Abstract
Objective
To determine the impact of hematopoietic deletion of nuclear factor− (erythroid-derived 2) like 2 factor (Nrf2) on the development of atherosclerosis and liver injury in an obese, hypercholesterolemic mouse model.
Methods and Results
Two-month-old male low-density lipoprotein receptor–deficient mice were lethally irradiated and transplanted with either wild type or Nrf2-deficient (Nrf2−/−) bone marrow cells. At 3 months of age, mice were placed on an obesogenic high-fat diet (HFD), high-cholesterol diet for 7 months. Despite no differences in body weight, body fat percentage, liver fat, plasma glucose, lipids, or insulin, the HFD-fed Nrf2−/− bone marrow recipients had increased proinflammatory vascular gene expression, a significant increase in atherosclerosis area (18% versus 28%; P=0.018) and lesion complexity, and a marked increase in liver fibrosis. The acceleration of vascular and liver injury may arise from enhanced macrophage migration, inflammation, and oxidative stress resulting from myeloid Nrf2 deficiency.
Conclusion
Myeloid-derived Nrf2 activity attenuates atherosclerosis development and liver inflammation and fibrosis associated with obesity. Prevention of oxidative stress in macrophage and other myeloid lineage cells may be an important therapeutic target to reduce inflammation-driven complications of obesity.
Keywords: atherosclerosis, nuclear factor− (erythroid-derived 2) like-2 factor, oxidative stress, inflammation, diabetes mellitus, obesity
Oxidative stress induced by aging, metabolic disease, and obesity is a key mechanism of tissue injury. Middle-aged (12–month-old) low-density lipoprotein receptor–deficient (Ldlr−/−) male mice develop both advanced atherosclerosis and all components of nonalcoholic steatohepatitis (NASH) when fed high-fat diet (HFD).1,2 Atherosclerosis and NASH developed in parallel in these mice, similar to humans with metabolic syndrome.3 Both complications were associated with marked tissue deficits in nuclear factor– (erythroid-derived 2) like 2 factor (Nrf2), a major transcriptional regulator of multiple antioxidant and detoxification enzymes.1,2 Loss of Nrf2 and antioxidant activity was implicated in atherosclerosis and NASH development, because oxidative stress is a known mediator of both pathologies.
Subsequent vascular studies of Nrf2 in animal models have been inconsistent. Mice with whole-body Nrf2 deletion demonstrated greater endothelial dysfunction compared with wild-type (WT) mice in response to HFD.4 Vascular upregulation of Nrf2 and its target genes in response to high glucose and H2O2was also blunted in aged rats and macaques, leading to increased vascular oxidative damage.5,6 These data indicate a critical role of Nrf2 in vascular injury. However, whole-body Nrf2 deletion in a nonobese mouse model of atherosclerosis, the apolipoprotein E–deficient mice, resulted in decreased atherosclerosis.7 This was attributed to altered liver and plasma cholesterol, decreased macrophage CD36, and decreased inflammasome activation resulting from systemic effects of Nrf2.7,8
By contrast, several investigations demonstrated that Nrf2 knockout markedly accelerated liver damage in response to HFD and other toxins.9,10 HFD-induced steatosis was associated with increased mitochondrial superoxide production2,11,12 and in some models, hepatic Nrf2 activity losses.2,9,13 Indeed, HFD-fed Nrf2−/− mice had more hepatic lipid and oxidative stress than WT mice and, when fed methionine–choline–deficient diet to induce NASH, a marked increase in inflammation.9,10,14 However, although informative, these studies did not address how Nrf2 loss in specific cell populations impacts liver injury.
Myeloid lineage cells differentiate into foam cells, which make up the cores of atherosclerotic lesions and Kupffer cells, which play a major role in NASH development.15,16 We, thus, hypothesize that Nrf2 deficiency in macrophages would lead to increased vascular and liver damage. To determine the role of hematopoietic Nrf2 in these processes, we transplanted bone marrow from Nrf2−/− mice into middle-aged male Ldlr−/− mice to generate mice with myeloid-specific Nrf2 deficiency. Our results indicate that myeloid Nrf2 deficiency accelerates both atherosclerosis and NASH development.
Materials and Methods
Animals, Diets, and Bone Marrow Transplantation
C57BL/6 background Ldlr−/− mice (Jackson Laboratory, #002207, Bar Harbor, ME) and Nrf2−/− mice (kind gift from Jeff Chan, University of California, Irvine, CA) were group housed under 12 hours light/12 hours dark and fed standard chow (8904, Harlan Teklad, Madison, WI). Baytril-treated mice aged 2 months were irradiated with 9 Gy using a 137Cs irradiator and 4 to 8 hours later injected with 4 million bone marrow cells from WT (C57BL/6J) or Nrf2−/− to generate WT and Nrf2−/− bone marrow transplant (BMT) mice. After 4 weeks, BMT mice were continued on chow or fed HFD (RD Western D12079B, Research Diets, New Brunswick, NJ; described in Table I in the online-only Data Supplement). After 7 months on diet, mice were weighed and analyzed for body composition, then sacrificed by CO2 overdose for assessment of en face atherosclerosis and liver histology as previously described.17 Thoracic aortae and liver samples for gene expression studies were collected from a second set of mice. All animal experiments were approved by The Methodist Hospital Research Institute Institutional Animal Care and Use Committees and complied with all federal, state, and local regulations and policies.
Cell Culture Experiments
Bone marrow for BMT and culture studies was collected from mouse femurs and tibia by syringe perfusion. Cells were cultured for 7 days in bone marrow–derived macrophage (BMDM) differentiation medium (DMEM, 20% fetal bovine serum, and 30% L929-cell conditioned medium) at 37°C in 5% CO2 and then assayed using the QCM Chemotaxis Cell Migration Assay, Fluorometric (ECM507, Chemicon International, Billerica, MA) according to the manufacturer’s instructions. For apoptosis assays, BMDMs were treated with 0, 1, 10, and 100 µmol/L H2O2 for 16 hours and caspase 3/7 activity was measured using ApoTox-Glo Triplex Assay Kits (Promega Corporation, Madison, WI). Peritoneal macrophage (PM) were isolated as previously described,18 lipopolysaccharide (LPS; 100 ng/mL) stimulated for 20 hours in RPMI 1640 media with 10% fetal bovine serum, and then harvested for RNA isolation.
