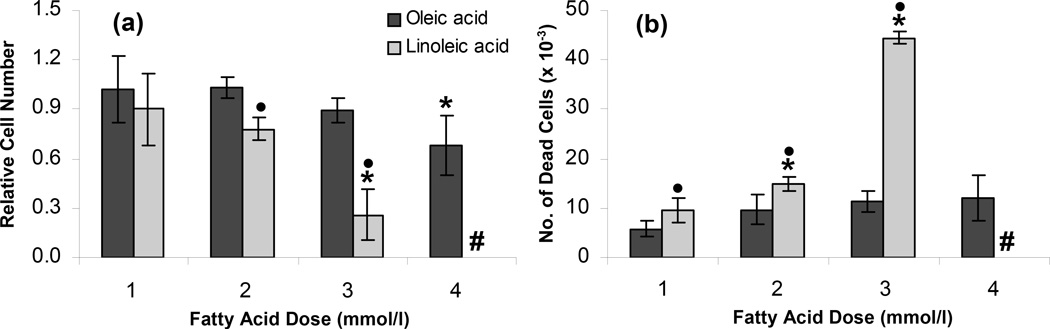
The cells were further characterized by measuring their proliferation and viability after culturing in the OA or LA containing medium. The cells attached to the cell culture plate surface after the 72 h FFA treatment were trypsinized and counted to provide an indication of cell proliferation during the treatments. Figure 3a shows that OA supplementation did not have a significant effect on the cell number relative to the control, except for the 4 mmol/l OA (p < 0.001). On the other hand, LA significantly reduced the relative cell number at the dose of 3 mmol/l (p < 0.001). Additionally, the floating dead cells were counted to provide an indication of the viability of the cells after the treatment and the cytotoxicity of the OA or LA doses. Figure 3b shows that OA did not induce significant cytotoxicity, and that the NT H35 cell viability for all OA doses was greater than 90%. On the other hand, LA significantly reduced the viability compared to an equivalent dose of OA (p < 0.001), and the cells treated with 4 mmol/l LA did not survive over the 72 h culture period.
Figure 3.
Effect of steatosis on (a) proliferation and (b) viability of H35 cells. All measurements were performed after the H35 cells were exposed to different FFA doses for 72 h. Error bars represent 95% confidence intervals. * p ≤ 0.05 compared to values for 0 mmol/l FFA dose. • p ≤ 0.05 compared to values for same OA dose. # Cells treated with 4 mmol/l LA did not survive over the 72 h culture period.
The above studies demonstrate that our reporter cell model recapitulates several important phenotypic and metabolic features of steatotic hepatocytes. We next set out to assess alterations in transcriptional dynamics induced by the accumulation of intracellular lipids using a recently constructed GFP reporter cell library in our laboratory that continuously reports on levels of transcriptional activation. However, such stable transfectants obtained by cloning, often show changes in the original characteristics such as the growth retardation and lower responsiveness against induction signal. Therefore, we characterized the effect of different doses of the selected fatty acids on TG accumulation and cell proliferation of the NFκB-transfected H35 cells in addition to the NT H35 cells. These data did not show any statistical differences compared to the data obtained for the NT H35 cells (reported in Figures 1a and 3a).
NFκB and HSE are transcription factors with well established roles in the response to inflammatory mediators. However, their role in integrating metabolic (intracellular fat) and inflammatory stimuli is unclear. By contrast, GRE is a transcription factor with a well established response to hormonal modulation, but is not well characterized in the context of steatosis. Therefore, NFκB, HSE, and GRE reporters were treated with medium containing different amounts of OA or LA as described above, and stimulated with either 10 ng/ml TNF-α or 4 mmol/l dexamethasone for 18 h and analyzed by flow cytometry to measure cellular GFP fluorescence. To adjust for nonspecific changes in cellular fluorescence caused by the accumulation of intracellular lipids or cytokine stimuli, reporter responses were normalized to the respective reporter cells treated with the experimental base medium (no FFA supplementation) and stimulated with either TNF-α or dexamethasone.
