Abstract
The adenosine triphosphate-binding cassette (ABC) transporter G5/G8 is critical in protecting the body from accumulating dietary plant sterols. Expressed in the liver and small intestine, it transports plant sterols into the biliary and intestinal lumens, thus promoting their excretion. The extent to which G5/G8 regulates cholesterol absorption remains unclear. G5/G8 is also implicated in reducing the absorption of dietary triacylglycerols (TAG) by unknown mechanisms. We hypothesized that G5/G8 suppresses the production of chylomicrons, and its deficiency would enhance the absorption of both dietary TAG and cholesterol. The aim of this study was to investigate the effects of G5/G8 deficiency on lipid uptake and secretion into the lymph under steady-state conditions. Surprisingly, compared with wild-type mice (WT) (n = 9), G5/G8 KO (n = 13) lymph fistula mice given a continuous intraduodenal infusion of [3H]-TAG and [14C]-cholesterol showed a significant (P < 0.05) reduction in lymphatic transport of both [3H]-TAG and [14C]-cholesterol, concomitant with a significant (P < 0.05) increase of [3H]-TAG and [14C]-cholesterol accumulated in the intestinal lumen. There was no difference in the total amount of radiolabeled lipids retained in the intestinal mucosa between the two groups. G5/G8 KO mice given a bolus of TAG showed reduced intestinal TAG secretion compared with WT, suggesting an independent role for G5/G8 in facilitating intestinal TAG transport. Our data demonstrate that G5/G8 deficiency reduces the uptake and secretion of both dietary TAG and cholesterol by the intestine, suggesting a novel role for the sterol transporter in the formation and secretion of chylomicrons.
Keywords: ABCG5/G8, Sterol, Cholesterol, Triacylglycerol, Absorption, Intestine, Lymph
Introduction
The small intestine is the primary site of lipid absorption. According to the 2000–2006 National Health and Nutrition Survey, an average American adult consumes about 80 g/day of fat (triacylglycerols or TAG), 300 mg of cholesterol, and 100–400 mg of plant sterols. While the small intestine absorbs large loads of TAG very efficiently, up to 500 g per day in humans [1], it only absorbs a fraction of cholesterol. Absorption of cholesterol in healthy individuals is 10–80 % [2–4] while absorption of plant sterols, which are structurally similar to cholesterol, are virtually unabsorbed [5]. For a long time, the existence of an intestinal transporter that limited the absorption of plant sterols was questioned but the limit is now known to be mediated by ATP-binding cassette (ABC) half-transporters G5/G8 [6].
Expressed in the liver and small intestine, G5 and G8 heterodimerize into a functional unit [7] to promote the secretion of sterols, with a preference for plant sterols over cholesterol [9]. In the liver, they are expressed on the apical membrane of hepatocytes [8] and secrete both cholesterol and plant sterols into the bile [10–12]. In the small intestine, they are presumed to be expressed on the apical brush border membrane of enterocytes and secrete cholesterol and plant sterols into the intestinal lumen [13]. Mutations in either G5 or G8 result in a loss of secretion [6, 14], clinically manifested as the autosomal recessive genetic disease of sitosterolemia [15]. Patients absorb plant sterols [16–18] and lose ability to secrete plant sterols into bile, resulting in drastically elevated plasma plant sterol levels which ultimately lead to extensive xanthomas, arthritis, and premature atherosclerosis. The pathophysiological defects underlying the disease highlight the importance of understanding the mechanism by which G5/G8 works to excrete sterols from the body.
In regards to sterol absorption, G5/G8 is well-established to limit the absorption of plant sterols [6, 11, 12, 19–21]. However, the extent to which G5/G8 limits cholesterol absorption is unclear. It is important to note that sitosterolemic patients have normal or slightly above-average plasma cholesterol levels [22] as well as normal cholesterol absorption [23, 24]. Interestingly, G5/G8 is also recently implicated in intestinal TAG metabolism, in which G5/G8 deficiency in mice was found to increase total TAG absorption and secretion by the intestine [25]. What is not known is whether G5/G8 serves to limit the absorption of other dietary lipids, not just of cholesterol, by affecting the production of chylomicrons.
The objective of the study in this report was to test the hypothesis that G5/G8 limits the production of chylomicrons in the small intestine by investigating the effects of G5/G8 deficiency on (1) the transport rate of dietary TAG and cholesterol into the lymph, and (2) the stage(s) of the absorptive process that may be affected, such as uptake, mucosal assimilation, or secretion into the lymph. We employed the well-established conscious lymph fistula model to directly analyze the steady-state transport rate of lipids across the enterocyte.
Materials and Methods
Animals
Male G5/G8 KO mice previously generated by Yu et al. [10] were kindly provided to us and were backcrossed >8 generations to yield animals that were nearly of a pure C57BL6/J background. Genotype was verified by PCR. Only 3–4 month old mice were used for the study. G5/G8 KO and WT mice were housed (3–4 mice per cage) in temperature-controlled (21 ± 1° C) cages under a normal 12:12 h light–dark cycle at the University of Cincinnati Laboratory Animal Medical Services. The mice had free access to water and standard rodent chow (LM-485 Mouse/Rat Sterilizable Diet, Harlan Laboratories).
Body Composition and Plasma Lipid Analysis
Mice were fasted overnight. Body weights were measured and body composition was determined by nuclear magnetic resonance (NMR) analysis. On a separate day, fasting blood samples were drawn from the tail vein into heparin capillary tubes. Fasting cholesterol and TAG were measured from plasma using Infinity cholesterol assay kits (Thermo-Fisher Scientific, Middletown, VA) and Total TAG assay kits (Randox Laboratories, Kearneysville, WV). Plasma sterol composition was analyzed by gas chromatography (GC) using 5-alpha cholestane as the internal standard. Plasma samples were heated in alcoholic KOH in glass test tubes at 65 °C for 2 h. Sterols were extracted with petroleum, dried under nitrogen and dissolved in hexane for GC analysis. Quantitative analyses were based on standard curves for cholesterol, sitosterol, and campesterol generated during the same GC run.
