Abstract
Background
Ependymoblastoma (EBL), ependymoma (EP), and primitive neuroectodermal tumors of the central nervous system not otherwise specified (CNS-PNET NOS) are pediatric brain tumors that can be differentiated by histopathology in the clinical setting. Recently, we described specific MRI features of EBL. In this study, we compare standardized MRI characteristics of EBL with EP and CNS-PNET NOS in a series comprising 22 patients in each group.
Methods
All 66 centrally reviewed cases were obtained from the database of the German multicenter HIT trials. We systematically analyzed the initial MRI scans at diagnosis according to standardized criteria, and paired comparison was performed for EBL and EP, as well as for EBL and CNS-PNET NOS.
Results
We found differences between EBL and EP regarding age at diagnosis, MR signal intensity, tumor margin and surrounding edema, presence and size of cysts, and contrast enhancement pattern. Although MRI appearance of EBL shares many features with CNS-PNET NOS, we revealed significant differences in terms of age at diagnosis, tumor volume and localization, tumor margins, edema, and contrast enhancement.
Conclusion
This is the first study that systematically compares multiple parameters of MRI in pediatric EBL with findings in EP and CNS-PNET NOS. Although a definite differentiation by means of MRI alone might not be feasible in the individual case, we identify significant differences between these tumor entities.
Keywords: CNS-PNET NOS, ependymoblastoma, ependymoma, magnetic resonance imaging, MRI
Ependymoblastoma (EBL) is a malignant CNS tumor with rapid growth and early cerebrospinal fluid dissemination. This rare tumor entity seems to affect mainly young children and is linked with a dismal prognosis. In the majority of cases, EBLs are hemispheric tumors, followed by infratentorial and spinal location.1 A manifestation outside the CNS is very rare but has been described for cases of congenital sacrococcygeal and ovarian tumors.2,3 Together with CNS neuroblastoma, CNS ganglioneuroblastoma, CNS primitive neuroectodermal tumors not otherwise specified (PNET NOS), and medulloepithelioma, EBL is listed as a subgroup of CNS-PNET, according to the 2007 World Health Organization (WHO) classification.4
EBL is defined as an embryonal tumor characterized by ependymoblastic rosettes, with multilayered proliferative tumor cells and a central lumen as histologic hallmarks. It was first described by Bailey and Cushing in 19265 as an ependyme-derived tumor entity that is distinct from ependymoma (EP). Over the past decade, some authors claimed that ependymoblastic rosettes are most frequently encountered in embryonal tumors with abundant neuropil and concluded that EBL might thus still be an imprecise diagnosis regarding histopathological classification.6 In this context, they proposed a novel histologic entity called “embryonal tumor with abundant neuropil and true rosettes” (ETANTR).7–9 However, we now know from molecular studies that CNS-PNET diagnosed as medulloepithelioma, EBL, or ETANTR with C19MC amplification and/or LIN28 expression comprise a distinct histogenetic diagnostic entity, representing a spectrum of embryonal tumors with multilayered rosettes.10–13
We recently described the MRI characteristics of EBL, and our data suggest that it might share imaging features with CNS-PNET NOS.14,15 However, a study that systematically compares imaging characteristics of EBL with other pediatric brain tumor entities has not yet been performed. With this study, we compare our recent collection of 22 EBLs with 22 cases of both EP and CNS-PNET NOS by paired analysis of defined MRI criteria. All 66 cases were obtained from the prospective German Society of Pediatric Oncology and Hematology multicenter trials HIT91, HIT-SKK92, and HIT2000 (HIT = German abbreviation for brain tumor), with central review for neuropathology, neuroradiology, and risk-adapted multimodal therapy.16
Materials and Methods
Study Population
MRI studies of 22 patients with EBL, EP, and CNS-PNET NOS, respectively, were obtained from the brain tumor database of the National Neuroradiological Reference Center for Pediatric Brain Tumors. Brain tumors were diagnosed between the years 2001 and 2013 and patients were registered to the HIT91, HIT-SKK92, and HIT2000 studies (ClinicalTrials.gov/NCT00303810). Due to the multicenter approach, MRI studies were performed with MR scanners of different manufacturers at 0.5- to 3.0-Tesla field strengths. A minimum of noncontrast T1-weighted images (T1WI), T2WI, and contrast-enhanced T1WI in at least 2 different planes and without severe motion artifacts were considered sufficient for inclusion in our study. Older cases with MRIs that had not yet been digitized for image analysis were excluded. Only cases with centrally confirmed histopathological diagnosis (T.P.) of the different tumor entities were included. Cases of CNS-(ganglio)neuroblastoma and pineoblastoma were excluded. There was only one case of medulloepithelioma in our database, showing insufficient image quality, not allowing for further systematic analysis of this tumor entity. All available cases with EBL that met the inclusion criteria were used for our study. With respect to EP and CNS-PNET NOS, 22 cases of each, following an alphabetical order of family names, were included in our digital database. All procedures were approved by the local and central ethics committee of the HIT studies and performed in accordance with the Helsinki Declaration of the World Medical Association.