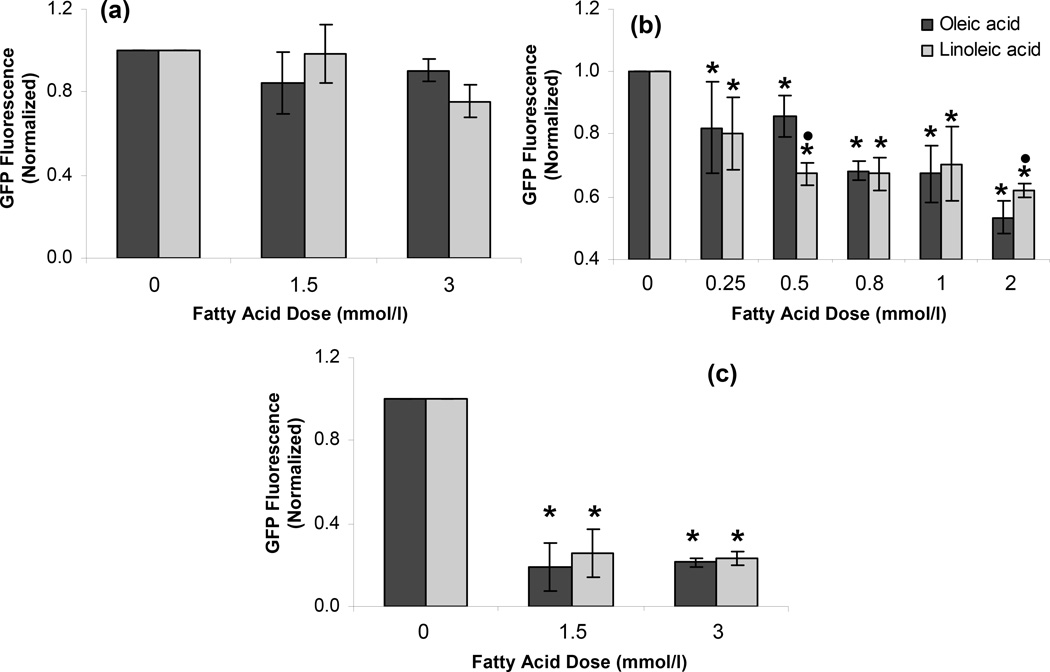
As shown in Fig. 4a, dexamethasone-induced GRE activation was not affected by the increased intracellular lipid accumulation. This result suggested that the fatty acid treatment and the subsequent intracellular lipid accumulation did not alter the fluorescence response of the reporter cells in a nonspecific manner. On the other hand, steatosis partially attenuated the NFκB reporter response to TNF-α in a dose dependent manner (Fig. 4b, p < 0.001). In contrast, as shown in Fig. 4c, TNF-α exposure resulted in a significant decline of the HSE reporter response in steatotic cells, compared to lean controls (p < 0.001).
Figure 4.
Effect of steatosis on (a) dexamethasone mediated activation of GRE and TNF-α mediated activation of (b) NFκB and (c) HSE in H35 cells. All reporter cells were exposed to different FFA doses for 72 h. GRE reporter cells were exposed to 4 mmol/l dexamethasone for 18 h before the flow cytometry. NFκB and HSE reporter cells were exposed to 10 ng/ml TNF-α for 18 h before the flow cytometry. Error bars represent 95% confidence intervals. * p ≤ 0.05 compared to values for 0 mmol/l FFA dose. • p ≤ 0.05 compared to values for same OA dose.
DISCUSSION
Fatty liver disease is a problem of growing clinical importance. The transition from reversible steatosis to progressive steatohepatitis, which depends critically on the response of lipid-laden cells to inflammatory mediators, represents a critical step in disease development. However, the integration of metabolic and inflammatory signals, which ultimately determine the response of steatotic cells to cytokine stresses, remains poorly understood at the level of transcriptional regulation. In this work, we describe construction and validation of a GFP reporter cell model of steatosis that is uniquely designed for non-destructive investigation of the transcriptional effects of combined intracellular lipid accumulation and inflammatory stress in living cells.
We created stable monoclonal GFP reporter cell lines to allow nondestructive monitoring of NFκB, HSE and GRE transcriptional activation in living cells. We quantified the relationship between extracellular fatty acid concentrations and total intracellular triglyceride content, and we demonstrated that the degree of steatosis can be controlled experimentally in a dose-dependent fashion, by modifying media composition. Next, we showed that increasing levels of steatosis are associated with characteristic increases in reactive oxygen species and decreases in mitochondrial membrane potential, both of which sensitize cells to subsequent stresses (Browning and Horton, 2004; Malhi et al., 2006). At the whole-cell level, we found steatosis to be associated with increased cell death and decreased proliferation, consistent with the acute liver injury and impaired compensatory proliferation that accompany steatohepatitis (Browning and Horton, 2004). By recapitulating the characteristic features of this disease process, as reported by other researchers (Cury-Boaventura and Curi, 2005; Gómez-Lechón et al., 2007; Malhi et al., 2006), our results validate the reporter cell model and establish it as a promising tool for studying transcriptional control in steatohepatitis. Finally, we compared the responses of reporter cells for NFκB, HSE, and GRE to their classical inducers under lean and fatty conditions and found that intracellular lipid accumulation was associated with dose-dependent impairment of NFκB and HSE but not GRE activation.
