Abstract
Background
CD3δ deficiency is a fatal form of severe combined immunodeficiency which can be cured by hematopoietic stem cell transplantation (HSCT). The presence of a thymus loaded with T cell progenitors in these patients may require special considerations in choosing the regimen of conditioning and the type of HSCT.
Objectives
To study the outcome of CD3δ deficiency using various modalities of stem cell transplantation.
Methods
We analyzed data on 13 patients with CD3δ deficiency who underwent HSCT in 7 centers. HSCT was performed using different sources of donor stem cells as well as various conditioning regimens.
Results
Two patients who received stem cells from matched related donors and survived, both needed substantial conditioning in order to engraft. Only one of six other patients who received a related mismatched donor (MMRD) transplant survived, two of them had no conditioning while the others received various combinations of conditioning regimens.
Three other patients received stem cells from a matched unrelated donor (MUD), survived and enjoyed full immune reconstitution.
Two other patients received unrelated cord blood without conditioning. One of them has had a partial but stable engraftment, while the other engrafted well but is only 12 months after HSCT. We also report here for the first time that patients with CD3δ deficiency can present with typical features of Omenn syndrome.
Conclusions
HSCT is a successful treatment for patients with CD3δ deficiency. The small number of patients in this report prevent definitive statements on the importance of survival factors, but several are suggested: 1) HLA matched donor transplants are associated with superior reconstitution and survival than mismatched donor transplants; 2) substantial conditioning appears necessary; 3) early diagnosis and absence of opportunistic infections.
Keywords: CD3delta, severe combined immunodeficiency, bone marrow transplant, stem cell transplant, myeloablative conditioning, engraftment
Introduction
Severe combined immune deficiency (SCID) consists of a group of inherited disorders characterized by profound T cell impairment leading to death in infancy unless treated with hematopoietic stem-cell transplantation (HSCT). Optimal outcomes are achieved by using a matched related donor (MRD). When such donors are unavailable alternative donors such as mismatched-related donors (MMRD) or matched unrelated donors (MUD) have been used 1-4. The overall experience over the past 20 years has shown that the survival rate with MMRD was around 50% while survival after MUD HSCT was 80% and better1. However, survival recorded in small single centre (and sometimes with short follow up) studies varied dramatically from 87% 5 to less than 30% 6 using haploidentical donors. In contrast, the outcome of patients after MUD-HSCT were consistently between 65-85% in single as well as multicentre studies1,4,7. These results highlight the importance of donor to recipient HLA proximity to the success of HSCT.
Other factors influencing outcome include the type of SCID. Patients with circulating B cells (T− B+ SCID) were reported to have a superior outcome than patients with no circulating B cells (T− B−)8. Patients with HLA-Class II deficiency also have inferior survival rates after bone marrow transplant (BMT) when compared to other subtypes of SCID9. Survival of patients with ADA deficiency has also been reported to be lower than other types of profound T cell deficiencies, especially if RID has not been used10.
These genotype- specific differences in outcome highlight the need for similar studies in each subgroup of patients with SCID. We studied here the outcome of patients with CD3δ deficiency, first described by Dadi et al11 in 2003 with a peculiar presentation. While circulating T cells carrying the αβ TCR or the γδ TCR were markedly reduced, the thymus was heavily populated with T cell progenitor cells. These cells appeared to contain increased levels of precursor T cell receptor α but reduced levels of CD4, CD8α and CD8β consistent with an arrest of differentiation in the CD4− CD8− double-negative stage of T-cell development11-13. Other types of SCID have typically completely dysplastic thymuses highlighted by a marked depletion of thymocytes in addition to lack of Hassall’s corpuscles14, raising the possibility that the abundance of T cell precursors in CD3δ recipients might influence the outcome of HSCT, especially if conditioning is not used.
Because of the extremely rare occurrence of CD3δ deficiency, we have gathered the total number of known CD3δ deficient children treated with HSCT in order to gain more information on their survival using various modes of transplantation.
Methods
Patients
We analyzed the data of 13 patients with CD3δ deficiency from 7 medical centers around the world including North America, Japan, Spain and Germany, who underwent HSCT. All patients had a molecular diagnosis of CD3δ deficiency. Questionnaires were filled by the managing physician and included epidemiological data clinical presenting symptoms, immune work up, HSCT data and outcome.
Evaluation of cellular and humeral immunity
Cell surface markers of peripheral blood cells, were evaluated by flow cytometry (Epics V; Coulter Electronics, Hialeah, FL or Becton Dickenson). Lymphocyte proliferation in response to phytohaemagglutinin (PHA) stimulation, using tritiated thymidine incorporation was determined as previously described1. The amount of signal joint (sj) T-cell receptor excision circles (TRECs) adjusted to CD4+ and CD8+ T subsets, was determined by real time quantitative PCR, as previously described. The number of TRECs in a given sample was compared to a value obtained with 10-fold serial dilutions of an internal standard provided by Dr. Daniel Douek (Vaccine Research Center, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA). Representatives of specific T-cell receptor Vβ families were detected and were quantified using quantitative PCR and flow cytometry (coulter, Elite) as previously described1.
