Abstract
Background
This study was initiated to test the feasibility and toxicity of a regimen that alternates the administration of weekly carboplatin and vincristine with temozolomide in the management of children with progressive and/or symptomatic low-grade glioma.
Methods
Eligible children received a 10-week induction regimen followed by six 10-week cycles of maintenance chemotherapy. Feasibility was evaluated with short-term and long-term endpoints. Short-term feasibility was evaluated by the ability to complete induction and 1 maintenance cycle in 24 weeks without >25% reduction in either carboplatin or temozolomide. Long-term feasibility was evaluated by the ability to administer induction and 4 maintenance cycles within 60 weeks without >25% reduction in either carboplatin or temozolomide. Efficacy was assessed by response to initial chemotherapy and 5-year event-free survival. Initial pathology was reviewed centrally.
Results
Sixty-six patients were enrolled on the study. It was feasible to deliver the regimen, and toxicity was acceptable. The only significant toxicities were hematologic. Both the short-term and long-term feasibility endpoints were met. The short-term feasibility success rate was 87% (95% CI: 77%–96%) and the long-term feasibility success rate was 79% (95% CI: 68%–90%). The 5-year event-free survival was 46% (95% CI: 33%–58%) and the 5-year survival was 87% (95% CI: 75%–93%).
Conclusion
It was feasible to deliver the combination of weekly carboplatin and vincristine alternating with temozolomide to children with progressive/symptomatic low-grade glioma with acceptable toxicities. This combination appears to be effective in delaying progression. Further trials are needed to establish the relative efficacy of this regimen compared with other regimens in use.
Keywords: chemotherapy, low-grade glioma, temozolomide
The long-term event-free survival (EFS) in children with low-grade glioma (LGG) of the central nervous system is excellent when these tumors are located in the cerebral or cerebellar hemispheres and are therefore amenable to complete or almost complete resection.1,2 When these tumors arise from central locations like the optic chiasm/hypothalamus and the thalamus, where resections are not feasible without significant morbidity, progression-free survival (PFS) has varied from 40% for hypothalamic tumors and 55% for thalamic tumors at 4 years in one study to 52% at 3 years for optic pathway glioma in another.2,11 Additional challenges include young age at diagnosis and a large tumor relative to the size of the brain. This generally precludes the immediate use of radiation therapy, which is considered definitive in progressive LGG. Fortunately several chemotherapy regimens with activity against these LGGs have been identified and included in the initial management to delay progression.3–14 The most commonly used regimen, which consists of carboplatin and vincristine, is generally prescribed for 18 months and is associated with a systemic hypersensitivity rate to carboplatin as high as 42%, resulting often in cessation of therapy.15–18 This regimen also requires frequent weekly visits to a medical facility for intravenous medications. Recent reports described the use of oral temozolomide (TMZ) in the treatment of progressive/symptomatic LGG.13,14 We initiated a pilot trial to test the feasibility and assess the toxicities of a regimen consisting of alternating courses of carboplatin/vincristine with monthly TMZ. If feasible, with acceptable toxicity, this regimen could be tested for efficacy in a larger prospective study. If effective, it could significantly decrease the frequency of visits to the hospital.
Patients and Methods
Eligibility
Children 10 years of age or younger with biopsy-proven LGGs involving brain or spinal cord that could not be resected due to tumor location and showed progression or caused symptoms at diagnosis were eligible for the study. Eligible patients should not have received any prior therapy except surgery at the time of enrollment on the study. Biopsy was not required when tumor was confined to the optic pathway (optic nerve and/or chiasm). Therapy was to be initiated within 28 days of biopsy, and a baseline MRI of the brain was performed within 30 days prior to the initiation of chemotherapy. Patients who were observed after initial biopsy and were later found to have progression and/or symptoms did not require repeat biopsy, but an MRI of the brain was to be done before enrollment to the study. Patients with metastatic disease were eligible for the study. Eligible histologies included grades I and II glioma. Neurofibromatosis type 1 patients were excluded from the study, as were patients with diffuse brainstem glioma. Patients with dorsally exophytic glioma who had >50% resection of the tumor excised were eligible only if there was progression of residual tumor. Pathology was centrally reviewed (A.A.) in patients who had a tissue biopsy, but confirmation by a central review was not required prior to enrollment.
