Abstract
Background
To analyze the relevance of dynamic susceptibility-weighted contrast-enhanced MRI (DSC-MRI) derived relative cerebral blood volume (rCBV) analysis for predicting response to bevacizumab (BEV) in patients with recurrent glioblastoma (rGB).
Methods
A total of 127 patients diagnosed with rGB receiving either bevacizumab (71 patients, BEV cohort) or alkylating chemotherapy (56 patients, non–BEV cohort) underwent conventional anatomic MRI and DSC-MRI at baseline and at first follow-up after treatment initiation. The mean rCBV of the contrast-enhancing tumor (cT1) as well as cT1 and fluid-attenuated inversion recovery (FLAIR) volumes at both time points were correlated with progression-free survival (PFS) and overall survival (OS) using Cox proportional hazard models, logisticregression, and the log-rank test.
Results
Baseline rCBV was associated with both PFS (hazard ratio [HR] = 1.3; P < .01) and OS (HR = 1.3; P < .01) in the BEV cohort and predicted 6-month PFS in 82% and 12-month OS in 79% of patients, whereas it was not associated with PFS (HR = 1.0; P = .70) or OS (HR = 1.0; P = .47) in the non-BEV cohort. Corresponding median OS and PFS rates in the BEV cohort for patients with rCBV-values less than 3.92 (optimal threshold from receiver operating characteristic [ROC] analysis of 12-month OS data) were 14.2 and 6.0 months, as compared to 6.6 and 2.8 months for patients with rCBV-values greater than 3.92 (P < .01, respectively). cT1 and FLAIR volumes at first follow-up were significant predictors of 6-month PFS and 12-month OS in the BEV cohort but not in the non-BEVcohort. Corresponding volumes at baseline were not significant in any cohort.
Conclusions
Pretreatment rCBV is a potential predictive imaging biomarker in BEV-treated rGB but not alkylating chemotherapy-treated rGB, which is superior to volumetric analysis of conventional anatomic MRI and predicts 6-month PFS and 12-month OS in 80% of BEV-treated patients.
Keywords: bevacizumab, cerebral blood volume, biomarker, glioblastoma, rCBV
Angiogenesis is a hallmark of cancer, especially glioblastoma, which is the most malignant and common primary brain tumor in adults.1 This complex and highly adaptive biological process is regulated through several pathways, with vascular endothelial growth factor (VEGF) as one key factor promoting survival, proliferation, migration, and permeability of endothelial cells.2 This discovery has prompted considerable interest in antiangiogenic treatments with bevacizumab (BEV), a humanized monoclonal antibody to the vascular endothelial growth factor (VEGF)A, being the most commonly used antiangiogenic agent in the setting of recurrent glioblastoma (rGB).1,2 Although antiangiogenic treatment causes a rapid decline in the contrast-enhancing tumor (cT1), only a fraction of patients show a meaningful decline in tumor burden because radiographic response may result partly from normalization of abnormally permeable vessels and not always indicate a true antitumoral effect.3–5 Therefore, it is critically important to identify radiological biomarkers that can predict treatment response and aid patient selection.
Relative cerebral blood volume (rCBV) imaging, which is derived from dynamic susceptibility-weighted contrast-enhanced MRI (DSC-MRI), allows characterization of the vasculature in the tumor microenvironment6 and has shown great promise for the evaluation of brain tumors.7–9 Preclinical studies suggest that rCBV imaging may have the potential to monitor changes in vascularity known to accompany antiangiogenic treatment;10,11 however, its role for predicting response to BEV treatment has not yet been fully evaluated.
The objective of the present study was to analyze the relevance of DSC-MRI-derived rCBV analysis for predicting response to BEV in patients with rGB.
Methods
Patients
Retrospective data evaluation was approved by the local ethics committee of the University of Heidelberg (ethics approval number: S-320/2012). A total of 127 patients diagnosed with rGB receiving either BEV (71 patients in the BEV cohort) or alkylating chemotherapy and never exposed to antiangiogenic treatment (56 patients in the non-BEV cohort) were included in this study. All participants met the following criteria: (i) pathologically confirmed GB with recurrence based on MRI in the period of July 2009 and January 2014 (only considering primary GB); (ii) regular treatment for GB recurrence with either BEV monotherapy (Avastin, Roche; 10 mg/kg of body weight) every 2 weeks per cycle in the BEV cohort or alkylating chemotherapy in the non-BEV cohort (refer to Supplementary Table S1 for a detailed listing), (iii) availability of MRI studies at baseline prior to initiating chemotherapy and at first follow-up after initiating chemotherapy that included conventional anatomic MRI and dynamic susceptibility-weighted contrast-enhanced MRI (DSC-MRI). Patients were excluded from this study if (i) they were exposed to antiangiogenic treatment at any time during their disease in the non-BEV cohort; (ii) or if a repeat surgery was performed prior to initiating treatment without measurable contrast-enhancement at baseline or between the baseline and first follow-up scan after initiating treatment; (iii) or if the MRI data were of insufficient quality due to motion artifacts or poor contrast injection.