Stem cell transplantation procedures
Procedures varied between clinical centers in this report and included transplants with matched related donor (MRD, 9/10 antigen match or better), mismatched related donor (MMRD), matched unrelated donor (MUD, 9/10 antigen match or better) or mismatched unrelated donor (MMUD). Stem cell sources included: Peripheral blood cells, bone marrow or cord blood. Conditioning, and GVHD prophylaxis, when given, also varied widely among centers.
Engraftment and chimerism studies
Neutrophil engraftment was considered to have occurred on the first of 3 consecutive days in which the absolute neutrophil count exceeded 0.5×109/L. Platelet engraftment was considered to have occurred on the first day of 7 consecutive days in which the platelet count exceeded 20× 109/L without platelet transfusion. Red cells engraftment was considered to have occurred on the last day that red blood cell transfusions were required.
Assessment of T cell receptor repertoire
TCR Vβ families within CD4+ or CD8+ subpopulations were assessed using flow cytometry (Beckman Coulter Immunotech)15.
Results
Patients
Thirteen patients treated in seven different clinical centers were included in this study. Seven patients were the first to be diagnosed with SCID in their family. Their age at time of diagnosis ranged from 1 week to 14 months (Table 1). The remaining 6 patients were diagnosed soon after birth because they were siblings or cousins of already diagnosed patients. Patients came from three ethnic groups including Caucasian in Mennonite communities (North American or European), Asian (Japanese), and Hispanic. All patients were found to have homozygous mutations in the CD3δ gene. Three different mutations have been identified which segregate according to ethnic origin suggesting a different founder effect in each group (Table 1). Interestingly, the patient treated in Germany harbored the same mutation detected in the Mennonites. This suggests a common ancestry and predicts that the mutation precedes the migration of Mennonites from Eastern Europe more than 400 years ago.
Table 1.
Demographic data, clinical presentation and genetic analysis
| Patient | Age of diagnosis | Gender/Ethnicity | Pre BMT features | Mutation analysis |
|---|---|---|---|---|
| 1 | Birth | F/Mennonite | None, cousin with SCID | c.202C>T |
| 2 | 14m | aM/Ecuadorian | Cryptosporidium, Salmonella Group C, FTT, pneumonitis, Omenn syndrome |
c.274+5G>A |
| 3 | 5m | aM/Ecuadorian | CMV pneumonitis, chronic diarrhea with adenovirus Omenn syndrome |
c.274+5G>A |
| 4 | 3m | bF/Japanese | PJP Pneumonitis, CMV, chronic diarrhea | c.275-2A>G |
| 5 | 1 week | M/Mennonite | Cutaneous candidiasis, oral thrush, otitis externa | c.202.C>T |
| 6 | 10m | F/Mennonite | Rotavirus, HHV6 Pneumonitis, FTT | c.202C>T |
| 7 | 13m | cM/Mennonite | Pulmonary aspergillosis, CMV hepatitis, FTT, oral thrush, Otitis |
c.202C>T |
| 8 | 6d | cF/Mennonite | None, sibling with SCID | c.202C>T |
| 9 | 9m | dF/Mennonite | PJP pneumonitis, chronic diarrhea, candida sepsis, FTT | c.202.C>T |
| 10 | Birth | dF/Mennonite | None, sibling with SCID | c.202C>T |
| 11 | Birth | dM/Mennonite | None, cousin with SCID | c.202C>T |
| 12 | Birth | bM/Japanese | None, sibling with SCID | c.275-2A>G |
| 13 | Birth | bM/Japanese | None, sibling with SCID | c.275-2A>G |
CMV: Cytomegalovirus; FTT: Failure to thrive; PJP: Pneumocystis Jiroveci Pneumonia; related patients as indicated
CMV: Cytomegalovirus; FTT: Failure to thrive; PJP: Pneumocystis Jiroveci Pneumonia; related patients as indicated
CMV: Cytomegalovirus; FTT: Failure to thrive; PJP: Pneumocystis Jiroveci Pneumonia; related patients as indicated
CMV: Cytomegalovirus; FTT: Failure to thrive; PJP: Pneumocystis Jiroveci Pneumonia; related patients as indicated
Patients (Pts) presented with typical features of SCID (Table 1) such as pneumonitis due to Pneumocystis jiroveci (Pt 4,9) or CMV infections (Pts 3,4,7), HHV6 (Pt 6), or Aspergillus (Pt 7). Five patients had chronic diarrhea (Pts 2,3,4,6,9) with isolates of adenovirus, rotavirus or Salmonella. Four patients had failure to thrive (FTT) (Pts 2,6,7,9), two had oral thrush (Pts 5,7) and one patient had systemic candidiasis (Pt 9). Pt 2 had Cryptosporidium in addition to viral pneumonitis and FTT. Pts 2, and 3 also had erythrodema as well as lymphadenopathy consistent with Omenn syndrome, suggesting the CD3δ mutations in these patients allowed for a leaky thymus.
Unlike other types of SCID, the thymus in CD3δ was detected by ultrasound and appeared heavily populated with early T cell progenitors, in contrast with the marked peripheral lymphopenia. This pattern could be clearly seen in patients who were diagnosed early before stress or immunosuppression affected the size of the thymus11,16.