Treatment
The treatment plan consisted of cycles of carboplatin/vincristine and TMZ given over a period of 70 weeks (see Table 1). After one cycle of induction with carboplatin/vincristine and TMZ (10 wk duration), response was assessed. During induction, carboplatin was prescribed weekly at a dose of 175 mg/m2 along with vincristine at a dose of 1.5 mg/m2 in weeks 0–3 followed by vincristine at the same dose in weeks 4 and 5. In week 6, TMZ at a dose of 200 mg/m2/d for 5 days was prescribed. Patients with stable disease or decreasing tumor size continued maintenance therapy with cycles of carboplatin/vincristine and TMZ to complete six 10-week cycles in the absence of progression (Table 1). The imaging studies prior to enrollment were repeated at the end of 10 weeks of therapy in order to assess response to initial treatment.
Table 1.
Treatment regimen
| Weeks |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| Induction | CV | CV | CV | CV | V | V | T | |||||||||||||
| Maintenance | CV | CV | CV | C | T | |||||||||||||||
Abbreviations: C, carboplatin; V, vincristine; T, temozolomide.
Six 10-week maintenance cycles.
Each maintenance cycle consisted of carboplatin prescribed at weekly doses of 175 mg/m2 for 4 weeks (wk 0–3). Vincristine was given weekly for 3 doses of 1.5 mg/m2 (maximal dose 2.0 mg) starting with the first dose of carboplatin. At week 6, TMZ was given at a dose of 200 mg/m2/d for 5 days. Every cycle of carboplatin or TMZ was scheduled to begin when the absolute neutrophil count was ≥1000/µL and platelet count ≥100 000/µL. If either the absolute neutrophil or platelet count was lower than the above parameters, chemotherapy was held and counts were repeated to determine whether the above-cited levels were obtained. If there was a >2-week delay in the resumption of either carboplatin or TMZ, the dose of the drug in the previous course was reduced by 25%. The weekly doses of carboplatin were not reduced once the cycle was started. We allowed decreasing the doses of both carboplatin and TMZ by 25% if necessary based on the above guidelines. However, 25% was the maximum reduction allowed for both carboplatin and TMZ.
If serum creatinine was greater than the upper limit of normal for age, creatinine clearance or glomerular filtration rate was obtained. If creatinine clearance was <75% of normal for age, then the carboplatin dose was held and creatinine clearance was assessed weekly until this level was reached. The dose was then reduced by 25%. If grades 3–4 hepatotoxicity developed, chemotherapy was held until toxicity decreased to grade 2. If the etiology of the toxicity was unexplained, then the dose of vincristine and carboplatin or TMZ (dependent on which was considered as the causative agent) was reduced by 25%. For grades 3–4 peripheral neuropathy, one subsequent dose of vincristine was held and then resumed at 1 mg/m2 (1.5 mg maximum) and then returned to full dose when symptoms resolved. For jaw pain, treatment with analgesics was recommended, but vincristine was not held. Children with any respiratory compromise did not receive carboplatin again and were off protocol therapy. These patients could receive TMZ off protocol every 28 days. Such patients were continued on protocol for follow-up.
Tumor response criteria were determined by changes in size using all 3-dimensional measurements: width (W), transverse (T), and length (L). Thus for all tumors, these 3 measurements were recorded, using either T1- or T2-weighted images (whichever gave the best estimate of tumor size). The cystic or necrotic components of a tumor were not considered in tumor measurements. Therefore, only the solid component of cystic/necrotic tumors was measured. If cysts/necrosis composed the majority of the lesion, the lesion was not “measurable” by the above methods. The following options were considered: if the cyst/necrosis was eccentric, the W, T, and L of the solid portion were measured, and the cyst/necrosis was excluded from measurement. If the cyst/necrosis was central but represented a small portion of the tumor (<25%), it was disregarded and the whole lesion was measured. If the cyst/necrosis was central but represented a large portion of the tumor, a solid aspect of the mass that could be reproducibly measured was identified. To assess response/progression, the ratio was calculated as follows: L × W × T (current scan) / L × W × T (reference scan). Development of new disease or progression in any established lesions was considered progressive disease, regardless of response in other lesions—for instance, when multiple lesions showed opposite responses, then progressive disease took precedence. The responses were defined as follows: complete response (CR ) = disappearance of all target lesions; partial response (PR) = 50% or greater decrease; stable disease (SD) = neither sufficient decrease nor sufficient increase to qualify for PD; progressive disease (PD) = 25% or more increase in size of the tumor.