Gene Expression
RNA was isolated using RNeasy kits (QIAGEN), reverse-transcribed with High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Carlsbad, CA), and amplified with Taqman PCR Core Reagent Kits and gene-specific primer/probe sets (Applied Biosystems), with expression values normalized to sample Ppia (Cyclophilin A) expression.
Protein Analyses
Peripheral blood mononuclear cells were isolated from 6 WT and 6 Nrf2−/− BMT mice, by red blood cell lysis, and processed for protein. peripheral blood mononuclear cells western blots were probed with antibodies for catalase (Abcam, Cambridge, MA) and β-actin (Santa Cruz Biotechnology) as a loading control and analyzed by densitometry using National Institutes of Health Image1.60 software.
Histology
En face atherosclerosis was analyzed as previously described.1 After atherosclerosis quantification, Sudan IV-stained vessels were destained in 80% ethanol, embedded in paraffin, sectioned, and stained to determine lesion complexity and composition by light microscopy with hematoxylin/eosin or immunofluorescence microscopy. Necrotic lipid cores (NLC) were quantified by light microscopy in 10 µm-serial sections obtained from the aortic arch and stained with hematoxylin/eosin.19 The area of lesion was defined by the internal elastic lamina and the luminal boundary, and data were calculated as percent NLCs in total lesion area in the sections quantified.20 Formalin-fixed liver tissue was stained with Masson Trichrome to detect collagen or hematoxylin/eosin for contrast. Steatosis, fibrosis, inflammation, and hepatocyte ballooning were assayed according to the NASH diagnosis guidelines of Kleiner et al.21 Vessels were paraffin-embedded for hematoxylin/eosin or dual immunofluorescence staining with F4/80 antibody (eBioscience, San Diego, CA) and Alexa Fluor 647–conjugated secondary antibody (Life Technologies, Carlsbad, CA), and Nrf2 antibody (Santa Cruz Biotechnology) and Alexa Fluor 555–cojugated secondary antibody (Invitrogen, Grand Island, NY). Immunofluorescent images were obtained at 600× magnification using a Nikon A1 confocal microscope and NIS-Elements Microscope Imaging Software.
Plasma Metabolite Analyses
Blood glucose levels were determined using OneTouch (Johnson and Johnson, Milpitas, CA) and plasma insulin was determined by ELISA (Rat/Mouse Insulin ELISA, Millipore, Billerica, MA). Sample aliquots were sent to the Mouse Metabolic Phenotyping Center at the University of Cincinnati for determination of plasma lipids, alanine amino transferase, and aspartate amino transferase levels.
Statistics
Mann-Whitney nonparametric analyses were employed to identify differences for the following pairwise group comparisons: Chow-fed WT versus Nrf2−/− BMT, HFD-fed WT versus Nrf2−/−BMT, Chow-fed versus HFD-fed WT BMT, Chow-fed versus HFD-fed Nrf2−/− BMT. Apoptosis data were analyzed by Kruskal-Wallis 1-way ANOVA with Dunn multiple comparisons tests. PM assay data were analyzed with unpaired Student t tests with Welch’s correction. A 2-tailed α=0.05 was used as the significance cutoff for all tests. Data are presented as mean±SEM and sample sizes are reported in figure legends. Significance is indicated as: *P<0.05, **P <0.01, ***P <0.005, †P<0.05, ††P<0.01, †††P<0.005, and 0.05>°*** P<0.1 (trend). Liver histology scores were reported as percentage of mice within a group presenting a given score of steatosis, ballooning, or fibrosis and were analyzed with Fisher exact test. GraphPad Prism 5.0 software was used for all statistical analyses.
Results
WT BMT and Nrf2−/− BMT Ldlr−/− Mice Have Similar Metabolic Phenotypes in Response to HFD Nrf2−/−
WT BMT-recipient Ldlr−/− mice demonstrated no gross phenotypic differences. Both groups gained similar body weight, body fat percentage, and liver fat and had similar increases in glucose, insulin, and plasma lipids (total cholesterol, triglycerides, and phospholipids) when fed HFD (Table), similar to previously published Ldlr−/− mouse dietary responses.1,22
Table.
WT BMT and Nrf2−/− BMT Mice Have Similar Metabolic Phenotypes With HFD
| Chow | HFD | |||
|---|---|---|---|---|
| WT (n=5) | Nrf2−/− (n=4) | WT (n=10) | Nrf2−/− (n=10) | |
| Body weight, g | 26.08 (0.212) | 27.98 (0.713)** | 34.94 (1.311)††† | 34.46 (1.400)† |
| Body fat, % | 8.05 (0.608) | 11.82 (2.566) | 24.15 (1.304)††† | 25.00 (3.040)†† |
| Liver fat, % | 4.87 (0.730) | 2.93 (0.410) | 15.35 (1.814)††† | 14.59 (1.859)†† |
| Fasted glucose, mg/dL | 81.00 (7.104) | 81.75 (7.510) | 93.90 (4.877) | 88.30 (5.793) |
| Fasted insulin, ng/mL | 1.00 (0.331) | 0.68 (0.148) | 1.45 (0.279) | 1.41 (0.370) |
| Triglycerides, mg/dL | 261.60 (44.435) | 151.59 (25.930) | 246.56 (30.361) | 269.64 (32.185)† |
| Total cholesterol, mg/dL | 630.07 (113.86) | 436.69 (103.43) | 1659.92 (186.70)††† | 1490.52 (156.87)† |
| Phospholipids, mg/dL | 466.12 (60.665) | 337.62 (39.835) | 636.51 (33.497)† | 676.95 (35.004)† |
WT indicates wild type; BMT, bone marrow transplant; Nrf2−/−, nuclear factor− (erythroid-derived 2) like 2 factor deficient; HFD, high-fat diet.
Whereas HFD increased weight, body, and liver fat, cholesterol and phospholipids, they were not different in HFD-fed Nrf2−/− and WT BMT mice. (Mean±SEM,
P<0.01 vs. WT for matched diet;
P<0.05,
P<0.01,
P<0.005 vs. chow for matched genotype).