Epidemiological and clinical characteristics of all participants are shown in Supplementary Table S2. The baseline characteristics of sex, age, extent of resection, adjuvant treatment at initial tumor presentation, and Karnofsky performance score (KPS) at treatment initiation were balanced between the BEV and non-BEV cohorts, whereas the number of recurrences prior to treatment were significantly lower in the non-BEV cohort (median of 2 recurrences in the BEV vs 1 recurrence in the non-BEV cohort, P < .01). Furthermore, the interval of baseline MRI to treatment initiation was significantly longer in the BEV cohort (median of 1.9 weeks for the BEV cohort and 0.9 weeks for the non-BEV cohort, P = .01), while the interval of treatment initiation to follow-up MRI was significantly shorter (median of 7.9 weeks for the BEV cohort and 10.9 weeks for the non-BEV cohort, P = .01). The aforementioned intervals reflect the off-label prescription practice for BEV in Germany because reimbursement by the patient's health insurance is granted only after following a case-by-case review and being subject to reevaluation after a limited number of treatment cycles.
Steroid use at baseline MRI was equally distributed between both cohorts (P = .16), whereas it was significantly lower in the BEV cohort at first follow-up (P < .01), which reflects the corticosteroid-sparing effects of BEV.12
Assessment of response to BEV/alkylating chemotherapy was performed according to the Response Assessment in Neuro-Oncology (RANO) working group criteria.3 Specifically, progressive disease (PD) was defined as ≥ 25% increase in the maximal cross-sectional tumor area or the appearance of any new enhancing lesion. For taking into account non-enhancing tumor progression, a significant increase (that we defined as ≥ 25% in the maximal cross-sectional area) in the nonenhancing lesions or the appearance of new lesions was also defined as PD (provided that the patient was on stable or increasing doses of corticosteroids and that the increase in fluid-attenuated inversion recovery (FLAIR) signal could not be attributed to comorbid events such as effects from surgery and chemoradiation, ischemia, epilepsy, or infection). Definite clinical deterioration not attributable to other causes apart from the tumor or changes in corticosteroid doses, failure to return for evaluation as a result of death or deteriorating condition, or clear progression of nonmeasurable disease were also defined as PD.3
At the time of last assessment (September, 2014), 94% of participants (67/71) in the BEV cohort and 95% of participants (53/56) in the non-BEV cohort showed tumor progression (P = .95 for group differences), and 77% of participants (55/71) in the BEV cohort and 82% (46/56) in the non-BEV cohort had died (P = .52 for group differences).
Magnetic Resonance Imaging
Images were acquired in the routine clinical workup using a 3 Tesla MR system (Magnetom Verio/Trio TIM, Siemens Healthcare) with a 12-channel head-matrix coil. Acquisition of the DSC-MRI sequence was performed as described previously.13 Prior dynamic imaging, a 0.1 mmol/kg prebolus dose of gadoterate meglumine (Gd-DOTA, DOTAREM, Guerbet, France) was administered to diminish T1 effects that might result from agent extravasation. DSC-PWI was obtained with a T2*-weighted gradient-echo EPI sequence during bolus injection of a standard dose (0.1 mmol/kg) of intravenous gadoterate meglumine. Twenty-six to 28 slices with a thickness of 5 mm were acquired with fat suppression (TE = 36 milliseconds [ms], TR = 2220 ms, FA = 90°, field of view = 240 × 240 mm, image matrix = 128 × 128 mm). In total, 50–75 dynamic measurements were performed. Subsequently, postcontrast T1-weighted 3D magnetization-prepared rapid acquisition gradient echo (cT1; TI = 1100 ms, TE = 4 ms, TR = 1710ms, and FA = 15°), and FLAIR (TI = 2400 ms; TE = 85 ms; TR = 8500 ms; section thickness, 5 mm; interslice gap, 5%) images were acquired.
Image Post Processing and Analysis
Postprocessing of DSC-MRI, cT1 and FLAIR data was performed with dedicated software (Olea Sphere v 2.3, Olea Medical). First, tumor segmentation on cT1 and FLAIR images at baseline and at first follow-up after BEV/alkylating chemotherapy treatment was performed according to a semiautomated region-growing segmentation method. This segmentation approach examines neighboring voxels of an initial seed point/voxel and determines whether the voxel neighbors should be added to the region of interest (ROI). Multiple seed points were used to account for multifocal or discontinuing lesions, and iteration of this process was performed on each slice of the cT1 images until the contrast-enhancing portion of the whole tumor was included, and macroscopic necrosis, cysts, and normal vessels were excluded from the segmented tumor. Similarly, segmentation of the tumor on FLAIR images was performed on each slice until the hyperintense FLAIR abnormality (excluding those resulting from obvious leukoaraiosis) was included. Tumor volume (cm³) was calculated as the sum over all slices of (ROI area × [slice thickness + gap]).