Evaluation of the immune system before HSCT
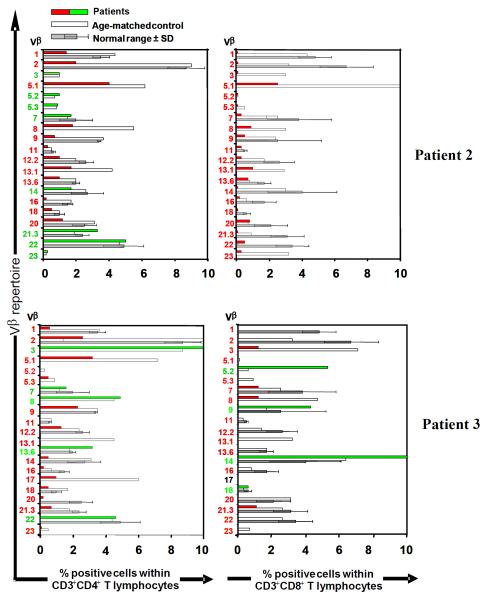
All patients with the exception of Pts 2 and 3, had extremely low (<500 cells/μl) numbers of circulating CD3+ T cells as originally described (Table 2). Pts 2 and 3 had a restricted T cell repertoire with clonally expanded T cells (Figure 1), commonly associated with Omenn syndrome and normal numbers of γδ T cells. In contrast, CD19+ B cells and CD56+ NK cells were comparable in number to controls. Mitogenic responses to in-vitro stimulation with PHA were markedly depressed in all eight patients tested. T cell receptor excision circles (TREC), a reflection of thymopoiesis were undetectable in all 4 patients tested. This was consistent with a maturational arrest of T cells at an early stage of differentiation. Indeed, a thymus biopsy performed in one patient as well as analysis of a thymus at autopsy on another patient revealed an arrest at the CD4/CD8 double negative stage of thymocyte development11,16.
Table 2.
Humoral and cell mediated immunity at presentation
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Normal range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute lymphocyte count (cells/μL) |
1050 | 3800 | 3600 | 758 | 3500 | 640 | 972 | 1530 | 1100 | 3400 | 580 | 996 | 1912 | 4000-10500 |
| Lymphocyte markers (%) | ||||||||||||||
| CD3+ | 0 | 14 | 30 | 1.7 | 1 | 0 | 27 | 1 | 2 | 0.4 | 0 | 0.18 | 0 | 51-77 |
| CD4+ | 0 | 7 | 9 | ND | 0 | 0 | 4 | 5 | 2 | 0 | 0 | ND | 0 | 35-56 |
| CD8+ | 0 | 3 | 9 | ND | 0 | 0 | 15 | <1 | 0.4 | 0.1 | 0 | ND | 23 | 12-28 |
| CD19+ | 66 | 54 | 53 | 67 | 51 | 86 | ND | 65 | 89 | 63 | 71 | 42 | 72 | 6-41 |
| CD56+ | 31 | 29 | 18 | 23 | 44 | 8 | ND | 33 | 7 | 33 | 24 | 57 | 27 | 4-18 |
| PHA | ND | 2a | 2.3a | ND | ND | 0a | 0b | 0b | 0a | 0.6a | 1a | ND | ND | |
| TREC (copies/0.5 μg DNA) | ND | UD | ND | ND | ND | ND | ND | ND | UD | UD | UD | ND | ND | >400 |
| IgE (U/L) | ND | 4000 | 2790 | ND | 120 | ND | ND | ND | <5 | ND | ND | ND | ND | <12 |
| Eosinophils (cell/uL) | 0 | 5200c | 5100 | 90 | 7000 | ND | 10 | 40 | 740 | 590 | 40 | 234 | 241 | 50-700 |
ND: not determined; UD: undetected
% of control stimulation index (Normal >50%),
counts per million (Normal >25,000),
Normal 70-500cells/ul
Figure 1. TCR Vβ repertoire with T cell subsets.
TCR Vβ repertoire within CD4+ and CD8+ T populations was determined by flow cytometry using a collection of anti-TCR Vβ antibodies. Data shown within range or over represented (green) or under represented (red) in comparison with a normal age-matched control (empty bars) and the normal range (grey bars ± SD)15.
Serum immunoglobulins appeared normal for age in all patients (data not shown), but IgE was markedly increased in Pt 2 and 3, but only moderately elevated in Pt. 5 (Table 2). Further, eosinophil counts were increased in all three patients. In patients 2 and 3 T cell repertoire was assessed using flow cytometry to determine representation of a panel of TCR Vβ families. In both patients TCR repertoire was restricted while displaying overrepresentation of several Vβ families (Figure 1).
HSCT procedure
The mean age of transplant was 7 months, ranging from 1-23 months (Table 3). Five patients received stem cells from MUDs, 3 from a bone marrow harvest (MUD-BM). Of these 3, two received a 6/6 HLA identical donor, while in the remaining patient a 9/10 antigen matched donor was used. Of the 2 other patients who received stem cells from unrelated donors, one was a 10/10 matched cord blood (MUD-CB) and the other had a 4/6 antigen mismatched cord blood (MMUD-CB). Eight patients received stem cells from related donors, of which two patients had a 9/10 (non DR) minor mismatch (MRD). In one transplant, bone marrow has been used while the other received peripheral blood stem cells. Six other patients received stem cells from true haploidentical related donors (MMRD). In four of these six cases peripheral blood stem cells were used, while the other two received bone marrow. In one patient, depletion was achieved by anti-CD2 and anti CD8 antibodies, in the others CD34+ cells were extracted with or without CD3 depletion. Two patients received stem cells after depletion of bone marrow with soybean agglutinin and E-rossetting (Pt 6,7).