Statistical Design and Analysis
The primary goals of this pilot study were to assess the short-term and long-term feasibility success rates of treatment with carboplatin, vincristine, and TMZ in children 10 years of age and younger with progressive or symptomatic LGG. Patients who terminated therapy due to tumor progression before the short-term feasibility evaluation endpoint were considered inevaluable for the feasibility endpoint but were included in the assessments of treatment efficacy. Short-term feasibility success occurred when induction and 1 maintenance cycle were completed within 24 weeks without >25% reduction in either carboplatin or TMZ. Long-term feasibility success occurred when induction and 4 maintenance cycles (ie, to start the fifth maintenance cycle) were completed within 60 weeks, using appropriate protocol-directed dose reductions for toxicity, provided that there was no more than 25% reduction in either carboplatin or TMZ. We calculated the proportion of eligible and evaluable patients whose treatment course satisfied each of these feasibility endpoints and used the standard normal approximation to the binomial distribution to calculate 95% CIs for the proportions. Treatment efficacy was characterized by response to induction therapy, 5-year EFS, and 5-year overall survival (OS). For EFS, an event was defined as the first occurrence of radiologically confirmed tumor recurrence or progression, a second malignancy, or death from any cause, and for OS an event was death from any cause. EFS and OS as a function of time since study enrollment were estimated using the Kaplan–Meier method where event time was defined as the time from study entry to event. Patients who had not experienced an event were considered censored at the date of last patient contact. Confidence intervals were calculated using the log-log transformation of the Kaplan–Meier estimate.19 The relationship of the presence of spinal cord metastases to risk for EFS event was assessed using proportional hazards regression where the presence of spinal cord metastases was the only factor in the model.19
As the study was designed, assuming a minimum of 50 patients evaluable for feasibility, the precision of estimates of treatment feasibility would be ±0.04 to ±0.06 (standard error) for feasibility rates between 0.70 and 0.90. Hence, an observed long-term feasibility rate of 0.8 would be consistent with the view that 69% or more of patients could receive the first 5 cycles of therapy within 60 weeks.
Toxicity was reported according to the National Cancer Institute Common Toxicity Criteria version 3. To characterize the adverse experience profile of this therapy, any toxicity that occurred at grade 3 or higher in at least 3% of all chemotherapy cycles was considered. The incidence of each of these complications was tabulated separately for induction and maintenance cycles.
Treatment decisions and identification of EFS events were made by the institutional investigator based on imaging and tests conducted at the treating site. When possible, however, images used by institutional investigators to assess protocol-defined response were submitted to the Quality Assurance Center in Rhode Island. There images were made available for review by 2 study committee members (M.C. and M.M.). A central review assessment of response was obtained for comparison with the institutional evaluation.
Results
Patients
Between July 2004 and August 2007, 66 patients were enrolled on the study. Data current to December 2013 were used for this report. One patient was deemed ineligible because therapy was not started on time as per protocol. The median age of the 65 eligible patients was 4.6 years (range, 0.4–9.8). Patient characteristics are summarized in Table 2. The majority of the tumors were located in the chiasmatic/hypothalamic region or optic nerve (n = 22). Ten patients with tumor confined to the optic pathway had no biopsy. Among the 58 patients who had spinal MRIs performed at the time of diagnosis, 12 had spinal cord involvement.
Table 2.
