Abstract
Background
Because World Health Organization (WHO) grades II and III meningiomas are relatively uncommon, there is limited literature on the descriptive epidemiology of these tumors, and the existing literature predates the 2000 WHO classification revisions. Our purpose was to provide a modern, population-based study of the descriptive epidemiology of WHO II and III meningiomas in the United States.
Methods
The Central Brain Tumor Registry of the United States (CBTRUS) was queried for intracranial meningiomas categorized by WHO grade for the 2004–2010 study period. Age-adjusted incidence (95% confidence interval in parentheses) per 100 000 population was calculated by age, sex, race, and ethnicity. Annual percent change (APC) was calculated using Joinpoint.
Results
From 2004 to 2010, the incidence of WHO II intracranial meningiomas increased from 0.28 (95% CI, 0.27–0.29) to 0.30 (95% CI, 0.28–0.32), representing an APC of 3.6% (95%CI, 0.8%–6.5%). Conversely, from 2000–2010, the incidence of WHO III meningiomas decreased from 0.13 (95% CI, 0.11–0.14) to 0.06 (95%CI, 0.06–0.07), representing an APC of −5.4% (95% CI, −6.8% to −4.0%). From 2004 to 2010, the overall proportion of WHO I, II, and III intracranial meningiomas was 94.6%, 4.2%, and 1.2%, respectively. For WHO II/III meningiomas, females in the 35–64 year age group had a higher incidence than males in the same age group, whereas males in the ≥75 year age group ≥ had a higher incidence. Black and Asian Pacific Islander races were both associated with the highest incidence of WHO II/III meningiomas. Hispanic ethnicity was not associated with any difference in incidence.
Conclusion
This study presents the most comprehensive evaluation of the modern descriptive epidemiology of WHO II and III meningiomas. Temporal trends likely reflect the 2000 WHO histological criteria revisions.
Keywords: atypical, anaplastic, epidemiology, malignant, meningioma
Meningiomas represent the most common primary brain tumor.1,2 Although the majority of meningiomas are benign, a small subset of tumors have atypical or anaplastic histology and behave more aggressively.3–7 Because higher-grade meningiomas are less common, prior incidence data have primarily come from hospital-based case studies, which may overestimate incidence due to referral bias.7–9 To date, there have been no population-based studies focused on the epidemiology of these tumors.
In 2000, significant revisions were made in the World Health Organization (WHO) histological classification of atypical (WHO II) and anaplastic (WHO III) meningiomas because histological criteria for these tumors were vague under the 1993 WHO grading system.10,11 For example, mitoses were categorized as “frequent” and “high” for WHO II and WHO III tumors, respectively.10 In addition, brain invasion was noted as a marker for malignancy. However, several studies subsequently demonstrated that brain invasion, in the absence of histologically aggressive features, is not a clinical predictor of malignant behavior.7,12,13 In the 2000 WHO classification, specific mitotic counts were provided for WHO II and III subtypes, and brain invasion was removed from the anaplastic criteria.11 Subsequent studies have demonstrated that the 2000 WHO classification system predicts clinical course better than the 1993 version.3,14 As a result of the WHO classification revisions, we are in need of updated incidence data for WHO II and III meningiomas. Therefore, we carried out a study to report the current population-based descriptive epidemiology of WHO II and III meningiomas in the United States).
Methods
Data Collection
Data were obtained from the Central Brain Tumor Registry of the United States (CBTRUS). CBTRUS contains the largest aggregation of primary brain tumor incidence data in the United States and incidence data from 50 central cancer registries (45 National Program of Cancer Registries [NPCRs]) and 5 Surveillance Epidemiology and End Results [SEERs]) representing ∼98% of the US population.2 Cancer registries in the United States are mandated to collect data on all tumors diagnosed in the United States, regardless of the type of medical facility at which these tumors were diagnosed. The Benign Brain Tumor Cancer Registries Amendment Act (Public Law 107-260) went into effect on January 1, 2004, and mandated the registration of all nonmalignant brain tumors in the United States. Therefore, 2004–2010 were used as the primary years of analysis for meningiomas of all WHO grades. Because incidence data on malignant meningiomas (WHO III) were available prior to 2004, additional data from 2000–2004 were utilized only for investigating temporal trends in the incidence of anaplastic meningiomas because accurate trends analysis requires a longer time period of study.
