Abstract
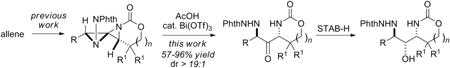
The synthesis of 1,3-diaminated stereotriads via the bis-aziridination of allenes is reported. The reactive 1,4-diazaspiro[2.2]pentane intermediates undergo a mild Brønsted acid-promoted rearrangement to yield 1,3-diaminated ketones in good yields with excellent stereocontrol. Directed reduction of the ketone can be achieved to yield a C-N/C-O/C-N stereotriad in high dr. The ability to transfer the axial chirality of the substrates to the products allows for the facile preparation of enantioenriched stereotriads from allenes in two simple steps.
Compounds containing 1,3-diamino-2-ol stereotriads are prevalent in a number of biologically active natural products and pharmaceuticals. These include the potent HIV protease inhibitor palinavir, aminated cyclohexanes that constitute components of streptomycin and other antibiotics, the natural product manzacidin B and key components of the antibiotic mannopeptimycins known as the hydroxyenduracididines (Figure 1).1
Figure 1.
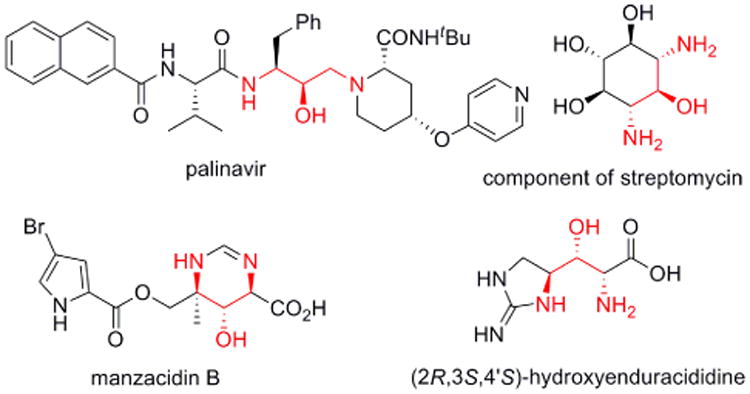
Biologically active 1,3-diamino-2-ols.
Due to the priviledged nature of these motifs, a number of methods have been developed for the stereocontrolled synthesis of 1,3-diaminated compounds.2-5 Typical approaches include the addition of enecarbamates to imines to yield anti 1,3-diamines,2 the diastereoselective reduction of ketimines,3 diastereoselective C-H amination4 and the addition of the α-carbanion of imines to N-protected imines.5 However, access to complex functionalized 1,3-diaminated compounds of the form C-N/C-X/C-N using well-established methodology is more limited. We hypothesized that a highly regio- and stereocontrolled allene oxidation could provide a new and convenient method to prepare C-N/C-O/C-N stereotriads in a few simple synthetic manipulations. Our previous work has already demonstrated the utility of allene bis-aziridination to 1,4-diazaspiro[2.2]pentanes (DASPs) 2 (Scheme 1) as a convenient scaffold to access vicinal diaminated synthetic motifs.6 In this communication, we report a complementary reactivity that allows for the conversion of these reactive intermediates to 3 and stereotriads of the form 4.
Scheme 1.
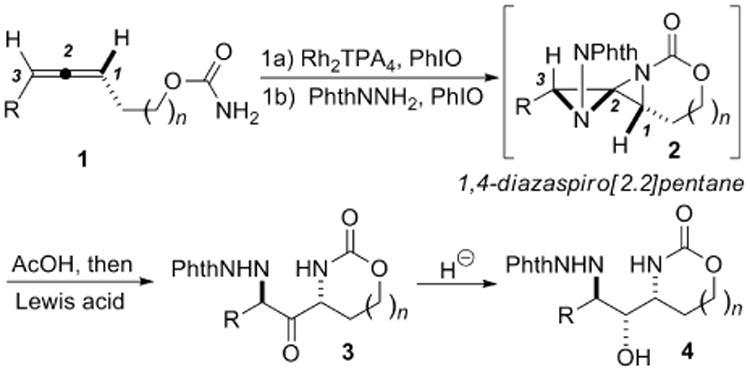
Bis-aziridination as a key step in the conversion of allenes to 1,3-diamino-2-ols via DASP intermediates.
