Abstract
Background
The study goal was to determine whether changes in relative cerebral blood volume (rCBV) derived from dynamic susceptibility contrast (DSC) MRI are predictive of overall survival (OS) in patients with recurrent glioblastoma multiforme (GBM) when measured 2, 8, and 16 weeks after treatment initiation.
Methods
Patients with recurrent GBM (37/123) enrolled in ACRIN 6677/RTOG 0625, a multicenter, randomized, phase II trial of bevacizumab with irinotecan or temozolomide, consented to DSC-MRI plus conventional MRI, 21 with DSC-MRI at baseline and at least 1 postbaseline scan. Contrast-enhancing regions of interest were determined semi-automatically using pre- and postcontrast T1-weighted images. Mean tumor rCBV normalized to white matter (nRCBV) and standardized rCBV (sRCBV) were determined for these regions of interest. The OS rates for patients with positive versus negative changes from baseline in nRCBV and sRCBV were compared using Wilcoxon rank-sum and Kaplan–Meier survival estimates with log-rank tests.
Results
Patients surviving at least 1 year (OS-1) had significantly larger decreases in nRCBV at week 2 (P = .0451) and sRCBV at week 16 (P = .014). Receiver operating characteristic analysis found the percent changes of nRCBV and sRCBV at week 2 and sRCBV at week 16, but not rCBV data at week 8, to be good prognostic markers for OS-1. Patients with positive change from baseline rCBV had significantly shorter OS than those with negative change at both week 2 and week 16 (P = .0015 and P = .0067 for nRCBV and P = .0251 and P = .0004 for sRCBV, respectively).
Conclusions
Early decreases in rCBV are predictive of improved survival in patients with recurrent GBM treated with bevacizumab.
Keywords: bevacizumab, MRI, overall survival, rCBV, recurrent glioblastoma
Each year, ∼17 000 patients are newly diagnosed with primary brain tumors, with glioblastoma multiforme (GBM) being the most common and most aggressive malignant primary brain tumor.1 For patients with glioblastoma, surgical resection followed by chemoradiation with concomitant and adjuvant temozolomide (TMZ) is the current standard of care. With this combination therapy, the median overall survival (OS) improved from 12.1 months with radiotherapy alone to 14.6 months,2 and somewhat longer for patients with glioblastoma containing a methylated promoter of O6-DNA methylguanine-methyltransferase (MGMT).3 For patients with recurrent glioblastoma, treatment with bevacizumab, a recombinant humanized monoclonal IgG1 antibody, which binds to human vascular endothelial growth factor (VEGF),4 is also standard of care in the US. In early studies patients treated with bevacizumab demonstrated an improved rate of progression-free survival (PFS) at 6 months compared with historical controls in clinical trials with no improvement in OS.5,6 However, in subsequent studies, the median OS for patients with high-grade gliomas treated with bevacizumab was 9–11 months from treatment initiation,7,8 which is superior to the historically documented survival of 3–6 months for recurrent malignant glioma.9 There is also evidence to suggest that the combination of bevacizumab and irinotecan might further improve outcomes in patients with recurrent malignant glioma.10 Also, results from a recent randomized controlled phase II trial (BELOB) demonstrated that bevacizumab in combination with lomustine resulted in an OS of 11–16 months compared with 8 months for either bevacizumab or lomustine alone.11 This suggests that OS is improved for at least a subpopulation of patients treated with bevacizumab alone or in combination.
The challenge therefore is to be able to predict which patients are most likely to derive benefit from anti-angiogenic treatment. For this purpose, standard anatomic imaging methods are proving insufficient.12 Specifically, standard assessments such as the Macdonald criteria,13 based on the measurement of contrast-enhancing tumor on MRI or CT, and the RANO criteria (Revised Assessment in Neuro-Oncology),14 which has newly incorporated fluid attenuated inversion recovery (FLAIR) imaging, are problematic. With bevacizumab, vascular permeability is decreased, with an ensuing decrease in the degree or extent of contrast enhancement or peritumoral edema. The resulting diminished enhancement on postcontrast T1-weighted images may not be indicative of a true biologic response but rather a pseudoresponse. Likewise, there is evidence that FLAIR imaging is also of limited value in prognosticating response to anti-angiogenic treatment in recurrent GBM.15 In addition, chemoradiation preceding bevacizumab administration may induce new or continued enhancement16 that may continue into early post-bevacizumab imaging evaluation and thus may wrongly indicate progression, a phenomenon termed pseudoprogression. For these reasons, physiologic imaging biomarkers, such as perfusion- and diffusion-based MRI, are being explored as potentially more direct biomarkers of treatment response.
The Radiation Therapy Oncology Group (RTOG) trial 0625 is a multicenter, randomized, phase II trial of bevacizumab with irinotecan or TMZ in recurrent GBM. The American College of Radiology Imaging Network (ACRIN) trial 6677 is the companion study that evolved with an advanced MRI component. Results from the portion of this study in which dynamic susceptibility contrast (DSC) MRI data were collected for the creation of relative cerebral blood volume (rCBV) maps are presented here. We address the hypothesis that early changes in posttreatment rCBV will be predictive of OS. This is the first study of its kind performed in a multicenter trial setting.