Postprocessing of DSC-MRI data included rigid-body registration to correct for motion artefacts. An arterial-input function (AIF) was determined automatically using cluster analysis techniques,14 and deconvolution of the AIF was performed using a time-insensitive block-circulant singular value decomposition.15 Mathematically leakage-corrected, whole-brain rCBV maps were generated by using an established tracer kinetic model applied to the first-pass data, with rCBV values computed pixel-by-pixel as the area under the concentration time curve (AUC) divided by the AUC of the AIF. (AIF pixels are assumed to be made of 100% blood if partial volume effects are neglected.)16 This approach eliminates the need to draw a reference ROI in the contralateral, nonaffected white matter for normalization of CBV values.
Following automatic coregistration of rCBV maps with corresponding cT1 images that included the previously segmented cT1 ROIs, a mean rCBV (calculated as the mean of all individual rCBV voxels from the segmented tumor on cT1-images) was determined from both baseline and first follow-up after initiation of BEV/alkylating chemotherapy treatment.
Statistical Analysis
Statistical analysis was performed using STATA version 12. Survival was calculated from initiation of BEV/alkylating chemotherapy treatment until death or last follow-up. Similarly, time to progression was calculated from initiation of BEV/alkylating chemotherapy treatment until tumor progression. Intragroup comparison of epidemiological, clinical, or imaging metrics between the BEV and non-BEV cohort was performed using a chi-square or 2-tailed t test. Spearman' rank correlation was used to examine whether dexamethasone prescription had any impact on rCBV values (assessing the influence of both baseline dexamethasone prescription vs baseline rCBV and dexamethasone prescription at follow-up vs follow-up rCBV). The influence of rCBV, cT1 volume, or FLAIR volume (including initial, residual, or changes between baseline and first follow-up) on progression-free survival (PFS) and overall survival (OS) was assessed with Cox regression analysis. Kaplan-Meier estimates and the log-rank test were used to assess and compare PFS and OS rates. Specifically, patients in the BEV cohort and non-BEV cohort were dichotomized on the basis of an rCBV-threshold that maximized the sum of sensitivity and specificity as a cutoff calculated from receiver operating characteristic (ROC) analysis of 12-month OS data. Univariate logistic regression analysis was performed to assess the predictive value of rCBV, cT1 volume and FLAIR volume (including initial, residual, and changes between baseline and first follow-up) on 6-month PFS and 12-month OS. We performed cross-validation of logistic-regression models with the “leave-one-out” (jackknife) procedure because performance of a predictive model is overestimated when it is simply determined on the sample of subjects used to construct the model (ie, it does not indicate how well the model will do when used to make new predictions for data not previously examined). P values < .05 were considered significant.
Results
Table 1 encompasses details on the analyzed imaging metrics. rCBV values at baseline were balanced between the BEV cohort (median, 4.3; range, 0.3–8.9) and non-BEV cohort (median, 3.2; range 0.6–12.8) (P = .22). In contrast, rCBV values at first-follow-up were significantly lower in the BEV cohort as compared with the non-BEV cohort (median change of −38% with a median rCBV of 2.7 in the BEV cohort and +34% with a median rCBV of 4.0 in the non-BEV cohort; P < .01, respectively). There was no significant association between rCBV values and dexamethasone prescription in any cohort at the time of baseline or follow-up MRI (P = .07 and .48 in the BEV cohort and P = .09 and .28 in the non-BEV cohort). Tumor volumes at baseline were well balanced between the BEV cohort and non-BEV cohort (median cT1 and FLAIR volumes of 8.3 and 100.4 in the BEV and 10.8 and 86.2 in the non-BEV cohort). In contrast, both cT1 and FLAIR volumes at first follow-up were significantly lower in the BEV cohort (median change of −52% and −40% to a median volume of 4.1% and 62.0%) as compared with the non-BEV cohort (median change of +61% and + 21% to a median volume of 18.2% and 132.4%) (P = .02 for cT1 volume) (P <.01 for FLAIR volume as well as cT1 and FLAIR volume change).
Table 1.
Analyzed imaging metrics in the bevacizumab and non- bevacizumab cohorts
| BEV Cohort Median (range) | Non-BEV Cohort Median (range) | P Value* | |
|---|---|---|---|
| rCBVbaseline | 4.3 (0.3–8.9) | 3.2 (0.6–12.8) | .22 |
| rCBVfirst follow-up | 2.7 (0.3–8.6) | 4.0 (0.7–10.9) | <.01 |
| rCBV change | −38% (−87% to +185%) | +34% (−70% to +324%) | <.01 |
| cT1 volumebaseline | 8.3 (0.6–54.0) | 10.8 (0.2–60.8) | .36 |
| FLAIR volumebaseline | 100.4 (7.5–384.5) | 86.2 (14.0–299.4) | .39 |
| cT1 volumefirst follow-up | 4.1 (0.1–126.5) | 18.2 (0.4–91.8) | .02 |
| FLAIR volumefirst follow-up | 62.0 (4.5–248.2) | 132.4 (28.7–326.6) | <.01 |
| cT1 volume change | −52% (−99% to +375%) | +61% (−68% to +2516%) | <.01 |
| FLAIR volume change | −40% (−88% to +817%) | +22% (−69% to +992%) | <.01 |
Abbreviations: BEV, bevacizumab; cT1, contrast-enhancing T1; FLAIR, fluid-attenuated inversion recovery; rCBV, relative cerebral blood volume.