A DASP such as 2 can essentially be viewed as a 1,3-diaminated ketone intramolecularly “protected” as an N,N-aminal (Scheme 1). We initially envisaged that a simple hydrolysis would yield the desired 1,3-diaminated ketone 3 directly from 2. However, this transformation was unsuccessful utilizing a variety of Lewis and Brønsted acids, irrespective of the presence or absence of water. In the case of mild acids, either no reaction was noted, or the DASP underwent ring-opening at C1 of 2.2a The use of stronger acids generally resulted in intractable mixtures of products.
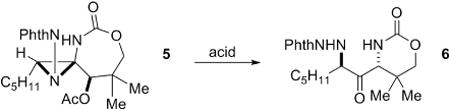
We postulated that the recalcitrance of the DASP to undergo hydrolysis might be due to the fact neither nitrogen atom is capable of resonance stabilization of an intermediate carbocation at C2. The relief of ring strain might render the heteroatoms better able to stabilize this carbocation, thus facilitating the desired reaction. In this vein, the acetate-opened DASP 5 was treated with a series of Lewis and Brønsted acids (Table 1). Weak Lewis acids, including CeCl3, Ti(OiPr)4 and ZnCl2 (entries 1-4), gave no reaction and the starting material was recovered unchanged. InCl3 (entry 6) gave slow conversion to the desired product, while Cu(OTf)2 and Sc(OTf)3 (entries 5 and 7) yielded an unidentified mixture of products. BF3OEt2, TsOH and Bi(OTf)3 (entries 8-12) all gave complete conversion of the starting material, with Bi(OTf)3 resulting in a 96% isolated yield of 6.
Table 1.
Optimizing the rearrangement of an N,N-spiroaminal to a 1,3-diaminated ketone.
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | additivesa | conversion | entry | additivesa | conversion |
| 1 | CeCl3•7H2O | 0% | 7 | Sc(OTf)3 | 100%b |
| 2 | NiCl2•6H2O | 0% | 8 | BF3OEt2 | 100% |
| 3 | Ti(OiPr)4 | 0% | 9 | TsOH | 100% |
| 4 | ZnCl2 | 0% | 10 | Bi(OTf)3 | 96%c |
| 5 | Cu(OTf)2 | 100%b | 11 | Bi(OTf)3 (0.2 equiv) | 100% |
| 6 | lnCl3 | 33% | 12 | Bi(OTf)3 (0.05 equiv) | 100% |
1.0 equiv unless otherwise indicated.
Complete conversion, several side products observed.
Isolated yield.
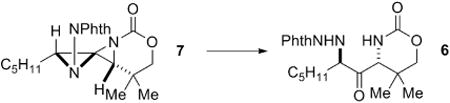
Bi(OTf)3 was chosen to further optimize the conversion of the DASP 7 directly to 6 (Table 2). The addition of Bi(OTf)3 alone (entry 1) did not promote the desired rearrangement. Treatment of 7 with AcOH, followed by addition of 5 mol % of Bi(OTf)3 (entry 2) gave complete conversion of the DASP to 6; however, the ring-opening with AcOH was slow. Interestingly, the ring-opening of 7 with TMSCl, followed by treatment with the Lewis acid (entry 3) gave only the ring-opened product and none of the desired rearrangement product. The same result was observed when thioacetic acid (entry 4) was employed as the nucleophile to open 7. These results suggested that the ester was playing an important role in promoting the rearrangement, and indeed, treatment of 7 with chloroacetic acid and Bi(OTf)3 also initiated the desired rearrangement to 6 (entry 5). We suspected that the role of Bi(OTf)3 might be to simply generate small amounts of TfOH, and indeed, the addition of a catalytic amount of TfOH (entry 6) to a mixture of 7 and AcOH did promote the desired rearrangement. Ultimately, in order to decrease the reaction time, AcOH was added to 7 at 35 °C and the mixture heated for 8 h prior to addition of the Bi(OTf)3 (entry 7) to give 6 in 88% isolated yield over the one-pot, two-step reaction.
