Abstract
Background
Brain tumor-initiating cells (BTICs) are stem-like cells hypothesized to form a disease reservoir that mediates tumor recurrence in high-grade gliomas. Oncolytic virotherapy uses replication-competent viruses to target and kill malignant cells and has been evaluated in clinic for glioma therapy with limited results. Myxoma virus (MyxV) is a safe and highly effective oncolytic virus (OV) in conventional glioma models but, as seen with other OVs, is only modestly effective for patient-derived BTICs. The objective of this study was to determine whether MyxV treatment against human BTICs could be improved by combining chemotherapeutics and virotherapy.
Methods
A 73-compound library of drug candidates in clinical use or preclinical development was screened to identify compounds that sensitize human BTICs to MyxV treatment in vitro, and synergy was evaluated mathematically in lead compounds using Chou-Talalay analyses. The effects of combination therapy on viral gene expression and viral replication were also assessed.
Results
Eleven compounds that enhance MyxV efficacy were identified, and 6 were shown to synergize with the virus using Chou-Talalay analyses. Four of the synergistic compounds were shown to significantly increase viral gene expression, indicating a potential mechanism for synergy. Three highly synergistic compounds (axitinib, a VEGFR inhibitor; rofecoxib, a cyclooxygenase-2 inhibitor; and pemetrexed, a folate anti-metabolite) belong to classes of compounds that have not been previously shown to synergize with oncolytic viruses in vitro.
Conclusions
This study has identified multiple novel drug candidates that synergistically improve MyxV efficacy in a preclinical BTIC glioma model.
Keywords: brain tumor-initiating cells, myxoma virus, oncolytic virotherapy, synergy
Glioblastoma multiforme (GBM) is a deadly brain cancer characterized by poor clinical outcome and resistance to conventional therapies.1,2 The current standard of care for GBMs includes surgical resection, radiotherapy, and chemotherapy using temozolomide (TMZ). However, virtually all patients succumb to recurrent disease.1,2
Brain tumor-initiating cells (BTICs) are stem-like cells hypothesized to drive GBM recurrence and treatment resistance,3–5 and have been putatively identified in human gliomas through in situ expression of neural stem cell markers.6,7 Recently, researchers investigating spontaneous murine high-grade gliomas have succeeded in tracing the emergence of a TMZ-resistant compartment that possessed distinctive stem-cell characteristics.4 Such studies highlight the importance of developing alternative therapies for targeting the BTIC compartment, which has repeatedly proven resistant to chemotherapy and radiation.8–11 Cultured patient-derived BTICs represent a powerful tool for evaluating the efficacy of novel GBM treatments. To maintain BTIC populations in culture, patient-derived surgical samples are grown as neurospheres in serum-free, growth factor-supplemented neural stem cell-promoting conditions.12,13 Culturing GBM specimens as neurospheres has been shown to retain the stem-like nature of the glioma, resulting in stable retention of original tumor gene expression patterns and in vivo growth characteristics when grafted into mice.14,15
One promising experimental approach to treating the BTIC compartment of GBMs is oncolytic virus (OV) therapy, which uses replication-competent viruses to selectively infect and kill malignant cells.16 Several OVs have been clinically evaluated for the treatment of malignant gliomas, including herpes simplex virus,17–19 adenovirus,20 reovirus,21 and Newcastle disease virus.22 These trials were designed to establish the safety of intracerebral OV injections, which were found to be safe. However, ancillary observations noted only a handful of clinical responses, suggesting that OV monotherapy is insufficient for achieving durable responses.16 It is conceivable that combination therapy approaches would promote enhanced clinical efficacy compared with virus alone.
We and others have previously assessed the effectiveness of OVs for treating the BTIC compartment23–27 and have shown that BTICs have variable levels of resistance to single agent OV treatment. One approach that may increase viral tropism for and enhanced killing of BTICs is the combined use of OV and drug therapy. This strategy has been previously shown to enhance OV efficacy in various models.28,29 The mechanisms of drug-virus interactions characterized to date tend to fall within 2 broad categories. First, there are drugs that act to modulate the vasculature and/or immune system to improve viral delivery and reduce immune-mediated viral clearance or improve acquired anti-tumor immune responses. Examples of these include gemcitabine,29 cyclophosphamide30 and cyclooxygenase-2 inhibitors.31 Alternatively, there are drugs that act on tumor cells to improve viral replication and/or virus-mediated cell killing, examples of which include histone deacetylase inhibitors32 and topoisomerase II poisons.27,33 Some drugs, such as rapamycin, have demonstrated both mechanisms of action, making them favorable choices for future combination therapy in the clinic.23,34,35
Myxoma virus (MyxV) is a promising oncolytic candidate that is highly effective in traditional xenograft models but shows limited efficacy as a monotherapy in other models, including syngeneic murine models and patient-derived BTIC models.23,34,36,37 While we have previously shown that the mTOR inhibitor rapamycin modestly improves MyxV efficacy against some patient-derived BTICs (an effect that is enhanced in some BTIC lines by TMZ23), the opportunity to develop more effective combinations remains open. Although drug screens have been performed to enhance OV therapy in the past, to our knowledge there has never been a screen designed to identify chemotherapeutics that enhance OV efficacy against BTICs. In this study, we manually screened a drug library containing 73 compounds in various stages of clinical development and identified multiple candidate compounds that enhance MyxV oncolytic efficacy. Candidate compounds were then tested for synergistic interactions with MyxV using the Chou-Talalay method.38,39 Not only did this approach confirm that several drugs with known ability to synergize with OVs were also effective with MyxV but, more importantly, we identified several novel candidates for OV combination therapy, some of which are in trials for glioma treatment (eg, bosutinib [NCT01331291] and axitinib [NCT01562197, NCT01508117]). These findings demonstrate that the drug screen approach may be an important tool for discovering novel OV combinatorial therapies for GBM.