Image Analysis
A retrospective view of MRI scans was performed by 2 neuroradiologists (M.W-M. and J.N.) in consensus. The same standardized MRI criteria for each of the 3 groups, adapted from the routine image evaluation of our Neuroradiological Reference Center, were analyzed. The tumor diameters were measured in 3 orthogonal dimensions (along the axial, coronal, and sagittal axes, in cm). Tumor volume was then calculated according to a common approximation formula (a × b × c × 0.5, in cm3). Tumor location was subgrouped into the following categories: supratentorial, infratentorial, brainstem/diencephalon; related to cortex or ventricles, basal ganglia, or intraventricular location; CNS dissemination without known primary. T1 and T2 signal intensity of the tumor was defined in relation to that of the cortex. Another criterion was the presence of cysts within the tumor, their localization (periphery or other location) and size (small/large, with a diameter >1 cm being considered as large). Registered were intratumoral hemorrhage, the homogeneity of the solid tumor (in T1WI and T2WI), and the delineation of the tumor from the adjacent brain tissue (well- or ill-defined borders). Also registered were possible peritumoral edema and the maximum extent of edema (in cm). An important parameter was the pattern of gadolinium enhancement within the tumors (intensity, percentage of enhancing solid volume, homogeneity), as was the presence or lack of diffusion restriction. Finally, the status of macroscopic meningeal dissemination (stage M2, M3 or M2+M3, according to the Chang classification17) at the time of diagnosis was recorded. When provided by the external referring centers, CT scans (with a focus on tumor calcifications) and MR spectroscopy data were also analyzed.
Statistical Analysis
After the analysis of these imaging parameters (encoding for either numerical or categorical variables), paired comparison was performed between (i) EBL and EP and (ii) EBL and CNS-PNET NOS, in order to test for differences of MRI characteristics between these groups. In case of numerical variables, the Shapiro–Wilk test was performed to test for normal distribution. t-Tests were then used for normally distributed variables, and the Mann–Whitney U test was used when values were not normally distributed. For categorical variables, a chi-square test and Fisher’s exact test (when 2 options were possible for each measurement) were performed. Bonferroni correction (doubling of P-values) was finally conducted for both numerical and categorical variables, in order to address the potential error that might result from multiple testing on our dataset. P < .05 was considered statistically significant. IBM SPSS for Mac v21 was used for all statistical analyses.
Results
Study Population
The mean age of patients with EBL was 2.1 ± 0.9 years (8 female, 14 male). For EP, mean age at diagnosis was 3.2 ± 1.6 years (14 female, 8 male). The mean age of patients with CNS-PNET NOS was 6.1 ± 4.9 years (8 female, 14 male). The findings for the 22 EBL cases have been recently described in greater detail.14,15 Tables 1 and 2 demonstrate the analyzed imaging parameters and a summary of the results for each group. P-values are listed for each category.
Table 1.
Overview of absolute and relative (%) frequency of MRI parameters in EBL, EP, and CNS-PNET NOS
| EBL, n = 22 | EP, n = 22 | CNS-PNET NOS, n = 22 | (a) P 1 vs 2 | (b) P 1 vs 3 | |
|---|---|---|---|---|---|
| Patient age, y, mean (SD) | 2.13 (0.91) | 3.20 (1.62) | 6.11 (4.88) | .022 | .002 |
| Tumor length, CC, cm (SD) | 5.55 (1.71) | 6.03 (2.18) | 3.75 (1.63) | ||
| Length, RL, cm (SD) | 5.25 (2.02) | 5.30 (2.07) | 3.84 (1.89) | ||
| Length, AP, cm (SD) | 5.93 (2.53) | 6.52 (3.17) | 4.34 (2.48) | ||
| Tumor volume, cm3 (SD) | 114.68 (100.63) | 145.52 (128.99) | 50.43 (61.39) | .992 | .028 |
| Edema width, cm (SD) | 0.9 (0.14) | 1.14 (0.64) | 0.96 (0.79) | – | – |
| Sex | 22 | 22 | 22 | .262 | 1.0 |
| Male | 14 (64%) | 8 (36%) | 14 (64%) | ||