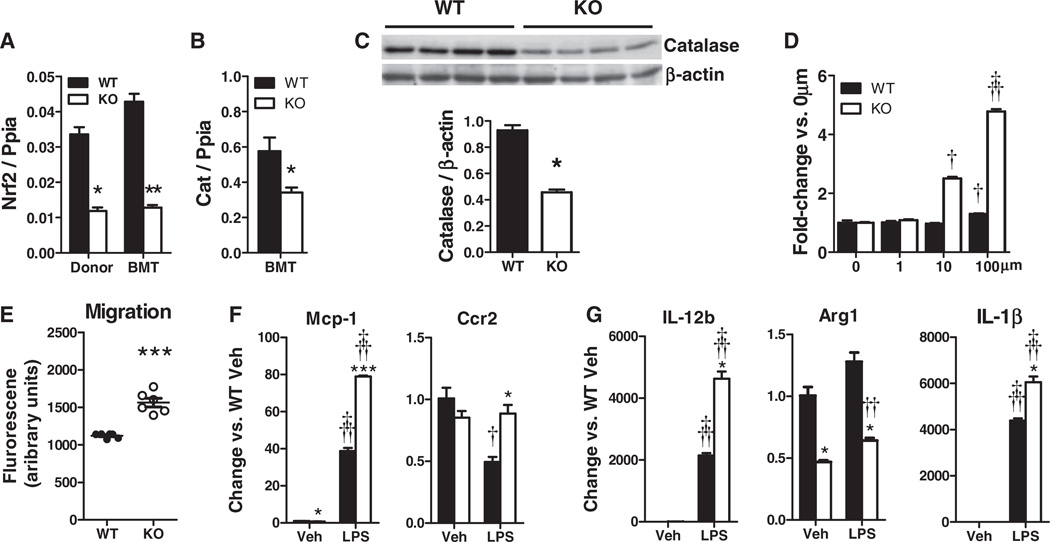
White blood cell abundance and diversity were not different in Nrf2−/− and WT BMT mice (Table II in the online-only Data Supplement). Nrf2 mRNA expression in peripheral blood mononuclear cells of Nrf2−/− BMT mice was similar to that found in Nrf2−/− mice (Figure 1A), indicating full bone marrow replacement, and resulted in corresponding decreases in the mRNA and protein expression of the major Nrf2-regulated antioxidant enzyme catalase, which is required for H2O2 detoxification (Figure 1B and 1C).
Figure 1.
Characterization of nuclear factor– (erythroid-derived 2) like 2 factor−deficient (Nrf2−/−) peripheral blood mononuclear cells (PBMCs) and bone marrow–derived macrophages (BMDMs). A, Nrf2 mRNA, (B) catalase mRNA, and (C) catalase protein are decreased in PBMCs of Nrf2−/− bone marrow transplantation (BMT) mice. D, Nrf2−/− BMDMs have greater susceptibility to apoptosis and Nrf2 deficiency increases macrophage migration in response to monocyte chemoattractant protein-1 (MCP-1). E and F, Gene expression for Nrf2−/− peritoneal macrophage (PM) treated with lipopolysaccharide (LPS) or vehicle. (Mean±SEM; A–C, n=4–8/group, *P<0.05, **P<0.01 vs. wildtype (WT); D, n=6, †P<0.05, †††P<0.005 vs. WT; E–G, n=3, *P<0.05, ***P<0.005 vs. WT for matched treatment; †P<0.05, ††P<0.01, †††P<0.005 vs. vehicle (Veh) for matched genotype). KO indicates knockout; IL, interleukin.
Nrf2 Deficiency Increases Macrophage Apoptosis, Migration, and Inflammatory Responses
Consistent with decreased Nrf2-mediated catalase expression, Nrf2−/− BMDMs exhibited greater apoptosis than WT BMDMs when challenged with H2O2 (Figure 1D). Nrf2−/− BMDM also displayed increased monocyte chemoattractant protein-1 (MCP-1)–induced migration than WT BMDMs (Figure 1E). LPS-induced Nrf2−/− PMs expressed substantially more Mcp-1 than WT PMs and were protected from an LPS-induced decrease in the expression of MCP-1 receptor Ccr2 (Figure 1F). Expression of Arg1, an anti-inflammatory enzyme that attenuates inducible nitric oxide synthase activity, was decreased in Nrf2−/− PMs, whereas LPS-induced expression of the proinflammatory cytokine genes Il12b and Il1b increased (Figure 1G), suggesting more proinflammatory basal and inducible phenotypes.
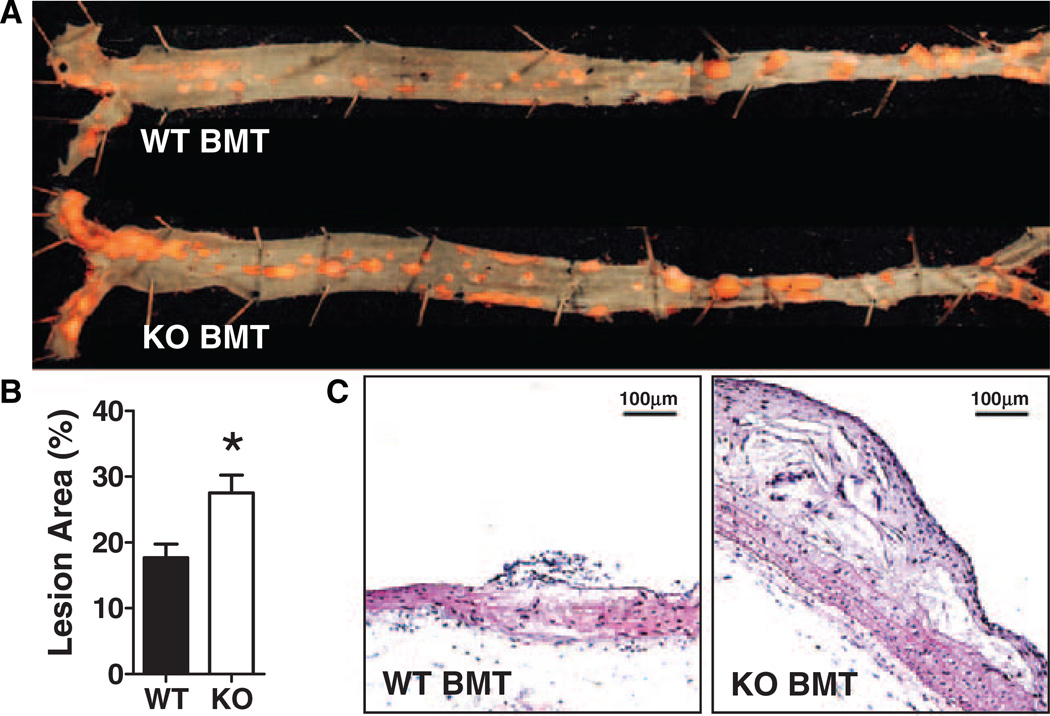
HFD-Fed Nrf2−/− BMT Ldlr−/− Mice Have Increased Atherosclerosis and Necrotic Lipid Core Development
Despite similar metabolic parameters, Nrf2−/− BMT Ldlr−/− mice had significantly more atherosclerotic lesion area than WT BMT Ldlr−/− mice (28% versus 18%, respectively; P=0.018; Figure 2A and 2B), with increased numbers of NLC in their aortic arch lesions (50% versus 14%, respectively; Figure 2C), suggesting that there was increased foam cell death in Nrf2−/− BMT mice, perhaps because of increased susceptibility to oxidative stress. Nrf2 was also highly expressed in the cap areas of accelerated lesions of middle-aged HFD-fed Ldlr−/− mice but decreased in the deeper lesions of these mice that give rise to NLC (Figure I in the online-only Data Supplement). However, Nrf2 was not detected in vascular macrophages of HFD-fed Nrf2−/− BMT Ldlr−/− mice, consistent with successful transplant of Nrf2−/− BMT.