Materials and Methods
Brain Tumor-initiating Cell Isolation and Culture
The Brain Tumour Initiating Core at the University of Calgary provided the human BTIC lines used in this study. BTIC lines had been established, as previously described,13 from tissue samples obtained through the University of Calgary Neurologic and Pediatric Tumor and Related Tissue Bank, following informed consent from glioma patients and approval by the University of Calgary Conjoint Health Research Ethics Board. BTICs were cultured as outlined in the Supplemental Methods and as described previously.23
Viruses Used
MyxV-dsRed consists of wild-type MyxV with a Discosoma red fluorescent protein (dsRED) cassette under the control of a late viral promoter.40 MyxV-FLuc contains both a green fluorescent protein and an enhanced firefly luciferase (FLuc) reporter under the control of an early viral promoter.23 The virus was propagated as described elsewhere41 and stored in aliquots at −80°C. Titration of viral stock was performed on baby green monkey kidney cells, using fluorescent foci-forming assays, as described in the Supplemental Methods.
Viral Replication Assays
Early viral gene expression was measured by infecting BTICs with MyxV-FLuc23 at the indicated multiplicity of infection (MOI) in clear-bottom, black-sided 96-well plates with the indicated concentrations of drugs. All treatments were performed in quadruplicate, and luminescence was measured as described in the Supplemental Methods.
Viability Assays
BTIC susceptibility to virus alone was assessed by plating cells (1 × 104 cells/well) in 96-well clear-bottom tissue culture plates and infecting with MyxV-dsRED at the indicated MOI. All treatments were performed in internal replicates of 6 and biological replicates of 3 unless otherwise noted. At the indicated time point, viability was measured using Alamar Blue (Invitrogen) according to manufacturer's instructions.
Small Molecule Inhibitor Drug Screen
The drug library, compiled by ChemieTek, consisted of 73 chemotherapeutic agents and small molecule inhibitors in various stages of preclinical and clinical development (Supplementary Table S1). The high-throughput screening procedure is described in the Supplemental Methods.
Chou-Talalay Analysis
Cells were plated at 1 × 104/well in 96-well plates and were treated as follows in internal replicates of 8: (i) no treatment; (ii) drug alone (1 μM, 3 μM, 10 μM for high-dose hits; 100 nM, 300 nM, and 1000 nM for low-dose hits); (iii) virus alone (1 MOI, 3 MOI, 10 MOI); and (iv) combination treatments including every combination of drug and virus concentrations listed. For each of the combination treatments, cells were pretreated with drug for 4 hours prior to infection with virus. Cells were incubated as described above for 48 hour, and viability was measured with Alamar Blue.
Combination indices (CIs) were calculated using the Chou-Talalay method,38,39 which stipulates that a CI > 1 indicates antagonism, a CI = 1 indicates an additive effect, and a CI < 1 indicates synergy (described fully in Supplemental Methods). Multiple studies have established that Chou-Talalay analysis is applicable to pharmacoviral interactions42,43 as well as more traditional drug-drug interactions.
Statistical Analysis
All statistical analyses were performed using Microsoft Excel and GraphPad Prism 6.0. Student' t tests were 2 sided and considered significant at P < .05.
Results
Patient-derived BTICs Are Moderately Susceptible to MyxV as a Monotherapy
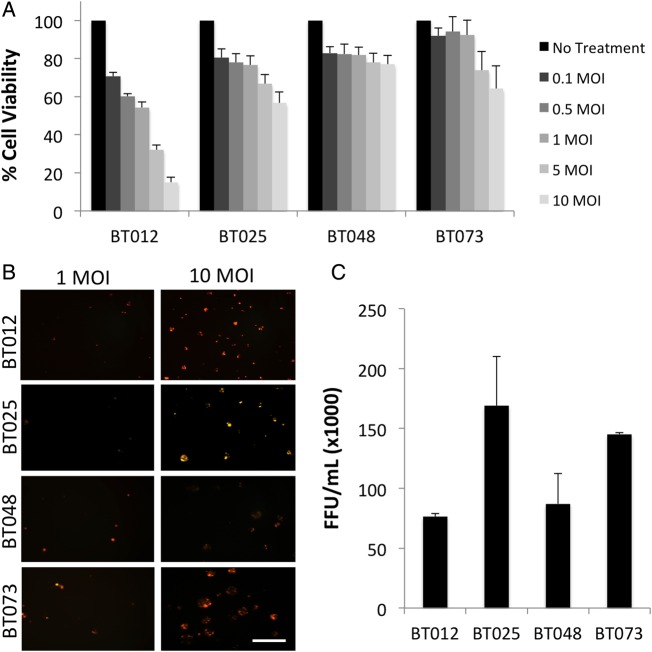
The viability of patient-derived BTIC cultures following MyxV infection was quantified using Alamar Blue at 48 hours post infection with 0–10 MOI of MyxV-dsRED (Fig. 1A). Susceptibility to MyxV-mediated cell death was variable, with BT012 cells demonstrating the greatest susceptibility to MyxV, and BT025, BT048, and BT073 cells demonstrating relatively limited susceptibility. Complete cell death (0% viability) was only observed in one BTIC line (BT012) at 72 hours post infection at the highest dose tested, 10 MOI (Supplementary Fig. S1). This confirmed previous observations that BTICs are only moderately susceptible to MyxV in vitro, which may underlie the limited efficacy of MyxV as a monotherapy in vivo.23
Fig. 1.