Materials and Methods
RTOG, in collaboration with ACRIN, both funded by the National Cancer Institute, conducted a prospective, randomized, phase II multicenter trial evaluating bevacizumab with either irinotecan or TMZ treatment in recurrent GBM (RTOG 0625/ACRIN 6677). Specifically, all patients received bevacizumab; each patient additionally received either irinotecan or TMZ, but not both. Data were then pooled from both trial arms for our analyses. Twenty-three institutions participated, each obtaining institutional review board approval before subject accrual and conducting the trial in compliance with the Health Insurance Portability and Accountability Act. Informed consent was obtained for all subjects.
Patients
Of 123 patients enrolled in RTOG 0625/ACRIN 6677, thirty-seven consented to at least one of the optional DSC-MRI scans, 21 of whom had data sufficient for analysis, defined as having both a baseline and at least one postbaseline scan with interpretable DSC-MRI data. Using these criteria, DSC-MRI data from n = 13, n = 17, and n = 13 patients were available from weeks 2, 8, and 16, respectively. The 21 patients were enrolled at 5 different sites.
Data Acquisition Methods
MRI equipment used in this study included both 1.5T scanners (Siemens Espree, Siemens Avanto, GE Signa Excite, GE HDx) and 3T scanners (GE HDx, GE Excite). As previously described in detail, conventional MRI included precontrast T1-weighted, T2-weighted, FLAIR, and diffusion-weighted imaging.15 After an intravenous injection of 0.1 mmol/kg of a standard gadolinium-based contrast agent, axial 2D spin-echo (2D-T1) and 3D volumetric (3D-T1) T1-weighted postcontrast images were acquired. Contrast agent administered for conventional postcontrast imaging served as a loading dose (“preload”) for subsequent DSC-MRI, diminishing the contribution from T1 changes that might occur with contrast agent extravasation through a disrupted blood–brain barrier.17–20 For DSC-MRI, gradient recalled echo (GRE)–echo planar images or spin echo (SE)–echo planar images were obtained with the following recommended parameters: GRE echo time (TE) = 30–40 ms; SE TE = 60–105 ms, repetition time = 1.3–1.5 s, flip angle = 90 degrees, slice thickness = 5 mm with a 0–2.5 mm gap, matrix = 128 × 128, field of view = 22–24 cm, and repetitions = 120. Images were acquired for 1 min before and 2 min after a 0.1 mmol/kg bolus injection of gadolinium contrast agent.
Image Analysis
The raw DSC-MRI time series were truncated to eliminate the first 5 time points prior to the MR signal reaching steady state. The truncated DSC-MRI time courses, S(t), were converted into concentration-time (ΔR2*(t)) curves using the following equation:
| (1) |
where SB is the mean baseline value. The rCBV maps, uncorrected for leakage effects, were computed from the integral of ΔR2*(t) described by the equation. Next, the rCBV maps were corrected for leakage effects18 and either normalized (nRCBV) (to normal appearing white matter) or standardized (sRCBV) using OsiriX open-source software with the IB Neuro plug-in (Imaging Biometrics LLC, Elm Grove WI). Standardization is a process by which rCBV values are mathematically transformed to a consistent intensity scale, regardless of MR scanner vendor, model, or field strength (1.5T or 3T).21 Contrast-enhancing regions of interest (ROIs) were determined using a semi-automatic method whereby a difference image was computed from standardized pre- and standardized postcontrast T1-weighted images and a threshold applied to automatically determine the ROI.22 These ROIs, which were determined anew for each time point, were applied to both nRCBV and sRCBV maps, which were coregistered to the T1-weighted images, from which mean values were extracted. OS was determined from study registration.
Statistical Analysis
Summary statistics including the mean and range were computed for both nRCBV and sRCBV. Receiver operating characteristic (ROC) curves were derived for the percent change from baseline of nRCBV and sRCBV at weeks 2, 8, and 16, using OS past 1 year (OS-1) as the reference standard. The area under the ROC curve (AUC) and the associated 95% confidence interval were computed empirically. The nonparametric Wilcoxon rank-sum test was used to test the hypothesis that nRCBV or sRCBV values were significantly different for patients who had expired versus those who remained alive at 1 year. In addition, Fisher's exact test was used to test the hypothesis that the proportion of patients alive at 1 year was significantly different among those with increased versus decreased nRCBV or sRCBV (relative to baseline) at week 2, 8, or 16. Kaplan–Meier survival estimates and log-rank tests were used to compare OS between patients with increased versus decreased nRCBV and sRCBV. All analyses were performed using SAS 9.3, with P < .05 considered statistically significant. Finally, as an initial test to determine if an association existed between either the nRCBV or sRCBV measures and the volume of enhancement, a Fisher's exact test was applied to the data collected at 2 weeks post-bevacizumab.