Annotation: *group differences evaluated using a 2-tailed t test; tumor volumes are given in cm.
Progression-free Survival and Overall Survival
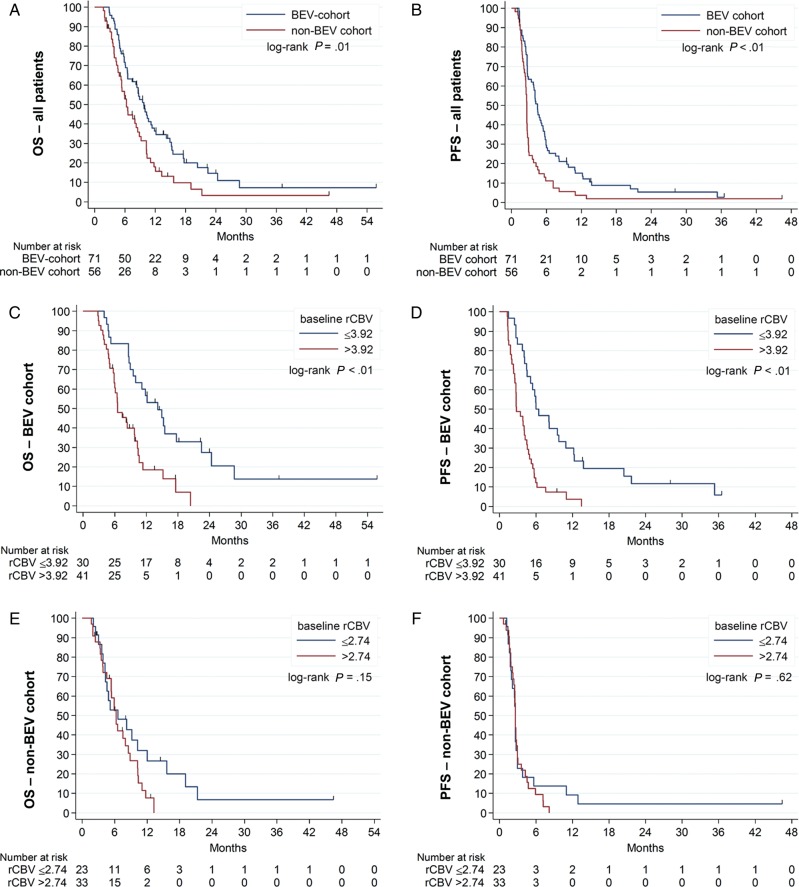
Median OS and PFS rates were significantly higher in the BEV cohort (9.7 and 4.5 months, respectively) as compared with the non-BEV cohort (6.3 and 2.6 months, respectively) (P = .01 for OS and P<0.01 for PFS) (Fig. 1A and B). Univariate Cox regression analysis demonstrated a significant association of baseline rCBV with PFS (hazard ratio [HR] = 1.33, P < .01) and OS (HR = 1.30, P < .01) in the BEV cohort, whereas there was no association with PFS (HR = 1.02, P = .70) or OS (HR = 1.04, P = .47) in the non-BEV cohort (Table 2). Using a cutoff of 3.92 (ie, the rCBV-value that maximizes the sum of sensitivity and specificity from ROC analysis of 12-month OS data), median OS and PFS rates for BEV-treated participants with a rCBV value of ≤3.92 were 14.2 and 6.0 months as compared with 6.6 and 2.8 months for BEV-treated patients with a rCBV value of >3.92 (P < .01, respectively) (Fig. 1C and D; Fig. 2).
Fig. 1.
Kaplan-Meier plot for overall survival (OS) and progression-free survival (PFS) (A and B) for all patients stratified by treatment administered (bevacizumab [BEV] vs non-BEV chemotherapy), (C and D) for patients in the BEV cohort stratified by the median baseline relative cerebral blood volume (rCBV), and (E and F) for patients in the non-BEV cohort stratified by the median baseline rCBV.
Table 2.