Patient characteristics
| n | % | |
|---|---|---|
| Sex | ||
| Male | 36 | 55.4 |
| Female | 29 | 44.6 |
| Race | ||
| White | 48 | 73.8 |
| Black | 6 | 9.2 |
| Chinese | 1 | 1.5 |
| Other | 8 | 12.3 |
| Unknown | 2 | 3.1 |
| Primary site from on-study form | ||
| Cerebral hemisphere | 3 | 4.6 |
| Basal ganglia/thalamus | 7 | 10.8 |
| Hypothalamus-optic | 15 | 23.1 |
| Optic nerve | 7 | 10.8 |
| Suprasellar | 7 | 10.8 |
| Midbrain/medulla/dorsal exophytic | 9 | 13.8 |
| Cerebellar hemisphere | 3 | 4.6 |
| Fourth ventricle | 6 | 9.2 |
| Third ventricle | 5 | 7.7 |
| Other | 3 | 4.6 |
| Extent of tumor resection from surgery prior to study entry | ||
| Biopsy only (<10%) | 20 | 30.8 |
| Partial resection (10%–49%) | 10 | 15.4 |
| Subtotal resection (50%–95%) | 14 | 21.5 |
| Near total (>95%) | 7 | 10.8 |
| Gross total (no visible tumor at time of surgery) | 1 | 1.5 |
| No surgery (optic pathway tumors) | 11 | 1.5 |
| Not available | 2 | 18.5 |
| Tumor size at study entry | ||
| Optic pathway tumors | 11 | 13.8 |
| <1.5 cm2 | 18 | 10.8 |
| ≥1.5 to <3 cm2 | 11 | 16.9 |
| ≥3 cm2 | 23 | 35.4 |
| 16.9 | ||
| Not available | 2 | 6 |
| Diagnosis from institutional pathology | ||
| Pilocytic astrocytoma | 41 | 63.1 |
| Fibrillary astrocytoma | 2 | 3.1 |
| Protoplasmic astrocytoma | 1 | 1.5 |
| Oligodendroglioma | 1 | 1.5 |
| Mixed glioma | 2 | 3.1 |
| Gangliogliomas | 3 | 4.6 |
| Other (pilomyxoid, low-grade astrocytoma) | 4 | 6.2 |
Among the patients who underwent biopsy, pilocytic astrocytoma was the most common pathology (n = 41). Pathology was available for central review in 35 of the 53 patients (66%) who underwent a surgical procedure. In all of those 35 cases, both institutional and central reviewers considered the patient to have LGG.
Toxicity
During induction therapy, grades 3 and 4 hematologic toxicities were encountered, with 48% of patients developing neutropenia, 9% anemia, 14% thrombocytopenia, and 9% febrile neutropenia without documented infection (see Table 3). Grade 3 or 4 neutropenia was observed in 32% of maintenance cycles; grade 3 or 4 thrombocytopenia and anemia occurred in 9% and 3% of maintenance cycles, respectively. There were 4 episodes of documented infection associated with grades 3–4 neutropenia. Only 7 of 65 patients (11%) on the study went off protocol therapy due to allergic reaction to carboplatin. Three of these patients were in maintenance cycle 3, one in cycle 4, two in cycle 5, and one in cycle 6. There were 5 patients who could not take oral TMZ at various points in the duration of treatment. There were 45 patients who had a dose reduction of carboplatin and 34 who had a dose reduction of TMZ. The median time to dose reduction for carboplatin from time of enrollment was 3.3 months (range, 2.3–15.7) and for TMZ 7.5 months (range, 2.3–16.3).
Table 3.
Common toxicity criteria version 3 coded grade 3 or higher adverse experiences that occurred in 3% or more of induction and maintenance reporting periods
| Organ System | Toxicity Type | Reporting Period |
|||
|---|---|---|---|---|---|
| Induction |
Maintenance Cycles |
||||
| Na | %b | N | % | ||
| Gastrointestinal | Abdominal pain | 2 | 3.1 | ||
| Ileus | 2 | 3.1 | |||
| Vomiting | 2 | 3.1 | 1 | 0.4 | |
| Metabolism/nutrition | Hypokalemia | 1 | 1.6 | 5 | 1.8 |
| Hyponatremia | 2 | 3.1 | 5 | 1.8 | |
| Investigations | Lymphocyte count decreased | 3 | 4.7 | 9 | 3.3 |
| Neutrophil count decreased | 31 | 48.4 | 87 | 32.0 | |
| Platelet count decreased | 9 | 14.1 | 27 | 9.9 | |
| White blood cell decreased | 7 | 10.9 | 20 | 7.4 | |
| Blood/lymphatic | Anemia | 6 | 9.4 | 9 | 3.3 |
| Febrile neutropenia | 6 | 9.4 | 6 | 2.2 | |
| Infections/infestations | Infections and infestations, organism not specified | 2 | 3.1 | 5 | 1.8 |
aNumber of cycles with the noted adverse experience.
bPercent of all cycles administered on which the noted adverse experience was reported.
Feasibility
Of the 65 eligible patients, 13 were inevaluable for feasibility: 11 patients progressed either during induction (n = 9) or the first cycle of maintenance (n = 2); 1 patient was lost to follow-up and 1 developed meningitis and was taken off protocol therapy.
Short term
Of the 52 evaluable patients, 4 required more than 24 weeks to complete induction and one maintenance cycle; 2 patients could not take oral TMZ; and 1 patient had prolonged neutropenia and seizures and did not take any TMZ during induction. The short-term feasibility success rate was 87% (95% CI: 77%–96%).