Brain tumor-initiating cells (BTICs) are moderately susceptible to MyxV infection and killing. (A) The viability of a panel of BTICs was assessed using Alamar Blue at 48 hours following MyxV infection, with absorbance values normalized to the untreated control for each BTIC line. Data are presented as mean ± SEM (n = 3, each with 6 internal replicates). (B) BTICs demonstrate variable late viral gene expression following infection with 1 or 10 MOI MyxV-dsRED. Viral red fluorescent protein expression was assessed 24 hours post infection with MyxV-dsRED. Cells were imaged using a Zeiss Axiovert microscope at 100X magnification with a rhodamine filter (scale bar = 50 μm). (C) Whole-cell viral titers were performed to assess viral replication at 72 hours post infection, demonstrating productive infection of all BTIC lines by MyxV. Data represent the mean number of foci-forming units (FFU) at 72 hours post infection, with the input virus subtracted (n = 2).
To verify that BTICs support a productive MyxV infection, we used a MyxV construct tagged with red fluorescent protein (RFP) under the control of a late viral promoter (MyxV-dsRED). As shown in Fig. 1B, all BTIC lines expressed RFP at 48 hours post infection, with BT048 expressing the lowest levels. To further confirm that infectious progeny virions were produced in these cells, we performed viral titers and found functional infectious virions in all BTIC lines (Fig. 1C). We selected the BT025 line for use in the drug screen because it was moderately resistant to MyxV-induced cell death (Fig. 1A) while still able to support productive viral replication (Fig. 1B and C).
Primary Screen of the Drug Library at 1μM Identifies 8 Lead Compounds
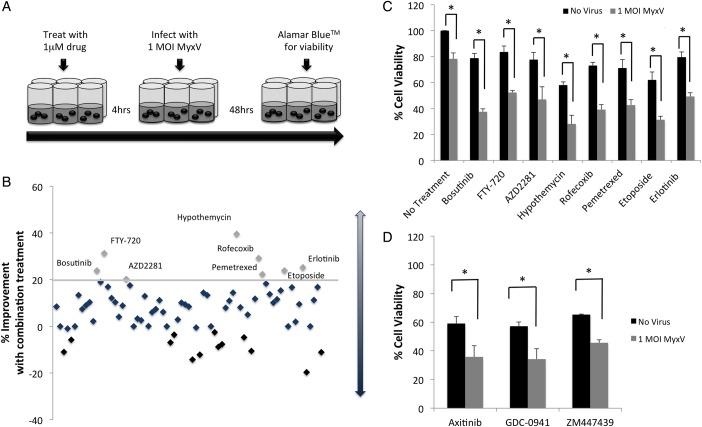
We used a diverse library of 73 compounds (Supplementary Table S1) and treated BT025 cells with MyxV-dsRED alone (1 MOI), drug alone (1 μM), or a combination of drug and virus (4 h preincubation with 1 μM drug prior to infection with 1 MOI MyxV-dsRED), and assessed viability using Alamar Blue 48 hours post infection (Fig. 2A).
Fig. 2.
Primary screen of 73 cytotoxic and small molecule inhibitors in combination with MyxV in BT025 reveals 8 candidate compounds for further characterization. (A) Schematic for primary drug screen. Brain tumor-initiating cells (BTICs) were incubated with 1 μM drug for 4 hours prior to infection with MyxV (1 MOI). Viability was assessed 48 hours post infection. (B) Summary of primary screen: drugs are positioned sequentially on x-axis relative to their number in Supplementary Table S1. Values on the y-axis indicate the percent difference in cell viability between drug alone and combination treatment. Combination treatments that caused a > 20% loss in cell viability than drug alone were considered candidate compounds (threshold for hits is indicated by horizontal grey line). The 8 candidate compounds identified are labeled by name. (C) viability was reassessed to validate these candidate compounds, and all 8 candidate compounds showed a significant (P < .05, *) combination effect compared with either drug alone or virus alone. Data are plotted as the mean viability ± SEM (n = 3). (D) Twenty-one potent drugs (those for which drug alone killed >70% of cells at 1 μM) were rescreened as shown in Supplementary Fig. S3A at 100 nM. Three of these drugs were shown to significantly (P < .05, *) improve cell killing compared with either treatment alone. Data are plotted as the mean viability ± SEM of 3 independent experiments, each with 6 internal replicates.