Univariate Cox regression analysis for progression-free survival and overall survival
| Progression-free Survival |
Overall Survival |
|||||||
|---|---|---|---|---|---|---|---|---|
| BEV Cohort |
Non-BEV Cohort |
BEV Cohort |
Non-BEV Cohort |
|||||
| P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | |
| Radiological parameters | ||||||||
| rCBVbaselinea | <.01 | 1.33 (1.17−1.52) | .70 | 1.02 (0.91−1.15) | <.01 | 1.30 (1.12−1.51) | .47 | 1.04 (0.93−1.17) |
| rCBVfirst follow-upa | <.01 | 1.34 (1.19−1.51) | .79 | 1.02 (0.90−1.16) | <.01 | 1.31 (1.13−1.52) | .24 | 1.09 (0.94−1.25) |
| rCBV changeb | .48 | 1.10 (0.74−1.65) | .68 | 0.93 (0.67−1.30) | .64 | 1.12 (0.71−1.77) | .07 | 0.69 (0.46−1.03) |
| cT1 volumebaselinec | .22 | 1.15 (0.92−1.45) | .41 | 1.09 (0.89−1.32) | .28 | 1.16 (0.89−1.50) | .01 | 1.26 (1.05−1.51) |
| FLAIR volumebaselinec | .91 | 1.00 (0.97−1.03) | .85 | 1.00 (0.95−1.04) | .89 | 1.00 (0.97−1.03) | .06 | 1.04 (1.00−1.09) |
| cT1 volumefirst follow-upc | <.01 | 1.28 (1.16−1.41) | <.01 | 1.21 (1.07−1.37) | <.01 | 1.39 (1.24−1.56) | <.01 | 1.28 (1.12−1.46) |
| FLAIR volumefirst follow-upc | <.01 | 1.07 (1.03−1.12) | .04 | 1.04 (1.00−1.08) | <.01 | 1.08 (1.03−1.13) | <.01 | 1.07 (1.03−1.11) |
| cT1 volume changed | <.01 | 2.26 (1.76−2.91) | .02 | 1.08 (1.01−1.14) | <.01 | 2.58 (1.96−3.40) | .21 | 1.05 (0.98−1.12) |
| FLAIR volume changed | .02 | 1.22 (1.04−1.43) | .42 | 1.06 (0.92−1.21) | .03 | 1.22 (1.02−1.46) | .94 | 1.01 (0.86−1.17) |
| Clinical parameters | ||||||||
| Agee | .14 | 1.02 (0.99−1.04) | .54 | 0.99 (0.97−1.02) | .15 | 1.02 (0.99−1.04) | .59 | 1.01 (0.98−1.04) |
| KPSf | .18 | 0.87 (0.70−1.07) | .17 | 0.88 (0.73−1.05) | .04 | 0.79 (0.62−1.00) | .02 | 0.78 (0.63−0.96) |
Abbreviations: BEV, bevacizumab; CI, confidence interval; cT1, contrast-enhancing T1; FLAIR, fluid-attenuated inversion recovery; HR, hazard ratio; OS, overall survival; KPS, Karnofsky performance score; PFS, progression-free survival; rCBV, relative cerebral blood volume.
Annotation: given HR correspond to:
aone-unit increase in rCBV.
b1% change in rCBV.
c10unit (10 cm³) increase in the tumor volume.
d1% change in tumor volume.
e1 year increase in age.
f10% increase in KPS.
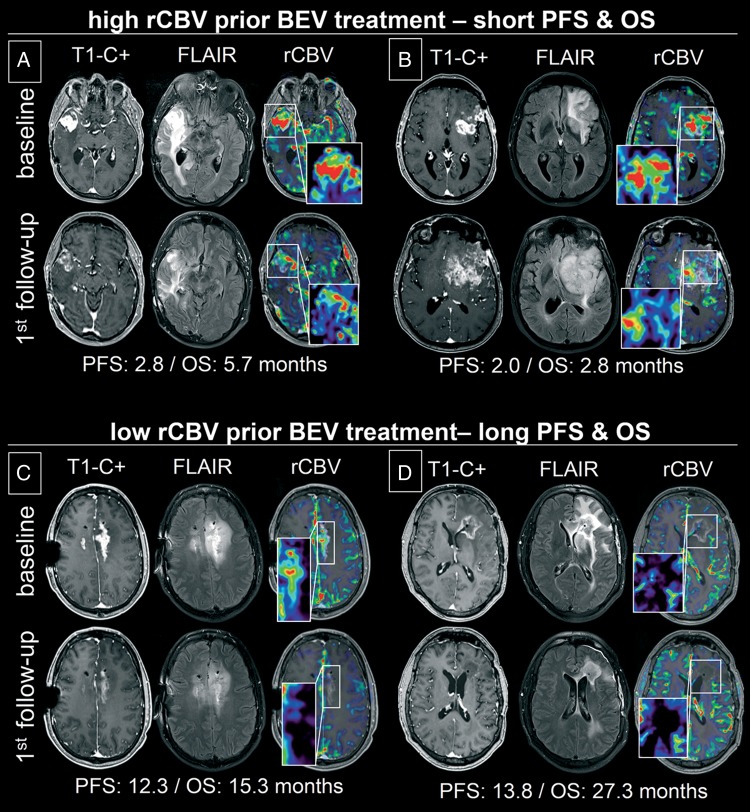
Fig. 2.
Contrast-enhancing T1 (T1-C+) images, fluid-attenuated inversion recovery (FLAIR) images, and relative cerebral blood volume (rCBV) maps (window center/width of 3.5/7.0) superimposed on corresponding T1-C+ images of 4 representative patients with recurrent glioblastoma (A-D) at baseline prior to initiating bevacizumab (BEV) treatment (upper row) and at first follow-up (lower row). Patients were stratified as nonresponder (A and B): progression after 2.8 months (second follow-up) for A and 2.0 months (first follow-up) for B) and responder (C and D: progression after 12.3 for C and 13.8 months for D) on the basis of baseline rCBV analysis (rCBV values of 6.4 and 5.9 for A and B and 3.5 and 1.2 for C and D). Corresponding rCBV-values, T1-C+, and FLAIR tumor volumes at first follow-up changed by −48%,−26%, and −20% for A; −44%, +180%, +73% for B; −55%, −62%, and −43% for C; and −60%, −86%, and −70% for D.