Table 2.
Investigation of the rearrangement reaction.
| ||
|---|---|---|
|
| ||
| entry | conditions | conversion |
| 1 | Bi(OTf)3(1.0 equiv) | 0% |
| 2 | AcOH, then Bi(OTf)3 (0.05 equiv)a | 100% |
| 3 | TMSCI, then Bi(OTf)3 (0.05 equiv)b | 0% |
| 4 | AcSH, then Bi(OTf)3 (0.05 equiv)b | 0% |
| 5 | CICH2CO2H, then Bi(OTf)3 (0.05 equiv) | 100% |
| 6 | AcOH, then cat. TfOH | 100% |
| 7 | AcOH at 35 °C, then Bi(OTf)3 (0.05 equiv) | 100%(88%)c,d |
40 h reaction time.
The product was the ring-opened DASP.
12 h reaction time.
Isolated yield with a dr > 19:1.
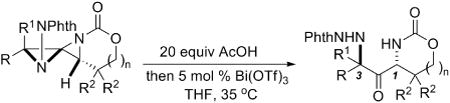
A variety of DASPs were converted to the corresponding 1,3-diaminated ketone products (Table 3) using the optimized reaction conditions. There was only a slight difference in yield between the use of the DASP 7 or the ring-opened DASP 5 (entries 1-2). DASPs formed from Z bicyclic methylene aziridines also underwent the desired rearrangement with high stereoselectivity (entries 3 and 4). The stereospecific nature of the reaction is illustrated by a comparison of the 1,3-diaminated ketone 6, obtained in 88% yield from the rearrangement of a DASP 7 derived from an E methylene aziridine, with the distinct product ketone 8c, obtained in 90% yield from the rearrangement of a DASP formed from a Z-methylene aziridine (Table 3, entries 2 and 4, respectively). The rearrangement was not affected by the absence of alkyl substitution in the tether (entries 5-9) or limited to the formation of six-membered rings, as a five-membered ring-containing product (entry 8) was obtained in 78% yield. The dr of the two nitrogen-bearing stereocenters was high in most cases (> 19:1) and none of the other diastereomer was detected by 1H NMR spectroscopy. Not surprisingly, if the product was allowed to remain under the acidic conditions for extended periods of time, significant isomerization to a mixture of diastereomers did occur.
Table 3.
Rearrangement of DASPs to 1,3-diaminated ketones.
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entrya | R, R1 | R2 | E/Zc | n | yield | dr |
| 1b | 5 C5H11, H | Me | E | 1 | 96% 6 | > 19:1 |
| 2 | 7a C5H11, H | Me | E | 1 | 88% 6 | > 19:1 |
| 3b | 7b H, CH2CO2Et | Me | Z | 1 | 65% 8b | 86:12 |
| 4 | 7c H, C5H11 | Me | Z | 1 | 90% 8c | > 19:1 |
| 5b | 7d C5H11, H | H | E | 1 | 89% 8d | > 19:1 |
| 6 | 7d C5H11, H | H | E | 1 | 81% 8d | > 19:1 |
| 7 | 7e Ph(CH2)2, H | H | E | 1 | 60% 8e | > 19:1 |
| 8b | 7f C5H11, H | H | E | 0 | 78% 8f | > 19:1 |
| 9 | 7g iBu, H | H | E | 1 | 57% 8g | > 19:1 |
| 10 | 7h iBu, H | Me | E | 1 | 77% 8h | > 19:1 |
The dr of the starting DASPs was > 19:1 in all cases.
The starting material was the acetate-opened DASP with a dr > 19:1.
Stereochemistry of the methylene aziridine used to form the DASP.
One convenient advantage of this methodology arises as a result of the ability to transfer the axial chirality of an enantioenriched allene to the 1,3-diaminated products.7 The fidelity of this transfer was verified by subjecting an enantioenriched DASP 9 to the reaction conditions for the rearrangement to 10 (eq 1). No degradation of the enantiopurity was indicated by chiral HPLC (see the Supporting Information for further details).