Long term
Of the 52 evaluable patients, 4 required more than 60 weeks to finish induction and 4 cycles of maintenance; 4 withdrew before completion of 4 maintenance cycles due to allergic reaction to carboplatin; and 3 patients could not take TMZ and withdrew from protocol therapy. The long-term feasibility success rate was 79% (95% CI: 68%–90%).
Treatment Efficacy
Of the 65 eligible patients, 3 were not evaluated for response during protocol therapy. An additional 5 patients did not have scans considered evaluable by the institutional investigator. Responses according to the treating investigators were: CR = 2 patients, PR = 15 patients, SD = 30 patients, and PD = 10 patients.
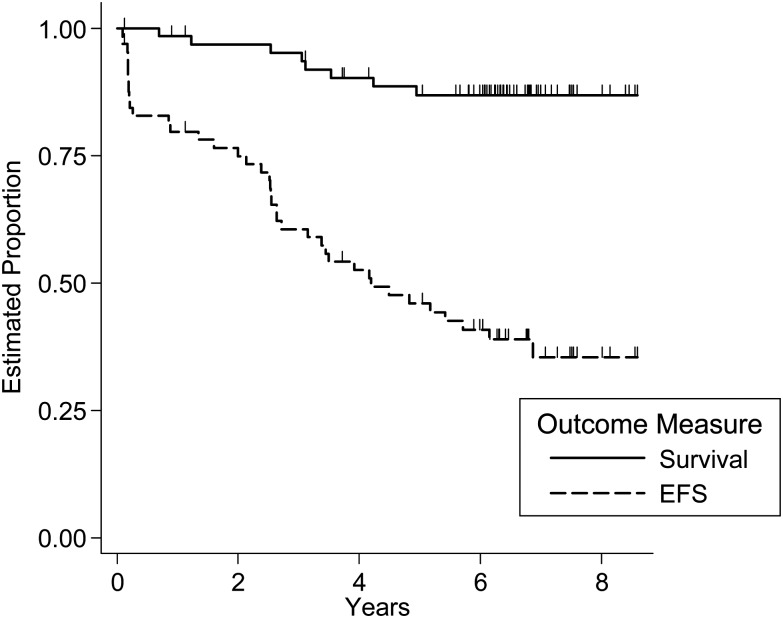
Figure 1 shows EFS and OS for the eligible patients. Thirty-nine patients experienced progression. There were 8 deaths. One patient who died was taken off protocol therapy during induction due to meningitis and died of cardiac arrest 3 years after enrollment. The 5-year EFS was 46% (95% CI: 33%–58%) and the 5-year survival was 87% (95% CI: 75%–93%). Spinal cord involvement was not related significantly to increased risk for EFS event (relative hazard rate for spinal cord involvement: 1.6; 95% CI: 0.75–3.7).
Fig. 1.
Event-free and overall survival of all eligible patients on the study.
Discussion
Children with centrally located LGG have an excellent OS but sustain significant morbidity related to neuroendocrine and visual functions as a result of the tumor and its progression and therapeutic interventions. The optimal chemotherapy regimen in terms of toxicity, ease of administration, duration of treatment, and effectiveness in delaying progression in these young patients is still elusive. In this pilot study we evaluated the feasibility and toxicity of a regimen alternating carboplatin and vincristine with TMZ.
This study was opened to all patients with progressive and/or symptomatic LGG. We excluded patients with neurofibromatosis type 1 due to a theoretical concern for exposure of these patients to the methylating agent, TMZ, which has the potential to cause second malignancies. The weekly carboplatin regimen was chosen over a monthly regimen, as the published results showed that the former had the best 3-year PFS, of 68%. The regimen was tolerated well, with 33 patients completing at least 70 weeks of therapy. More importantly, the study met the requirements for the short-term and long-term feasibility endpoints. The short-term and long-term feasibility success rates were 87% (95% CI: 77%–96%) and 79% (95% CI: 68%–90%), respectively. The degree and type of toxicities were expected. Compared with the study by Ater and colleagues,12 where the regimen containing weekly carboplatin and vincristine given over 60 weeks had a cumulative carboplatin dose of 7000 mg/m2, the present regimen had a prescribed total cumulative dose of 4900 mg/m2 given over 72 weeks.12 The regimen in this study also reduced the number of visits for intravenous infusions significantly to 30/72 (42%) weeks compared with 42/60 (70%) weeks in the study by Ater et al.12
Only 11% of patients developed allergy to carboplatin resulting in cessation of carboplatin therapy. This is similar to the incidence in the Children's Oncology Group study A9952. This is much lower than the incidence of 41.9% described by Lafay-Cousin from the Canadian Pediatric Brain Tumor Consortium.15 Increased cumulative exposure and weekly frequency of systemic carboplatin infusions are implicated in hypersensitivity reactions to carboplatin.15–18 The regimen in this study lowers both the cumulative exposure and the weekly frequency of carboplatin. However, the incidence of allergy is no different from the A9952 study.