Lymph and Duodenal Cannulation
Before surgery, the mice were fasted overnight with free access to water. Anesthesia comprised of ketamine (80 mg/kg) and xylazine (20 mg/kg) was administered intraperitoneally. Under anesthesia, the main mesenteric lymph duct was cannulated with a polyvinylchloride tube (0.20 mm inner diameter; 0.50 mm outer diameter) according to procedures first described by Bollman et al. [26] which were later adapted by us for the mouse [27–29]. Due to the delicate nature of the mouse lymph duct, the cannula was secured with a drop of cyanoacrylate glue (Krazy Glue, Columbus, OH, USA) instead of a suture. A second PVC tube (0.5 mm inner diameter, 0.8 mm outer diameter) was introduced through the fundus of the stomach into the duodenum and secured by a purse-string suture. The fundal incision was closed by the cyanoacrylate glue. Following surgery, a 5 % salineglucose solution containing 145 mmol/L NaCl, 4 mmol/L KCl, and 0.28 mol/L glucose was infused into the duodenal cannula at a constant rate of 0.3 mL/h to replenish fluids and electrolytes lost from lymph drainage. The animals were allowed to recover overnight in restraining cages maintained at 30° C to prevent hypothermia caused by surgical stress and single-animal housing. Despite being restrained, the animals still had considerable freedom to move. All procedures were performed in accordance with the University of Cincinnati Internal Animal Care and Use Committee and in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Preparation of Lipid Infusate
Lipid emulsions were prepared containing 4 µmol triolein [labeled with [9,10-3H(N)]-triolein, 1 µCi (Perkin-Elmer, Waltham, MA)] 0.78 µmol cholesterol [labeled with [1,2-14C(N)]-cholesterol, 0.1 µCi (Perkin-Elmer, Waltham, MA)], 0.78 µmol egg phosphatidylcholine, and 5.7 µmol sodium taurocholate (Sigma, St. Louis, MO, USA), sonicated in 0.3 ml phosphate-buffered saline (pH 6.4). Homogeneity was verified by determining the radioactivity sampled from the top, middle, and bottom of the emulsion. Emulsions were considered homogeneous if the counts did not vary >1 %. The lipid dose was chosen based on the average daily fat and cholesterol intake for mice fed a standard rodent chow diet (4 % fat, 0.02 % cholesterol by weight). The total amount of fat and cholesterol delivered over the course of the 6 h study approximated the total daily physiologic intake of lipids.
Experimental Procedure
The morning following surgery, the 5 % glucose-saline solution was replaced with the lipid emulsion described above. The emulsion was continuously infused into the duodenum for 6 h at a constant rate of 0.3 mL/h. Lymph samples were collected every hour into pre-cooled conical centrifuge tubes. Since the animals are conscious during lipid infusion, our experiments lack the complications of anesthesia which is known to dampen the production of chylomicrons [30]. At the end of the 6 h, mice were anesthetized for tissue and organ removal. The stomach, small intestine, colon, and their luminal contents were collected for lipid analysis. The mucosa tissue of the small intestine was left intact, cut into 4 equal length segments, and labeled M1–M4 (M1—duodenum, M2 and M3—two equal segments of the jejunum, M4—ileum). The intestinal segments were homogenized using a Polytron homogenizer. The luminal contents of the stomach, small intestine, and colon were collected from three rinses of 10 mM NaTC. Radioactivity of each sample was measured by beta-scintillation. Total lymph TAG and cholesterol were measured using Total TAG assay kits (Randox Laboratories, Kearneysville, WV, USA) and Infinity cholesterol assay kits (Thermo-Fisher Scientific, Middletown, VA, USA).
Absorptive and Transport Index Calculations
Absorptive and lymph transport indexes were calculated as previously described [31]. The absorptive index represents the percentage of infused lipid taken up by the enterocyte and is calculated from the following equation: absorptive index = 100 % − % total dose recovered in the lumen. The lymphatic transport index represents the percentage of absorbed lipid secreted into the lymph and takes into consideration the uptake of lipid by the small intestine. This index is very useful to characterize the ability of the small intestine to transport lipid into lymph as chylomicrons and is calculated from the equation: Lymphatic transport index = % infused dose recovered in lymph/absorptive index × 100.
Intestinal TAG Secretion Studies
Plasma TAG in mice (fasted overnight) was measured using a total TAG assay kit (Randox Laboratories, Antrim, UK). Intestinal TAG secretion was measured a week later to minimize stress-induced variables caused by handling. To measure intestinal TAG secretion, mice (fasted overnight) were given an intraperitoneal injection of Poloxamer 407 at 1 mg/g body weight, which was previous shown to be optimal for mice to block lipoprotein lipase activity [32]. Immediately after injection, the mice were gavaged with 1 µCi [3H]-triolein in 150 µL olive oil. Tail blood was drawn immediately and hourly for 4 h following gavage into heparin capillary tubes. Radioactivity in the plasma samples was measured by scintillation spectroscopy. The intestinal secretion rate was derived from the average amount of radioactivity secreted over 4 h.
Statistical Analysis
The data shown are mean values ± standard errors (SE). To compare groups throughout the 6 h infusion period, two-way repeated measures analysis of variance (ANOVA) with Tukey post-test analysis were used. For comparison of data that have 2 independent variables, 2-way ANOVA was used. A t test was used for comparisons of only 2 groups. Statistical analyses were performed using GraphPad Prism v6. Data were considered significant if P < 0.05.
Results
Body and Plasma Parameters of G5/G8 KO and WT Mice
No significant differences were found in body weight, fat and lean mass, fasting plasma cholesterol and TAG levels between G5/G8 KO and WT mice (Table 1) although G5/G8 KO mice tended to have a lower body weight with less fat mass. G5/G8 KO mice had elevated plasma levels of sitosterol and campesterol compared with WT, in ratios similar to previous reports in chow-fed mice [10, 25].
Table 1.