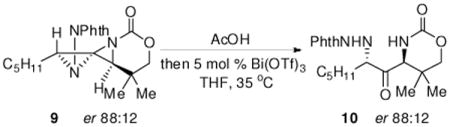 |
(1) |
The next issue was to determine if stereocontrolled reduction of the ketone could be achieved. The use of Na(OAc)3BH (STAB-H) for the reduction of 6 gave the C-N/C-O/C-N stereotriad 11 in 3:1 dr when THF was employed as the solvent and in 9:1 dr in 70% yield when CH2Cl2 was utilized for the reaction (Scheme 2).8 Treatment of 11 with an excess of p-nitrobenzoyl chloride gave a crystalline derivative 12 (Scheme 2), containing the three heteroatoms in a 1,2-syn:2,3-anti relationship. This indicates that the reduction could be controlled either by chelation involving the NNPhth group, or by Felkin-Ahn control via the carbamate. Changing the bulk of the side chain (Scheme 2, compounds 13-14) did not affect the dr. Removing the steric bulk in the carbamate ring, as in 15, gave decreased stereoselectivity, indicating that Felkin-Ahn control is likely operative.
Scheme 2.
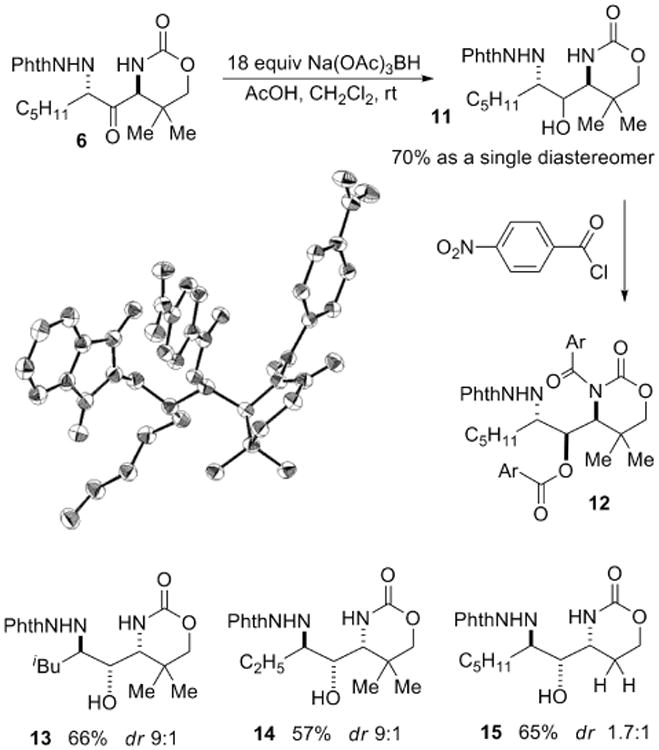
Reduction of aminoketones and determination of the relative stereochemistry of the C-N/C-O/C-N stereotriad.
We were pleased to find that the conversion of allenic carbamates 16 and 17 to the resulting 1,3-diaminated ketones 6 and 8d could be achieved in a single pot (Scheme 3). Although the yields were only moderate (approximately 80% per chemical step), the reaction introduces significant complexity into a simple, readily available substrate. The major loss in yield occurs in the first allene aziridination, where the formation of the Z methylene aziridine competes with that of the desired E methylene aziridine. Our ongoing work in the design of new catalysts with improved selectivity in the allene aziridination should alleviate this issue. Regardless, the Z methylene aziridine reacts much more slowly in the subsequent reactions and can be easily separated from the final desired product. The diastereoselectivity of the isolated products was greater than 19:1, comparable to the dr observed in the step-wise conversion of the isolated DASPs to 6 and 8d.
Scheme 3.
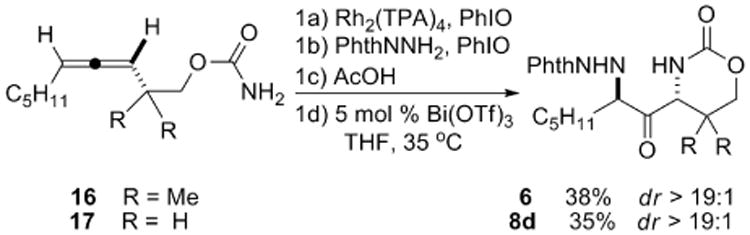
One-pot conversion of allenic carbamates to 1,3-diaminated ketones.