The 5-year EFS was 46% and the 5-year survival was 87%, suggesting that this is an effective regimen in delaying progression of these tumors. Stable disease was the most common response in 30 of the 60 patients, once again underscoring that arrest of progression is as important in this type of tumor as CR or PR. One of the regimens most commonly used in North America is weekly treatment with carboplatin and vincristine.3,4 In the initial report with newly diagnosed diencephalic LGG using the weekly carboplatin and vincristine regimen, the 3-year PFS was 68%.4 This regimen was given over 94 weeks. A shortened version (60 wk) was compared with a regimen using 4 drugs: vincristine, 6-thioguanine, procarbazine, and CCNU (TPCV).12 The TPCV regimen was superior in preventing tumor progression in a higher proportion of patients than was carboplatin and vincristine.12 The 5-year EFS was 39% for the carboplatin/vincristine regimen compared with 52% for TPCV. The TPCV regimen, however, had slightly more toxicity when the allergic reaction to carboplatin was excluded. Gnekow and colleagues20 showed a 5-year PFS of 47% with a regimen consisting of weekly vincristine and carboplatin given every 3 weeks. Three-fourths of the patients who received chemotherapy did not require radiation therapy. A phase II study of vinblastine in patients who had previously received 1–3 regimens of chemotherapy with or without radiation showed a 5-year PFS of 42%.7 Other regimens include irinotecan and bevacizumab, and cisplatin with etoposide.8–10 Although cisplatin (lower dose) and etoposide in 37 patients resulted in a 3-year EFS of 65%, there still is concern for the incidence of ototoxicity and the resulting hearing loss in children who are likely to be visually compromised at the time of diagnosis. Researchers with the French Society of Pediatric Oncology described their experience with the combination of procarbazine, carboplatin, vincristine, etoposide, cisplatin, and cyclophosphamide. They showed a 34% PFS rate at 5 years in a group of children less than 5 years old.11 The current study compares very well with the above regimens in terms of outcome, toxicity, ease of administration at home, and the total duration of therapy.
Recent data showed that duplication of the BRAF proto-oncogene at 7q34, activating mutations involving codon 600 in exon 15 of BRAF, V600E, and other mutations involving the mitogen-activated protein kinase kinase (MEK)1/2 of the mitogen-activated protein kinase (MAPK) pathway are important in the pathogenesis of LGG.21–31 The immediate downstream phosphorylation target of BRAF is MEK1/2 of the MAPK pathway. Effective blockade of phosphorylation of MEK1/2 therefore provides an avenue of investigation to target progressive LGG in central locations. Efforts to identify effective agents that can block the MAPK pathway are under way. Such agents perhaps in combination with chemotherapy may lead to long-term stabilization of these tumors with minimal side effects.
In conclusion, it is feasible to deliver the combination of carboplatin and vincristine alternating with TMZ to children with progressive/symptomatic LGG with acceptable toxicities. Approximately 60% of this regimen does not require a visit to the hospital for intravenous infusions. However, whether this indicates a better quality of life for the child and the family is unknown at this time and is beyond the scope of this manuscript. The risk for allergic reactions is low, as shown in this study, and is similar to the incidence of allergy described by Ater et al.12 Concomitant use of TMZ and its potential impact on immunosuppression may have also contributed to the lower rate of allergy to carboplatin in this study. Also within the confines of the study design, there is a suggestion that this combination is effective in delaying progression. Further trials are needed to establish the relative efficacy of this regimen compared with other regimens in use and establish its priority in the armamentarium of chemotherapy regimens to delay the use of radiation therapy. Methylating agents like TMZ and procarbazine should be used with caution. There is a concern for secondary malignancies with the use of TMZ in general and especially in patients with neurofibromatosis type 1.
The future holds great promise for an increasing role for biologic agents, but until then, effective chemotherapy regimens with reasonable toxicity profiles and ease of administration will continue to play an important role in the management of children with centrally located LGG.