The cT1 volume at baseline was not predictive of PFS or OS in the BEV cohort, whereas it was predictive of OS (but not PFS) in the non-BEV cohort on univariate Cox regression analysis. The FLAIR volumes at baseline were not predictive of PFS or OS in any cohort (Table 2).
Univariate Cox regression analysis also demonstrated a significant association of rCBV at first follow-up with PFS (HR = 1.34, P < .01) and OS (HR = 1.31, P < .01) in the BEV cohort but not in the non-BEV cohort, whereas the relative change in rCBV was not associated with PFS or OS in any cohort (Table 2).
Both the residual and change in cT1 and FLAIR volume at first follow-up were significantly associated with PFS and OS, whereas the change in cT1 yielded the highest HR for PFS and OS (2.26 and 2.58, respectively) (Table 2). Similarly, in the non-BEV cohort, residual cT1 and FLAIR volumes at first-follow up were also significantly associated with both PFS and OS.
Logistic Regression Analysis
We evaluated the predictive value of rCBV, cT1 volume, and FLAIR volume (including initial, residual, or changes between baseline and first follow-up) on 6-month PFS and 12-month OS (Table 3). Overall, 70%/89% of participants in the BEV/non-BEV cohort showed progression within 6 months, and 69%/86% of participants in the BEV/non-BEV cohort died within 12 month after treatment initiation.
Table 3.
Univariate logistic regression analysis for predicting 6-month progression-free survival and 12-month overall survival
| Variable | 6-month Progression-free Survival |
12-month Overall Survival |
||||||
|---|---|---|---|---|---|---|---|---|
| BEV Cohort |
Non-BEV Cohort |
BEV Cohort |
Non-BEV Cohort |
|||||
| P Value* | Correctly Classified | P Value* | Correctly Classified | P Value* | Correctly Cassified | P Value* | Correctly Classified | |
| CBVbaseline | .01 | 81.7% | .80 | n.s. | <.01 | 78.9% | .36 | n.s. |
| rCBVfirst follow-up | <.01 | 77.5% | .86 | n.s. | <.01 | 70.4% | .27 | n.s. |
| rCBV change | .86 | n.s. | .77 | n.s. | .42 | n.s. | .08 | n.s. |
| cT1 volumebaseline | .42 | n.s. | .58 | n.s. | .43 | n.s. | .22 | n.s. |
| FLAIR volumebaseline | .92 | n.s. | .94 | n.s. | .74 | n.s. | .80 | n.s. |
| cT1 volumefirst follow-up | .01 | 74.7% | .17 | n.s. | .04 | 67.6% | .18 | n.s. |
| FLAIR volumefirst follow-up | .04 | 67.6% | .09 | n.s. | .05 | 67.6% | .06 | n.s. |
| cT1 volume change | .07 | n.s. | .17 | n.s. | .02 | 71.8% | .17 | n.s. |
| FLAIR volume change | .35 | n.s. | .06 | n.s | .40 | n.s. | .52 | n.s. |
Abbreviations: BEV, bevacizumab; cT1, contrast-enhancing tumor; FLAIR, fluid-attenuated inversion recovery; n.s., not significant; OS, overall survival; PFS, progression-free survival; rCBV, relative cerebral blood volume.
Annotation: * logistic regression was performed with the “leave-one-out” (jackknife) cross-validation procedure.
For the BEV cohort, rCBV at baseline and first follow-up, as well as the cT1 and FLAIR volumes at first-follow up, were significant predictors of 6-month PFS and 12-month OS (Table 3). Overall, baseline rCBV demonstrated the best performance and correctly predicted 6-month PFS in 81.7% and 12-month OS in 78.9% of participants (P = .01 and <0.01, respectively). None of the analyzed imaging metrics was a significant predictor of 6-month PFS or 12-month OS in the non-BEV cohort.
Following BEV-failure, the majority of participants received supportive care only (55% in the BEV cohort and 50% in the non-BEV cohort), whereas chemotherapy was administered for the majority of remaining cases (34% in the BEV cohort and non-BEV cohort, respectively) (Supplementary Table S3 encompasses detailed information on postprogression therapy). There was no significant difference in the probability of receiving postprogression therapy between participants in the BEV cohort and non-BEV cohort (P = .81).
Discussion
This study demonstrates that pretreatment rCBV analysis qualifies as a potential predictive imaging biomarker for BEV-treated rGB (but not for alkylating chemotherapy-treated rGB) that is superior to volumetric analysis of conventional anatomic MRI and correctly predicts 6-month PFS and 12-month OS in 80% of BEV-treated patients.