Body weight, composition and fasting plasma parameters of G5/G8 KO compared with WT control mice
| WT | G5/G8 KO | |
|---|---|---|
| Body weight (g) | 32.3 ± 0.8 | 30.1 ± 0.6 |
| Fat mass (g) | 3.7 ± 0.5 | 2.6 ± 0.4 |
| Lean mass (g) | 26.4 ± 0.5 | 25.1 ± 0.4 |
| Plasma cholesterol (mg/dL) | 87 ± 6.3 | 87.5 ± 8.3 |
| Plasma sitosterol (mg/dL) | – | 25 ± 4.7 |
| Plasma campesterol (mg/dL) | – | 14.1 ± 3.9 |
| Plasma TAG (mg/dL) | 62.3 ± 6.5 | 54.5 ± 5.6 |
“–” sterols were not detectable by GC
Lymphatic TAG and Cholesterol Transport Rate
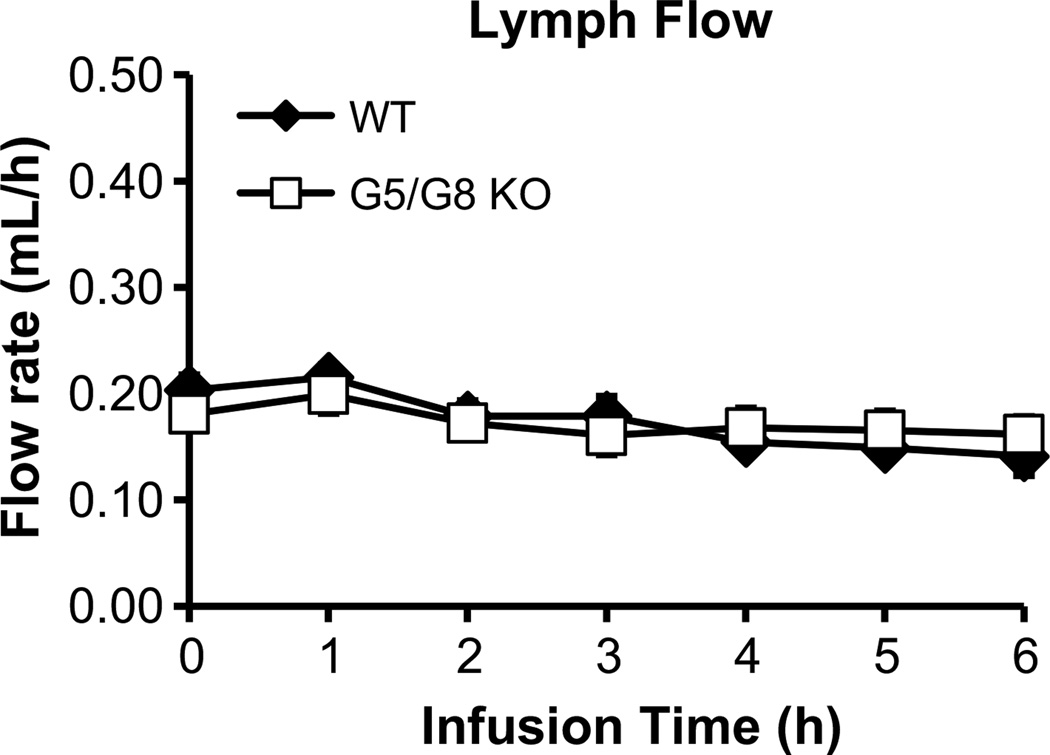
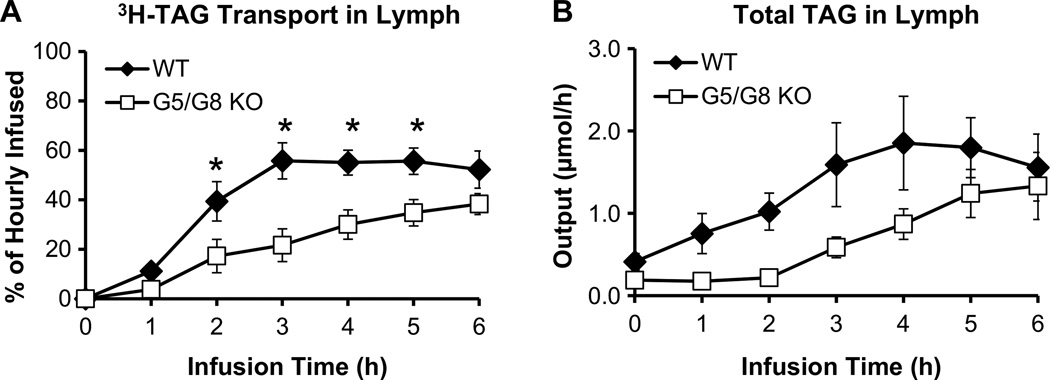
Infusion of the lipid emulsion at 0.3 mL/h, produced a steady lymph flow of 0.17–0.20 mL/h for both groups of mice (Fig. 1). This showed that lymph flow would not be a variable affecting chylomicron output [33]. In WT mice, the lymphatic [3H]-TAG transport rate reached a steady state of 58 % of the hourly dose after 3 h (Fig. 2a), which agreed with previous findings that steady-state absorption is achieved by 4 h [34–36]. In contrast, G5/G8 KO mice showed a significant (P < 0.05) reduction in the lymphatic [3H]-TAG transport rate compared with WT, which was evident at 2 h and maximal at 3 h of infusion (Fig. 2a). The difference tapered at the end of the infusion period, suggesting that G5/G8 KO mice experienced a delay in reaching maximal TAG transport rates. Output of TAG mass in the lymph was also reduced in G5/G8 KO mice (Fig. 2b) and paralleled the output of radiolabeled tracer. These results were surprising because G5/G8 KO mice were previously reported to have increased absorption of TAG [25].
Fig. 1.
Lymph flow was maintained at a steady continuous rate throughout the 6 h infusion period for both G5/G8 KO (n = 13) and wild-type control (n = 9) mice. Mesenteric lymph flow was measured at hourly intervals immediately after lipid infusion for 6 h. Values are means ± SE
Fig. 2.