We did not carry out detailed mechanistic studies on this unusual rearrangement. However, the stereoselective nature of the reaction, the necessity for an ester moiety at C1 and the relative stereochemistry of the 1,3-diaminated chiral centers suggests that a mechanism similar to that illustrated in Scheme 4 may be operative. A double displacement at C1 of the DASP via anchimeric assistance would yield retention of the stereochemistry at this carbon, while promoting the desired hydrolysis at C2.
Scheme 4.
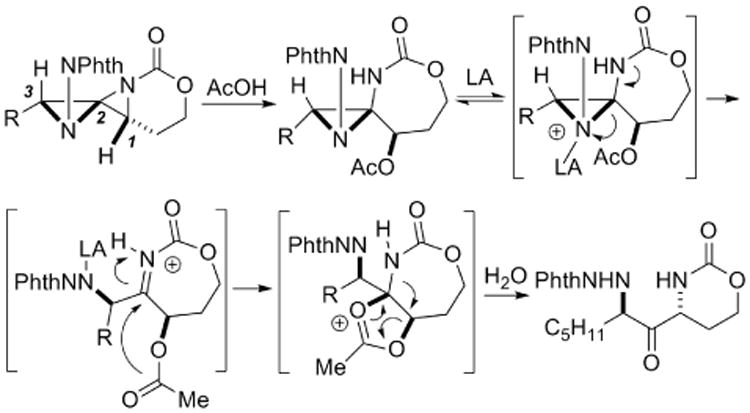
Possible mechanism for the rearrangement.
In conclusion, allene bis-aziridination to 1,4-diazaspiro[2.2]pentanes can serve as a key step in the stereoselective syntheses of 1,3-diaminated ketones and their corresponding C-N/C-O/C-N stereotriads via stereoselective reduction with STAB-H. The factors that control the stereoselective reductions of these 1,3-diaminated ketones will be further investigated to provide access to other C-N/C-X/C-N stereoisomers. Studies to apply allene oxidation methods to the total synthesis of biologically active molecules, including manzacidin B and (2R,3S,4′S)-hydroxyenduracididine (Figure 1) are underway and will be reported in due course.
Supplementary Material
Acknowledgments
The authors thank Dr. Ilia A. Guzei of the University of Wisconsin-Madison for assistance with X-ray crystallography. Funding was provided by start-up funds from the University of Wisconsin-Madison. The NMR facilities at UW-Madison are funded by the NSF (CHE-9208463, CHE-9629688) and NIH (RR08389-01).
Footnotes
Supporting Information Available: Experimental details and characterization data for all new compounds, as well as X-ray crystallographic information for 12. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Beaulieu P, Lavallee P, Abraham A, Anderson PC, Boucher C, Bousquet Y, Duceppe J, Gillard J, Gorys V, Grand-Maitre C, Grenier L, Guindon Y, Guse I, Plamondon L, Soucy F, Valois S, Wernic D, Yoakim C. J Org Chem. 1997;62:3440. [Google Scholar]; (b) Shinada T, Ikebe E, Oe K, Namba K, Kawasaki M, Ohfune Y. Org Lett. 2010;12:2170. doi: 10.1021/ol0704789. [DOI] [PubMed] [Google Scholar]; (c) Arya DP, editor. Aminoglycoside Antibiotics. John Wiley & Sons; Hoboken, NJ: 2007. [Google Scholar]; (d) He H, Williamson RT, Shen B, Graziani EI, Yang HY, Sakya SM, Petersen PJ, Carter GT. J Am Chem Soc. 2002;124:9729. doi: 10.1021/ja020257s. [DOI] [PubMed] [Google Scholar]
- 2.For selected references on the synthesis of 1,3-diamines, see: Dagousset G, Drouet F, Masson G, Zhu J. Org Lett. 2009;11:5546. doi: 10.1021/ol9023985.Vesely J, Ibrahem I, Rios R, Zhao G, Xu Y, Cordova A. Tetrahedron Lett. 2007;48:2193.Zhao C, Liu L, Wang D, Chen Y. Eur J Org Chem. 2006;13:2977.Merla B, Risch N. Synthesis. 2002;10:1365.Wu P, Lin D, Lu X, Zhou L, Sun J. Tetrahedron Lett. 2009;50:7249.Matsubara R, Kobayashi S. Accts Chem Res. 2008;41:292. doi: 10.1021/ar700098d.Robak MT, Herbage MA, Ellman JA. Chem Rev. 2010;110:3600. doi: 10.1021/cr900382t.Lanter JC, Chen H, Zhang X, Sui Z. Org Lett. 2005;7:5905. doi: 10.1021/ol0525258.