In addition to validating the model, our studies also revealed several interesting differences between ω-6 (oleic acid) and ω-9 (linoleic acid) fatty acids. First, for equivalent doses of OA and LA, LA resulted in a larger impact on ROS generation, MMP, proliferation, and cellular death. One possible explanation for the differential effects of LA versus OA might be due to the unique potential of LA to be metabolized into arachidonic acid. The polyunsaturated fatty acid arachidonic acid has been shown to cause toxicity in rat hepatocytes, particularly when accompanied by high levels of cytochrome P4502E1 (CYP2E1). This toxicity was shown to involve lipid peroxidation and oxidative stress which activated p38 mitogen-activated protein kinase (MAPK) pathway and resulted in decreased mitochondrial membrane potential and decreased NFκB activation, effects which together were associated with cell injury and death (Wu and Cederbaum, 2003).
Under normal conditions, the liver has a tremendous capacity for regeneration after injury; however this compensatory proliferation is compromised in steatotic livers (Robertson et al., 2001). At a molecular level, steatosis has been associated with increased IκBα and a corresponding decreased activation of NFκB, inhibition of cyclin D1 and Bcl-xL expression and increased apoptotic death (DeAngelis et al., 2005). Cyclins are cellular proteins involved in cell cycle control and it was hypothesized that the intracellular ROS may interfere with cyclins (Pizzimenti at al., 2002). The increase in the generation of intracellular ROS and the subsequent decreased proliferation observed in our cell culture model can thus be explained on the basis of these previous studies (DeAngelis et al., 2005; Pizzimenti at al., 2002).
It has been proposed that the progression from reversible steatosis to chronic steatohepatitis is caused by a “second hit” (Day and James, 1998). Cytokines such as TNF-α represent important potential mediators of such a “second hit”. TNF-α is one of several adipokines/cytokines and chemokines known to be liberated from adipose tissue of obese individuals. These mediators have direct intracellular stress activation and pro-apoptotic effects, but also indirectly amplify and propagate the inflammatory process by recruiting and activating additional immune cells (Diehl et al., 2005; Lalor et al., 2007). One of the principle intracellular targets of TNF-induced signaling is the transcription factor NFκB. However, the role of transcription factors in combined steatosis and cytokine stress remains unclear, as evidenced by numerous conflicting reports on whether steatosis and its sequelae are associated with increases or decreases in NFκB activity (Bianchi et al., 2002; Hennig et al., 1996; Robin et al., 2005). For example, alcohol given to ob/ob mice increased plasma TNF-α, heat shock protein-70, and decreased NFκB protein levels as well as its DNA binding, possibly unleashing the apoptotic effects of this cytokine (Robin et al., 2005). On the other hand, Hennig et al. showed that LA activated NFκB in cultured endothelial cells (Hennig et al., 1996), and Bianchi et al. demonstrated that arachidonic acid activated an inactive NFκB complex in human HepG2 hepatoma cells (Bianchi et al., 2002). These conflicting results could be attributed to the fact that fatty acid metabolism may be cell type specific and/or that these experiments used different FFA molecules with different doses and exposure times. In addition, some of these experiments used animal models, which involve combined effects of multiple cell types and systemic mediators.
Most reports of NFκB activation or deactivation attribute their results to the excess intracellular ROS generated due to FFA exposure. It has been argued that the effects might be biphasic with a threshold effect, where low-to-moderate levels of ROS activate NFκB, but larger levels inhibit or deactivate NFκB (Marshall et al., 2000; Michiels et al., 2002). In this work, we have described a tool that can be used for high throughput investigation of steatosis and transcriptional regulation and also has the potential to reveal spatial and temporal heterogeneity in the response. Initial results from our steatotic reporter cells indicate that steatosis leads to increases in ROS and decreased activation of NFκB. In addition, our results indicate that HSE activation, which has diverse cytoprotective roles and was recently shown to be activated by TNF-α (Feldstein et al., 2003; Schett et al, 1998), was also attenuated. Importantly, the observed responses were specific to NFκB and HSE and were not observed for GRE, which suggests that they are not simply artifacts involving direct effects of intracellular fat accumulation on GFP expression or fluorescence. While, additional studies will be necessary to more exhaustively investigate these relationships, our initial studies demonstrate that the reporters developed and validated in this work offer an opportunity to compare transcriptional activation in live cells with different degrees of steatosis after stimulation with the inflammatory cytokine. More broadly, the establishment of techniques for creating steatotic reporter cells provides a foundation for future studies comparing lean and steatotic responses to cytokine stress and opens the potential for high-throughput investigations in microfluidic arrays, and detailed spatio-temporal studies of local steatotic and inflammatory responses.