Histology and behavior codes from International Classification of Diseases for Oncology,15 (ICD-O-3), 3rd Edition, were used to classify WHO grades as follows: WHO I (9530/0, 9531/0, 9532/0, 9533/0, 9534/0, 9537/0), WHO II (9530/1, 9531/1, 9532/1, 9533/1, 9534/1, 9537/1, 9538/1, 9539/1), and WHO III (9530/3, 9531/3, 9532/3, 9533/3, 9534/3, 9535/3, 9537/3, 9538/3, 9539/3). Hemangiopericytoma (9510) was not included. ICD-O-3 histology and behavior codes were crossed with ICD-O-3 site codes C70.0 (cerebral meninges) and C70.9 (meninges not otherwise specified). C70.1 (spinal meninges) was excluded. Given that the ratio of spinal-to-cerebral meningiomas was ∼1:20 during the study years and that atypical and anaplastic meningiomas are much less common in the spine, meningiomas classified under C70.9 are much more likely to be cerebral rather than spinal and were therefore included. In the temporal trends, a conservative estimate of incidence is provided, which does not include site code C70.9 (meninges not otherwise specified).
There was no age exclusion. Only WHO II and III meningiomas with histological confirmation were included; those reported with radiographic diagnosis were excluded. A prior study demonstrated strong agreement (>90%) between CBTRUS data and patient medical records.16 Patients with multiple meningiomas were only counted as one occurrence in the incidence data with demographic data obtained from the first tumor. Only 0.8% of patients with WHO II and III meningiomas in the CBTRUS database have multiple tumors. Data regarding the presence of genetic syndromes were not available. Age, sex, racial, and ethnic demographic information was obtained from the CBTRUS database. Population data from the United States Census Bureau were used to calculate incidence rates and were obtained from the National Cancer Institute SEER program (http://seer.cancer.gov).
Statistical Analysis
SEER*Stat 8.1.5 (National Cancer Institute) was used to calculate age-adjusted incidence rates. Joinpoint Regression Program 4.0.1 (National Cancer Institute) was used to calculate the annual percentage change (APC) in incidence during the study period (http://surveillance.cancer.gov/joinpoint). All age-adjusted incidence rates are per 100 000 population. A 95% confidence interval (CI) is provided with each incidence rate, APC, and incidence rate ratio (IRR) statistic.
Results
Overall Incidence Rates
From 2004–2010, there were 6047 intracranial WHO II meningiomas, resulting in an average annual age-adjusted incidence of 0.28 (95% CI, 0.27–0.29) (Table 1). The age-adjusted incidence rate using a conservative estimate was 0.24 (95% CI, 0.23–0.25). From 2000–2010, there were 3004 WHO III meningiomas, resulting in an average annual age-adjusted incidence of 0.09 (95% CI, 0.09–0.10) (Table 2). The age-adjusted incidence rate using a conservative estimate was 0.07 (95% CI, 0.07–0.08). From 2004 to 2010, the overall proportions of WHO I, II, and III intracranial meningiomas were 94.6%, 4.2%, and 1.2%, respectively.
Table 1.
Age-adjusted incidence rate (per 100 000 population) of World Health Organization grade II intracranial meningiomas by year
| Estimate |
Conservative Estimate* |
|||||
|---|---|---|---|---|---|---|
| Count | Rate (95% CI) | APC (95% CI) | Count | Rate (95% CI) | APC (95% CI) | |
| Total | 6047 | 0.28 (0.27–0.29) | – | 5191 | 0.24 (0.23–0.25) | – |
| 2004 | 752 | 0.26 (0.24–0.28) | 3.6 (0.8–6.5) | 648 | 0.22 (0.20–0.24) | 3.0 (−0.5–6.6) |
| 2005 | 792 | 0.27 (0.25–0.28) | 702 | 0.24 (0.22–0.25) | ||
| 2006 | 731 | 0.24 (0.23–0.26) | 633 | 0.21 (0.20–0.23) | ||
| 2007 | 879 | 0.28 (0.27–0.30) | 745 | 0.24 (0.22–0.26) | ||
| 2008 | 929 | 0.30 (0.28–0.32) | 819 | 0.26 (0.25–0.28) | ||
| 2009 | 1005 | 0.32 (0.30–0.34) | 856 | 0.27 (0.25–0.29) | ||
| 2010 | 959 | 0.30 (0.28–0.32) | 788 | 0.24 (0.23–0.26) | ||
Abbreviations: APC, annual percentage change; CI, confidence interval.
*Conservative estimate does not include site code C70.9 (meninges NOS).
Table 2.