- 3.Martjuga M, Belyakov S, Liepinsh E, Suna E. J Org Chem. 2011;76:2635–2647. doi: 10.1021/jo1025767. and references therein. [DOI] [PubMed] [Google Scholar]
- 4.For selected references, see: Espino CG, Wehn PM, Chow J, Du Bois J. J Am Chem Soc. 2001;123:6935.Kurokawa T, Kim M, Du Bois J. Angew Chem Int Ed. 2009;48:2777. doi: 10.1002/anie.200806192.Liang C, Robert-Peillard F, Fruit C, Mueller P, Dodd RH, Dauban P. Angew Chem Int Ed. 2006;45:4641. doi: 10.1002/anie.200601248.
- 5.For selected references, see: Hou X, Luo Y, Yuan K, Dai L. J Chem Soc Perk Trans I. 2002;12:1487.Merla B, Risch N. Synthesis. 2002;10:1365.Kiss L, Mangelinckx S, Sillanpaeae R, Fueloep F, De Kimpe N. J Org Chem. 2007;72:7199. doi: 10.1021/jo0710634.
- 6.(a) Rigoli JW, Boralsky LA, Hershberger JC, Meis AR, Marston D, Guzei IA, Schomaker JM. J Org Chem. 2012 doi: 10.1021/jo3000282. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Grigg RD, Schomaker JM, Timokhin V. Tetrahedron. 2011;67:4318. [Google Scholar]; (c) Boralsky LA, Marston D, Grigg RD, Hershberger JC, Schomaker JM. Org Lett. 2011;13:1924. doi: 10.1021/ol2002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For selected references on allenes and examples of the transfer of the axial chirality of an allene to point chirality, see: Ogasawara M. Tetrahedron-Asymmetry. 2009;20:259.Kim H, Williams LJ. Curr Opin Drug Disc. 2008;11:870.Ma SM. Pure Appl Chem. 2006;78:197.Krause N, Hoffmann-Röder A. Tetrahedron. 2004;60:11671.Krause NA, Hashmi SK, editors. Modern Allene Chemistry. Wiley-VHC; Weinheim: 2004. Brawn RA, Panek JS. Org Lett. 2009;11:4362. doi: 10.1021/ol901692n.Yue Z, Williams LJ. J Org Chem. 2009;74:7707. doi: 10.1021/jo901320f. and references therein.Shangguan N, Kiren S, Williams LJ. Org Lett. 2007;9:1093. doi: 10.1021/ol063143k.Lotesta SD, Hou YQ, Williams LJ. Org Lett. 2007;9:869. doi: 10.1021/ol063087n.Joyasawal S, Lotesta SD, Akhmedov NG, Williams LJ. Org Lett. 2010;12:988. doi: 10.1021/ol902984e.
- 8.(a) Huang Y, Li M, Yu X, Yuan Z. Zhongguo Yiyao Gongye Zazhi. 2008;39:695. [Google Scholar]; (b) Abdel-Magid AF, Mehrman SJ. Org Process Res Devel. 2006;10:971. [Google Scholar]; (c) Gribble GW, Abdel-Magid AF. Sodium Triacetoxyborohydride in the Encyclopedia of Reagents for Organic Synthesis. 2001 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