Table 3.
Stem cell transplantation procedures
| Patient | Age at 1st BMT |
Type of BMT |
HLA Match |
Conditioning |
GVHD Prophylaxis |
||||
|---|---|---|---|---|---|---|---|---|---|
| 1st HSCT | 2nd HSCT | 3rd HSCT | 1st HSCT | 2nd HSCT | 3rd HSCT | ||||
| 1 | 35 d | MRD, BM | MRD, BM, Same donor |
9/10 | Flu 16mg/kg | Flu 150mg/m2, Cam 0.8mg/kg, Melf 140mg/m2 |
Tacrolimus | ||
| 2 | 23 m | MRD, PBSC, CD34+ |
9/10 | Flu 150mg/m2, Bu 140mg/m2, ATG 12.5mg/kg |
Prednisone | ||||
| 3 | 8m | MMRD, PBSC, CD34+ |
Haplo 3/6 |
Flu 150mg/m2, Melf 280/m2, ATG 12.5mg/kg |
none | ||||
| 4 | 4m | MMRD, PBSC, CD2−/CD8− |
Haplo 3/6 |
None | Prednisone | ||||
| 5 | 6 m | MMRD, PBSC, CD34+ CD3− |
MMRD, PBSC, CD34+/CD3−, Cryopreserved, Same donor |
MMRD, BM, CD3-−/CD19−, Same donor |
Haplo 5/10 |
Bu 16mg/kg, Flu 160mg/m2, ATG 10mg 1kg |
CyA | ||
| 6 | 12m | MMRD, PBSC | MMRD, PBSC Same donor |
Haplo 3/6 |
Flu4mg/kg, Cam 24mg |
Bu 16mg/kg, Flu 4mg/kg, Cam 24 mg |
MTX, Tacrolimus (2nd HSCT) |
||
| 7 | 14m | MMRD, BM S+E Maternal |
MMRD, BM S+E Paternal |
MMRD, BM S+E Paternal |
Haplo 3/6 |
ATG 30mg/kg | ATG 80mg/kg, Cyclo 100mg/kg |
ATG 80mg, Cyclo 120mg/kg, TBI |
None |
| 8 | 2 m | MMRD, BM S+E |
MMRD, BM, S+E same donor |
Haplo 3/6 |
Cyclo 200mg/kg ATG 80mg/kg |
None | |||
| 9 | 16 m | MUD, BM | 6/6 | Bu 16mg/kg, Cyclo200mg/kg |
Prednisone, CyA | ||||
| 10 | 4 m | MUD, BM | 6/6 | Bu 16mg/kg, Cyclo 200mg/kg |
Prednisone, CyA | ||||
| 11 | 4 m | MUD, BM | 9/10 | Bu 16mg/kg, Cyclo 200mg/kg |
Prednisone, CyA | ||||
| 12 | 1m | MMUD, Cord | 4/6 | None | MTX, Tacrolimus | ||||
| 13 | 25 d | MUD, Cord | 6/6, 10/10 |
None | MTX, Tacrolimus | ||||
ATG: Anti Thymocyte Globulin; BM: Bone Marrow; Bu: Busulfan; Cam: Campath; CyA: Cyclosporine A; Cyclo: Cyclophophomide; Flu: Fludarabine; HI: Haploidentical; Melf: Melfalane; MTX: Methotrexate; MRD: Matched Related Donor (matched was defined as 9/10 antigen or better); MUD: Matched Unrelated donor; MMRD: Mismatched Related Donor; MMUD: Mismatch Unrelated Donor; PBL: Peripheral Blood; PBSC: Peripheral Blood Stem Cell; TBI: Total body radiation; S+E: Soybean agglutinin and sheep erythrocyte rosettting.
Conditioning was applied in 10/13 patients. Patients with MUD-BM received myeloablation with busulfan and cyclophosphamide. The two patients who received cord blood were not conditioned. Of the six patients who received MMRD, one had no conditioning (Pt 4) while the rest received fludarabine, melphalan, and ATG (Pt 3); busulfan, fludarabine and ATG (Pt 5); fludarabine and CAMPATH (Pt 6); ATG (Pt 7), or cyclophosphamide with ATG (Pt 8). The patients who had a 9/10 MRD-HSCT were conditioned with fludarabine (Pt 1) or with fludarabine, busulfan and ATG (Pt 2). Five patients failed to engraft after the first HSCT. Pt 1 received a second transplant from the same donor after more aggressive conditioning with fludarabine, CAMPATH and melphalan. Four other patients who received HSCT from a MMRD (haploidentical) needed a second (Pt 6,8) or even a third transplant (Pt 5,7) (Table 3).
GVHD prophylaxis was used in all but 3 protocols (Pt 3, 7, 8). Patients with MUD-BM received prednisone and cyclosporine-A, while the two patients who received cord blood were treated with methotrexate and tacrolimus. In the MMRD transplants, prednisone was used as sole medication in Pt 4, cyclosporine-A was used as the sole drug in Pt 5, while the combination of methotrexate and tacrolimus was used in Pt 6. MRD recipients got either tacrolimus(Pt 1) or prednisone(Pt 2).