| Female | 8 (36%) | 14 (64%) | 8 (36%) | ||
| Localization | 22 | 22 | 22 | 1.00 | .048 (.122**) |
| Ventricle supratentorial | 2 (9%) | 3 (14%) | 0 (0.0%) | ||
| Associated to ventricle, supratentorial | 1 (5%) | 2 (9%) | 0 (0.0%) | ||
| Cortex, frontal | 6 (27%) | 5 (23%) | 7 (32%) | ||
| Cortex parietal | 7 (32%) | 8 (36%) | 2 (9%) | ||
| Cortex, occipital | 0 | 0 | 0 | ||
| Cortex temporal | 0 (0.0%) | 0 (0.0%) | 1 (5%) | ||
| Basal ganglia | 0 (0.0%) | 1 (5%) | 3 (14%) | ||
| Cortex, infratentorial | 1 (5%) | 0 (0.0%) | 0 (0.0%) | ||
| Ventricle, infratentorial | 3 (14%) | 3 (14%) | 0 (0.0%) | ||
| Brainstem | 2 (9%) | 0 (0.0%) | 5 (23%) | ||
| No primary | 0 (0.0%) | 0 (0.0%) | 4 (18%) | ||
| Cysts | 22 | 22 | 18** | .11 | .696 |
| Yes | 11 (50%) | 18 (82%) | 6 (33%) | ||
| No | 11 (50%) | 4 (18%) | 12 (67%) | ||
| Cyst periphery | 22 | 22 | 18 | .001 | 1 |
| Yes | 6 (27%) | 18 (82%) | 5 (28%) | ||
| No | 16 (73%) | 4 (18%) | 13 (72%) | ||
| Cyst size | 11 | 18 | 6 | .042 | .56 |
| Big | 2 (18%) | 12 (67%) | 3 (50%) | ||
| Small | 9 (82%) | 6 (33%) | 3 (50%) | ||
| Intratumoral hemorrhage | 22 | 22 | 18 | .406 | .37 |
| Yes | 17 (77%) | 12 (55%) | 10 (56%) | ||
| No | 5 (23%) | 10 (45%) | 8 (44%) | ||
| Signal homogeneity (in T1WI and T2WI) | 22 | 22 | 18 | .362 | 1 |
| Homogeneous | 2 (9%) | 0 (0.0%) | 0 (0.0%) | ||
| Predominantly homogeneous | 3 (14%) | 8 (36%) | 3 (17%) | ||
| Predominantly inhomogeneous | 9 (41%) | 6 (27%) | 9 (50%) | ||
| Inhomogeneous | 8 (36%) | 8 (36%) | 6 (33%) | ||
| Margin | 22 | 22 | 18 | .001 | .001 |
| Well defined | 19 (86%) | 6 (27%) | 3 (17%) | ||
| Predominantly well defined | 3 (14%) | 10 (45%) | 6 (33%) | ||
| Ill defined | 0 (0.0%) | 6 (27%) | 9 (50%) | ||
| T2 signal | 22 | 22 | 18 | 1 | .172 |
| Hyperintense | 1 (5%) | 1 (5%) | 4 (22%) | ||
| Isointense | 2 (9%) | 4 (18%) | 4 (22%) | ||
| Hypointense | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| All appear | 19 (86%) | 17 (77%) | 10 (56%) | ||
| T2 predominant signal | 22 | 22 | 18 | .032 | .96 |
| Hyperintense | 10 (45%) | 2 (9%) | 9 (50%) | ||
| Isointense | 12 (55%) | 20 (91%) | 8 (44%) | ||
| Hypointense | 0 (0.0%) | 0 (0.0%) | 1 (6%) | ||
| Edema | 22 | 22 | 18 | .002 | .002 |
| Yes | 2 (9%) | 13 (59%) | 11 (61%) | ||
| No | 20 (91%) | 9 (41%) | 7 (39%) | ||
| T1 signal | 22 | 22 | 18 | .096* | 1 |
| Hyperintense | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Isointense | 6 (27%) | 8 (36%) | 5 (28%) | ||
| Hypointense | 9 (41%) | 2 (9%) | 10 (56%) | ||
| All appear | 7 (32%) | 12 (55%) | 3 (17%) | ||
| T1 predominant signal | 22 | 22 | 18 | .02 | 1 |
| Hyperintense | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Isointense | 10 (45%) | 19 (86%) | 8 (44%) | ||
| Hypointense | 12 (55%) | 3 (14%) | 10 (56%) | ||
| CE intensity | 22 | 22 | 18 | .14 | .854 |
| None | 5 (23%) | 1 (5%) | 7 (39%) | ||
| Slight | 6 (27%) | 2 (9%) | 4 (22%) | ||
| Moderate | 8 (36%) | 12 (55%) | 3 (17%) | ||
| Intense | 3 (14%) | 7 (32%) | 4 (22%) | ||
| CE homogeneity | 17 | 21 | 11 | .001 | .01 |
| Homogeneous | 1 (6%) | 2 (10%) | 0 (0.0%) | ||
| Predominantly homogeneous | 13 (76%) | 2 (10%) | 2 (18%) | ||
| Predominantly inhomogeneous | 2 (12%) | 10 (48%) | 2 (18%) | ||
| Inhomogeneous | 1 (6%) | 7 (33%) | 7 (64%) | ||
| CE percent of solid tumor | 17 | 21 | 11 | .001 | .038 |
| 1%–25% | 9 (53%) | 2 (10%) | 3 (27%) | ||
| 26%–50% | 7 (41%) | 0 (0.0%) | 2 (18%) | ||
| 51%–75% | 1 (6%) | 4 (19%) | 1 (9%) | ||
| 76%–100% | 0 (0.0%) | 15 (71%) | 5 (45%) | ||
| Diffusion restriction | 22 | 22 | 18 | .4 | 1 |
| Yes | 14 (64%) | 12 (55%) | 11 (61%) | ||
| No | 0 (0.0%) | 3 (14%) | 1 (6%) | ||
| No DWI available | 8 (36%) | 7 (32%) | 6 (33%) | ||
| Confirmed by ADC | 22 | 22 | 18 | 1 | 1 |
| Yes | 11 (50%) | 10 (45%) | 8 (44%) | ||
| No | 0 (0.0%) | 0 (0.0%) | 1 (6%) | ||
| No ADC available | 11 (50%) | 12 (55%) | 9 (50%) |
Abbreviations: CC, cranio-caudal; RL, right–left; AP, anteroposterior; CE, contrast enhancement; ADC, apparent diffusion coefficient; DWI, diffusion-weighted image.