Results
For the 21 analyzable patients, the average age was 53.5 ± 12.4 years (median, 55; range, 23–74); 11 (52%) were male; all were caucasian; and 13 (62%) had Karnofsky performance scores of 70–80. Upon study completion, 8 (38%) were alive at 1 year (OS-1).
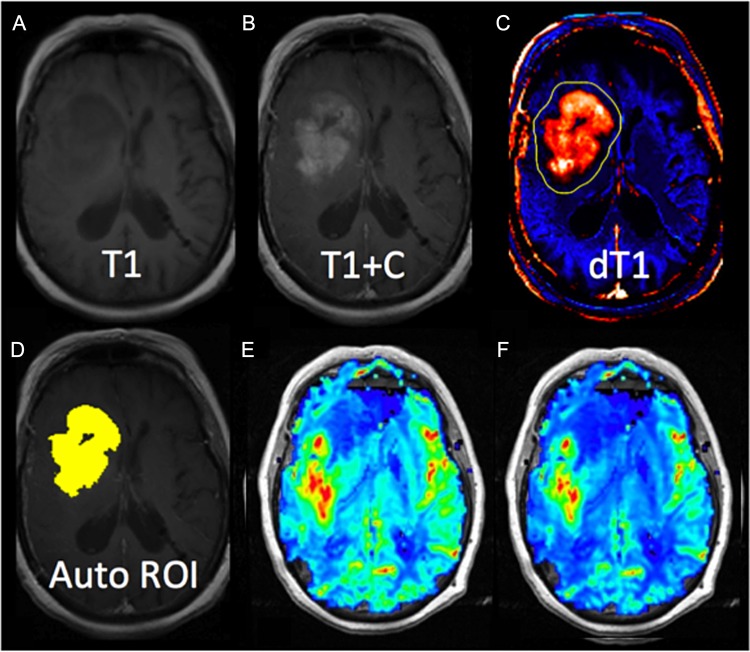
Figure 1 provides an example of pre- and postcontrast T1-weighted images (T1, T1 + C) and a computed delta T1 map (dT1) from which an automated ROI is determined. Also shown are the corresponding nRCBV and sRCBV maps for this patient. Delta T1 maps facilitate accurate determination of enhancing tumor ROIs when there is precontrast T1 hyperintensity and diminished contrast enhancement secondary to bevacizumab. The dT1 maps have the added advantage of enabling automatic determination of enhancing tumor ROI, since they are derived from standardized images mapped to a calibrated image intensity scale.22
Fig. 1.
Example results from a representative case. Shown are (A and B) the pre- and postcontrast T1-weighted images, (C) the delta T1 (dT1) map, (D) an image with the automatically determined ROI highlighted in yellow, and (E and F) the normalized and standardized RCBV maps from the same slice.
As shown in Table 1, on average both nRCBV and sRCBV decreased at weeks 2, 8, and 16, relative to the baseline value, with the only exception being nRCBV at week 16 (10.08% ± 60.07%). However, comparing subjects who survived past 1 year with those who did not, the survivor group tended to exhibit a much larger decrease in rCBV than the nonsurvivor group, with these effects reaching statistical significance for nRCBV at week 2 (P = .0451) and sRCBV at week 16 (P = .014).
Table 1.
Summary statistics and comparison of nRCBV and sRCBV percent change from baseline at weeks 2, 8, and 16 stratified by survival status
| Scan Time Point | N | Normalized RCBV (nRCBV) |
Standardized RCBV (sRCBV) |
||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (range) | P-valuea | Mean (SD) | Median (range) | P-valuea | ||
| Week 2 | |||||||
| RCBV (% change) | 13 | −17.13 (54.46) | −11.60 (−89.79, 81.85) | – | −16.06 (54.08) | −37.41 (−91.46, 86.77) | – |
| Alive at 1 y | 5 | −52.94 (33.48) | −64.68 (−89.79, −11.60) | .0451 | −56.41 (21.57) | −51.73 (−91.46, −37.41) | .0653 |
| Dead at 1 y | 8 | 5.24 (54.38) | 0.33 (−78.55, 81.85) | 9.15 (53.47) | 9.14 (−61.01, 86.77) | ||
| Week 8 | |||||||
| RCBV (% change) | 17 | −10.27 (55.17) | −37.39 (−96.09, 81.48) | – | −12.51 (52.40) | −13.20 (−93.38, 133.82) | – |
| Alive at 1 y | 6 | −13.89 (53.79) | −38.93 (−65.18, 68.75) | .8836 | −25.17 (23.55) | −23.48 (−51.29, 8.17) | .7325 |
| Dead at 1 y | 11 | −8.30 (58.41) | −3.00 (−96.09, 81.48) | −5.60 (62.99) | −13.20 (−93.38, 133.82) | ||
| Week 16 | |||||||
| RCBV (% change) | 13 | 10.08 (60.07) | 2.99 (−54.33, 142.00) | – | −2.77 (31.74) | −7.47 (−60.85, 60.64) | – |
| Alive at 1 y | 7 | −17.01 (41.95) | −37.81 (−53.65, 54.73) | .1375 | −22.15 (20.19) | −20.45 (−60.85, 0) | .0140 |
| Dead at 1 y | 6 | 41.69 (65.75) | 46.76 (−54.33, 142.00) | 19.85 (28.09) | 22.43 (−12.77, 60.64) | ||
aP-value is from a 2-sided Wilcoxon rank-sum test.