Figure 2.
Atherosclerosis is increased in nuclear factor– (erythroid-derived 2) like 2 factor−deficient (Nrf2−/−) bone marrow transplantation (BMT) vs wild-type (WT) mice. A, Representative aortae stained with Sudan IV; (B) Quantification of en face atherosclerotic area (mean±SEM for n=7–8; *P=0.018). C, Hematoxylin-eosin−stained lesion cross sections detect complex lesions in Nrf2−/− BMT and fatty streaks in WT BMT mice. KO indicates knockout.
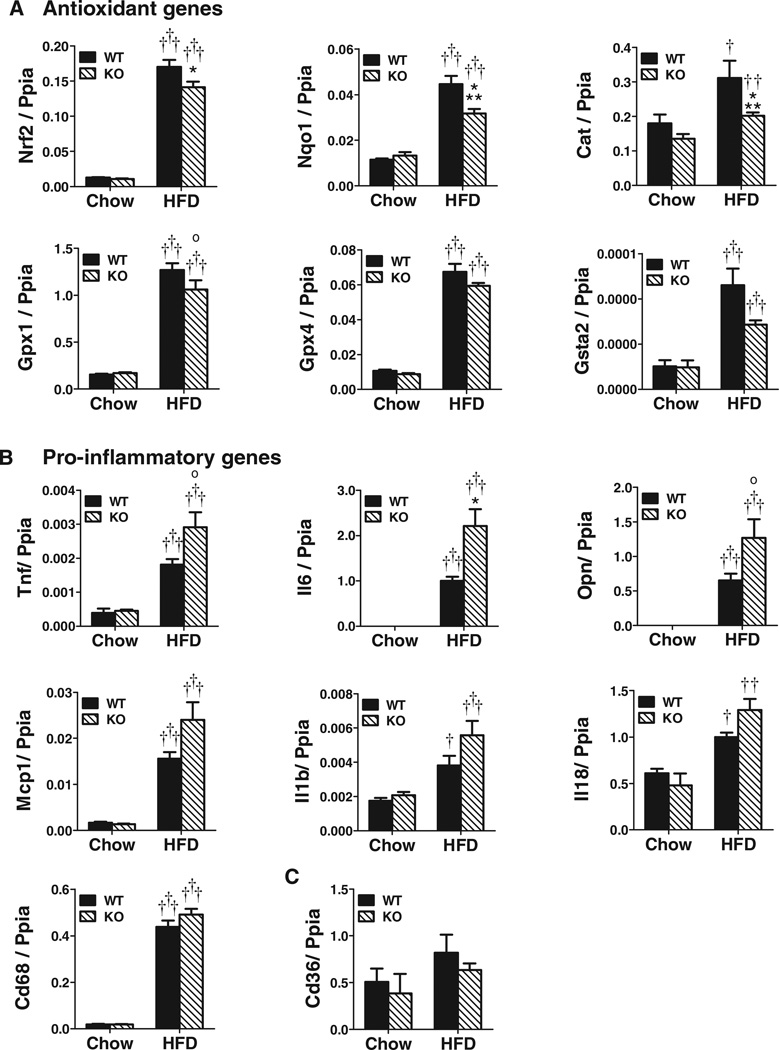
Vessels of HFD-Fed Nrf2−/− BMT Mice Have Reduced Antioxidant Genes and Increased Proinflammatory Genes
Vascular expression of Nrf2 and several Nrf2-regulated antioxidant enzymes, including NAD(P)H dehydrogenase, quinone 1 (Nqo1), catalase (Cat) and glutathione peroxidase 1 (Gpx1), were decreased in HFD-fed Nrf2−/− versus WT BMT mice (Figure 3A), with trends for decreased Gpx4 and glutathione S-transferase alpha 2 (Gsta2). Other Nrf2 target genes, such as superoxide dismutase 2 (Sod2), heme oxygenase 1 (Hmox1), and thioredoxin reductase (Txnrd1), were not different (data not shown), suggesting differential macrophage contributions to vascular antioxidant enzyme expression. Vascular expression of Park7, which protects Nrf2 from degradation, and MafG and MafK, Nrf2 transcriptional partners, were also not different (data not shown). HFD also increased vascular expression of multiple proinflammatory genes more in Nrf2−/− BMT vs. WT BMT mice (Figure 3B), including Tnf, Il6, and Opn, with trends for Mcp1, Il1b and Il18 (Figure 3B). Cd68 expression, a marker of macrophage accumulation, increased with HFD but was not different between HFD-fed Nrf2−/− BMT vs. WT BMT mice, nor was vascular expression of Cd36, a major cholesterol uptake protein implicated in previous Nrf2 studies (Figure 3C). This general decrease in antioxidant enzyme expression coupled with increased proinflammatory cytokine expression, could contribute to the increased atherosclerotic phenotype of HFD-fed Nrf2−/− BMT vs. WT BMT mice.