This table shows P-values after paired comparison of imaging parameters in (a) EBL vs EP and (b) EBL vs CNS-PNET NOS. Note that relative frequencies do not equal 100% in some cases, due to mathematical rounding. Statistically significant differences (P < .05) are in bold print.
*Values with .05<P < .1 after Bonferroni correction are considered to show a strong tendency toward significant results.
**Calculation after exclusion of disseminated disease without known primary tumor.
Table 2.
Supplementary data of EBL, EP, and CNS-PNET NOS cases regarding spinal MRI, MR spectroscopy, and CT findings
| EBL, n = 22 | EP, n = 22 | CNS-PNET NOS, n = 22 | (a) P 1 vs 2 | (b) P 1 vs 3 | |
|---|---|---|---|---|---|
| Spinal MRI available | 22 | 22 | 18 | ||
| Yes | 7 (32%) | 12 (55%) | 12 (67%) | ||
| No | 3 (14%) | 2 (9%) | 3 (17%) | ||
| Not sufficient | 12 (55%) | 8 (36%) | 3 (17%) | ||
| Meningeal dissemination* | 22 | 22 | 22 | .66 | .246 |
| Unknown (M0 or M1) | 17 (77%) | 20 (91%) | 14 (64%) | ||
| M2 | 0 (0.0%) | 1 (5%) | 1 (5%) | ||
| M3 | 3 (14%) | 0 (0.0%) | 0 (0.0%) | ||
| M2 + M3 | 2 (9%) | 1 (5%) | 7 (32%) | ||
| MR spectroscopy available | 22 | 22 | 18 | ||
| Yes | 3 (14%) | 0 (0.0%) | 3 (17%) | ||
| No | 19 (86%) | 22 (100%) | 15 (83%) | ||
| CT available | 22 | 22 | 18 | ||
| Yes | 5 (23%) | 3 (14%) | 5 (28%) | ||
| No | 17 (77%) | 19 (86%) | 13 (72%) | ||
| Calcifications (CT) | 5 | 3 | 5 | .392 | .334 |
| Yes | 3 (60%) | 0 (0.0%) | 0 (0.0%) | ||
| No | 2 (40.0%) | 3 (100%) | 5 (100%) |
Note that relative frequencies do not equal 100% in some cases, due to mathematical rounding.
This table shows P-values after paired comparison of imaging parameters in (a) EBL vs EP and (b) EBL vs CNS-PNET NOS.
*Macroscopic dissemination (detectable with MRI).
Ependymoblastoma Compared With Ependymoma
We found a significantly different age distribution between EBL and EP (P = .02), with EBL occurring at a younger age (mean age, 2.1 y vs 3.2 y in EP). There was a male predominance in the EBL group (m/f ratio of 1.75/1), whereas EP occurred more frequently in girls (m/f ratio of 1/1.75, P = .262); however, this difference was not statistically significant. The localization of the 2 tumor types also did not differ significantly. EPs showed heterogeneous signal intensity on T1WI slightly more often, whereas EBLs appeared rather iso- or hypointense (P = .096). EBLs were rather hypointense on T1WI, compared with EPs, which appeared more often isointense (P = .02). T2 signal was predominantly hyperintense in EBLs, whereas EPs tended to have a predominantly isointense signal (P = .032). EBL had more clearly defined borders to the adjacent tissue (P = .001). If there were cysts present in the tumor periphery (P = .001) and if the cysts had a diameter >1 cm (P = .04), the tumor was more likely to be an EP. Although peritumoral edema was rare in both groups, it was found more frequently in EP (P = .002). With respect to contrast enhancement, EBL showed more homogeneous enhancement (P = .001), with less percentage of enhancing solid compounds (P = .001) compared with EP. Typical MRIs of EBL and EP are shown in Figs. 1 and 2, respectively.
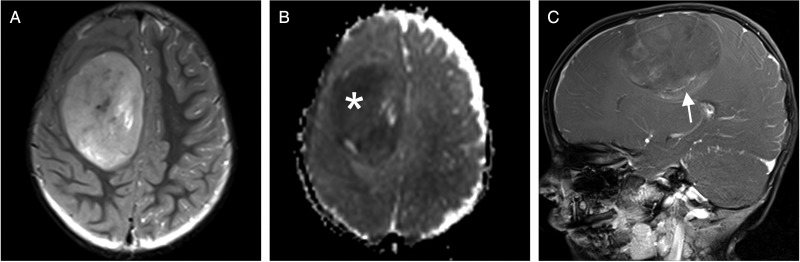
Fig. 1.
Example of MRI in EBL (3T Siemens Trio). (A) T2WI shows a hyperintense, well-demarcated tumor in the right hemisphere with no surrounding edema. (B) There is low signal in the apparent diffusion coefficient map, indicative of high cellularity (white asterisk). (C) Mild to moderate inhomogeneous enhancement can be found after gadolinium administration on T1WI (white arrow).
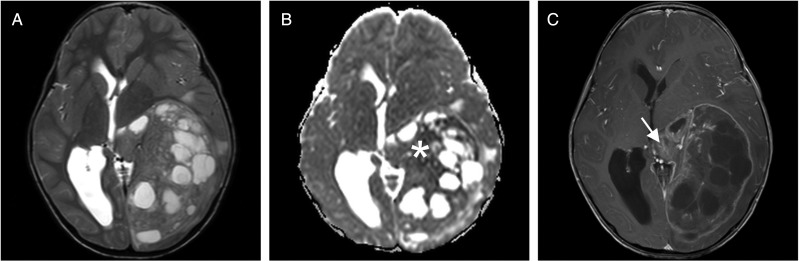
Fig. 2.