G5/G8 KO mice (n = 13) display lower TAG transport into the lymph compared to WT control mice (n = 9). Lymphatic output of a [3H]-TAG and b total TAG are expressed as a percentage of the infused hourly dose and molar output, respectively. Lymph fistula mice were intraduodenally infused with a lipid emulsion containing [3H]-TAG and [14C]-cholesterol for 6 h. Values are means ± SE
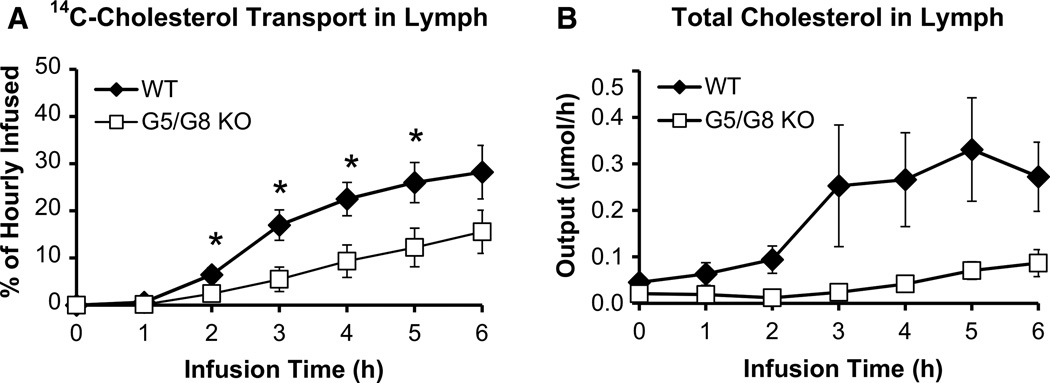
G5/G8 KO mice also showed a significant (P < 0.05) reduction in the lymphatic transport rate of [14C]-cholesterol compared with WT, which was evident at 2 h (Fig. 3a). While WT mice achieved a lymphatic [14C]-cholesterol transport rate of 25 % of the hourly dose at 6 h, G5/G8 KO mice only achieved 15 % of the hourly dose. These results correlated with the output of total lymph cholesterol (Fig. 3b), although G5/G8 KO mice displayed greater variability. Thus, the reduction in lymphatic cholesterol transport by G5/G8 KO mice applied to both exogenous (radiolabeled) and endogenous plus exogenous (total) cholesterol outputs.
Fig. 3.
G5/G8 KO mice (n = 13) displayed lower cholesterol transport into the lymph compared to WT control mice (n = 9). Lymphatic output of a [14C]-cholesterol and b total cholesterol are expressed as a percentage of the hourly infused dose and molar output, respectively. Lymph fistula mice were intraduodenally infused with a lipid emulsion containing [3H]-TAG and [14C]-cholesterol for 6 h. Values are means ± SE
Distribution of Radiolabeled TAG and Cholesterol Along Various Segments of the Small Intestine
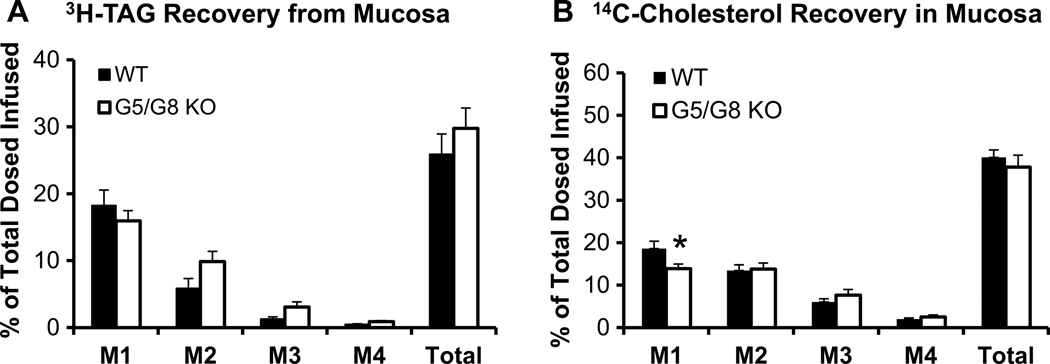
Figure 4 shows the distribution of the amount of [3H]-TAG and [14C]-cholesterol in the mucosa of intestinal segments, M1–M4 (M1 representing the duodenum, M2 and M3 the jejunum, M4 the ileum). There was no difference between the groups in the amount of [3H]-TAG in any of the segments (Fig. 4a). There was significantly less (P < 0.05) [14C]-cholesterol in only the duodenum of G5/G8 KO mice (Fig. 4b, M1). Thus, although G5/G8 KO mice had decreased lymphatic outputs of [3H]-TAG and [14C]-cholesterol, the data show that the lipids did not accumulate in the mucosa.
Fig. 4.
Distribution of a [3H]-TAG and b [14C]-cholesterol along the small intestine divided into 4 segments of equal length, from proximal to distal: M1, M2, M3, and M4 (M1—duodenum, M2 and M3—jejunum, M4—ileum). Radioactivity was determined by scintillation counter. *P < 0.05. Values are means ± SE
Total Recovery of Radiolabeled TAG and Cholesterol at the End of 6 h
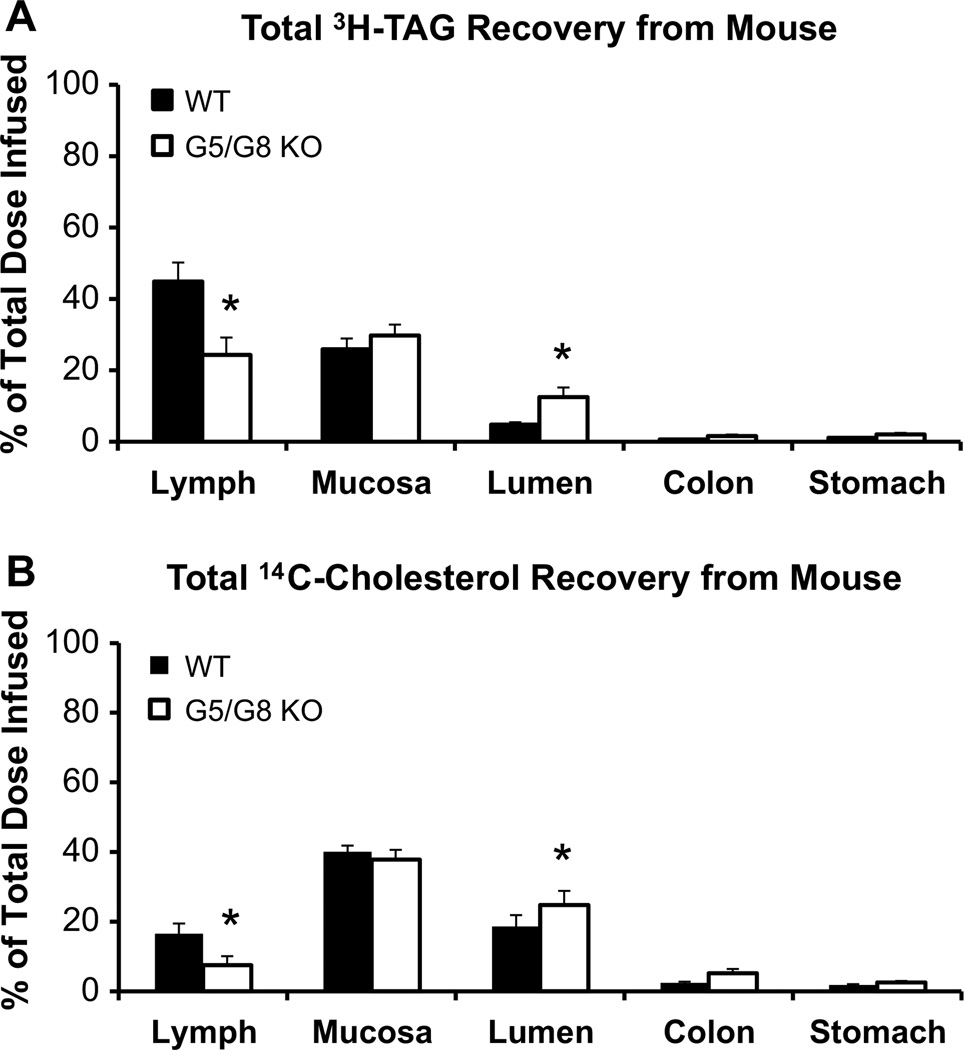
At the end of the 6 h, G5/G8 KO mice showed a significant (P < 0.05) reduction of [3H]-TAG in the lymph, no difference in the mucosa, and an increased (P < 0.05) amount recovered from the intestinal lumen (Fig. 5a). Very little [3H]-TAG refluxed into the stomach and little was recovered from the colon, indicating that most of the TAG we infused did not pass on into the feces during the experiment (Fig. 5a).