The viability data generated for all 73 drugs alone and in combination with MyxV-dsRED are shown in Supplementary Fig. S2. Data are summarized as the difference in viability between combination-treated cells and drug–alone-treated cells on a scatterplot for all 73 compounds (Fig. 2B). Drugs for which combination treatment did not enhance MyxV efficacy were clustered around 0%, while combination treatments that were less effective than drug alone were represented as negative values. Since virus alone leads to a ∼20% reduction in the viability of cells (indicated by the solid grey line), combination treatments that reduced viability >20% than by drug alone were considered candidate compounds for further investigation. Based on this criterion, 8 potential candidates were selected for further characterization. All 8 drugs showed a significant (P < .05) combination effect when compared with either drug or virus alone (Fig. 2C). These drugs and their known activities are listed in Supplementary Table S1. Drugs that were identified as candidate compounds at 1 μM in the first-pass screen are referred as “high-dose candidate compounds.”
Secondary Screen at 100nM Identifies 3 Additional Lead Compounds
Based on the primary screen (Supplementary Fig. S2), we observed that 21 compounds caused a > 70% reduction in BT025 viability at 1 μM in the absence of virus. This level of cytotoxicity was thought to obscure the opportunity to discover potential combination effects. Subsequently, these drugs were rescreened at a lower dose (100 nM) in 3 independent trials. Data for all 21 drugs are shown in Supplementary Fig. S3. From this screen at 100 nM, 3 additional drugs were identified for which combination treatment significantly (P < .05) improved the efficacy of MyxV compared with drug alone or virus alone (see Fig. 2D). For ease of identification, drugs that were identified as candidate compounds at 100 nM in the secondary screen were referred to as low-dose candidate compounds.
As shown in Supplementary Fig. S3A, several drugs were highly cytotoxic even at 100 nM. These drugs were rescreened in a third and final screen at a concentration of 10 nM (Supplementary Fig. S3B); however, no further candidate compounds were identified. Lastly, to validate the Alamar Blue assay, several drugs were shown to reduce absolute cell number in combination with MyxV using trypan blue staining. A representative example at 48 hour post infection is shown in Supplementary Fig. S3C.
Six Candidate Compounds Synergize With MyxV at the Lowest Dose Tested
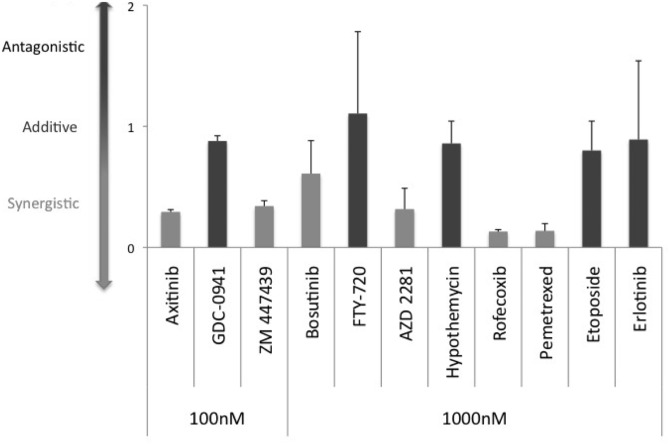
To determine the nature of the interaction observed with our 11 drug-virus combinations, Chou-Talalay analysis was performed. This analysis considers the concentration of drug or virus required to achieve a 50% loss of viability as a monotherapy (IC50) and compares this with the dose of each required to achieve 50% loss of viability in the combination treatment condition. By comparing the theoretical versus observed concentrations of drug and virus required to achieve a 50% loss of viability, a CI can be calculated, and drug-virus interactions can be classified as synergistic, additive, or antagonistic.
As part of this analysis, dose-response curves of cell viability were generated using drug-alone, virus-alone, and drug-virus combinations for each of the 11 candidate compounds with a range of drug concentrations (1 μM, 3 μM, and 10 μM for the high-dose compounds; 0.1 μM, 0.3 μM, and 1 μM for the low-dose compounds) and virus concentrations (1 MOI, 3 MOI, 10 MOI) (Supplementary Fig S4A–J). Based on these dose-response curves, the IC50 values for the virus (IC50MyxV) were calculated, both alone and in combination with each dose of each drug (Supplementary Table S2). As expected, for the majority of drugs, the IC50MyxV decreased when the virus was used in combination with the candidate compounds. CI values were calculated for MyxV in combination with each dose of drug. Fig. 3 shows the combination indices for the lowest dose tested of each drug. According to the Chou-Talalay method,38 a drug interaction is considered synergistic if CI < 1, additive if CI = 1, and antagonistic if CI > 1. By this method, 6 of the original 11 candidate compounds were shown to be synergistic. Supplementary Table S3 shows the CI values for all 3 doses of each drug tested. To determine whether the induction of apoptosis was a possible mechanism of synergy, the 6 compounds shown to be synergistic with MyxV were screened alone and in combination with MyxV for changes in caspase-3/-7 activation. However, no change in caspase-3/-7 activation was observed for any combination-treated group (Supplemental Fig. S10), suggesting that other mechanisms of cell death may be more important in this model.
Fig. 3.