MRI study of a large supratentorial EP (3T Philips Achieva). (A) The tumor has an inhomogeneous signal in T2WI with multiple cysts and minimal surrounding edema. (B) Apparent diffusion coefficient map shows restricted diffusion in parts of the tumor (white asterisk). (C) After gadolinium administration, there is strong enhancement of the capsule and of some parts of the solid tumor component (white arrow).
Ependymoblastoma Compared With CNS-PNET NOS
EBL shared many imaging features with the group of CNS-PNET NOS. It appears that the tumor volume of EBL at diagnosis is larger (P = .03) than that of CNS-PNET NOS, although the latter can also manifest as bulky hemispheric masses. Both EBL and CNS-PNET NOS showed a male predominance with m/f ratio of 1.75/1. In our study population, EBL occurred at significantly younger ages (mean, 2.1 y, P = .002), whereas CNS-PNET NOS was diagnosed at a wider age range from childhood to adolescence (mean, 6.1 y). We found a statistically significant difference in terms of tumor location (P = .048); however, after exclusion of disseminated CNS-PNET NOS without known primary tumor, the difference between EBL and CNS-PNET NOS locations did not reach the significance level (P = .122). Tumor margins were better defined in EBL (P = .001), but mild surrounding edema occurred more frequently in CNS-PNET NOS (P = .002). After gadolinium administration, enhancement appeared more inhomogeneous in CNS-PNET NOS compared with EBL (P = .01). The percentage of enhancing solid tumor was less in EBL (P = .038). None of the other MRI features significantly differed between the 2 groups. MRIs of a typical CNS-PNET NOS are shown in Fig. 3.
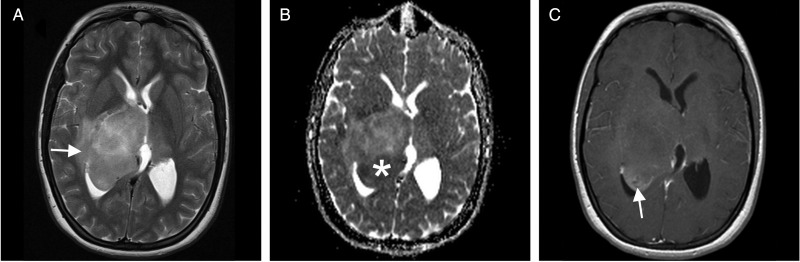
Fig. 3.
Exemplary MRI findings in CNS-PNET NOS (1.5T Siemens Symphony). (A) T2WI shows an iso- to hyperintense central mass with moderate peripheral edema (white arrow). (B) Some parts of the tumor show low apparent diffusion coefficient, suggestive of high cellularity (white asterisk). (C) Only the posterior portions of the tumor moderately enhance on T1WI after gadolinium administration (white arrow).
Discussion
In this study, we first systematically compared MRI characteristics of EBL with EP and CNS-PNET NOS, respectively. We thus provide important information regarding differentiation of these tumor entities by means of MRI.
MRI Characteristics and Comparison of Ependymoblastoma and Ependymoma
The described MRI characteristics of EBL are based on the largest series comprising 22 centrally reviewed EBL cases, which have been recently described in detail by our group.14,15 In contrast to the limited radiological data of EBL, imaging features of EP are well documented in the literature, since EP represents the third most frequent intracranial neoplasm in children after pilocytic astrocytoma and medulloblastoma.18
In our study, EBL occurred in very young children (mean age, 2.1 y) and with a male predisposition, whereas EP had a slightly (but significantly) higher age at diagnosis and girls were affected more frequently. It has been shown that age at diagnosis in EP varies with location of the tumor.19 In line with our finding, the mean age of EP is reported to be 4.1 years in the literature. With respect to sex distribution, Ellison and colleagues found 57% males and 43% females in a series of 229 pediatric EP.20 According to the German Childhood Cancer Registry, the m/f ratio in EP is 1.5/1, in contrast to 1.75/1 in EBL, indicating that sex might not be a strong parameter to differentiate EBL from EP.18
About 60% of EPs have an infratentorial location within the posterior fossa or spinal canal, whereas 40% occur supratentorially.21 According to our brain tumor database (data not shown), 2/3 of EP cases have an infratentorial, and 1/3 a supratentorial location. In the present series, 19 EPs were located supratentorially, and only 3 cases had an origin in the posterior fossa. Thus, tumor location does not seem to be a differentiating factor between EBL and EP in our study; however, it is well known that infratentorial EP tends to noninvasively extend through the foramina of the fourth ventricle into the perimedullary cisterns and upper cervical spinal canal (“plastic” growth).22,23 None of our 6 infratentorial EBLs (4 in the fourth ventricle or cerebellum, 2 in the brainstem) showed this behavior, suggesting that growth pattern might be of help in morphologically differentiating EP from EBL in cases with infratentorial origin.