Engraftment of the hematopoietic system
In the MUD-BM group engraftment was rapid with normal neutrophil counts achieved by 8-12 days post transplant, platelets supplementation was no longer needed 12-35 days after HSCT and red cell transfusion were not needed after 7-30 days (Table 4). The second transplant in PT 1 resulted in a rapid engraftment of neutrophils and platelets within 20 and 24 days post transplant respectively. Multilineage engraftment in Pts 6,7,8 was not achieved and there was no chimerism data for Pts 4, 12 and 13 (Table 4). Achievement of chimerism was virtually 100% in Pts 2,3,5,9,10,11 all of whom received a myeloablative conditioning regimen with either busulfan or fludarabine. Engraftment of the second transplant in Pt 1 also showed 100% donor cells 1 year after the second HSCT. Pt 8 had only 7% donor cells among peripheral mononuclear cells, but T cells were 95% of donor origin (Table 4).
Table 4.
Engraftment and outcome
| Patient | Neutrophils >500 (days after transplant) |
Platelets (days after transplant) |
Red cells (last transfusion/days) |
Chimerism (% donor) |
Time from BMT |
Outcome | Cause of death |
|---|---|---|---|---|---|---|---|
| 1 | 20 | 24 | 30 | 100 | 13m | Alive & well | |
| 2 | 10 | 12 | ND | 100 | 1y | Alive & well | |
| 3 | 12 | 13 | 26 | 100 | 28d | Died | CMV liver failure |
| 4 | ND | ND | ND | ND | 8m | Died | CMV Encephalitis |
| 5 | 18 | 29 | 21 | 100 | 14m | Died | GvHD |
| 6 | ND | ND | ND | Failed to engraft |
Died | GvHD, DIC, HHV6 |
|
| 7 | ND | ND | ND | Engrafted | 14m | Died | CMV Encephalitis, Infection, respiratory failure |
| 8 | ND | ND | ND | 7 ( T cell 95%, B 1%) |
17y | Alive & well | |
| 9 | 12 | 12 | 7 | >95 | 18y | Alive & well | |
| 10 | 12 | 14 | 30 | >95 | 7y | Alive & well | |
| 11 | 8 | 35 | 25 | >95 | 3y | Alive & well | |
| 12 | ND | ND | ND | ND | 4y | Alive & well | |
| 13 | ND | ND | ND | ND | 1y | Alive & well |
Immune reconstitution
Eight of thirteen patients survived and had evidence of immune reconstitution (Table 5). Patients who received MUD BM transplant showed full reconstitution upto 20 years after HSCT (Pt 9,10,11). The number of CD3+ T cells, CD19+ B cells and CD56+ NK cells were normal as were the proportions of CD4+ and CD8+ cells. Mitogenic responses to PHA (Table 5) and anti-CD3 (not shown), T cell repertoire as determined by detection of TCR Vβ families and TREC levels were all comparable to normal controls. Humoral immunity was also normal in all three patients including a robust ability to produce specific antibodies. Immune reconstitution in the MUD-CB case (Pt 13), did not achieve normal levels of circulating CD3 and CD4 cells one year post transplant. However, PHA responses were normal (Table 5) and immunoglobulin levels appear normal. TCRVβ, TRECs and specific antibody production in response to vaccinations were not assessed in this patient. The patient who received a MMUD-CB (Pt 12) had low number of circulating CD3+ and CD4+ T cells but normal responses to PHA, 4 years after transplant. Immunoglobulin levels appear normal but antibody responses to vaccines have not been assessed. The sole survivor of a haploidentical MMRD transplant (Pt 8) has low CD3+ cells 16 years after transplant. PHA responses are normal but T cell receptor repertoire and TRECs were not analyzed, making it difficult to fully appreciate immune reconstitution in this patient. Serum immunoglobulin levels and specific antibodies were normal (Table 5).
Table 5.