Six of 11 candidate compounds are synergistic with MyxV in BT025 cells. Combination index (CI) values for drug-virus interactions were calculated for all 11 compounds based upon multiple dose response curves (Supplementary Fig. S4). CI values were calculated, as described in the Materials & Methods, and plotted as shown. According to the Chou-Talalay method, the interaction is considered synergistic when CI < 1. Six of the 11 drugs interacted synergistically with MyxV. Data are plotted as the mean CI for each combination treatment ± SEM of 2 independent CI calculations.
Five Candidate Compounds Significantly Increase Viral Gene Expression
To determine whether the combination effect was due to an increase in viral infection, MyxV-FLuc, which contains a highly sensitive firefly luciferase reporter construct under the control of an early viral promoter, was utilized. The cytotoxicity of the FLuc-expressing virus was shown to be comparable to MyxV-dsRed (Supplementary Fig. S6). This method of assaying preliminary viral replication is sensitive, as can be seen by the directly proportional response of infecting BT025 at varying MOIs and the observed luminescence (r2 = 0.998; Supplementary Fig. S6B).
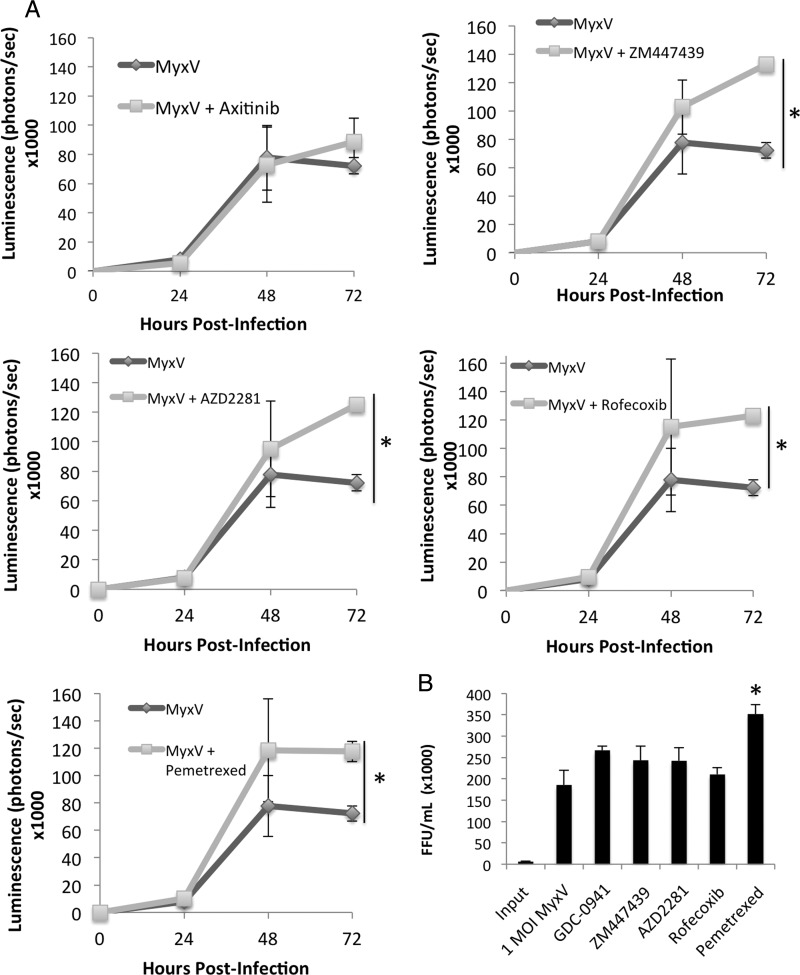
To determine whether combination treatment with any of the 11 candidate compounds increased viral gene expression, viral luminescence in MyxV-treated and combination-treated BT025 cultures was monitored at 0, 24, 48, and 72 hours post infection (Fig. 4A and Supplementary Fig. S7. In the MyxV-only condition, viral gene expression increased steadily over time (Fig. 4A), plateauing after 48 hours. Interestingly, 5 of the 6 synergistic candidate compounds demonstrated a significant (P < .05) increase in viral luciferase expression compared with the virus-only control by 72 hours post infection (Fig. 4A), suggesting a possible mechanism for the interactions between drug and virus.
Fig. 4.
Viral gene expression in combination-treated BT025 cultures increases over time and is enhanced by combination treatment with 5 of 6 synergistic compounds. (A) Brain tumor-initiating cells were infected with MyxV-FLuc with and without concomitant drug treatment, and luminescence was quantified at 0–72 hours post infection. Five drugs were shown to significantly increase (P < .05, *) viral luminescence at the 72 hour time point Data are plotted as the mean viability ± SEM of 3 independent experiments, each with 6 internal replicates. Data for all 11 drugs are shown in Supplementary Fig. S6. (B) Only pemetrexed increased whole cell virus titers at 72 hours post infection. Data are plotted as the mean viability ± SEM of 2 independent experiments, each with 2 internal replicates.