Fig. 5.
Total recovery of a [3H]-TAG and b [14C]-cholesterol in WT control (n = 9) and G5/G8 KO (n = 13) mice at the end of the 6 h lipid infusion. Lymph refers to the total radiolabeled lipid collected from the lymph over the 6-h infusion period. Mucosa refers to radioactivity recovered after homogenization of the small intestine. Lumen refers to small intestinal luminal contents that were washed and collected at the end of infusion. Very low amounts of [3H]-TAG and [14C]-cholesterol were recovered from the colon and stomach, indicating little excretion and reflux, respectively. Radioactivity was determined by scintillation counter. *P < 0.05. Values are means ± SE
The total recovery of [14C]-cholesterol was very similar to the total recovery of [3H]-TAG (Fig. 5b). G5/G8 KO mice showed a significant (P < 0.05) reduction of [14C]-cholesterol in the lymph, no difference in the intestinal mucosa, and an increased (P < 0.05) amount recovered from the intestinal lumen compared with WT. Very low amounts of [14C]-cholesterol were found in the lumens of the stomach and colon from both groups (Fig. 5b).
When quantifying the efficiency of lipid uptake and secretion by the intestines of the animals (Table 2), we found that G5/G8 KO mice had a lower (P < 0.05) absorptive index for [3H]-TAG, indicating that the intestines of G5/G8 KO mice had a reduced capacity to take up [3H]-TAG. G5/G8 KO mice also had a lower (P < 0.05) lymphatic transport index for [3H]-TAG than WT. This finding is important because it shows that TAG is not only less readily taken up but also its subsequent secretion is poorer in G5/G8 KO mice compared with WT. As with TAG, G5/G8 KO mice had a significantly (P < 0.05) lower absorptive index as well as a lower (P < 0.05) lymphatic transport index for [14C]-cholesterol than WT, indicating that G5/G8 KO mice had a compromised ability to absorb cholesterol and transport the absorbed cholesterol into lymph. Taken together, the data clearly indicates that a loss of G5/G8 function causes impairment in both the uptake and lymphatic transport of dietary TAG and cholesterol into lymph. How G5/G8 is affecting the mechanism of uptake and the packaging of absorbed lipids into chylomicrons warrants further study.
Table 2.
Comparison of lipid absorptive and lymph transport indexes for [3H]-TAG and [14C]-cholesterol in WT control (n = 9) and G5/G8 KO mice (n = 13)
| TAG | Cholesterol | |||
|---|---|---|---|---|
| Absorptive index | Lymphatic transport index | Absorptive index | Lymphatic transport index | |
| WT | 95.2 ± 0.7 | 47.0 ± 5.3 | 77.1 ± 3.4 | 20.6 ± 3.1 |
| G5/G8 KO | 87.5 ± 2.7* | 26.8 ± 4.9* | 67.4 ± 4.8* | 9.5 ± 2.9* |
P < 0.05.
Values are means ± SE
Intestinal TAG Secretion
Since G5/G8 KO mice were previously shown to have an increased intestinal secretion of TAG [25], we determined the intestinal secretion of TAG in our G5/G8 KO mice using a different cohort of animals. In contrast, G5/G8 KO mice showed lower (P < 0.05) intestinal secretion of [3H]-TAG (n = 6, 10.1 ± 1.3 % total dose/h) compared with WT (n = 6, 15.3 ± 1.5 % total dose/h). Fasting plasma TAG and cholesterol were similar between the two groups (Table 1).
Discussion
In the present study, we found that G5/G8 deficiency in mice decreases the transport rate of both dietary TAG and cholesterol into the lymph. The overall reduction of lipids delivered to the lymph was accompanied by their accumulation in the intestinal lumen. Interestingly, the lipids did not accumulate in the intestinal mucosa. The data show that G5/G8 deficiency suppresses the transport of TAG and cholesterol from lumen to lymph, suggesting a novel role of G5/G8 in facilitating the production of chylomicrons.
G5/G8 deficiency did not affect the body weight, body composition, or fasting plasma TAG and cholesterol levels, although G5/G8 KO mice tended to have a lower body weight with less fat mass (Table 1). Under steady state conditions of lipid transport, G5/G8 KO mice were delayed in reaching maximal TAG transport rates into the lymph (Fig. 2) as well as showed decreased overall transport of TAG from lumen to lymph (Fig. 5a; Table 1). G5/G8 KO mice were no different from WT mice in the mucosal accumulation of TAG (Fig. 4a), suggesting that G5/G8 deficiency did not increase the intracellular TAG pools of the enterocyte. This is unlike the case of CD36 deficiency where TAG accumulated in the mucosa, manifesting in large cytosolic lipid droplets and reducing intestinal TAG secretion [27, 37]. Overall the data suggest that dietary TAG uptake into the enterocyte and its secretion into the lymph are limited in the state of G5/G8 deficiency.