Immune Reconstitution after HSCT
| Patient | 1 | 2 | 8 | 9 | 10 | 11 | 12 | 13 | Normal range |
|---|---|---|---|---|---|---|---|---|---|
| Absolute lymphocyte count (cells/μl) | 2724 | 2450 | 930 | 2440 | 2450 | 3400 | 1241 | 2220 | 1200-6000 |
| Time of evaluation after BMT | 1y | 16m | 16y | 20y | 7y | 3y | 4y | 1y | |
| Lymphocyte markers (%) | |||||||||
| CD3+ | ND | 60 | 42 | 75 | 74 | 84 | 34 | 22.5 | 51-77 |
| CD4+ | 21 | 39 | 32 | 47 | 36 | 47 | 24 | 18 | 35-56 |
| CD8+ | 23 | 21 | 9 | 26 | 27 | 31 | 20 | 6 | 21-28 |
| CD56+ | ND | 9 | 43 | 12 | 11 | 4 | 23 | 21 | 6-41 |
| CD19+ | 38 | 29 | 13 | 10 | 14 | 8 | 41 | 58.1 | 4-18 |
| TCR Vβ | ND | ND | ND | Normal | Normal | Normal | ND | ND | |
| PHA | ND | 77b | 105,543c | 68b | 69b | 65b | 285a | 307a | |
| TREC (copies/0.5 μDNA) | ND | ND | ND | Normal | Normal | Normal | ND | ND | >400 |
| Serum Ig (g/l) | |||||||||
| IgG | 7.3 | 8.1 | 12.50 | 10 | 8.5 | 7.2 | 7.71 | 8.5 | 2.3-14.1 |
| IgA | 0.13 | 0.65 | 2.47 | 0.9 | 1.5 | 0.4 | 1.51 | 3 | 0-0.8 |
| IgM | 1.38 | 0.84 | 0.8 | 1.5 | 0.9 | 0.5 | 1.19 | 7.5 | 0-1.7 |
| Tetanus (Iu/ml) | ND | ND | Positive | 2.26 | >7 | 3.5 | ND | ND | >0.13 |
| Polio | ND | ND | Positive | 1:64 | ND | ND | ND | ND | >1:16 |
| Isohemagglutinin | ND | ND | 1:32 | 1:128 | 1:64 | 1:02 | ND | ND | >1:64 |
ND: Not Done
stimulation index (normal 254-388),
% of control stimulation index (Normal >50%),
counts per million (Normal >25,000)
Pts 1 and 2 who received (minor mismatch) MRD have shown signs of engraftment with lower than normal numbers of circulating CD3+ as well as CD4+ T cells. Responses to stimulation with PHA were not performed in Pt 1 and were normal in Pt 2. Serum immunoglobulin levels were normal in both patients but specific antibody levels have not been assessed yet.
Complications after HSCT
Acute GVHD occurred in most patients. Pt 5 had a severe fatal course of GVHD with liver involvement. Pts 9,10,11,12 had mainly skin and gut GVHD graded II-III which was reversed by steroid treatment. Chronic GVHD developed in 2 patients. Pt 1 had a more severe course and is still receiving immunosuppression. Pt 9 has residual vitiligo and hyperpigmentation. Infectious complications after HSCT included CMV infection in 3 patients which were all fatal; although these patients all presented with CMV disease at the time of diagnosis; another patient suffered HHV-6 infection, while one patient had a perineal abscess with Enterobacter cloache. Other complications included autoimmune oophoritis in one patient.
Long term outcome after HSCT
Survival rate was 62% with 8/13 patients alive to date. Five patients who received a HLA haploidentical depleted donor transplant had died; Pt 3 died of CMV related hepatic failure. Pt 4 had failed to engraft and died at 8 months from CMV encephalitis, while Pt 5 died at 14 months from sequels of severe GVHD after 3 transplants. Pt 6 died of GVHD and disseminated intravascular coagulation while, Pt 7 died of CMV encephalitis and respiratory failure. Pts 2,9,10,11,12 remain well with no treatment. Pt 9, now 20 years after HSCT was able to complete her pregnancy and deliver a healthy baby girl. Pt 1 has lost her first graft and suffered from chronic GVHD. She recently received a second bone marrow transplant from the same donor and is doing well. Her autoimmune oophoritis was well controlled.
Discussion
Patients with CD3δ deficiency typically present with a profound T cell lymphopenia, lacking both T cell carrying the αβ or the γδ T cell receptor11. This is in contrast with mice that underwent CD3δ gene deletion, who are markedly leaky for γδ T cells1-4. Unlike other types of SCID, the thymus in CD3δ deficiency is easily detected by ultrasound or chest radiography11,17. Thymic tissue shows marked accumulation of early T cell progenitors, mostly CD4/CD8 double negative cells, and a lack of Hassall’s corpuscles. As expected the thymus appeared more depleted of thymocytes in autopsies of patients due to stress and/or the use of immunosuppressive drugs14. To date the best hope for cure in these patients remains a hematopoietic stem cell transplantation.
However, the presence of residual T cell progenitors as well as normally developed B cells and NK cells raised a dilemma of what strategy should be used for stem cell therapy.
We have therefore studied a group of patients with CD3δ who received different modalities of HSCT. Although the small number of patients did not allow for a detailed statistical analysis some trends emerged.
Five of six patients who received HSCT from a related haploidentical donor died. Three of five presented with CMV disease and one presented with HHV6 pneumonitis which possibly influenced their poor outcome. The fourth patient (pt. 5), in spite of conditioning with busulfan, fludarabine and ATG, showed no signs of T cell engraftment. Subsequently two additional attempts at immune reconstitution were made using stem cells without conditioning failed, and the patient died of GVHD. This case highlights the difficulty in engrafting T cells in patients with CD3δ deficiency.
A similar poor outcome has been already observed before, in three other patients who died after a haplo-identical donor transplantation16. Two of them were very young at diagnosis and transplant, the first died 25 days after transplant from disseminated adenovirus at the age of two months. The second patient was diagnosed at 6 days but died of disseminated aspergillosis at 6 months after BMT16. In sharp contrast, all 5 patients who received an unrelated matched bone marrow or cord blood transplant survived although 4 of these were healthy babies diagnosed because of a previously affected family member. Nevertheless, the largest study of MUD-BMT showed no difference in outcome among patients younger or older than 3 months of age, unlike results from a single center experience using MMRD18. Patients who were treated with MUD-BM showed a robust immune reconstitution which has lasted for more than 20 years in one patient. Moreover, the conditioning regimen with busulfan and cyclophosphamide did not compromise this patient’s ability to conceive. Interestingly, the two patients who were transfused with unrelated matched or mismatched cord blood appeared to engraft without conditioning. Immune reconstitution in these patients should be interpreted cautiously because of the relatively short follow-up time. So far the number of T cells remains low up to 4 years after transplant although PHA responses and serum immunoglobulins appear normal, but specific antibodies, T cell repertoire and TREC’s were not assessed. Long term follow-up should determine whether conditioning is required for effective long term engraftment in patients with CD3δ deficiency who receive cord blood.