Figure 3.
High-fat diet− (HFD) fed nuclear factor– (erythroid-derived 2) like 2 factor−deficient (Nrf2−/−) bone marrow transplant (BMT) mice reveal enhanced proatherosclerotic gene expression in comparison with wild-type (WT) BMT mice. Expression of (A) antioxidant genes, (B) proinflammatory genes, and (C) Cd36. (Mean±SEM, n=4–9/group; *P<0.05, ***P<0.005 and 0.05>°P<0.1 (trend) vs. WT for matched diet, †P<0.05, ††P<0.01, †††P<0.005 vs. chow for matched genotype). Nqo1 indicates NAD(P)H dehydrogenase, quinone 1; Gpx4, glutathione peroxidase 4; KO, knockout; Mcp1, monocyte chemoattractant protein-1; Tnf, transforming growth factor; OPN, osteopontin.
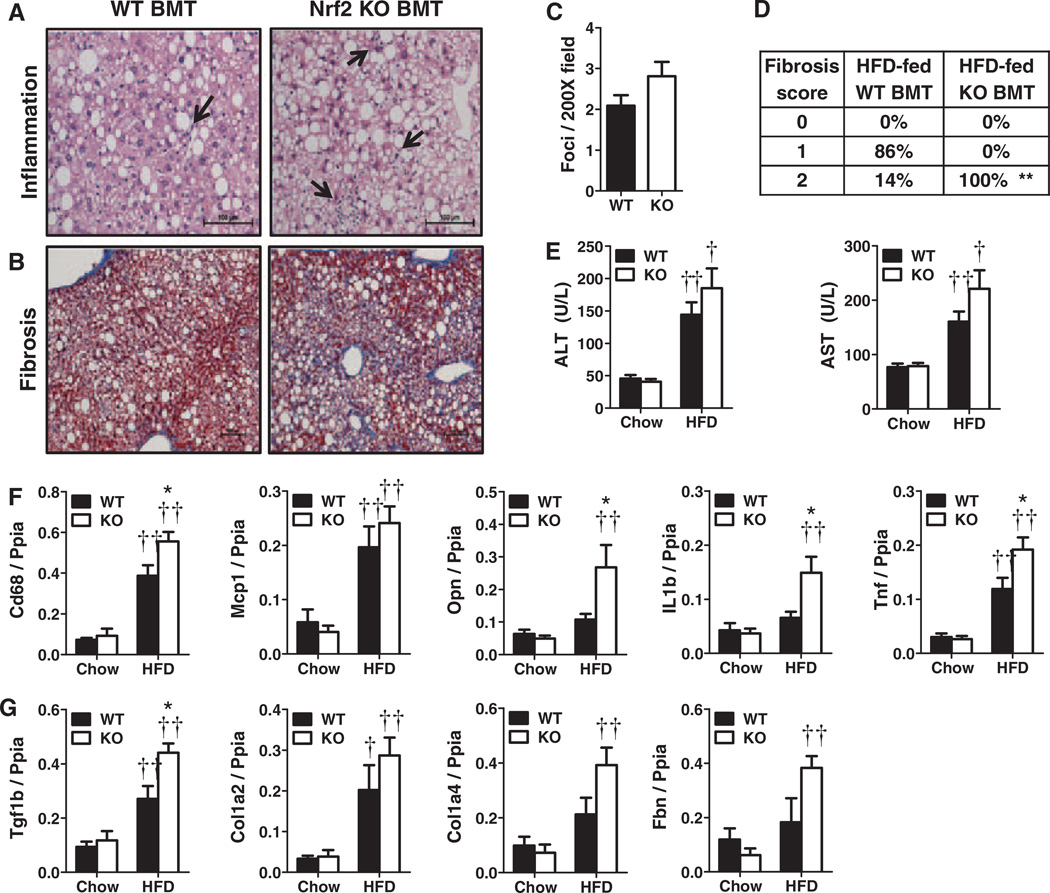
Myeloid Nrf2 Deficiency Increases HFD-Induced Liver Inflammation and Fibrosis
HFD-fed Nrf2−/− BMT and WT BMT Ldlr−/−mice developed similar amounts of liver fat assessed by nuclear magnetic resonance (Table). Although ballooning degeneration was similar, Nrf2−/− BMT mice had slightly more micro- and macrovesicular steatosis by histological assessment (Figure II in the online-only Data Supplement). Liver Nrf2, Sod2 and Cat mRNA expression similarly increased in both groups of HFD-fed mice (Figure II in the online-only Data Supplement). Livers of HFD-fed Nrf2−/− BMT mice, however, exhibited a trend towards increased inflammatory cell foci (Figures 4A and 4C; P=0.11), and more fibrosis than WT BMT mice (Figure 4B and 4D), in agreement with a trend toward increased blood transaminase levels (Figure 4E) suggestive of greater liver injury in HFD-fed Nrf2−/− BMT mice. Livers of HFD-fed Nrf2−/− BMT mice also displayed significantly more expression of several proinflammatory marker genes (Cd68, Opn, Il1b and Tnf) compared to WT BMT mice (Figure 4F). In agreement with histological fibrosis results, Nrf2−/− BMT mice also revealed increased hepatic expression of the profibrotic cytokine transforming growth factor β1 (Tgfb1) as well as several fibrotic proteins, including type 1 and 4 collagens (Col1a2 and Col4a1) and fibronectin (Fbn) (Figure 4G). There were no differences in expression of hepatic Cd36, 3-hydroxy-3-methylglutaryl-coenzyme A reductase, or other lipid metabolism genes (data not shown).
Figure 4.
(Nrf2−/−) bone marrow transplant (BMT) increases inflammation and fibrosis in liver. Representative histology of (A) inflammatory foci (arrows), and (B) fibrosis. C, Inflammatory foci per ×200 field (n=3–7/group); (D) hepatic fibrosis scores (n=4–7/group); (E) plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST); Hepatic (F) proinflammatory and (G) fibrotic gene expression. (Mean±SEM, n=4–8/group; *P<0.05, **P<0.01 vs. WT for matched diet, †P<0.05, ††P<0.01 vs. chow for matched genotype). KO indicates knockout; HFD, high-fat diet; IL, interleukin; Tnf, tumor necrosis factor; Tgf1b, transforming growth factor.