Funding
This work was not supported by funding.
Conflict of interest statement. None.
References
- 1.Pollack IF. Brain tumors in children. N Engl J Med. 1994;331(22):1500–1507. [DOI] [PubMed] [Google Scholar]
- 2.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15(8):2792–2799. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Lange B, Ater J, et al. Carboplatin and vincristine for recurrent and newly diagnosed low-grade gliomas of childhood. J Clin Oncol. 1993;11(5):850–856. [DOI] [PubMed] [Google Scholar]
- 4.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. [DOI] [PubMed] [Google Scholar]
- 5.Petronio J, Edwards MS, Prados M, et al. Management of chiasmal and hypothalamic gliomas of infancy and childhood with chemotherapy. J Neurosurg. 1991;74(5):701–708. [DOI] [PubMed] [Google Scholar]
- 6.Prados MD, Edwards MS, Rabbitt J, et al. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32(3):235–241. [DOI] [PubMed] [Google Scholar]
- 7.Bouffet E, Jakacki R, Goldman S, et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30(12):1358–1363. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52(7):791–795. [DOI] [PubMed] [Google Scholar]
- 9.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20(20):4209–4216. [DOI] [PubMed] [Google Scholar]
- 10.Massimino M, Spreafico F, Riva D, et al. A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. J Neurooncol. 2010;100(1):65–71. [DOI] [PubMed] [Google Scholar]
- 11.Laithier V, Grill J, Le Deley MC, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy—results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21(24):4572–4578. [DOI] [PubMed] [Google Scholar]
- 12.Ater JL, Zhou T, Holmes E, et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children's Oncology Group. J Clin Oncol. 2012;30(21):2641–2647. doi:10.1200/JCO.2011.36.6054. Epub 2012 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaw SL, Coleman LT, Downie PA, et al. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer. 2007;49(6):808–811. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children's Oncology Group. Cancer. 2007;110(7):1542–1550. [DOI] [PubMed] [Google Scholar]
- 15.Lafay-Cousin L, Sung L, Carret AS, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma. Cancer. 2008;112(4):892–899. [DOI] [PubMed] [Google Scholar]
- 16.Chang SM, Fryberger S, Crouse V, et al. Carboplatin hypersensitivity in children. Cancer. 1995;75(5):1171–1175. [DOI] [PubMed] [Google Scholar]
- 17.Yu DY, Dahl GVH, Shames RS, et al. Weekly dosing of carboplatin increases risk of allergy in children. J Pediatr Hematol Oncol. 2001;23(6):349–352. [DOI] [PubMed] [Google Scholar]
- 18.Schiavetti A, Varrasso G, Maurizi P, et al. Hypersensitivity to carboplatin in children. Med Pediatr Oncol. 1999;32(3):183–185. [DOI] [PubMed] [Google Scholar]
- 19.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons; 2002. [Google Scholar]
- 20.Gnekow AK, Falkenstein F, von Hornstein S, et al. Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro Oncol. 2012;14(10):1265–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Deshmukh H, Gutmann RJ, et al. Alterations of BRAF and HIPK2 loci predominate in sporadic pilocytic astrocytoma. Neurology. 2009;73(19):1526–1531. doi:10.1212/WNL.0b013e3181c0664a. Epub 2009 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones DT, Kocialkowski S, Liu L, et al. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28(20):2119–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cin H, Meyer C, Herr R, et al. Oncogenic FAM131B-BRAF fusion resulting from 7q34 deletion comprises an alternative mechanism of MAPK pathway activation in pilocytic astrocytoma. Acta Neuropathol. 2011;121(6):763–774. [DOI] [PubMed] [Google Scholar]
- 26.Sievert AJ, Jackson EM, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19(3):449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bar EE, Lin A, Tihan T, et al. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67(9):878–887. [DOI] [PubMed] [Google Scholar]
- 28.Jacob K, Albrecht S, Sollier C, et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer. 2009;101(4):722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin A, Rodriguez FJ, Karajannis MA, et al. BRAF alterations in primary glial and glioneuronal neoplasms of the central nervous system with identification of 2 novel KIAA1549:BRAF fusion variants. J Neuropathol Exp Neurol. 2012;71(1):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forshew T, Tatevossian RG, Lawson AR, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218(2):172–181. [DOI] [PubMed] [Google Scholar]
- 31.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]