These results were surprising because G5/G8 deficiency was previously found to increase absorption and secretion of TAG [25]. However, when we measured intestinal TAG secretion into the plasma, we observed decreased intestinal TAG secretion in G5/G8 KO mice, which is consistent with our lymphatic transport results. The difference in results may be attributed to the genetic background of the mice used in the two studies (129S6SvEv × C57BL/6 J strain used in their study versus a nearly pure C57BL/6 J background used in our study), which could potentially introduce phenotypic variability [38, 39].
Interestingly, G5/G8 deficiency also decreased the lymphatic transport rate, as well as mass output, of cholesterol (Fig. 3). This decrease was observed concomitant with an increase of lipid accumulated in the intestinal lumen (Fig. 5b; Table 1), suggesting that G5/G8 deficiency also limited the uptake and secretion of dietary cholesterol. A study by Wang et al. [20] had reported that G8 KO mice had increased cholesterol transport into the lymph. There are two key differences in our study design that may explain the difference in our observations. Firstly, to account for variations in bile composition, the Wang et al. [40] diverted the bile duct in animals and supplied “model bile” comprised of taurocholate and phospholipid. The lipid infusate included only a trace amount of radiolabeled cholesterol. In a system where luminal cholesterol is deficient, it is possible that G5/G8 may efflux cholesterol better under these conditions, especially in the presence of bile salt and phospholipid, which are good cholesterol acceptors. Secondly, they supplied medium-chain fatty acids as the vehicle and hence, TAG-rich chylomicrons are minimally produced in their system. Unknown is whether cholesterol transport is altered with the addition of dietary TAG in G5/G8 KO animals. The question of whether cholesterol transport can be altered in the presence of TAG was also posed by Sontag et al. [41], which they believed cholesterol absorption rates are different in delivery vehicles using medium-chain TAG versus long-chain TAG.
Because G5/G8 is presumed to efflux sterols into the intestinal lumen, its deficiency would expectedly accumulate cholesterol in the mucosa. Also, since G5/G8 is expressed differentially along the length of the intestine [42], which is thought to contribute to differential degrees of absorption [20, 21, 43], we speculated that G5/G8 deficiency would accumulate lipids differently in the various intestinal segments. However, we found that G5/G8 deficiency did not affect the mucosal accumulation of cholesterol, except slightly in the duodenum (Fig. 4b). These results complement the report from Wang et al. [20], who found no differences in [14C]-cholesterol retained in any of the intestinal segments between G8 KO and WT mice after measuring acute uptake 45 min after lipid dose. Nyugen et al. [21]reported that G5/G8 KO mice accumulated [14C]-cholesterol in the jejunum; however, this was seen after a bolus dose of mixed sterols. It is also important to note that acute conditions differ from steady-state conditions in terms of measuring regional differences in mucosal uptake [44]. Regardless of slight differences in regional uptake, the data from this study and other laboratories clearly demonstrate that G5/G8 deficiency does not significantly impact the accumulation of lipids in the mucosa.
Since the transport processes of both TAG and cholesterol are affected from lumen to lymph, the findings illustrate a critical role for G5/G8 in the formation and secretion of chylomicrons. G5/G8 KO mice do not have altered gene expressions of microsomal transfer protein (MTP) and apolipoprotein B, NPC1L1 protein levels [45] nor have markers that would indicate ER stress [21], all of which would affect chylomicrons assembly. Interestingly, a recent report by Nguyen et al. [21] also observed G5/G8 KO mice having reduced cholesterol transport into the lymph, concomitant with reduced TAG associated with chylomicrons. After analyzing chylomicron composition, they concluded that G5/G8 KO mice had abnormal chylomicron assembly. They speculated that the plant sterols in their lipid infusate caused the reduction in lymph cholesterol. However, we did not infuse plant sterols and still found a dramatic reduction in the lymphatic transport of TAG and cholesterol in G5/G8 KO mice, suggesting that the effect was independent of plant sterols. However, since the mice were maintained on a chow diet prior to fasting, we cannot exclude the possibility that plant sterols derived from the chow may have caused interference.
Reports on cholesterol absorption in G5/G8 KO mice have varied and may be attributed to the methods used. Earlier studies found no difference in fractional cholesterol absorption between G5/G8 KO and WT mice [10, 19]. However the method used in these reports, the fecal dual-isotope ratio method [46], may be inappropriate for G5/G8 KO mice because the method depends critically on a non-absorbable radiolabeled plant sterol marker [3, 45], which is well-absorbed in G5/G8 deficient mice [20]. Hence, we used the conscious lymph fistula model to directly determine the absorption of dietary lipids into the lymph, as well as to measure their accumulation in the intestinal lumen and mucosa under steady-state conditions of lipid transport.
G5/G8 is implicated in the transintestinal cholesterol efflux (TICE) pathway, which is responsible for the reverse transport of cholesterol from the plasma to the basolateral membrane of the enterocyte, its efflux into the intestinal lumen, and subsequent elimination into the feces [40, 47]. However, G5 deficiency was found to only affect 4 % of the daily TICE contribution to cholesterol excretion [48], suggesting a minor role for G5/G8 in this reverse transport. Also, the rates of TICE were found to range between 1–2 nmol/min × 100 g body weight [40], which is considerable less (~10 fold) than the steady-state transport rate of cholesterol into the lymph (Fig. 3b). Although our study was not designed to examine TICE, we believe that TICE has limited contribution to our findings.
Our study has potential limitations. Firstly, the animals used in the study were maintained on standard rodent chow which has a considerable amount of plant sterols and accumulate in the plasma of G5/G8 KO mice. As a result, the plant sterols derived from plasma may interfere with the transport processes of TAG or cholesterol in the intestine, in our study as well as of other investigators [10, 20, 25]. Secondly, G5/G8 KO mice are known to have less biliary cholesterol [10], which may be a variable in our analysis. However, the cholesterol provided in our lipid emulsion per hour is substantially more (~12 fold) than that of bile, assuming a gallbladder volume of 16.5 µL of a WT mouse [49] with a biliary cholesterol concentration of 4 µmol/mL [10], making the contribution of biliary cholesterol minimal in our study.