The ROC AUCs and associated 95% CIs, using OS-1 as the reference standard, for nRCBV and sRCBV at weeks 2, 8, and 16 are shown in Table 2. If the lower 95% confidence limit of a marker is at least 0.5, the marker is considered capable of differentiating between 1-year survivors and nonsurvivors. Based on this criterion, percent change of nRCBV (AUC, 0.850; 95% CI: 0.624–1.000) and sRCBV (AUC, 0.825; 95% CI: 0.577–1.000) at week 2 and percent change of sRCBV at week 16 (AUC, 0.905; 95% CI: 0.736–1.000) are good prognostic markers for OS-1. Neither marker measured at the week 8 scan was found to be useful for prognosis of OS-1.
Table 2.
AUC (95% CI) of the ROC curve of percent change from baseline of nRCBV and sRCBV, using OS-1 as the reference standard
| N | nRCBV | sRCBV | |
|---|---|---|---|
| Week 2 | 13 | 0.850 (0.624, 1.000) | 0.825 (0.577, 1.000) |
| Week 8 | 17 | 0.470 (0.171, 0.768) | 0.561 (0.277, 0.844) |
| Week 16 | 13 | 0.762 (0.435, 1.000) | 0.905 (0.736, 1.000) |
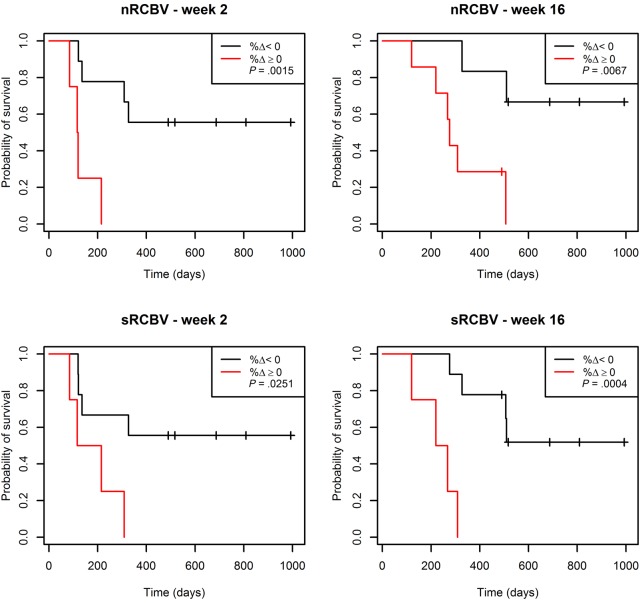
We further separated patients into 2 groups by percent change of nRCBV or sRCBV—one group with positive change (increase) from baseline, and the other group with negative change (decrease) from baseline. As shown in Table 3, patients with a positive change in nRCBV and sRCBV at weeks 2 and 16 had much worse OS than participants with a negative change. For example, none of the patients (0/4) with positive changes at week 2 survived past year 1, compared with 56% of patients (5/9) with a negative change. The group with a positive change had significantly shorter survival time compared with the group with a negative change at both week 2 and week 16 (P = .0015 and P = .0067 for nRCBV and P = .0251 and P = .0004 for sRCBV, respectively). The week 2 and week 16 Kaplan–Meier survival curves for nRCBV and sRCBV are shown in Fig. 2. In all cases, the week 8 survival results showed a similar trend but failed to reach statistical significance. Using Fisher's exact test, no associations between enhancing volumes and either nRCBV or sRCBV were found at the 2-week time point (P = 1.0).
Table 3.
Association between the percent change from baseline at weeks 2, 8, and 16 for nRCBV and sRCBV and OS-1, or time to death
|
nRCBV |
sRCBV |
|||||
|---|---|---|---|---|---|---|
| Week 2 | Increase (n = 4) | Decrease (n = 9) | P-value | Increase (n = 4) | Decrease (n = 9) | P-value |
| OS-1 (n = 5) | 0 (0%) | 5 (56%) | .1049a | 0 (0%) | 5 (56%) | .1049a |
| Time to death (n = 13) | – | – | .0015b | – | – | .0251b |
| Week 8 | Increase (n = 6) | Decrease (n = 11) | P-value | Increase (n = 6) | Decrease (n = 11) | P-value |
| OS-1 (n = 6) | 2 (33%) | 4 (36%) | 1.000a | 1 (17%) | 5 (45%) | .3334a |
| Time to death (n = 17) | – | – | .1672b | – | – | .1862b |
| Week 16 | Increase (n = 7) | Decrease (n = 6) | P-value | Increase (n = 4) | Decrease (n = 9) | P-value |
| OS-1 (n = 7) | 2 (29%) | 5 (83%) | .1026a | 0 (0%) | 7 (78%) | .0210a |
| Time to death (n = 13) | – | – | .0067b | – | – | .0004b |
aP-value is from 2-sided Fisher's exact test.
bP-value is from 2-sided log-rank test.