Several studies assessed the early changes in perfusion (by means of rCBV) and permeability (by means of the volume transfer constant [Ktrans], an estimate related to microvascular permeability) on DSC and DCE-MRI for predicting response to BEV in rGB and produced controversial results. A study by Zhang et al showed that the early reduction in Ktrans (measured 96 h after treatment initiation) was neither associated with PFS nor OS.17 This was confirmed by a subsequent study of Verhoeff et al showing that the extent of early reduction in rCBV or Ktrans (measured 4 days prior and 3 and 21 days following treatment initiation with BEV and dose-intense temozolomide) had no impact on disease outcome in recurrent high-grade glioma (including 15 rGB and 8 anaplastic astrocytoma cases).18 These 2 studies contradicted the results reported by Sorensen et al on cediranib (a pan-VEGF inhibitor) in 31 patients with recurrent GB. A greater reduction in Ktrans and a greater increase in rCBV (measured 1 day prior and 1 day after application of a single dose of cediranib) was associated with significantly longer OS and PFS.19 Overall, it is difficult to draw conclusions from these limited and heterogeneous patient populations that have, in part, contradicting results.
Verhoeff et al also evaluated perfusion and permeability prior to initiation of BEV treatment and showed that baseline rCBV was associated with OS. This initial observation was further strengthened in a recently published study by Schmainda et al who performed rCBV analysis prior to and at first follow-up after BEV treatment initiation in a rather heterogeneous population of recurrent high-grade gliomas (23 primary GBs, 4 secondary GBs, 6 anaplastic astrocytomas, and 3 anaplastic oligodendrogliomas). rCBV-analysis at baseline prior to BEV treatment stratified OS but not PFS, whereas rCBV analysis at first follow-up stratified both OS and PFS.20 The results from these promising initial studies with a more heterogeneous patient cohort, including both anaplastic gliomas as well as primary and secondary GBs, led to the present confirmatory series with a larger sample size, a control group, and a focus on primary rGB. With the results from the present study, we now provide evidence that pretreatment rCBV is not a prognostic, but rather a potential predictive, imaging biomarker for BEV-treated rGB because it was associated exclusively with treatment outcome in the BEV cohort (but not in the non-BEV cohort). These results are particularly important since there are currently— apart from the assessment of the lower Gaussian apparent diffusion coefficient (ADC) curve21,22 that requires sophisticated postprocessing—no acceptable pretreatment imaging biomarkers for judicious selection of patients with rGB who may achieve maximum benefit from BEV. Therefore, it would be of interest to also determine if a combined analysis of rCBV and the lower ADC curve may further improve the stratification of patients in their response to BEV treatment.
Beside its focus on imaging metrics, our study also showed that median PFS and OS rates were significantly higher for patients in the BEV cohort with an increase of 1.9 and 3.4 months, respectively, over patients in the non-BEV cohort, which adds to other reports in in the literature suggesting that BEV treatment may translate into increased OS in the setting of rGB.23,24
Despite the straightforward results of the present study, we must acknowledge that the retrospective nature of the present study has certain limitations regarding patient selection and distribution of epidemiological and clinical characteristics between the BEV cohort and non BEV cohort. Specifically, one potential confounding issue is the phenomenon of early or delayed radiochemotherapy treatment-related imaging changes (ie, pseudoprogression/radiation necrosis), which presents as increasing lesion volume or new contrast enhancement on MRI suggestive for tumor progression. Because the enhancement seen in pseudoprogression/radiation necrosis can be mistaken for tumor progression, patients may be routed to BEV as treatment for tumor recurrence that has been observed to decrease the permeability of not only tumor-related leaky vasculature but also of radiation-induced leaky vasculature.25 The absence of histological confirmation, which would be the gold-standard for ruling out pseudoprogression/radiation necrosis,26 is another limitation of the present study. However, considering the literature available on rCBV-thresholds that are suggestive of radiation necrosis (rCBV < 1.5–1.87,27) it is rather unlikely that the long-term survival in our study was related to the presence of radiation necrosis (median rCBV of 2.5 for patients surviving >12 months following BEV treatment).
A methodological limitation of the present study was the use of 2 different MR systems; despite comparative assignment of patients to the different scanner types and identical sequence parameters, results might have been biased without the use of additional normalization of rCBV-maps (eg, Gaussian normalization).28 Whether all of these limitations influenced the analyzed outcome measures remains to be proven. Post hoc analysis of DSC-MRI from the ongoing EORTC-26101 trial, which compares BEV + lomustine with lomustine alone in patients with rGB at first recurrence, may allow prospective validation of whether rCBV is a predictive imaging biomarker in the setting of BEV-treated rGB.29
In conclusion, we have demonstrated the potential utility of pretreatment rCBV analysis as a predictive imaging biomarker for BEV-treated rGB (but not for alkylating chemotherapy-treated rGB), which is superior to volumetric analysis of conventional anatomic MRI and correctly predicts 6-month PFS and 12-month OS in 80% of BEV-treated patients. Thus, pretreatment rCBV analysis deserves validation in prospective randomized trials because it may allow judicious preselection of patients who could obtain maximum benefit from BEV-treatment.
Supplementary Material
Funding
Martha Nowosielski holds a DOC-fFORTE fellowship with the Austrian Academy of Science at the Department of Neurology, Innsbruck Medical University. Benedikt Wiestler is a scholar of the DKTK Heidelberg School of Oncology Postdoctoral Program.