Most EP is reported to be hypo- to isointense on unenhanced T1WI and hyperintense on T2WI.24 Our comparative analysis revealed that there are some significant differences in T1 and T2 signal intensity of the solid tumor parts; however, it still seems challenging to distinguish EBL and EP by means of MR signal characteristics alone. Foci of heterogeneous signals within the solid compounds of these tumors are suggestive of hemorrhage, necrosis, or calcifications in both EBL and EP. Calcifications are reported in 40%–80% of supra- and infratentorial cases of EP.25,26 In our EBL series, 3 of the 5 available CT scans showed calcifications, suggesting similar findings in EBL and EP.
According to our present study, a characteristic that could help differentiate EBL from EP may be that cysts (and especially large cysts with a diameter of >1 cm) were more frequently seen in EP (P = .04). It has been shown that supratentorial EP often contains cystic components, whereas infratentorial EP is a rather solid neoplasm.25,27 Gadolinium enhancement of EP is reported to be variable, with moderate enhancement in most cases.21,28,29 Another relevant result of our data may be that a higher proportion of solid tumor mass of EP enhances gadolinium; furthermore, enhancement of EP is rather inhomogeneous compared with EBL. Since EBL and nonmyxopapillary EP are tumors with high cellularity, all EBL and the majority of our EP cases (80%) showed diffusion restriction. Some cases of nonanaplastic (infratentorial) EP do not show restricted diffusion, suggesting that this subtype might be more easily differentiated from EBL.30,31 Thus, the diagnostic value of DWI is rather the differentiation of EBL and EP from other tumor entities with generally lower cellularity, such as low-grade astrocytoma. Previous work shows that cutoff values for apparent diffusion coefficient can help to discern pediatric low-grade from high-grade tumors; however, there is a substantial overlap between tumor types.32
MRI Characteristics and Comparison of Ependymoblastoma and CNS-PNET NOS
Histologically designated as a subgroup of CNS-PNET, EBL seems to share most MRI features with CNS-PNET NOS. In contrast to medulloblastoma, the group of CNS-PNET NOS are rather uncommon, representing only 11%–12% of pediatric embryonal tumors and 3%–7% of all pediatric brain tumors.4,18 The exact frequency of EBL is unknown. In a recent meta-analysis, Ding et al33 found only 71 published EBL cases between the years 1970 and 2012. In the present study, we found a significant difference regarding location of EBL and CNS-PNET NOS (P = .048). It must be noted that CNS-PNET NOS can occur as disseminated disease without a primary tumor (4 cases in our study), and thus differences in location were not significant after exclusion of these cases. As opposed to CNS-PNET NOS, there was no evidence of disseminated EBL without known primary tumor in our study population. Interestingly, we found 6 infratentorial EBLs (4 in the fourth ventricle and cerebellum, 2 originating from the brainstem); these cases may be morphologically confused with other tumor types such as medulloblastoma, malignant glioma, plexus carcinoma, and atypical teratoid rhabdoid tumor. According to the literature, extracerebellar CNS-PNET usually occurs in children younger than 5 years.18,34 EBL patients were significantly younger in our study, with a mean age of 2.1 years at manifestation, whereas CNS-PNET NOS could be found in a wider age range (mean, 6.1 y). According to the German Childhood Cancer Registry, mean age of CNS-PNET was lower than in our study (3.9 y) but still higher compared with our EBL cases.18
MRI appearance of CNS-PNET is described as often very large heterogeneous-appearing hemispheric masses.34,35 Interestingly, tumor size of EBL was even larger in our study, compared with that of CNS-PNET NOS (P = .028). The larger volume and the younger age at manifestation may reflect that EBL comprises a highly aggressive and rapidly growing subgroup within the PNET tumors, with a worse response to therapy and a dismal prognosis.1,36 Furthermore, pediatric brain tumors can be very large in infants, where cranial sutures give way to intracranial pressure, with subsequent clinical symptoms occurring relatively late.
With respect to MR signal characteristics, we found a rather heterogeneous signal in unenhanced T1WI and T2WI for both EBL and CNS-PNET NOS. It has been described that necrosis and intratumoral hemorrhage are frequently seen in CNS-PNET.34,35 Correspondingly, we found signs of hemorrhage in 77% of EBL and 56% of CNS-PNET NOS, respectively. Calcifications can be found in up to 70% of CNS-PNET.34 In our collection, calcifications were present in 60% of EBL but in none of the CNS-PNET NOS. There were, however, only 5 CT scans available in each group. Tumor borders in CNS-PNET are usually sharp, with minimal or absent surrounding edema.35,37 Although we found that tumor margins of EBLs were even better defined and accompanied by less surrounding edema, these features may not be of help in distinguishing the 2 tumor types. Contrast enhancement of CNS-PNET is usually variable and heterogeneous and the tumors show diffusion restriction.34,35,37 In our study, enhancement pattern in CNS-PNET NOS was rather heterogeneous, with a significantly higher percentage of enhancing tumor compared with EBL. We found diffusion restriction in 100% of our EBL cases and in 89% of CNS-PNET NOS, again reflecting high cellularity in both entities. In summary, it seems challenging to reliably differentiate EBL from CNS-PNET NOS using MRI. A hemispheric brain tumor that is morphologically compatible with CNS-PNET may be an EBL, especially in case of a very large tumor with a manifestation at <3 years of age.