Fig. 2.
Kaplan–Meier curves for OS stratified by percent change (%Δ) in nRCBV and sRCBV at 2 and 16 weeks.
Discussion
This study represents the first multicenter trial to demonstrate that rCBV, and not a particular implementation of DSC-MRI, can serve as a useful biomarker for the prediction of response to anti-angiogenic therapy. Specifically, a decreasing rCBV, measured at either 2 or 16 weeks post treatment initiation, predicted a clear improvement in OS for patients. Improvement in OS suggests that bevacizumab not only improves the postcontrast imaging appearance secondary to reduction of blood–brain barrier permeability (pseudoresponse), but additionally causes a positive biologic effect in at least a subset of patients. Thus, early changes in rCBV appear to be helpful for predicting which patients will derive benefit from bevacizumab treatment.
These results are consistent with a previous single-center retrospective study demonstrating the utility of rCBV for predicting response to bevacizumab.23 The rCBV values measured between 20 and 60 days after treatment initiation were shown to be predictive of PFS and OS. However, in seeming contradiction to the current study, no significant difference in OS was demonstrated between patients whose rCBV increased versus those for which rCBV decreased. Yet, the results from this previous study were obtained at 3–9 weeks post treatment initiation, which more closely match the data from the 8-week time point of this study. Recall that at 8 weeks no significant difference in OS based on changes in rCBV was predicted. Though the two studies are consistent, it is unclear as to why changes in rCBV measured at 8 weeks, compared with the 2- and 16-week time points, did not predict OS. In the current study it may be due to the small number of patients. However, in the previous single-center study, 36 patients were included. Alternatively, the explanation may have a biologic basis, possibly explained by the theory of vascular normalization.24 It is a point that deserves further investigation.
Several previous studies have also used DSC-MRI derived parameters to predict response to cediranib, a pan-VEGF receptor tyrosine kinase inhibitor.25,26 In one study,25 increases in tumor cerebral blood flow, derived from DSC-MRI, were shown to be associated with improved OS for patients with newly diagnosed GBM. Similarly, in a second study, a durable increase in cerebral blood flow as measured with arterial spin labeling was associated with improved PFS and OS in patients with recurrent GBM treated with cediranib.26 While these results seem at odds with the results of the current study, where rCBV decreases are associated with OS, they may in fact be consistent given the theory of vascular normalization.24 With vascular normalization it is theorized that with an effective anti-angiogenic therapy inefficient blood vessels are pruned, resulting in a decrease in blood volume, which then enables a more efficient (ie, possibly increased) blood flow. Whether differences can be attributed to vascular normalization or differences in the treatment being evaluated (cediranib vs bevacizumab), it is a question worth pursuing in future studies.
The results of this study are also consistent with several additional studies, which did not address response to a targeted therapy but do demonstrate that rCBV was predictive of survival in general. For example, Law et al27 showed that the median time to progression for patients with gliomas was significantly less if nRCBV was >1.75, a finding independent of pathologic findings.28 Similarly, Chaskis et al29 demonstrated that rCBV correlated with grade and survival with a median OS of 6 months for those with hyperperfusion (rCBV > gray matter) versus 63 months otherwise. A significant negative correlation was demonstrated between elevated rCBV and survival especially for rCBV ratios (ie, normalized to gray matter) >1.7. Likewise, Lev et al30 showed that the degree of nRCBV elevation, using a threshold of 1.5, was a stronger predictor of both tumor grade and OS than degree of enhancement.31 In yet another study, pretreatment maximum rCBV in patients with high-grade gliomas showed improved survival for those with maximum nRCBV <2.332 and was shown to be an independent prognostic factor in patients with high-grade astrocytoma.
Yet, there are a few reports that suggest that rCBV is not predictive of survival.33–39 However, there are some important differences between these few studies and the current and previous studies showing an ability to predict survival. For example, in some cases rCBV was not directly computed. Rather, a “perfusion-related” value was determined, which was based on signal heuristic analysis, an approach that has been shown to be problematic.40 Other studies did not account for contrast agent extravasation or “leakage” effects, which are well known for their potential to confound the DSC-MRI rCBV measurements in brain tumors. This may also explain why in one study,37 increases rather than decreases in rCBV showed a correlation with longer survival. When contrast agent leakage is not corrected, the rCBV can be underestimated and the underestimation increases with higher-grade tumors.18 Therefore, the rCBV of the high-grade tumors could have been more severely underestimated, showing the inverse correlation reported. However, in a similar study where an association between increased rCBV and improved OS was also reported, it was stated that leakage correction was applied. But as described, gamma-variate fitting was undertaken before a leakage correction algorithm was applied. Gamma-variate fitting does not compensate for leakage effects that might occur throughout the signal time course.40 Rather it simply forces the post-bolus baseline to zero and makes any subsequent applications of leakage correction algorithms ineffective.