Age-adjusted incidence rate (per 100 000 population) of World Health Organization grade III intracranial meningiomas by year
| Estimate |
Conservative Estimatea |
|||||
|---|---|---|---|---|---|---|
| Count | Rate (95% CI) | APC (95% CI) | Count | Rate (95% CI) | APC (95% CI) | |
| TOTAL | 3004 | 0.09 (0.09–1.00) | 2388 | 0.07 (0.07–0.08) | ||
| 2000 | 347 | 0.13 (0.11–0.14) | −5.4 (−6.8 to −4.0) | 274 | 0.10 (0.09–0.11) | −5.4 (−6.7 to −4.0) |
| 2001 | 304 | 0.11 (0.10–0.12) | 236 | 0.09 (0.08–0.10) | ||
| 2002 | 288 | 0.10 (0.09–0.11) | 237 | 0.08 (0.07–0.10) | ||
| 2003 | 291 | 0.10 (0.09–0.12) | 220 | 0.08 (0.07–0.09) | ||
| 2004 | 283 | 0.10 (0.09–0.11) | 234 | 0.08 (0.07–0.09) | ||
| 2005 | 274 | 0.09 (0.08–0.11) | 221 | 0.08 (0.07–0.09) | ||
| 2006 | 244 | 0.08 (0.07–0.09) | 189 | 0.06 (0.05–0.07) | ||
| 2007 | 291 | 0.09 (0.08–0.11) | 232 | 0.07 (0.07–0.09) | ||
| 2008 | 231 | 0.07 (0.06–0.08) | 188 | 0.06 (0.05–0.07) | ||
| 2009 | 243 | 0.08 (0.07–0.09) | 192 | 0.06 (0.05–0.07) | ||
| 2010 | 208 | 0.06 (0.06–0.07) | 165 | 0.05 (0.04–0.06) | ||
Abbreviations: APC, annual percentage change; CI, confidence interval.
aConservative estimate does not include site code C70.9 (meninges NOS).
Temporal Trends
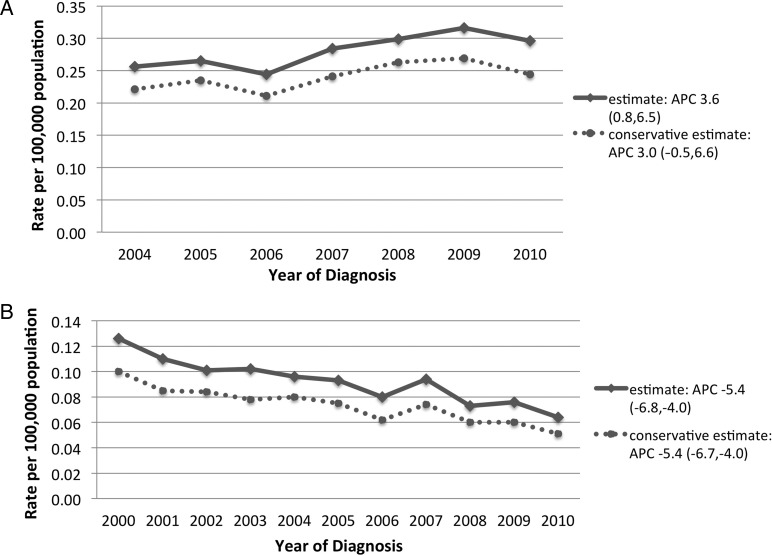
From 2004, the overall incidence of WHO II meningiomas increased from 0.26 (95% CI, 0.24–0.28) to 0.30 (95% CI, 0.28–0.32) in 2010, which represented an APC of 3.6% (95% CI, 0.8–6.5) (Fig. 1A). From 2000, the overall incidence of WHO III meningiomas decreased steadily from 0.13 (95% CI, 0.11–0.14) in 2000 to 0.06 (95% CI, 0.06–0.07) in 2010, resulting in an APC of −5.4% (95% CI, −6.8 to −4.0) (Fig. 1B).
Fig. 1.
Age-adjusted incidence of World Health Organization (WHO) grades II (A) and III (B) intracranial meningiomas. APC = annual percent change (expressed as percent with 95% confidence interval).