To assess whether the drugs that increase viral gene expression dampen the intracellular antiviral response, levels of phosphorylated and total STAT1 were assessed 24 hours post treatment by immunoblot. Although MyxV in all cases appeared to dampen constitutive Stat1 phosphorylation on serine 727, combination-treated BTICs appeared to have lower levels of phosphorylated STAT1 than virus-only treated cells (Supplemental Fig. S7), suggesting that drug treatment may decrease intracellular antiviral signaling and promote oncolysis.
To assess whether an increase in viral replication mirrored the increase in viral infection and early gene expression, BTIC cells and supernatant were harvested 72 hours after treatment, and whole-cell titers were performed on the samples. As shown in Fig. 4B, only pemetrexed increased viral replication above baseline levels (P < .01).
Axitinib Synergizes With MyxV in 3 Other BTIC Lines
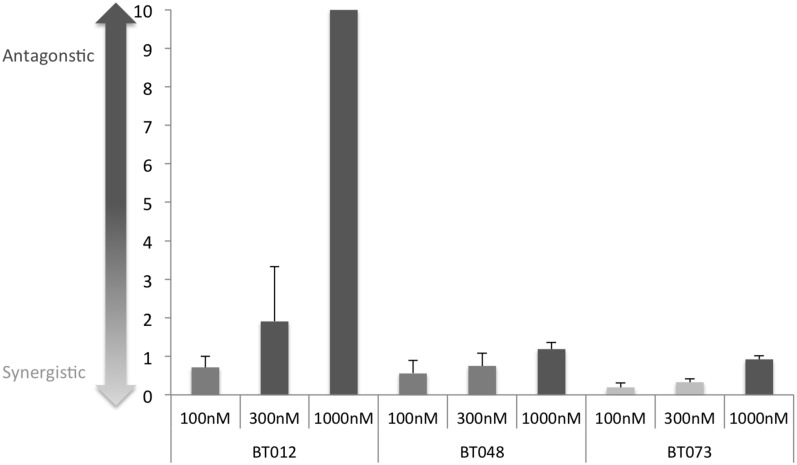
To determine whether the combination effect observed following drug pretreatment was unique to BT025, we assessed BTIC viability in 3 other patient-derived BTIC lines: BT012, BT048, and BT073 (Supplemental Fig. S8). Axitinib (compound D8) was shown to increase cell killing significantly in all 3 BTIC lines tested. This interaction was shown to be synergistic at several doses in BT073 (Fig. 5). The dose response data for this analysis are shown in Supplementary Fig. S9. We also investigated whether the mechanism of synergy with axitinib was dependent upon apoptosis. As shown in Supplemental Fig. S10, neither caspase-3/-7 activation (A) nor PARP cleavage (B) was observed in treatment with MyxV, axitinib, or combination treatment in BT025, suggesting that this synergistic interaction is independent of apoptosis. Because axitinib is upstream of phosphatidylinositol 3-kinase (PI-3K), we hypothesized that direct PI-3K inhibition may also have a synergistic effect with MyxV. Using 2 other PI-3K inhibitors (LY294002 and ZSTK474), we also observed a significant increase in BTIC killing compared with either drug alone or virus alone, suggesting that PI-3K inhibition may represent an important mechanism for achieving synergy with MyxV.
Fig. 5.
Axitinib demonstrates synergism in 3 additional brain tumor-initiating cell (BTIC) lines. (A) Combination indices (CI) for drug-virus interactions were calculated for axitinib in 3 additional BTIC lines (BT012, BT048, BT073) based upon multiple dose response curves (Supplementary Fig. S6). CI values were calculated as described in the Supplemental Methods. Data are plotted as the mean CI for each combination treatment ± SEM for 2 independent CI calculations.
Discussion
As investigators prepare to evaluate MyxV in the clinical setting, preclinical studies must realistically assess the limitations of the virus as a monotherapy and explore opportunities to enhance viral oncolytic efficacy. Identifying compounds that act synergistically with MyxV may be an effective strategy for optimizing tumor cell killing. We performed a single-dose in vitro screen of 73 chemotherapeutics and small molecule inhibitors against BT025 brain tumor-initiating cells to identify compounds that synergize with MyxV. Unlike previous, much larger pharmacoviral screens (>10 000 drugs44), our library contained only well-characterized drug candidate compounds including widely used chemotherapeutics and small molecule inhibitors that are in preclinical or clinical development. This approach eliminated reagents with unknown or prohibitive toxicity profiles.
We found 11 clinically relevant compounds that significantly increased the efficiency of MyxV cell killing; 6 of these were shown to be synergistic, as determined by Chou-Talalay analyses. We demonstrated that viral infection, viral replication, viral gene expression, and intracellular antiviral signaling pathways (eg, phospho-STAT1) can be modulated by these compounds, although these effects may be underestimated due to BTIC cell death at later time points. Two of the candidate compounds we identified (ie, the phosphatidylinositol 3-kinase (PI-3K) inhibitor GDC-0941 and the topoisomerase II poison etoposide) have previously been shown to improve the efficacy of oncolytic herpes simplex virus in glioma stem cell models.27,43 Thus, these compounds served as de facto positive controls within our drug library, and their identification by our screen provides confidence in its validity. Despite being previously shown to enhance MyxV-mediated cell killing,23 rapamycin (Drug 67 in Supplementary Fig. S2) showed only a modest (∼10%) improvement in killing of cultured BTICs, a difference that was below our threshold for designating a candidate compound of interest in this study. By setting the threshold high, we endeavored to identify compounds that offer a substantial improvement over rapamycin for in vitro combination therapy.