Histopathological Considerations
The WHO classification defines several histopathological variants of EP.4 Besides myxopapillary EP (WHO grade I), intracranial pediatric EP is basically divided into classical (grade II) and anaplastic (grade III) tumors. In the present series of EP, the majority of the tumors (20 cases) were designated grade III, whereas only 2 EP cases were grade II lesions. However, there is considerable histopathological variation between and within tumors, which may lead to difficulties in grading EP correctly. This is reflected by strikingly disconcordant studies of children with intracranial EP that report ratios of grade II to grade III tumors between 17:1 and 1:7.20,38 However, histopathological grading is not predictive for outcomes in most pediatric trial cohorts.
Together with medulloepithelioma, the proposed novel entity (ETANTR) and EBL have been recently shown to be comprised by a common molecular and diagnostic entity.13,39 To address these molecular findings in our study, we included only histologically designated EBL cases that tested positive for LIN28 expression. According to the 2007 WHO classification, EBL is at present defined as a variant of CNS-PNET with distinctive ependymoblastic rosettes.4 Correspondingly, all 22 tumors of our case series were designated EBL (grade IV) by central neuropathological review (T.P.). With our study, we further define EBL from the radiological perspective.
Conclusion
We first systematically analyzed and compared MRI characteristics of pediatric EBL with EP and CNS-PNET NOS in a series of 22 centrally reviewed cases of each group. A definite differentiation of these entities with MRI seems to be difficult; however, we identified particular imaging features that might help distinguish these histologically distinct tumor types.
Funding
This work was supported by the Deutsche Kinderkrebsstiftung (German Childhood Cancer Foundation).
Acknowledgments
We are grateful to György A. Homola and Johannes Hain for their help with manuscript editing and statistics.
Conflict of interest statement. None declared.
References
- 1.Gerber NU, von Hoff K, von Bueren AO, et al. Outcome of 11 children with ependymoblastoma treated within the prospective HIT-trials between 1991 and 2006. J Neurooncol. 2011;102(3):459–469. [DOI] [PubMed] [Google Scholar]
- 2.Morovic A, Damjanov I. Neuroectodermal ovarian tumors: a brief overview. Histol Histopathol. 2008;23(6):765–771. [DOI] [PubMed] [Google Scholar]
- 3.Santi M, Bulas D, Fasano R, et al. Congenital ependymoblastoma arising in the sacrococcygeal soft tissue: a case study. Clin Neuropathol. 2008;27(2):78–82. [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, et al. (eds.) WHO Classification of Tumours of the Central Nervous System. Lyon: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey P, Cushing H. A Classification of the Tumors of the Glioma Group on a Histogenetic Basis With a Correlated Study of Prognosis. Philadelphia: J. B. Lippincott Co.; 1926. [Google Scholar]
- 6.Judkins AR, Ellison DW. Ependymoblastoma: dear, damned, distracting diagnosis, farewell!*. Brain Pathol. 2010;20(1):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gessi M, Giangaspero F, Lauriola L, et al. Embryonal tumors with abundant neuropil and true rosettes: a distinctive CNS primitive neuroectodermal tumor. Am J Surg Pathol. 2009;33(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhart CG, Brat DJ, Cohen KJ, et al. Pediatric neuroblastic brain tumors containing abundant neuropil and true rosettes. Pediatr Dev Pathol. 2000;3(4):346–352. [DOI] [PubMed] [Google Scholar]
- 9.Dunham C, Sugo E, Tobias V, et al. Embryonal tumor with abundant neuropil and true rosettes (ETANTR): report of a case with prominent neurocytic differentiation. J Neurooncol. 2007;84(1):91–98. [DOI] [PubMed] [Google Scholar]
- 10.Ceccom J, Bourdeaut F, Loukh N, et al. Embryonal tumor with multilayered rosettes: diagnostic tools update and review of the literature. Clin Neuropathol. 2014;33(1):15–22. [DOI] [PubMed] [Google Scholar]
- 11.Korshunov A, Remke M, Gessi M, et al. Focal genomic amplification at 19q13.42 comprises a powerful diagnostic marker for embryonal tumors with ependymoblastic rosettes. Acta Neuropathol. 2010;120(2):253–260. [DOI] [PubMed] [Google Scholar]
- 12.Korshunov A, Sturm D, Ryzhova M, et al. Embryonal tumor with abundant neuropil and true rosettes (ETANTR), ependymoblastoma, and medulloepithelioma share molecular similarity and comprise a single clinicopathological entity. Acta Neuropathol. 2014;128(2):279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spence T, Sin-Chan P, Picard D, et al. CNS-PNETs with C19MC amplification and/or LIN28 expression comprise a distinct histogenetic diagnostic and therapeutic entity. Acta Neuropathol. 2014;128(2):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nowak J, Seidel C, Berg F, et al. MRI characteristics of ependymoblastoma: results from 22 centrally reviewed cases. AJNR Am J Neuroradiol. 2014;35(10):1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowak J, Seidel C, Pietsch T, et al. Ependymoblastoma of the brainstem: MRI findings and differential diagnosis. Pediatr Blood Cancer. 2014;61(6):1132–1134. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich C, von Bueren AO, von Hoff K, et al. Treatment of young children with CNS-primitive neuroectodermal tumors/pineoblastomas in the prospective multicenter trial HIT 2000 using different chemotherapy regimens and radiotherapy. Neuro Oncol. 2013;15(2):224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent JP, Chang CH, Cohen ME. A classification system for primitive neuroectodermal tumors (medulloblastoma) of the posterior fossa. Cancer. 1985;56(7 Suppl):1807–1809. [DOI] [PubMed] [Google Scholar]
- 18.German Childhood Cancer Registry. Annual Report 2012. http://www.kinderkrebsregister.de/fileadmin/kliniken/dkkr/pdf/jb/jb2012/jb2012_final_s.pdf.