Conflict of interest statement. P.K. reports no disclosures; B.W. reports no disclosures; S.B. reports no disclosures; A.W. reports no disclosures; M.N. reports no disclosures; S.H. reports no disclosures; H.P.S. reports disclosures outside the submitted work: grants from Siemens, personal fees from Siemens, Curagita, and Codivien; W.W. reports disclosures outside the submitted work: personal fees from Roche, MSD, and Prime Oncology, grants from Apogenix, Boehringer Ingelheim, MSD, and Roche; M.B. reports disclosures outside the submitted work: personal fees from Codman, Roche, Guerbet, Novartis, and Bayer Healthcare, grants from Guerbet, Hopp Foundation, Novartis, and Bayer Healthcare; A.R. reports no disclosures.
Supplementary Material
References
- 1.Wong ET, Brem S. Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Canc Netw. 2008;6(5):515–522. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. [DOI] [PubMed] [Google Scholar]
- 3.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 4.Leu K, Pope WB, Cloughesy TF, et al. Imaging biomarkers for antiangiogenic therapy in malignant gliomas. CNS Oncol. 2013;2(1):33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowosielski M, Wiestler B, Goebel G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014;82(19):1684–1692. [DOI] [PubMed] [Google Scholar]
- 6.Hu LS, Eschbacher JM, Dueck AC, et al. Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol. 2012;33(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong DS, Kim ST, Kim EH, et al. Diagnostic dilemma of pseudoprogression in the treatment of newly diagnosed glioblastomas: the role of assessing relative cerebral blood flow volume and oxygen-6-methylguanine-DNA methyltransferase promoter methylation status. AJNR Am J Neuroradiol. 2011;32(2):382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology. 2008;247(2):490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugahara T, Korogi Y, Tomiguchi S, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol. 2000;21(5):901–909. [PMC free article] [PubMed] [Google Scholar]
- 10.Keunen O, Johansson M, Oudin A, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA. 2011;108(9):3749–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pechman KR, Donohoe DL, Bedekar DP, et al. Characterization of bevacizumab dose response relationship in U87 brain tumors using magnetic resonance imaging measures of enhancing tumor volume and relative cerebral blood volume. J Neurooncol. 2011;105(2):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vredenburgh JJ, Cloughesy T, Samant M, et al. Corticosteroid use in patients with glioblastoma at first or second relapse treated with bevacizumab in the BRAIN study. Oncologist. 2010;15(12):1329–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kickingereder P, Wiestler B, Sahm F, et al. Primary Central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging. Radiology. 2014;2723:843–850. [DOI] [PubMed] [Google Scholar]
- 14.Mouridsen K, Christensen S, Gyldensted L, et al. Automatic selection of arterial input function using cluster analysis. Magn Reson Med. 2006;55(3):524–531. [DOI] [PubMed] [Google Scholar]
- 15.Wu O, Ostergaard L, Weisskoff RM, et al. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med. 2003;50(1):164–174. [DOI] [PubMed] [Google Scholar]
- 16.Ostergaard L, Weisskoff RM, Chesler DA, et al. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med. 1996;36(5):715–725. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Kreisl T, Solomon J, et al. Acute effects of bevacizumab on glioblastoma vascularity assessed with DCE-MRI and relation to patient survival. Paper presented at: The International Society for Magnetic Resonance in Medicine, Hawaii, USA, 2009. [Google Scholar]
- 18.Verhoeff JJ, Lavini C, van Linde ME, et al. Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol. 2010;21(8):1723–1727. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen AG, Batchelor TT, Zhang WT, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmainda KM, Prah M, Connelly J, et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 2014;16(6):880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellingson BM, Sahebjam S, Kim HJ, et al. Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. AJNR Am J Neuroradiol. 2014;35(4):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pope WB, Kim HJ, Huo J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology. 2009;252(1):182–189. [DOI] [PubMed] [Google Scholar]
- 23.Taal W, Oosterkamp HM, Walenkamp AM, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]
- 24.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 25.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kickingereder P, Dorn F, Blau T, et al. Differentiation of local tumor recurrence from radiation-induced changes after stereotactic radiosurgery for treatment of brain metastasis: case report and review of the literature. Radiat Oncol. 2013;8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song YS, Choi SH, Park CK, et al. True progression versus pseudoprogression in the treatment of glioblastomas: a comparison study of normalized cerebral blood volume and apparent diffusion coefficient by histogram analysis. Korean J Radiol. 2013;14(4):662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellingson BM, Zaw T, Cloughesy TF, et al. Comparison between intensity normalization techniques for dynamic susceptibility contrast (DSC)-MRI estimates of cerebral blood volume (CBV) in human gliomas. J Magn Reson Imaging. 2012;35(6):1472–1477. [DOI] [PubMed] [Google Scholar]
- 29.European Organisation for Research and Treatment of Cancer - EORTC. Bevacizumab and Lomustine for Recurrent GB. Available at: http://clinicaltrials.gov/show/NCT01290939. Accessed February 21, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.