Discussion
This investigation provides evidence that myeloid Nrf2 deficiency enhances both HFD-induced atherosclerosis area and complexity, and liver inflammation and fibrosis. Enhanced atherosclerosis development and liver injury in this study are likely mediated by increased migration, inflammation, and oxidative stress responses of Nrf2-deficient inflammatory cells. These results strongly suggest that Nrf2 activity in macrophages, Kupffer cells and perhaps in other myeloid lineage cell types plays an important role in the attenuation of these 2 common obesity-associated pathologies. Taken together, these results may have therapeutic implications to ameliorate vascular and liver complications of metabolic syndrome driven by inflammation and oxidative stress.
Macrophages are critical mediators of atherosclerosis that take up oxidized cholesterol in the vessel wall to form foam cells, whose subsequent deaths lead to NLC formation. The dynamics of NLC formation and resolution are major determinants of atherosclerosis extent and lesion complexity. Reducing macrophage apoptosis, by deletion of the proapoptotic factor C/EBP homologous protein23,24 or by deletion of Stat1 to prevent endoplasmic reticulum stress, attenuates plaque necrosis.25 Conversely, changes that promote apoptosis, including deletion of the insulin receptor or Bcl-2, even early in the atherosclerotic process, increase NLC formation and lesion complexity.26,27 We previously showed that HFD-fed young Ldlr−/− that develop fatty streaks have increased vascular expression of Nrf2-regulated antioxidant genes, whereas HFD-fed middle-aged Ldlr−/− mice that develop advanced lesions have decreased vascular Nrf2 expression corresponding to the onset of NLC development, suggesting that vascular oxidative stress promotes the observed NLC formation.1 Our current results link macrophage Nrf2 deficiency with increased foam cell death, a major cause of NLC formation. We find that Nrf2-deficient macrophages have increased apoptosis in response to H2O2-induced oxidative stress, consistent with a report that cells from Nrf2−/− mice have an impaired antioxidant response, decreased expression of prosurvival factors, and increased apoptosis and decreased survival when exposed to oxidative stress28 and electronegative LDL,29 despite no differences in basal levels of reactive oxygen species in Nrf2-deficient cells. Similarly, in a mouse emphysema model, Nrf2−/− mice receiving WT BMTs had improved pulmonary function and decreased apoptosis of their alveolar macrophages.30 Several reports, thus, indicate that Nrf2 deficiency is associated with enhanced macrophage apoptosis to oxidative stress.
We and others have shown that impairment of macrophage migration and proinflammatory responses decreases atherosclerosis.31–33 Enhanced macrophage migration and inflammation may contribute to increased atherosclerosis in Nrf2−/− BMT mice. Vascular expression of the macrophage chemoattractant osteopontin was higher in Nrf2−/− BMT than WT BMT mice. LPS-treated Nrf2−/− macrophages also expressed more MCP-1 than WT macrophages, without a corresponding downregulation of the MCP-1 receptor C-C chemokine receptor type 2, indicating that Nrf2−/− macrophages may be both more chemoattractive and responsive to migration factors than WT macrophages, as observed in our in vitro studies. Cellular oxidative stress generates H2O2, which promotes cell migration; thus, reduced antioxidant responses are implicated in cell movement.34 Nrf2−/− BMT mice have reduced expression of several antioxidant genes in response to HFD, including catalase, which can attenuate vascular injury. In Apoe−/− mice, catalase overexpression reduced atherosclerosis and was associated with both decreased vascular oxidative stress and reduced acellular area in aortic sinus lesions, indicative of decreased macrophage death.35 Catalase overexpression is also associated with decreased vascular macrophage accumulation in a hindlimb ischemia model and with impaired MCP-1–directed macrophage migration in vitro.36 Other studies have demonstrated that Nrf2 affects the migration of multiple cancer cell types and leukocytes in arthritis.37,38
Our atherosclerosis results differ from those reported for Apoe−/−Nrf2−/− mice, which developed less atherosclerosis than Apoe−/− mice.7,8,39 This difference likely results from the mouse models. Ldlr−/− mice require HFD to develop the hypercholesterolemia required to drive atherosclerosis. However, HFD also induces central adiposity and metabolic syndrome, and obesity-associated oxidative stress may drive target organ damage in Ldlr−/− mice. Apoe−/− mice develop spontaneous hypercholesterolemia in the absence of HFD and do not become grossly obese even when fed HFD.40 Indeed, apolipoprotein E overexpression is reported to induce metabolic syndrome.41 Nrf2 may also be differentially regulated in Apoe−/− and Ldlr−/− mice, because apolipoprotein E has been reported to decrease hepatic Nrf2 expression,42 whereas differences between whole-body and bone marrow Nrf2 deficiency may also contribute to phenotype differences. For example, 1 study found that Apoe−/−Nrf2−/− mice have decreased plasma and liver cholesterol, which was proposed to partially account for the decreased atherosclerosis in these mice.7 We did not observe significant differences in plasma cholesterol, triglycerides, or phospholipids in our Nrf2−/− BMT Ldlr−/− mice, suggesting that hepatocyte Nrf2 deficiency contributes to these lipid changes. Another study found smaller plaques in Apoe−/−Nrf2−/− mice, which were associated with decreased uptake of modified LDL by isolated macrophages.39 Both studies attributed the decreased atherosclerosis observed in Apoe−/−Nrf2−/− mice to decreased macrophage CD36 expression.7 However, Apoe−/−Cd36−/−steroid receptor RNA activator−/− mice do not have decreased lesions or foam cell formation versus control mice, suggesting that reduced macrophage CD36 expression is unlikely to attenuate atherosclerosis.43
Nrf2 is also reported to regulate inflammasome-mediated interleukin-1β secretion. Macrophages of Apoe−/−Nrf2−/− mice express less interleukin-1β, which is associated with decreased atherosclerosis without altered plasma cholesterol.8 NLR family, pyrin domain containing 3 inflammasome-deficiency also attenuated atherosclerosis in apolipoprotein E −/−–deficient mice, suggesting that inflammasome activation by cholesterol impacts this process.44 Despite this potential effect on Nrf2−/− macrophage interleukin-1β, we found that in Nrf2−/− BMT mice HFD markedly increased vascular expression of other inflammatory cytokine genes known to promote atherosclerosis, including Tnf, Opn, and Il6, with trends for Mcp1. Thus, HFD and myeloid Nrf2 deficiency appears to strongly drive multiple inflammatory responses in the vasculature of Ldlr−/− mice.