In conclusion, although the exact mechanism is still unknown of how G5/G8 handles TAG and cholesterol in the intestine, our studies show that a loss of G5/G8 function causes a backup of lipid trafficking across the enterocyte and subsequent production of chylomicrons. Instead of accumulating in the mucosa, the lipids are rate-limited and accumulate in the intestinal lumen. Our findings demonstrate a novel role for G5/G8 and provide another insight into the complex intracellular processes that orchestrate with G5/G8 transporters in the delivery of dietary lipids.
Acknowledgments
This work was supported by National Institutes of Health Grants DK092138 and DK059630 (University of Cincinnati Mouse Metabolic Phenotyping Center) to Dr. Patrick Tso and American Heart Association Grant 13PRE17140013 to Linda Zhang.
Abbreviations
- ABC
Adenosine triphosphate-binding cassette
- GC
Gas chromatography
- KO
Knockout
- MTP
Microsomal transfer protein
- NMR
Nuclear magnetic resonance
- NPC1L1
Niemann-pick C1-like 1
- PCR
Polymerase chain reaction
- PVC
Polyvinyl chloride
- TAG
Triacylglycerol
- TICE
Transintestinal cholesterol efflux
- WT
Wild-type
Footnotes
Conflict of interest None.
References
- 1.Kasper H. Faecal fat excretion, diarrhea, and subjective complaints with highly dosed oral fat intake. Digestion. 1970;3(6):321–322. doi: 10.1159/000197052. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Ahrens EH. An evaluation of the relative merits of two methods of measuring the balance of sterols in man: isotopic balance versus chromatographic analysis. J Clin Invest. 1966;45(9):1503–1515. doi: 10.1172/JCI105457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundy SM, Ahrens EH. Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J Lipid Res. 1969;10(1):91–107. [PubMed] [Google Scholar]
- 4.Bosner MS, Ostlund RE, Osofisan O, Grosklos J, Fritschle C, Lange LG. Assessment of percent cholesterol absorption in humans with stable isotopes. J Lipid Res. 1993;34(6):1047–1053. [PubMed] [Google Scholar]
- 5.Salen G, Ahrens EH, Grundy SM. Metabolism of β-sitosterol in man. J Clin Invest. 1970;49(5):952–967. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290(1):1171–1175. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 7.Graf GA, Cohen JC, Hobbs HH. Missense mutations in ABCG5 and ABCG8 disrupt heterodimerization and trafficking. J Biol Chem. 2004;279(23):24881–24888. doi: 10.1074/jbc.M402634200. [DOI] [PubMed] [Google Scholar]
- 8.Graf GA, Li WP, Gerald RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. J Clin Invest. 2002;110(5):659–669. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temel RE, Gebre AK, Parks JS, Rudel LL. Compared with Acyl-CoA: cholesterol O-acyltransferase (ACAT) 1 and lecithin: cholesterol acyltransferase, ACAT2 displays the greatest capacity to differentiate cholesterol from sitosterol. J Biol Chem. 2003;278(48):47594–47601. doi: 10.1074/jbc.M308235200. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99(25):16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110(5):671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klett EL, Lu K, Kosters A, Vink E, Lee MH, Altenburg M, Shefer S, Batta AK, Yu H, Chen J, Klein R, Looije N, Oude-Elferink R, Groen AK, Maeda N, Salen G, Patel SB. BMC Med. 2004;2(1):5. doi: 10.1186/1741-7015-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tachibana S, Hirano M, Hirata T, Matsuo M, Ikeda I, Ueda K, Sato R. Cholesterol and plant sterol efflux from cultured intestinal epithelial cells Is mediated by ATP-binding cassette transporters. Biosci Biotechnol Biochem. 2007;71(8):1886–1895. doi: 10.1271/bbb.70109. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27(1):79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya AK, Connor WE. β-Sitosterolemia and xanthomatosis: a newly described lipid storage disease in two sisters. J Clin Invest. 1974;53(4):1033–1043. doi: 10.1172/JCI107640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin HJ, Wang C, Salen G, Lam KC, Chan TK. Sitosterol and cholesterol metabolism in a patient with coexisting phytosterolemia and cholestanolemia. Metabolism. 1983;32(2):126–133. doi: 10.1016/0026-0495(83)90216-0. [DOI] [PubMed] [Google Scholar]
- 17.Salen G, Shore V, Tint GS, Forte T, Shefer S, Horak I, Horak E, Dayal B, Nguyen L, Batta AK. Increased sitosterol absorption, decreased removal, and expanded body pools compensate for reduced cholesterol synthesis in sitosterolemia with xanthomatosis. J Lipid Res. 1989;30(9):1319–1330. [PubMed] [Google Scholar]
- 18.Salen G, Shefer S, Nguyen L, Ness GC, Tint GS, Shore V. Sitosterolemia. J Lipid Res. 1992;33(7):945–955. [PubMed] [Google Scholar]
- 19.Plösch T, Bloks VW, Terasawa Y, Berdy S, Siegler K, Van Der Sluijs F, Kema IP, Groen AK, Shan B, Kuipers F, Schwartz M. Sitosterolemia in ABC-transporter G5-deficient mice Is aggravated on activation of the liver-X receptor. Gastroenterology. 2004;126(1):290–300. doi: 10.1053/j.gastro.2003.10.074. [DOI] [PubMed] [Google Scholar]
- 20.Wang HH, Patel SB, Carey MC, Wang DQ. Quantifying anomalous intestinal sterol uptake, lymphatic transport, and biliary secretion in Abcg8−/− mice. Hepatology. 2007;45(4):998–1006. doi: 10.1002/hep.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen TM, Sawyer JK, Kelley KL, David MA, Kent CR, Rudel LL. ACAT2 and ABCG5/G8 are both required for efficient cholesterol absorption in mice: evidence from thoracic lymph duct cannulation. J Lipid Res. 2012;53(8):1598–1609. doi: 10.1194/jlr.M026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwiterovich PO, Bachorik PS, Smith HH, McKusick VA, Connor WE, Teng B, Sniderman AD. Hyperapobetalipoproteinaemia in two families with xanthomas and phytosterolaemia. The Lancet. 1981;317(8218):466–469. doi: 10.1016/s0140-6736(81)91850-x. [DOI] [PubMed] [Google Scholar]
- 23.Salen G, Tint GS, Shefer S, Shore V, Nguyen L. Increased sitosterol absorption is offset by rapid elimination to prevent accumulation in heterozygotes with sitosterolemia. Arterioscler Thromb Vasc Biol. 1992;12(5):563–568. doi: 10.1161/01.atv.12.5.563. [DOI] [PubMed] [Google Scholar]