To further support the notion that substantial conditioning was required, one patient who received a related HLA-matched donor (9/10 match) bone marrow transplant after mild conditioning, failed to engraft. More aggressive conditioning appeared to have resulted in engraftment. The need for substantial conditioning in patients with SCID who receive a related match donor transplant is unusual and reserved to patients who have a large number of circulating autologous T cells or maternal engraftment. Engraftment in this series was superior in individuals who received myeloablative conditioning regimens. The reason for this phenomenon is not completely understood and should be the subject of future studies. It has been clearly shown though that in the absence of CD3δ, the thymus gland in patients is heavily populated with surviving immature thymocytes. It is possible that one reason for the accumulation of thymocytes is their resilence to apoptosis which perhaps renders them less sensitive to non myeloablative treatments such as ATG.
Interestingly, two patients in this series (Pt 2, 3) presented with typical features consistent with Omenn syndrome. The unique mutation in these patients may have resulted in a leaky thymus as well as the delayed diagnosis in patient 2 (manuscript in preparation). This is the first description of Omenn syndrome in patients with CD3δ deficiency.
The major complications observed included infections and GVHD. Pts 9,10,11,12 had grade II-III skin and gut GVHD which was reversed after treatment with pulse steroids. Two patients developed chronic GVHD (Pt 1,12) of which one (Pt 1) was still treated with immunosuppression. The most significant infections at the time of diagnosis were with CMV, which were fatal in 3 patients.
We present the largest series of patients with CD3δ deficiency that underwent stem cell transplantation and their outcome. Donor-recipient HLA proximity appeared critical as 6/6 patients who received 0-1 antigen mismatched donors as compared with only 2 of 7 patients with more than one antigen mismatch survived. MUD-BM transplant with myeloablative conditioning therapy resulted in excellent long term immune reconstitution. MUD-cord blood appeared to also be effective treatment although full immune reconstitution and long term engraftment have not been established yet. Related matched transplants appear to need substantial conditioning for full engraftment, while the MMRD transplants failed to engraft and led almost invariably to death of these patients, although it must be noted that 4 of the 6 recipients of the MMRD transplants versus only 1 of 5 recipients of MUD transplants had CMV or other life-threatening infectious disease at the time of diagnosis and transplant. Also, of the 6 children diagnosed at birth because of a previously affected family member only 1 received a MMRD transplant (and is a long term survivor) while 4 of the remaining 5 were recipients of a MUD transplant (all survivors). However, we have previously demonstrated that age or severe infections before BMT did not significantly affect survival in patients who received a MUD transplant1. This may not be the case in MMRD transplants according to experience in a single center claiming superior outcome in patients who receive MMRD transplants before the age of 3.5 months18. Nevertheless, no patient in this series received MUD-BM before the age of 4 months. Moreover, immune reconstitution in the MUD treated patients is sustained for many years and remains solid, including a full TCR repertoire and TREC levels, while the sole survivor after MMRD has lymphopenia and her thymic function as well as T cell repertoire are unknown.
In conclusion, with the limitation of a small cohort of patients we observed several factors which might affect survival in CD3δ. 1. HLA matched donors are preferred to mismatched donors. 2. Substantial conditioning is necessary even in RID transplants. 3. Early diagnosis and absence of opportunistic infections.Additional patients are needed to confirm these findings and an updated web-based facility for these and similar rare SCID cases would greatly facilitate therapeutic decisions worldwide.
Clinical Implications.
We tried to identify the optimal stem cell transplantation strategy for CD3δ deficiency. Several factors appear important for full reconstitution, including close HLA matching, conditioning and early diagnosis.
Patients with CD3δ deficiency may achieve full immune reconstitution with HSCT when treated at an early age with a close HLA match after conditioning.
Acknowledgements
This work is supported by the Canadian Centre for Primary Immunodeficiency and the Jeffrey Modell Foundation, and the Donald and Audrey Campbell Chair in Immunology, the NIH-NIAID U54-AI082978, and The David Center, Texas Children’s Hospital.
Funding: This work was supported by The Canadian Centre for Primary Immunodeficiency, The Canadian Immunodeficiency Society, The Jeffrey Modell Foundation and The Donald and Audrey Campbell Chair in Immunology.