Atherosclerosis and fatty liver disease often occur together and are associated with similar risk factors. For example, elevated liver enzymes in the setting of obesity are a risk factor for both coronary artery disease and NASH.44 Kupffer cells, specialized liver macrophages, are key drivers of liver inflammation.42 Despite similar fat content, livers of HFD-fed Nrf2−/− versus WT BMT mice revealed a trend toward more inflammatory cell foci coupled with significantly increased expression of CD68 and several proinflammatory genes. These changes were accompanied by a 2-fold increase in fibrosis and increased expression of fibrosis genes, consistent with evidence that hepatic inflammation leads to fibrosis.45 Steatosis is proposed as the first hit in fatty liver disease, whereas oxidative stress represents a second hit that promotes progression from nonalcoholic fatty liver disease to NASH, characterized by inflammation and fibrosis.46 Stellate cells, the primary source of liver collagen, are quiescent under normal conditions but can become activated, proliferate, and increase collagen production in response to inflammation and oxidative stress. Thus, it appears likely that increased HFD-induced liver inflammation, resulting from Nrf2 deletion in Kupffer cells and invading macrophages, may activate stellate cells. Nrf2 activation has been reported to inhibit α-smooth muscle actin and transforming growth factor-1β expression and function in a mouse liver fibrosis model,47 further suggesting that Nrf2 activity plays an important role in the attenuation of liver fibrosis. Meher et al48 showed that myeloid Nrf2 deficiency is not sufficient to protect mice from HFD-induced insulin resistance, consistent with our findings that insulin or glucose levels were not different between WT BMT and Nrf2−/− BMT mice (Table). However in their study, Nrf2−/− BMT mice had less weight gain and fat mass with HFD, which may be responsible for their lack of effects of myeloid Nrf2- blation on adipose tissue inflammation.48
In summary, we observed that the loss of antioxidant responses in hematopoietic cells leads to increased macrophage migration, inflammation, and oxidative stress. These proinflammatory changes likely contributed to NLC formation and accelerated atherosclerosis and to accelerated hepatic fibrosis, both of which are highly uncommon in obese mouse models. This escalation of 2 major pathologies in HFD-fed Nrf2−/− BMT mice underscores the importance of Nrf2 to protect tissues from leukocyte-mediated inflammatory responses in the face of overwhelming oxidative stress. Taken together these data indicate that loss of Nrf2 in inflammatory cells increases the severity of common obesity-associated complications, such as NASH and atherosclerosis, and that treatments designed to enhance intracellular antioxidant pathways specifically in inflammatory cells might have beneficial effects in this setting.
Supplementary Material
Acknowledgments
The authors are very grateful to Jeff Chan (University of California, Irvine) for providing Nrf2−/− mice. Lipid analyses were performed by the University of Cincinnati Mouse Metabolic Phenotyping Core DK059630.
Sources of Funding
Funding for these experiments was provided by the MacDonald Foundation (W.A. Hsueh) and R24DK087723 (W.A. Hsueh). A.A. Gupte was supported by a postdoctoral award from American Heart Association.
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.300345/-/DC1.
Disclosures
None.
References
- 1.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Praticò D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104:e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 2.Gupte AA, Liu JZ, Ren Y, Minze LJ, Wiles JR, Collins AR, Lyon CJ, Pratico D, Finegold MJ, Wong ST, Webb P, Baxter JD, Moore DD, Hsueh WA. Rosiglitazone attenuates age- and diet-associated nonalcoholic steatohepatitis in male low-density lipoprotein receptor knockout mice. Hepatology. 2010;52:2001–2011. doi: 10.1002/hep.23941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Targher G, Bertolini L, Padovani R, Rodella S, Arcaro G, Day C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J Hepatol. 2007;46:1126–1132. doi: 10.1016/j.jhep.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Ungvari Z, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol. 2011;300:H1133–H1140. doi: 10.1152/ajpheart.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ungvari Z, Bailey-Downs L, Gautam T, Sosnowska D, Wang M, Monticone RE, Telljohann R, Pinto JT, de Cabo R, Sonntag WE, Lakatta EG, Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, Losonczy G, Ballabh P, de Cabo R, Sonntag WE, Csiszar A. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol. 2011;301:H363–H372. doi: 10.1152/ajpheart.01134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, Wang X, Castellani LW, Reue K, Lusis AJ, Araujo JA. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhry S, Nazmy MH, Meakin PJ, Dinkova-Kostova AT, Walsh SV, Tsujita T, Dillon JF, Ashford ML, Hayes JD. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radical Biology & Medicine. 2011;48:357–371. doi: 10.1016/j.freeradbiomed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto H, Okada K, Shoda J, Warabi E, Ishige K, Ueda T, Taguchi K, Yanagawa T, Nakahara A, Hyodo I, Ishii T, Yamamoto M. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;298:G283–G294. doi: 10.1152/ajpgi.00296.2009. [DOI] [PubMed] [Google Scholar]
- 11.Videla LA, Rodrigo R, Orellana M, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci. 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Tabbi-Anneni I, Gunda V, Wang L. Transcription factor Nrf2 regulates SHP and lipogenic gene expression in hepatic lipid metabolism. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1211–G1221. doi: 10.1152/ajpgi.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita K, Tamiya G, Ando S, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wouters K, van Gorp PJ, Bieghs V, Gijbels MJ, Duimel H, Lütjohann D, Kerksiek A, van Kruchten R, Maeda N, Staels B, van Bilsen M, Shiri-Sverdlov R, Hofker MH. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 2008;48:474–486. doi: 10.1002/hep.22363. [DOI] [PubMed] [Google Scholar]