- 19.McGuire CS, Sainani KL, Fisher PG. Incidence patterns for ependymoma: a Surveillance, Epidemiology, and End Results study. J Neurosurg. 2009;110(4):725–729. [DOI] [PubMed] [Google Scholar]
- 20.Ellison DW, Kocak M, Figarella-Branger D, et al. Histopathological grading of pediatric ependymoma: reproducibility and clinical relevance in European trial cohorts. J Negat Results Biomed. 2011;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spoto GP, Press GA, Hesselink JR, et al. Intracranial ependymoma and subependymoma: MR manifestations. AJNR Am J Neuroradiol. 1990;11(1):83–91. [PMC free article] [PubMed] [Google Scholar]
- 22.Osborn AG. Osborn's Brain: Imaging, Pathology, and Anatomy. Salt Lake City: Amirsys; 2013:503. [Google Scholar]
- 23.Poretti A, Meoded A, Huisman TA. Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging. 2012;35(1):32–47. [DOI] [PubMed] [Google Scholar]
- 24.Mermuys K, Jeuris W, Vanhoenacker PK, et al. Best cases from the AFIP: supratentorial ependymoma. Radiographics. 2005;25(2):486–490. [DOI] [PubMed] [Google Scholar]
- 25.Morrison G, Sobel DF, Kelley WM, et al. Intraventricular mass lesions. Radiology. 1984;153(2):435–442. [DOI] [PubMed] [Google Scholar]
- 26.Osborn AG, Daines JH, Wing SD. The evaluation of ependymal and subependymal lesions by cranial computed tomography. Radiology. 1978;127(2):397–401. [DOI] [PubMed] [Google Scholar]
- 27.Armington WG, Osborn AG, Cubberley DA, et al. Supratentorial ependymoma: CT appearance. Radiology. 1985;157(2):367–372. [DOI] [PubMed] [Google Scholar]
- 28.Furie DM, Provenzale JM. Supratentorial ependymomas and subependymomas: CT and MR appearance. J Comput Assist Tomogr. 1995;19(4):518–526. [DOI] [PubMed] [Google Scholar]
- 29.McConachie NS, Worthington BS, Cornford EJ, et al. Review article: computed tomography and magnetic resonance in the diagnosis of intraventricular cerebral masses. Br J Radiol. 1994;67(795):223–243. [DOI] [PubMed] [Google Scholar]
- 30.Kan P, Liu JK, Hedlund G, et al. The role of diffusion-weighted magnetic resonance imaging in pediatric brain tumors. Childs Nerv Syst. 2006;22(11):1435–1439. [DOI] [PubMed] [Google Scholar]
- 31.Rasalkar DD, Chu WC, Paunipagar BK, et al. Paediatric intra-axial posterior fossa tumours: pictorial review. Postgrad Med J. 2013;89(1047):39–46. [DOI] [PubMed] [Google Scholar]
- 32.Porto L, Jurcoane A, Schwabe D, et al. Differentiation between high and low grade tumours in paediatric patients by using apparent diffusion coefficients. Eur J Paediatr Neurol. 2013;17(3):302–307. [DOI] [PubMed] [Google Scholar]
- 33.Ding D, Zhao A, Qiu B, et al. Ependymoblastoma with cystic change in a child. J Neurosurg Pediatr. 2014;13(6):658–665. [DOI] [PubMed] [Google Scholar]
- 34.Borja MJ, Plaza MJ, Altman N, et al. Conventional and advanced MRI features of pediatric intracranial tumors: supratentorial tumors. AJR Am J Roentgenol. 2013;200(5):W483–W503. [DOI] [PubMed] [Google Scholar]
- 35.Osborn AG. Osborn's Brain: Imaging, Pathology, and Anatomy. Salt Lake City: Amirsys; 2013:569–572. [Google Scholar]
- 36.Gessi M, von Bueren AO, Treszl A, et al. MYCN amplification predicts poor outcome for patients with supratentorial primitive neuroectodermal tumors of the central nervous system. Neuro Oncol. 2014;16(7):924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poussaint TY. Magnetic resonance imaging of pediatric brain tumors: state of the art. Top Magn Reson Imaging. 2001;12(6):411–433. [DOI] [PubMed] [Google Scholar]
- 38.Tihan T, Zhou T, Holmes E, et al. The prognostic value of histological grading of posterior fossa ependymomas in children: a Children's Oncology Group study and a review of prognostic factors. Mod Pathol. 2008;21(2):165–177. [DOI] [PubMed] [Google Scholar]
- 39.Spence T, Perotti C, Sin-Chan P, et al. A novel C19MC amplified cell line links Lin28/let-7 to mTOR signaling in embryonal tumor with multilayered rosettes. Neuro Oncol. 2014;16(1):62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]