Abbreviations
- ATG
Anti thymocyte globulin
- BMT
Bone marrow transplant
- CMV
Cytomegalovirus
- DIC
Disseminated Intravascular Coagulopathy
- FTT
Failure to thrive
- GvHD
Graft versus host disease
- HHV6
Human Herpesvirus Six
- HLA
Human leukocyte antigen
- HSCT
Hematopoietic stem cell transplantation
- MMRD
Mismatched related donor
- MRD
Matched related donor
- MUD
Matched unrelated donor
- NK
Natural killer
- PCR
Polymerase chain reaction
- PHA
Phytohaemagglutinin
- PJP
Pneumocystis jiroveci pneumonia
- Pt
Patient
- RID
Related identical donor
- SCID
Severe combined immunodeficiency
- TCR
T cell receptor
- TREC
T cell receptor excision circle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grunebaum E, Mazzolari E, Porta F, Dallera D, Atkinson A, Reid B, et al. Bone marrow transplantation for severe combined immune deficiency. JAMA. 2006;295:508–18. doi: 10.1001/jama.295.5.508. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–655. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 3.Tsuji Y, Imai K, Kajiwara M, Aoki Y, Isoda T, Tomizawa D, et al. Hematopoietic stem cell transplantation for 30 patients with primary immunodeficiency diseases: 20 years experience of a single team. Bone Marrow Transplant. 2006;37:469–477. doi: 10.1038/sj.bmt.1705273. [DOI] [PubMed] [Google Scholar]
- 4.Dalal I, Reid B, Doyle J, Freedman M, Calderwood S, Saunders F, et al. Matched unrelated bone marrow transplantation for combined immunodeficiency. Bone Marrow Transplant. 2000;25:613–621. doi: 10.1038/sj.bmt.1702215. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak CC, Hung GY, Horn B, Dunn E, Oon CY, Cowan MJ. Megadose CD34+ cell grafts improve recovery of T cell engraftment but not B cell immunity in patients with severe combined immunodeficiency disease undergoing haplocompatible nonmyeloablative transplantation. Biol Blood Marrow Transplant. 2008;14:1125–33. doi: 10.1016/j.bbmt.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Caillat-Zucman S, Le Deist F, Haddad E, Gannagé M, Dal Cortivo L, Jabado N, et al. Impact of HLA matching on outcome of hematopoietic stem cell transplantation in children with inherited diseases: a single-center comparative analysis of genoidentical, haploidentical or unrelated donors. Bone Marrow Transplant. 2004;33:1089–95. doi: 10.1038/sj.bmt.1704510. [DOI] [PubMed] [Google Scholar]
- 7.Gennery AR, Slatter MA, Grandin L, Taupin P, Cant AJ, Veys P, et al. Transplantation of hematopoietic stem cells and long term survival for primary immunodeficiencies in Europe: entering a new century, do we do better? J Allergy Clin Immunol. 2010;126(3):602–10. doi: 10.1016/j.jaci.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91:3646–53. [PubMed] [Google Scholar]
- 9.Klein C, Cavazzana-Calvo M, Le Deist F, Jabado N, Benkerrou M, Blanche S, et al. Bone marrow transplantation in major histocompatibility complex class II deficiency: a single-center study of 19 patients. Blood. 1995;85:580–7. [PubMed] [Google Scholar]
- 10.Antoine C, Müller S, Cant A, Cavazzana-Calvo M, Veys P, Vossen J, et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968-99. Lancet. 2003;361:553–60. doi: 10.1016/s0140-6736(03)12513-5. [DOI] [PubMed] [Google Scholar]
- 11.Dadi HK, Simon AJ, Roifman CM. Effect of CD3delta deficiency on maturation of alpha/beta and gamma/delta T-cell lineages in severe combined immunodeficiency. N Engl J Med. 2003;349:1821–8. doi: 10.1056/NEJMoa031178. [DOI] [PubMed] [Google Scholar]
- 12.Siegers GM, Swamy M, Fernández-Malavé E, Minguet S, Rathmann S, Guardo AC, et al. Different composition of the human and the mouse gammadelta T cell receptor explains different phenotypes of CD3gamma and CD3delta immunodeficiencies. J Exp Med. 2007;204:2537–44. doi: 10.1084/jem.20070782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recio MJ, Moreno-Pelayo MA, Kiliç SS, Guardo AC, Sanal O, Allende LM, et al. Differential biological role of CD3 chains revealed by human immunodeficiencies. J Immunol. 2007;178:2556–64. doi: 10.4049/jimmunol.178.4.2556. [DOI] [PubMed] [Google Scholar]
- 14.Roifman CM. Studies of patients’ thymi aid in the discovery and characterization of immunodeficiency in humans. Immunol Rev. 2005;203:143–55. doi: 10.1111/j.0105-2896.2005.00236.x. [DOI] [PubMed] [Google Scholar]
- 15.van den Beemd R, Boor PP, van Lochem EG, Hop WC, Langerak AW, Wolvers-Tettero IL, et al. Flow cytometric analysis of the Vbeta repertoire in healthy controls. Cytometry. 2000;40:336–45. doi: 10.1002/1097-0320(20000801)40:4<336::aid-cyto9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.de Saint Basile G, Geissmann F, Flori E, Uring-Lambert B, Soudais C, Cavazzana-Calvo M, et al. Severe combined immunodeficiency caused by deficiency in either the delta or the epsilon subunit of CD3. J Clin Invest. 2004;114:1512–7. doi: 10.1172/JCI22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takada H, Nomura A, Roifman CM, Hara T. Severe combined immunodeficiency caused by a splicing abnormality of the CD3delta gene. Eur J Pediatr. 2005;164:311–4. doi: 10.1007/s00431-005-1639-6. [DOI] [PubMed] [Google Scholar]
- 18.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu Rev Immunol. 2004;22:625–44. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]