In the current study, leakage effects were handled by using both a contrast agent preload, to diminish leakage effects, and a postprocessing correction algorithm applied to the rCBV maps, to correct for any remaining leakage effects.18 Several previous studies have demonstrated the utility of such methods to diminish leakage effects, with the preload and postprocessing correction methods working together to increase the accuracy of the final rCBV maps17,19,20,41 and give the best distinction between tumor and treatment effects20 and between high-grade tumor and reference brain.40 Using a similar approach in the current study, changes in rCBV were clearly predictive of OS when measured at 2 and 16 weeks post treatment initiation. These findings suggest that a similar approach should be followed when using rCBV as a predictive tool in brain tumors.
Both normalized and standardized rCBV maps (nRCBV, sRCBV) were shown to be predictive of OS, suggesting that they provide comparable information. However, an advantage of standardizing rCBV maps is that it precludes the need for the additional manual step of drawing reference ROIs to which the maps are normalized. This in turn decreases the potential for user error and interobserver differences while improving workflow. Standardization of rCBV maps may therefore provide an easier and more reliable solution for future clinical trials.
The use of rCBV as a biomarker for vascular volume in tumor tissue is supported by several previous studies performed in both animal models and patients. Measures of rCBV compared well with histologic measures of fractional blood volume as determined in rat brain tumor models.42–44 Direct comparison between the rCBV when using a gadolinium contrast agent, similar to the approach used in this study, and rCBV measured with an iron oxide contrast agent, which is not confounded by contrast agent extravasation, showed excellent agreement.41 The agreement was best when the effects of gadolinium contrast agent extravasation were addressed by using both a preload of contrast agent and a postprocessing leakage correction algorithm. The agreement was diminished when either but not both leakage correction steps were used. Accordingly, in the current study, both the contrast agent preload and postprocessing correction algorithm were applied. Finally, studies performed in patients where the rCBV measures were spatially correlated with tissue biopsy samples demonstrated that rCBV was able to distinguish vascular from avascular tissue with high accuracy.45 Similarly, in a comparison study, there were only two approaches for obtaining rCBV that were able to distinguish high-grade tumor from reference brain, one of which is the approach used in this study.40
A limitation of the current study is the low number of patients enrolled in the advanced imaging arm (ACRIN 6677) of the parent RTOG study. However, even with this limited number, the results are very compelling, as indicated by the statistically significant differences found in OS between those with increasing versus decreasing rCBV values. So at minimum these studies provide strong evidence to support the continued evaluation of rCBV in larger multicenter trials.
An initial analysis to determine whether the rCBV changes tracked enhancement changes was performed at the 2-week time point with no statistical association determined. This is consistent with previous studies that evaluated changes in contrast-enhancement and FLAIR-hyperintense volumes, and found no predictive value for these measures in the context of bevacizumab treatment for recurrent GBM.25 In addition, no statistically significant differences in enhancing and FLAIR volumes were found between the low and high rCBV groups. However, in a recent study using multicenter clinical trial data (AVF3708g, the BRAIN trial), a statistically significant difference in PFS and OS was observed if pretreatment or 6 week posttreatment enhancing volumes were above or below an empirically determined threshold, or volume changes were >25%.46 Taken together, these results suggest that enhancement volume and rCBV may be independent biomarkers, both deserving further study, or as recently proposed may be useful in a tiered system of evaluation.47
Of significance for future clinical trials, this previous study46 also demonstrated that the Gaussian-normalized T1 subtraction maps improved both the visualization and the quantitative distinction among the various groups compared with conventional segmentation methods. This finding lends additional support to the approach in this study to use dT1 (standardized T1 subtraction maps) to delineate enhancing tumor volume. However, an added advantage to the dT1 approach is that the standardization step22 precludes the need for any user manipulation and enables the automation of ROI delineation. This decreases the potential for observer error, which also makes it an easy and reliable approach amenable for use in clinical trials.