Interestingly, our screen identified several classes of drugs not previously shown to synergize with any OVs in vitro. Notably, our 3 most synergistic drugs (axitinib [a tyrosine kinase inhibitor that inhibits vascular endothelial growth factor receptor {VEGF-R}, c-Kit, and others], rofecoxib [a cyclooxygenase-2 {COX-2} inhibitor], and pemetrexed [a folate antimetabolite]) represent classes of compounds that have not been previously shown to act directly upon tumor cells to enhance OV efficacy. Although inhibitors of the COX-231 and VEGF45 pathways have been investigated in the context of OV therapy as immunomodulatory and vascular normalizing agent, respectively, this is the first time these compounds have been shown to improve OV efficacy in tumor cells directly. This is exciting because these drugs may have additional positive effects on oncolytic therapy in vivo, improving BTIC tumor susceptibility while increasing viral delivery or modulating immune responses. Axitinib, which is synergistic in multiple BTIC lines, is of particular interest, given that this compound is already in phase II clinical trials for gliomas (NCT01562197 and NCT01508117). We are currently investigating the mechanisms of these virus-drug interactions in vitro and in vivo, and we believe these drug classes are excellent candidates for further preclinical and clinical evaluation.
Several interesting patterns emerged from this screen. For instance, several of the compounds observed to interact either additively or synergistically with MyxV (including GDC-0941, axitinib, hypothemycin, and erlotinib) target the PI-3K/Akt/mTOR signaling pathway at either the receptor or intracellular level. This is consistent with previous reports from our lab and others indicating that targeting the PI-3K pathway with rapamycin or other inhibitors is a viable approach for improving viral oncolysis.23,34,35 The PI-3K pathway is known to promote cellular proliferation and survival, which suggests that inhibiting this pathway prior to viral infection may be important for directing the infected cell's fate towards death. In support of this hypothesis, we demonstrated that direct inhibition of PI-3K by 2 additional compounds also improves MyxV efficacy in BTICs. Dysregulation of the PI-3K/Akt/mTOR pathway is particularly common in high-grade gliomas,46–48 making this a rational and appropriate target for combination therapy. There is also evidence for COX-2 inhibition affecting this pathway since COX-2 inhibition has been shown to impair Akt phosphorylation and lead to growth arrest.49 The antifolate pemetrexed has also been shown to inhibit mTOR signaling in a lymphoblastic leukemia model.50 The elucidation of such patterns highlights an important strength of the drug screen approach for identifying candidates for combination therapy with OVs, namely the capacity to identify overarching patterns of drug-virus interactions.
Our in vitro pharmacoviral screen is an important first step for elucidating potential drug-virus combinations to develop an effective, multipronged approach to OV therapy. In determining which drugs to pursue further, a number of features should be considered including (i) the ability to achieve synergy at doses that correspond to clinically achievable plasma concentrations of the drugs; (ii) the ability of the drug to cross the blood-brain barrier in preclinical models; (iii) the presence of ongoing or completed clinical trials in GBM to verify blood-brain barrier penetration in humans; (iv) acceptable toxicity profiles; (v) oral bioavailability; and (vi) the potential for additional beneficial clinical effects. Based upon these criteria, optimal therapeutic regimens for combinatorial OV therapy can be strategically developed.
Supplementary Material
Funding
This work was supported by grants (to P.A.F.) from the V Foundation and the Terry Fox Foundation. B.A.M. was funded by a graduate studentship from the Canadian Institutes of Health Research. F.J.Z. was funded by Alberta Innovates-Health Solutions, a Vanier Scholarship from Canadian Institutes of Health Research, and the Izaak Walton Killam scholarship from the University of Calgary. G.M.'s lab was funded by National Institutes of Health grants R01 AI080607 and R01 CA138541.
Supplementary Material
Acknowledgments
The authors would like to thank the Brain Tumour Initiating Core, led by Drs. Sam Weiss and Gregory Cairncross (University of Calgary, Calgary, Alberta), for generously providing the brain tumor-initiating cell lines.
Conflict of interest statement: None declared.