CONCLUSION
This work describes construction and validation of an in vitro model of steatosis, specifically designed for studying transcriptional activation in living cells during inflammatory stress. By culturing H35 rat hepatoma reporter cells in medium containing OA or LA, we created dose-dependent and species-independent levels of steatosis, but interestingly found that fatty acid species differentially affected the mitochondrial membrane potential, accumulation of intracellular reactive oxygen reactive species, proliferation, cell survival, and activation of NFκB and HSE transcriptional reporters. Taken together, these studies establish that reporter cells are valuable tools for investigating the response of steatosis to inflammatory stress at the level of transcriptional regulation, and that this experimental tool will uniquely contribute to our understanding the progression of fatty liver disease at the molecular level. In addition, this model offers a general tool for developing therapeutic modulators of metabolism and stress response pathways to prevent and possibly reverse the progression of steatosis, thereby avoiding long term consequences such as cirrhosis and hepatocellular carcinoma.
ACKNOWLEDGEMENTS
This work was funded by the National Institutes of Health under the Bioengineering Research Partnership, BRP (5R01AI063795) and P41 (EB002503) programs. The authors thank Drs. Sihong Wang and Yaakov Nahmias for discussions and Mr. Luke Selby for technical assistance.
REFERENCES
- Angulo P. Medical progress: nonalcoholic fatty liver disease. N Eng J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Bécuwe P, Bianchi A, Didelot C, Barberi-Heyob M, Dauça M. Arachidonic acid activates a functional AP-1 and an inactive NF-κB complex in human HepG2 hepatoma cells. Free Rad Biol Med. 2003;35:636–647. doi: 10.1016/s0891-5849(03)00387-3. [DOI] [PubMed] [Google Scholar]
- Bergamini CM, Gambetti S, Dondi A, Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr Pharm Des. 2004;10:1611–1626. doi: 10.2174/1381612043384664. [DOI] [PubMed] [Google Scholar]
- Berthiaume F, MacDonald AD, Kang YH, Yarmush ML. Control analysis of mitochondrial metabolism in intact hepatocytes: effect of Interleukin-1β and Interleukin-6. Metabolic Eng. 2003;5:108–123. doi: 10.1016/s1096-7176(03)00010-7. [DOI] [PubMed] [Google Scholar]
- Bianchi A, Bécuwe P, Frank P, Dauça M. Induction of MnSOD gene by arachidonic acid is mediated by reactive oxygen species and p38 MAPK signaling pathway in human HepG2 hepatoma cells. Free Rad Biol Med. 2002;32:1132–1142. doi: 10.1016/s0891-5849(02)00834-1. [DOI] [PubMed] [Google Scholar]
- Bianchi A, Dewailly E, Gautier H, Merlin JL, Slomianny C, Dauça M, Bécuwe P. Decrease of human hepatoma cell growth by arachidonic acid is associated with an accumulation of derived products from lipid peroxidation. Biochimie. 2004;86:633–642. doi: 10.1016/j.biochi.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Euro J Clinic Invest. 2002;32:S14–S23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for Interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- Cury-Boaventura MF, Curi R. Regulation of reactive oxygen species (ROS) production by C18 fatty acids in jurkat and raji cells. Clinic Sci. 2005;108:245–253. doi: 10.1042/CS20040281. [DOI] [PubMed] [Google Scholar]
- Das UN. Essential fatty acids: biochemistry, physiology, and pathology. Biotech J. 2006;1:420–439. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- Day CP, James O. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- DeAngelis RA, Markiewski MM, Taub R, Lambris JD. A high-fat diet impairs liver regeneration in C57BL/6 mice through overexpression of the NF-κB inhibitor, IκBα. Hepatology. 2005;42:1148–1157. doi: 10.1002/hep.20879. [DOI] [PubMed] [Google Scholar]