- 17.Collins AR, Meehan WP, Kintscher U, Jackson S, Wakino S, Noh G, Palinski W, Hsueh WA, Law RE. Troglitazone inhibits formation of early atherosclerotic lesions in diabetic and nondiabetic low density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:365–371. doi: 10.1161/01.atv.21.3.365. [DOI] [PubMed] [Google Scholar]
- 18.Li YM, Baviello G, Vlassara H, Mitsuhashi T. Glycation products in aged thioglycollate medium enhance the elicitation of peritoneal macrophages. J Immunol Methods. 1997;201:183–188. doi: 10.1016/s0022-1759(96)00224-4. [DOI] [PubMed] [Google Scholar]
- 19.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, Rabadán-Diehl C, Goldberg IJ. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007;100:1415–1427. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- 20.Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. doi: 10.1385/1-59259-340-2:293. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsukano H, Gotoh T, Endo M, Miyata K, Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K, Mizuta H, Oike Y. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2011;30:1925–1932. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 25.Lim WS, Timmins JM, Seimon TA, Sadler A, Kolodgie FD, Virmani R, Tabas I. Signal transducer and activator of transcription-1 is critical for apoptosis in macrophages subjected to endoplasmic reticulum stress in vitro and in advanced atherosclerotic lesions in vivo. Circulation. 2008;117:940–951. doi: 10.1161/CIRCULATIONAHA.107.711275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubota T, Kubota N, Moroi M, Terauchi Y, Kobayashi T, Kamata K, Suzuki R, Tobe K, Namiki A, Aizawa S, Nagai R, Kadowaki T, Yamaguchi T. Lack of insulin receptor substrate-2 causes progressive neointima formation in response to vessel injury. Circulation. 2003;107:3073–3080. doi: 10.1161/01.CIR.0000070937.52035.25. [DOI] [PubMed] [Google Scholar]
- 27.Thorp E, Li Y, Bao L, Yao PM, Kuriakose G, Rong J, Fisher EA, Tabas I. Brief report: increased apoptosis in advanced atherosclerotic lesions of Apoe−/− mice lacking macrophage Bcl-2. Arterioscler Thromb Vasc Biol. 2009;29:169–172. doi: 10.1161/ATVBAHA.108.176495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merchant AA, Singh A, Matsui W, Biswal S. The redox-sensitive transcription factor, Nrf2, regulates murine hematopoietic stem cell survival independent of ROS levels. Blood. 2011;118:6572–6579. doi: 10.1182/blood-2011-05-355362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedrosa AM, Faine LA, Grosso DM, de Las Heras B, Boscá L, Abdalla DS. Electronegative LDL induction of apoptosis in macrophages: involvement of Nrf2. Biochim Biophys Acta. 2010;1801:430–437. doi: 10.1016/j.bbalip.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Ishii Y, Itoh K, Morishima Y, Kimura T, Kiwamoto T, Iizuka T, Hegab AE, Hosoya T, Nomura A, Sakamoto T, Yamamoto M, Sekizawa K. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol. 2005;175:6968–6975. doi: 10.4049/jimmunol.175.10.6968. [DOI] [PubMed] [Google Scholar]
- 31.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawson TC, Kuziel WA, Osahar TA, Maeda N. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 1999;143:205–211. doi: 10.1016/s0021-9150(98)00318-9. [DOI] [PubMed] [Google Scholar]
- 33.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 34.Park JG, Oh GT. The role of peroxidases in the pathogenesis of atherosclerosis. BMB Rep. 2011;44:497–505. doi: 10.5483/bmbrep.2011.44.8.497. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 36.Hodara R, Weiss D, Joseph G, Velasquez-Castano JC, Landázuri N, Han JW, Yoon YS, Taylor WR. Overexpression of catalase in myeloid cells causes impaired postischemic neovascularization. Arterioscler Thromb Vasc Biol. 2011;31:2203–2209. doi: 10.1161/ATVBAHA.111.233247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maicas N, Ferrándiz ML, Brines R, Ibáñez L, Cuadrado A, Koenders MI, van den Berg WB, Alcaraz MJ. Deficiency of Nrf2 accelerates the effector phase of arthritis and aggravates joint disease. Antioxid Redox Signal. 2011;15:889–901. doi: 10.1089/ars.2010.3835. [DOI] [PubMed] [Google Scholar]
- 38.Rachakonda G, Sekhar KR, Jowhar D, Samson PC, Wikswo JP, Beauchamp RD, Datta PK, Freeman ML. Increased cell migration and plasticity in Nrf2-deficient cancer cell lines. Oncogene. 2010;29:3703–3714. doi: 10.1038/onc.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS ONE. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreyer SA, Lystig TC, Vick CM, LeBoeuf RC. Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis. 2003;171:49–55. doi: 10.1016/j.atherosclerosis.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Karagiannides I, Abdou R, Tzortzopoulou A, Voshol PJ, Kypreos KE. Apolipoprotein E predisposes to obesity and related metabolic dysfunctions in mice. FEBS J. 2008;275:4796–4809. doi: 10.1111/j.1742-4658.2008.06619.x. [DOI] [PubMed] [Google Scholar]
- 42.Graeser AC, Boesch-Saadatmandi C, Lippmann J, Wagner AE, Huebbe P, Storm N, Hoppner W, Wiswedel I, Gardemann A, Minihane AM, Doring F, Rimbach G. Nrf2-dependent gene expression is affected by the proatherogenic apoE4 genotype-studies in targeted gene replacement mice. J Mol Med. 2011;89:1027–1035. doi: 10.1007/s00109-011-0771-1. [DOI] [PubMed] [Google Scholar]
- 43.Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008;78:185–196. doi: 10.1093/cvr/cvm093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2011;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomobe K, Shinozuka T, Kuroiwa M. Nomura YmeAge-related changes of Nrf2 and phosphorylated GSK-3beta in a mouse model of accelerated aging (SAMP8) Arch Gerontol Geriatr. 2011;54:e1–e7. doi: 10.1016/j.archger.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med. 2009;87:679–695. doi: 10.1007/s00109-009-0464-1. [DOI] [PubMed] [Google Scholar]
- 47.Sun LY, Bokov AF, Richardson A, Miller RA. Hepatic response to oxidative injury in long-lived Ames dwarf mice. FASEB J. 2011;25:398–408. doi: 10.1096/fj.10-164376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meher AK, Sharma PR, Lira VA, Yamamoto M, Kensler TW, Yan Z, Leitinger N. Nrf2 deficiency in myeloid cells is not sufficient to protect mice from high-fat diet-induced adipose tissue inflammation and insulin resistance. Free Radic Biol Med. 2012;52:1708–1715. doi: 10.1016/j.freeradbiomed.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.