References
- 1.Kanu OO, Mehta A, Di C, et al. Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets. 2009;13(6):701–718. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 2012;149(1):36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Nishimura MC, Bumbaca SM, et al. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 2010;17(4):362–375. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Song T, Yang L, et al. Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. J Exp Clin Cancer Res. 2008;27:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pallini R, Ricci-Vitiani L, Banna GL, et al. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin Cancer Res. 2008;14(24):8205–8212. [DOI] [PubMed] [Google Scholar]
- 8.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Wakeman TP, Lathia JD, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28(1):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101(39):14228–14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JJ, Stechishin O, Chojnacki A, et al. Proliferation of human glioblastoma stem cells occurs independently of exogenous mitogens. Stem Cells. 2009;27(8):1722–1733. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. [DOI] [PubMed] [Google Scholar]
- 15.Wakimoto H, Mohapatra G, Kanai R, et al. Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol. 2012;14(2):132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zemp FJ, Corredor JC, Lun X, et al. Oncolytic viruses as experimental treatments for malignant gliomas: using a scourge to treat a devil. Cytokine Growth Factor Rev. 2010;21(2–3):103–117. [DOI] [PubMed] [Google Scholar]
- 17.Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9(6):398–406. [DOI] [PubMed] [Google Scholar]
- 18.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7(10):859–866. [DOI] [PubMed] [Google Scholar]
- 19.Harrow S, Papanastassiou V, Harland J, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11(22):1648–1658. [DOI] [PubMed] [Google Scholar]
- 20.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10(5):958–966. [DOI] [PubMed] [Google Scholar]
- 21.Forsyth P, Roldan G, George D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther. 2008;16(3):627–632. [DOI] [PubMed] [Google Scholar]
- 22.Freeman AI, Zakay-Rones Z, Gomori JM, et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther. 2006;13(1):221–228. [DOI] [PubMed] [Google Scholar]
- 23.Zemp FJ, Lun X, McKenzie BA, et al. Treating brain tumor-initiating cells using a combination of myxoma virus and rapamycin. Neuro Oncol. 2013;15(7):904–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheema TA, Wakimoto H, Fecci PE, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc Natl Acad Sci USA. 2013;110(29):12006–12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Hengel SK, Balvers RK, Dautzenberg IJ, et al. Heterogeneous reovirus susceptibility in human glioblastoma stem-like cell cultures. Cancer Gene Ther. 2013;20(9):507–513. [DOI] [PubMed] [Google Scholar]
- 26.Allen C, Opyrchal M, Aderca I, et al. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther. 2012;20(4):444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheema TA, Kanai R, Kim GW, et al. Enhanced antitumor efficacy of low-dose Etoposide with oncolytic herpes simplex virus in human glioblastoma stem cell xenografts. Clin Cancer Res 2011;17(23):7383–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ottolino-Perry K, Diallo JS, Lichty BD, et al. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18(2):251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wennier ST, Liu J, McFadden G. Bugs and drugs: oncolytic virotherapy in combination with chemotherapy. Curr Pharm Biotechnol. 2012;13(9):1817–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lun XQ, Jang JH, Tang N, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Research. 2009;15(8):2777–2788. [DOI] [PubMed] [Google Scholar]
- 31.Chang CL, Ma B, Pang X, et al. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Ther. 2009;17(8):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Otsuki A, Patel A, Kasai K, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16(9):1546–1555. [DOI] [PubMed] [Google Scholar]
- 33.Graat HC, Witlox MA, Schagen FH, et al. Different susceptibility of osteosarcoma cell lines and primary cells to treatment with oncolytic adenovirus and doxorubicin or cisplatin. Br J Cancer. 2006;94(12):1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lun X, Alain T, Zemp FJ, et al. Myxoma virus virotherapy for glioma in immunocompetent animal models: optimizing administration routes and synergy with rapamycin. Cancer Res. 2010;70(2):598–608. [DOI] [PubMed] [Google Scholar]
- 35.Stanford MM, Barrett JW, Nazarian SH, et al. Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells. J Virol. 2007;81(3):1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zemp FJ, McKenzie BA, Lun X, et al. Resistance to oncolytic myxoma virus therapy in nf1(-/-)/trp53(-/-) syngeneic mouse glioma models is independent of anti-viral type-I interferon. PloS one. 2013;8(6):e65801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lun X, Yang W, Alain T, et al. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65(21):9982–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. [DOI] [PubMed] [Google Scholar]
- 39.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 40.Wennier ST, Liu J, Li S, et al. Myxoma virus sensitizes cancer cells to gemcitabine and is an effective oncolytic virotherapeutic in models of disseminated pancreatic cancer. Mol Ther. 2012;20(4):759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smallwood SE, Rahman MM, Smith DW, et al. Myxoma virus: propagation, purification, quantification, and storage. Curr Protoc Microbiol. 2010;Chapter 14:Unit 14A 11:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin SF, Gao SP, Price DL, et al. Synergy of a herpes oncolytic virus and paclitaxel for anaplastic thyroid cancer. Clin Cancer Res. 2008;14(5):1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kanai R, Wakimoto H, Martuza RL, et al. A novel oncolytic herpes simplex virus that synergizes with phosphoinositide 3-kinase/Akt pathway inhibitors to target glioblastoma stem cells. Clin Cancer Res. 2011;17(11):3686–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diallo JS, Le Boeuf F, Lai F, et al. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol Ther. 2010;18(6):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kottke T, Chester J, Ilett E, et al. Precise scheduling of chemotherapy primes VEGF-producing tumors for successful systemic oncolytic virotherapy. Mol Ther. 2011;19(10):1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanu OO, Hughes B, Di C, et al. Glioblastoma Multiforme Oncogenomics and Signaling Pathways. Clin Med Oncol. 2009;3:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 49.Uddin S, Ahmed M, Hussain A, et al. Cyclooxygenase-2 inhibition inhibits PI3 K/AKT kinase activity in epithelial ovarian cancer. Int J Cancer. 2010;126(2):382–394. [DOI] [PubMed] [Google Scholar]
- 50.Racanelli AC, Rothbart SB, Heyer CL, et al. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Re. 2009;69(13):5467–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.