- 24.Lutjohann D, Bjorkhem I, Beil UF, von Bergmann K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J Lipid Res. 1995;36(8):1763–1773. [PubMed] [Google Scholar]
- 25.Mendez-Gonzales J, Julve J, Rotllan N, Llaverias G, Blanco-Vaca F, Escola-Gil JC. ATP-binding cassette G5/G8 deficiency causes hypertriglyceridemia by affecting multiple metabolic pathways. Biochim Biophys Acta. 2011;1811(12):1186–1193. doi: 10.1016/j.bbalip.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Bollman JL, Cain JC, Grindlay JH. Techniques for the collection of lymph from the liver, small intestine or thoracic duct of the rat. J Lab Clin Med. 1948;33(1):1349–1352. [PubMed] [Google Scholar]
- 27.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, von Lehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131(4):1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo CM, Nordskog BK, Nauli AM, Zheng S, vonLehmden SB, Yang Q, Lee D, Swift LL, Davidson NO, Tso P. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G344–G352. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]
- 29.Kohan AB, Howles PN, Tso P. Methods for studying rodent intestinal lipoprotein production and metabolism. Current Protocols in Mouse Biology. 2012;2:219–230. doi: 10.1002/9780470942390.mo120049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redgrave TG. Formation of cholesteryl ester-rich particulate lipid during metabolism of chylomicrons. J Clin Invest. 1970;49(3):465–471. doi: 10.1172/JCI106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergstedt SE, Hayashi H, Kritchevsky D, Tso P. A comparison of absorption of glycerol tristearate and glycerol trioleate by rat small intestine. Am J Physiol. 1990;259(3):G386–G393. doi: 10.1152/ajpgi.1990.259.3.G386. [DOI] [PubMed] [Google Scholar]
- 32.Wout ZGM, Pec EA, Maggiore JA, Williams RH, Palicharla P, Johnston TP. Poloxamer 407-mediated changes in plasma cholesterol and triglycerides following intraperitoneal injection to rats. PDA J Pharm Sci Tech. 1992;46:192–200. [PubMed] [Google Scholar]
- 33.Tso P, Pitts V, Granger DN. Role of lymph flow in intestinal chylomicron transport. Am J Physiol. 1985;249(1):G21–G28. doi: 10.1152/ajpgi.1985.249.1.G21. [DOI] [PubMed] [Google Scholar]
- 34.Clark SB. Chylomicron composition during duodenal triglyceride and lecithin infusion. Am J Physiol. 1978;235:E183–E190. doi: 10.1152/ajpendo.1978.235.2.E183. [DOI] [PubMed] [Google Scholar]
- 35.Mansbach CM, II, Arnold A. Steady-state kinetic analysis of triacylglycerol delivery into mesenteric lymph. Am J Physiol. 1986;251(2 Pt 1):G263–G269. doi: 10.1152/ajpgi.1986.251.2.G263. [DOI] [PubMed] [Google Scholar]
- 36.Tso P, Balint JA, Rodgers JB. Effect of hydrophobic surfactant (Pluronic L-81) on lymphatic lipid transport in the rat. Am J Physiol. 1980;239:G348–G353. doi: 10.1152/ajpgi.1980.239.5.G348. [DOI] [PubMed] [Google Scholar]
- 37.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115(5):1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Funkat A, Massa CM, Jovanovska V, Proietto J, Andrikopoulos S. Metabolic adaptations of three inbred strains of mice (C57BL/6, DBA/2, and 129T2) in response to a high-fat diet. J Nutr. 2004;134(12):3264–32694. doi: 10.1093/jn/134.12.3264. [DOI] [PubMed] [Google Scholar]
- 39.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57(1):65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 40.Van der Velde AE, Vrins CL, van der Oever K, Kunne C, Elferink RP, Kuipers F, Groen AK. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133(3):967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Sontag TJ, Chellan B, Getz GS, Reardon CA. Differing rates of cholesterol absorption among inbred mouse strains yield differing levels of HDL-cholesterol. J Lipid Res. 2013;54(9):2515–2524. doi: 10.1194/jlr.M040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang DQ-H, Paigen B, Carey MC. Genetic factors at the enterocyte level account for variations in intestinal cholesterol absorption efficiency among inbred strains of mice. J Lipid Res. 2001;42(11):1820–1830. [PubMed] [Google Scholar]
- 43.Duan LP, Wang HH, Wang DQ. Cholesterol absorption is mainly regulated by the jejunal and ileal ATP-binding cassette sterol efflux transporters Abcg5 and Abcg8 in mice. J Lipid Res. 2004;45(7):1312–1323. doi: 10.1194/jlr.M400030-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Clark SB, Lawergren B, Martin JV. Regional intestinal absorptive capacities for triolein: an alternative to markers. Am J Physiol. 1973;225(3):574–585. doi: 10.1152/ajplegacy.1973.225.3.574. [DOI] [PubMed] [Google Scholar]
- 45.Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Genetic inactivation of NPC1L1 protects against sitosterolemia in mice lacking ABCG5/ABCG8. J Lipid Res. 2009;50(2):293–300. doi: 10.1194/jlr.M800439-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Borgstrom B. Quantitative aspects of the intestinal absorption and metabolism of cholesterol and β-sitosterol in the rat. J Lipid Res. 1968;9(4):473–481. [PubMed] [Google Scholar]
- 47.Van der Velde AE, Vrins CL, van den Oever K, Seemann I, Elferink RP, van Eck M, Kuipers F, Groen AK. Regulation of direct transintestinal cholesterol excretion in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(1):G203–G208. doi: 10.1152/ajpgi.90231.2008. [DOI] [PubMed] [Google Scholar]
- 48.Van der Veen JN, van Dijk TH, Vrins CL, van Meer H, Havinga R, Bijsterveld K, Tietge UJ, Groen AK, Kuipers F. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J Biol Chem. 2009;284(29):19211–19219. doi: 10.1074/jbc.M109.014860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graewin SJ, Kiely JM, Lee KH, Svatek CL, Nakeeb A, Pitt HA. Nonobese diabetic mice have diminished gallbladder motility and shortened crystal observation time. J Gastrointest Surg. 2004;8(7):824–830. doi: 10.1016/j.gassur.2004.06.014. [DOI] [PubMed] [Google Scholar]