- Diehl AM, Li ZP, Lin HZ, Yang SQ. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–306. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato MT, Lahoz A, Jiménez N, Pérez G, Serralta A, Mir J, Castell JV, Gómez-Lechón MJ. Potential impact of steatosis on cytochrome P450 enzymes of human hepatocytes isolated from fatty liver grafts. Drug Metab Disp. 2006;34:1556–1562. doi: 10.1124/dmd.106.009670. [DOI] [PubMed] [Google Scholar]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;42:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- Finstad HS, Kolset SO, Holme JA, Wiger R, Farrants AO, Blomhoff R, Drevon CA. Effect of n-3 and n-6 fatty acids on proliferation and differentiation of promyelocytic leukemic HL-60 cells. Blood. 1994;84:3799–3809. [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Morales A, Kaplowitz N, Fernandez-Checa JC. Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol. 1995;48:825–834. [PubMed] [Google Scholar]
- Gilmore TD, Herscovitch M. Inhibitors of NF-κB signaling: 785 and counting. Oncogene. 2006;25:6887–6899. doi: 10.1038/sj.onc.1209982. [DOI] [PubMed] [Google Scholar]
- Gómez-Lechón MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chemico-Biological Interactions. 2007;165:106–116. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Helling TS. Liver failure following partial hepatectomy. HPB. 2006;8:165–174. doi: 10.1080/13651820510035712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig B, Toborek M, Joshi-Barve S, Barger SW, Barve S, Mattson MP, McClain CJ. Linoleic acid activates nuclear transcription factor-κB (NFκB) and induces NF-κB-dependent transcription in cultured endothelial cells. Am J Clin Nutr. 1996;63:322–328. doi: 10.1093/ajcn/63.3.322. [DOI] [PubMed] [Google Scholar]
- Hensley K, Kotake Y, Sang H, Pye QN, Wallis GL, Kolker LM, Tabatabaie T, Stewart CA, Konishi Y, Nakae D, Floyd RA. Dietary choline restriction causes complex I dysfunction and increased H2O2 generation in liver mitochondria. Carcinogenesis. 2000;21:983–989. doi: 10.1093/carcin/21.5.983. [DOI] [PubMed] [Google Scholar]
- Itoh N, Matsuda T, Ohtani R, Okamoto H. Angiotensinogen Production by Rat Hepatoma Cells is Stimulated by B Cell Stimulatory Factor 2/Interleukin-6. FEBS Lett. 1989;244:6–10. doi: 10.1016/0014-5793(89)81150-0. [DOI] [PubMed] [Google Scholar]
- King KR, Wang S, Irimia D, Jayaraman A, Toner M, Yarmush ML. A high-throughput microfluidic real-time gene expression living cell array. Lab Chip. 2007;7:77–85. doi: 10.1039/b612516f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KR, Wang S, Jayaraman A, Yarmush ML, Toner M. Microfluidic flow-encoded switching for parallel control of dynamic cellular microenvironments. Lab Chip. 2008;1:107–116. doi: 10.1039/b716962k. [DOI] [PubMed] [Google Scholar]
- Lalor PF, Faint J, Aarbodem Y, Hubscher SG, Adams DH. The role of cytokines and chemokines in the development of steatohepatitis. Semin Liver Dis. 2007;27:173–193. doi: 10.1055/s-2007-979470. [DOI] [PubMed] [Google Scholar]
- Lieber CS. CYP2E1: from ASH to NASH. Hepatol Res. 2004;28:1–11. doi: 10.1016/j.hepres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Lo CJ, Chiu KC, Fu M, Lo R, Helton S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NFκB activity. J Surg Res. 1999;82:216–222. doi: 10.1006/jsre.1998.5524. [DOI] [PubMed] [Google Scholar]
- Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- Mannaerts GP, Van Veldhoven PP, Casteels M. Peroxisomal lipid degradation via betaand alpha-oxidation in mammals. Cell Biochem Biophys. 2000;32:73–87. doi: 10.1385/cbb:32:1-3:73. [DOI] [PubMed] [Google Scholar]
- Marshall HE, Merchant K, Stamler JS. Nitrosation and oxidation in the regulation of gene expression. FASEB J. 2000;14:1889–1900. doi: 10.1096/fj.00.011rev. [DOI] [PubMed] [Google Scholar]
- Michiels C, Minet E, Mottet D, Raes M. Regulation of gene expression by oxygen: NF-κB And HIF-1, two extremes. Free Rad Bio Med. 2002;33:1231–1242. doi: 10.1016/s0891-5849(02)01045-6. [DOI] [PubMed] [Google Scholar]
- Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-kappa B inhibition by omega-3 fatty acids modulates lps-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284:L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- Ovelgonne H, Bitorina M, Van Wijk R. Stressor-specific Activation of Heat Shock Genes in H35 Rat Hepatoma Cells. Toxicol Appl Pharmacol. 1995;135:100–109. doi: 10.1006/taap.1995.1212. [DOI] [PubMed] [Google Scholar]
- Pitot HC, Peraino C, Morse PA, Jr, Potter VR. Hepatomas in Tissue Culture Compared with Adapting Liver In Vivo. Natl Cancer Inst Monogr. 1964;13:229–242. [PubMed] [Google Scholar]
- Pizzimenti S, Laurora S, Briatore F, Ferretti C, Dianzani MU, Barrera G. Synergistic effect of 4-Hydroxynonenal and PPAR ligands in controlling human leukemic cell growth and differentiation. Free Radic Biol Med. 2002;32:233–245. doi: 10.1016/s0891-5849(01)00798-5. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gestrointest Liver Phsyiol. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- Richards CD, Langdon C, Pennica D, Gauldie J. Murine Cardiotrophin-1 Stimulates the Acute-phase Response in Rat Hepatocytes and H35 Hepatoma Cells. J Interferon Cytokine Res. 1996;16:69–75. doi: 10.1089/jir.1996.16.69. [DOI] [PubMed] [Google Scholar]
- Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis: II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- Robin MA, Demeilliers C, Sutton A, Paradis V, Maisonneuve C, Dubois S, Poirel O, Lettéron P, Pessayre D, Fromenty B. Alcohol increases tumor necrosis factor α and decreases nuclear factor-κB to activate hepatic apoptosis in genetically obese mice. Hepatology. 2005;42:1280–1290. doi: 10.1002/hep.20949. [DOI] [PubMed] [Google Scholar]
- Ross JA, Moses AGW, Fearon KCH. The anti-catabolic effects of n-3 fatty acids. Curr Opin Clin Nutr Metab Care. 1999;2:219–226. doi: 10.1097/00075197-199905000-00005. [DOI] [PubMed] [Google Scholar]
- Schett G, Redlich K, Xu Q, Bizan P, Gröger M, Tohidast-Akrad M, Kiener H, Smolen J, Steiner G. Enhanced expression of heat shock protein 70 (HSP70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of Hsp70 expression and Hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J Clin Invest. 1998;102:302–311. doi: 10.1172/JCI2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XR, Torphy TJ, Grisowold DE, Shealy D. Coming of age: anti-cytokine therapies. Mol Interv. 2002;2:36–46. doi: 10.1124/mi.2.1.36. [DOI] [PubMed] [Google Scholar]
- Thompson DM, King KR, Wieder KJ, Toner M, Yarmush ML, Jayaraman A. Dynamic gene expression profiling using a microfabricated living cell array. Anal Chem. 2004;76:4098–4103. doi: 10.1021/ac0354241. [DOI] [PubMed] [Google Scholar]
- Toborek M, Barger SW, Mattson MP, Barve S, McClain CJ, Hennig B. Linoleic acid and TNF-α cross-amplify oxidative injury and dysfunction of endothelial cells. J Lipid Res. 1996;37:123–135. [PubMed] [Google Scholar]
- Toborek M, Blanc EM, Kaiser S, Mattson MP, Hennig B. Linoleic acid potentiates TNF-mediated oxidative stress, disruption of calcium homeostasis, and apoptosis of cultured vascular endothelial cells. J Lipid Res. 1997;38:2155–2167. [PubMed] [Google Scholar]
- Ulloa L, Tracey KJ. Cytokine profile: a code for sepsis. Trends in Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- Weider KJ, King KR, Thompson DM, Zia C, Yarmush ML, Jayaraman A. Optimization of reporter cells for expression profiling in a microfluidic device. Biomed Microdevices. 2005;7:213–222. doi: 10.1007/s10544-005-3028-3. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Role of p38 MAPK in CYP2E1-dependent arachidonic acid toxicity. J Biol Chem. 2003;278:115–1124. doi: 10.1074/jbc.M207856200. [DOI] [PubMed] [Google Scholar]
- Yu AS, Keeffe EB, Mulhall BP, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease: an overview. Rev Gastroenterol Disord. 2002;2:11–19. [PubMed] [Google Scholar]
- Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-α expression by preventing NF-κB activation. J Am Coll Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]