This trial offers evidence for use of rCBV as a biomarker to predict response to bevacizumab. Measures of rCBV or its changes could be used to identify patients responding poorly to standard treatment with bevacizumab and steer them to clinical trials. Or it may be used to identify patients responding well to bevacizumab, thereby suggesting a more aggressive approach in dealing with potential side effects. As such, rCBV may offer a solution to the recent call for the development of new biomarkers for bevacizumab.48 As discussed, the need for such a biomarker has become even more critical given the recent FDA withdrawal of approval for bevacizumab in patients with metastatic breast cancers as well as negative results from trials such as those using bevacizumab in colorectal cancers or as first-line therapy in glioblastoma.49 Consequently, these recent developments have diminished the initial hope that VEGF-targeted therapies would be broadly effective and have brought the current and future growth of bevacizumab use to a standstill, which can potentially be overcome with the identification of predictive biomarkers. In this regard, molecular biologic research may give us a renewed understanding of resistance and therapeutic escape as well as the potential for new biomarkers. For example, it has been demonstrated that the VEGF–VEGF receptor 2–neuropilin-1 (NRP-1) axis in glioblastoma stem cells is maintained in an autocrine manner by continuous secretion of VEGF ligand, allowing for promotion of GBM tumor growth, invasiveness, and enhanced resistance to some treatments.50 Yet, while biomarkers such as plasma VEGF-A levels and tumor expression of NRP-1 showed some evidence of being able to predict benefit of bevacizumab, the findings were inconclusive. Alternatively, the results of this study clearly support rCBV as a predictive biomarker for bevacizumab treatment in patients with brain tumors. Still, it seems that the utility of rCBV as a biomarker may be further expanded and understood by continued parallel investigations of promising molecular biomarkers such as NRP-1 as well.
Finally, the results of this trial may also have implications for our understanding of how bevacizumab works in recurrent glioblastoma. For example, there are several articles that suggest that bevacizumab acts simply as a “super steroid” with a decrease in tumor contrast agent enhancement without a true biologic effect,51 that any antitumor effect is hard to see,12,52 or worse, that bevacizumab causes the tumor to become more invasive.53,54 While each of these possibilities may be true in certain patients, the results from this study suggest that there may be a subset of patients identifiable by use of DSC-MRI in whom treatment with bevacizumab diminishes CBV and leads to a survival benefit.
Summary
This study demonstrates that a DSC-MRI protocol with leakage correction and a fixed method of image acquisition and analysis can be deployed successfully in a multicenter clinical trial. Although the number of patients in this study was relatively small, the results support the concept of rCBV as a biomarker of treatment response in patients with recurrent GBM. It therefore provides the justification for the performance of a larger prospective, possibly adaptive, clinical trial where the role of rCBV may be more fully defined.
Funding
ACRIN received funding from the National Cancer Institute through grants U01-CA080098 and U01-CA079778.
Acknowledgments
We acknowledge Imaging Biometrics LLC for technical support, software development, and assistance with data analysis.
Conflict of interest statement. K.M.S. has an ownership interest in Imaging Biometrics LLC.
References
- 1.Woehrer A, Bauchet L, Barnholtz-Sloan JS. Glioblastoma survival: has it improved? Evidence from population-based studies. Current opinion in neurology. 2014;276:666–674. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in randomized phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet. 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 3.Hegi ME, Diserens A-C, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 4.Moen MD. Bevacizumab in previously treated glioblastoma. Drugs. 2010;70(2):181–189. [DOI] [PubMed] [Google Scholar]
- 5.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 6.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110(1):173–180. [DOI] [PubMed] [Google Scholar]
- 8.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91(3):329–336. [DOI] [PubMed] [Google Scholar]
- 9.Gruber ML, Buster WP. Temozolomide in combination with irinotecan for treatment of recurrent malignant glioma. Am J Clin Oncol. 2004;27:33–38. [DOI] [PubMed] [Google Scholar]
- 10.Xu T, Chen J, Lu Y, et al. Effects of bevacizumab plus irinotecan on response and survival in patients with recurrent malignant glioma: a systematic review and survival-gain analysis. BMC Cancer. 2010;10:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 12.Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. AJNR Am J Neuroradiol. 2008;29(3):419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macdonald DR, Cascino TL, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 14.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 15.Boxerman JL, Zhang Z, Safriel Y, et al. Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study. Neuro Oncol. 2013;15(7):945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donahue KM, Krouwer HGJ, Rand SD, et al. Utility of simultaneously acquired gradient-echo and spin-echo cerebral blood volume and morphology maps in brain tumor patients. Magn Reson Med. 2000;43:845–853. [DOI] [PubMed] [Google Scholar]
- 18.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol. 2006;27(4):859–867. [PMC free article] [PubMed] [Google Scholar]
- 19.Schmainda KM, Rand SD, Joseph AM, et al. Characterization of a first-pass gradient-echo spin-echo method to predict brain tumor grade and angiogenesis. AJNR Am J Neuroradiol. 2004;25(9):1524–1532. [PMC free article] [PubMed] [Google Scholar]
- 20.Hu LS, Baxter LC, Pinnaduwage DS, et al. Optimized preload leakage-correction methods to improve the diagnostic accuracy of dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in posttreatment gliomas. AJNR Am J Neuroradiol. 2010;31(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedekar D, Jensen TR, Schmainda KM. Standardization of relative cerebral blood volume (rCBV) image maps for ease of both inter and intra-patient comparisons. Magn Reson Med. 2010;64(3):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedekar D, Jensen T, Rand S, et al. Delta T1 method: an automatic postl-contrast RO1 selection technique for brain tumors. Paper presented at: International Society for Magnetic Resonance in Medicine, 18th Annual Meeting 2010; Stockholm, Sweden. [Google Scholar]
- 23.Schmainda KM, Prah M, Connelly J, et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014;16(6):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–989. [DOI] [PubMed] [Google Scholar]
- 25.Batchelor TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110(47):19059–19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen AG, Emblem KE, Polaskova P, et al. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2012;72(2):402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Law M, Oh S, Babb JS, et al. Low-grade gliomas: dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging—prediction of patient clinical response. Radiology. 2006;238(2):658–667. [DOI] [PubMed] [Google Scholar]
- 28.Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247(2):490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaskis C, Stadnik T, Michotte A, et al. Prognostic value of perfusion-weighted imaging in brain glioma: a prospective study. Acta Neurochir (Wien). 2006;148(3):277–285. [DOI] [PubMed] [Google Scholar]
- 30.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol. 2004;25(2):214–221. [PMC free article] [PubMed] [Google Scholar]
- 31.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity and predictive values of perfusion MR imaging and proton MR spectroscopic imaigng compared to conventional imaging. Am J Neuroradiol. 2003;24(10):1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 32.Hirai T, Murakami R, Nakamura H, et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol. 2008;29(8):1505–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caseiras GB, Chheang S, Babb J, et al. Relative cerebral blood volume measurements of low-grade gliomas predict patient outcome in a multi-institution setting. Eur J Radiol. 2010;73(2):215–220. [DOI] [PubMed] [Google Scholar]
- 34.Oh J, Henry RG, Pirzkall A, et al. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging. 2004;19(5):546–554. [DOI] [PubMed] [Google Scholar]
- 35.Leimgruber A, Ostermann S, Yeon EJ, et al. Perfusion and diffusion MRI of glioblastoma progression in a four-year prospective temozolomide clinical trial. Int J Radiat Oncol Biol Phys. 2006;64(3):869–875. [DOI] [PubMed] [Google Scholar]
- 36.Chang SM, Nelson S, Vandenberg S, et al. Integration of preoperative anatomic and metabolic physiologic imaging of newly diagnosed glioma. J Neurooncol. 2009;92(3):401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galban CJ, Chenevert TL, Meyer CR, et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15(5):572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saraswathy S, Crawford FW, Lamborn KR, et al. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J Neurooncol. 2009;91(1):69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawford FW, Khayal IS, McGue C, et al. Relationship of pre-surgery metabolic and physiological MR imaging parameters to survival for patients with untreated GBM. J Neurooncol. 2009;91(3):337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paulson ES, Schmainda KM. Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology. 2008;249(2):601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boxerman JL, Prah DE, Paulson ES, et al. The Role of preload and leakage correction in gadolinium-based cerebral blood volume estimation determined by comparison with MION as a criterion standard. AJNR Am J Neuroradiol. 2012;33(6):1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pathak AP, Schmainda KM, Ward BD, et al. MR-derived cerebral blood volume maps: issues regarding histological validation and assessment of tumor angiogenesis. Magn Reson Med. 2001;46(4):735–747. [DOI] [PubMed] [Google Scholar]
- 43.Badruddoja MA, Krouwer HG, Rand SD, et al. Antiangiogenic effects of dexamethasone in 9L gliosarcoma assessed by MRI cerebral blood volume maps. Neuro Oncol. 2003;5(4):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dennie J, Mandeville JB, Boxerman JL, et al. NMR imaging of changes in vascular morphology due to tumor angiogenesis. Magn Reson Med. 1998;40(6):793–799. [DOI] [PubMed] [Google Scholar]
- 45.Hu L, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast perfusion MR imaging measurements. Am J Neuroradiol. 2009;30(3):552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boxerman JL, Zhang Z, Schmainda KM, et al. Early post-bevacizumab change in rCBV from DSC-MRI predicts overall survival in recurrent glioblastoma whereas 2D-T1 response stauts does not: results from the aCRIN 6677/RTOG 0625 multi-center study. Paper presented at: Radiologic Society of North America 2014; Chicago. [Google Scholar]
- 48.Maru D, Venook AP, Ellis LM. Predictive biomarkers for bevacizumab: are we there yet? Clin Cancer Res. 2013;19(11):2824–2827. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2048–2049. [DOI] [PubMed] [Google Scholar]
- 50.Hamerlik P, Lathia JD, Rasmussen R, et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209(3):507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagane M, Nishikawa R. Bevacizumab for glioblastoma-a promising drug or not? Cancers. 2013;5(4):1456–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verhoeff JJ, van Tellingen O, Claes A, et al. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Claes A, Gambarota G, Hamans B, et al. Magnetic resonance imaging-based detection of glial brain tumors in mice after antiangiogenic treatment. Int J Cancer. 2008;122(9):1981–1986. [DOI] [PubMed] [Google Scholar]
- 54.de Groot JF, Fuller G, Kumar AJ, et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12(3):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]