Incidence by Age and Sex
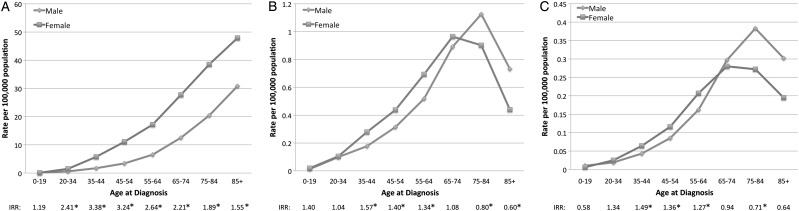
For males, the incidence of WHO II meningiomas increased from a nadir of 0.01 (95% CI, 0.01–0.02) in the 0–19 year age group to a peak of 1.13 (95% CI, 1.02–1.24) in men aged 75–84 years (Table 3). For females, the incidence increased from a nadir of 0.02 (95% CI, 0.01–0.03) in the 0–19 year age group to a peak of 0.97 (95%, 0.90–1.04) in women aged 65–74 years. The incidence of WHO II meningiomas was significantly higher in females in the 35–64 year age group, whereas the incidence was significantly higher in males aged ≥75 years (Fig. 2B).
Table 3.
Age-adjusted incidence rate (per 100 000 population) of World Health Organization grade II intracranial meningiomas by age and sex (2004–2010)
| Age (y) | Male |
Female |
||
|---|---|---|---|---|
| Count | Rate (95% CI) | Count | Rate (95% CI) | |
| 0–19 | 40 | 0.01 (0.01–0.02) | 52 | 0.02 (0.01–0.03) |
| 20–34 | 207 | 0.10 (0.09–0.11) | 213 | 0.10 (0.09–0.12) |
| 35–44 | 258 | 0.18 (0.16–0.20) | 411 | 0.28 (0.25–0.31) |
| 45–54 | 466 | 0.31 (0.29–0.34) | 668 | 0.44 (0.41–0.47) |
| 55–64 | 569 | 0.52 (0.48–0.56) | 815 | 0.69 (0.65–0.74) |
| 65–74 | 561 | 0.89 (0.82–0.97) | 715 | 0.97 (0.90–1.04) |
| 75–84 | 416 | 1.13 (1.02–1.24) | 472 | 0.90 (0.82–0.99) |
| 85+ | 80 | 0.73 (0.58–0.91) | 104 | 0.44 (0.36–0.53) |
Abbreviations: CI, confidence interval; y, years.
Fig. 2.
Age-adjusted incidence of WHO grades I (A), II (B), and III (C) intracranial meningiomas by age at diagnosis and sex. IRR = incidence rate ratio (female:male); * = statistically significant difference in incidence rates between sexes.
For males, the incidence of WHO III meningiomas increased from a nadir of 0.01 (95% CI, 0.01–0.01) in the 0–19 year age group to a peak of 0.38 (95% CI, 0.32–0.45) in men aged 75–84 years (Table 4). For females, the incidence increased from a nadir of 0.01 (95% CI, 0.00–0.01) in the 0–19 year age group to a peak of 0.28 in the 65–74 year age group. The incidence of WHO III meningiomas was significantly higher in females in the 35–64 year age group, whereas the incidence was higher in males aged ≥75 years (Fig. 2C).
Table 4.
Age-adjusted incidence rate (per 100 000 population) of World Health Organization grade III intracranial meningiomas by age and sex (2004–2010)
| Age (y) | Male |
Female |
||
|---|---|---|---|---|
| Count | Rate (95% CI) | Count | Rate (95% CI) | |
| 0–19 | 29 | 0.01 (0.010–0.01) | 16 | 0.01 (0.00–0.01) |
| 20–34 | 39 | 0.02 (0.01–0.03) | 50 | 0.03 (0.02–0.03) |
| 35–44 | 63 | 0.04 (0.03–0.06) | 95 | 0.06 (0.05–0.08) |
| 45–54 | 126 | 0.09 (0.07–0.10) | 178 | 0.12 (0.10–0.13) |
| 55–64 | 177 | 0.16 (0.14–0.19) | 242 | 0.21 (0.18–0.23) |
| 65–74 | 186 | 0.30 (0.26–0.34) | 208 | 0.28 (0.24–0.32) |
| 75–84 | 142 | 0.38 (0.32–0.45) | 144 | 0.27 (0.23–0.32) |
| 85+ | 33 | 0.30 (0.21–0.42) | 46 | 0.20 (0.14–0.26) |
Abbreviations: CI, confidence interval; y, years.
The crossover in incidence rates by sex of WHO II and III meningiomas was not seen when examining WHO I meningiomas. Instead, females had a significantly higher incidence rate at all ages > 20 years (Fig. 2A).
Incidence by WHO Grade
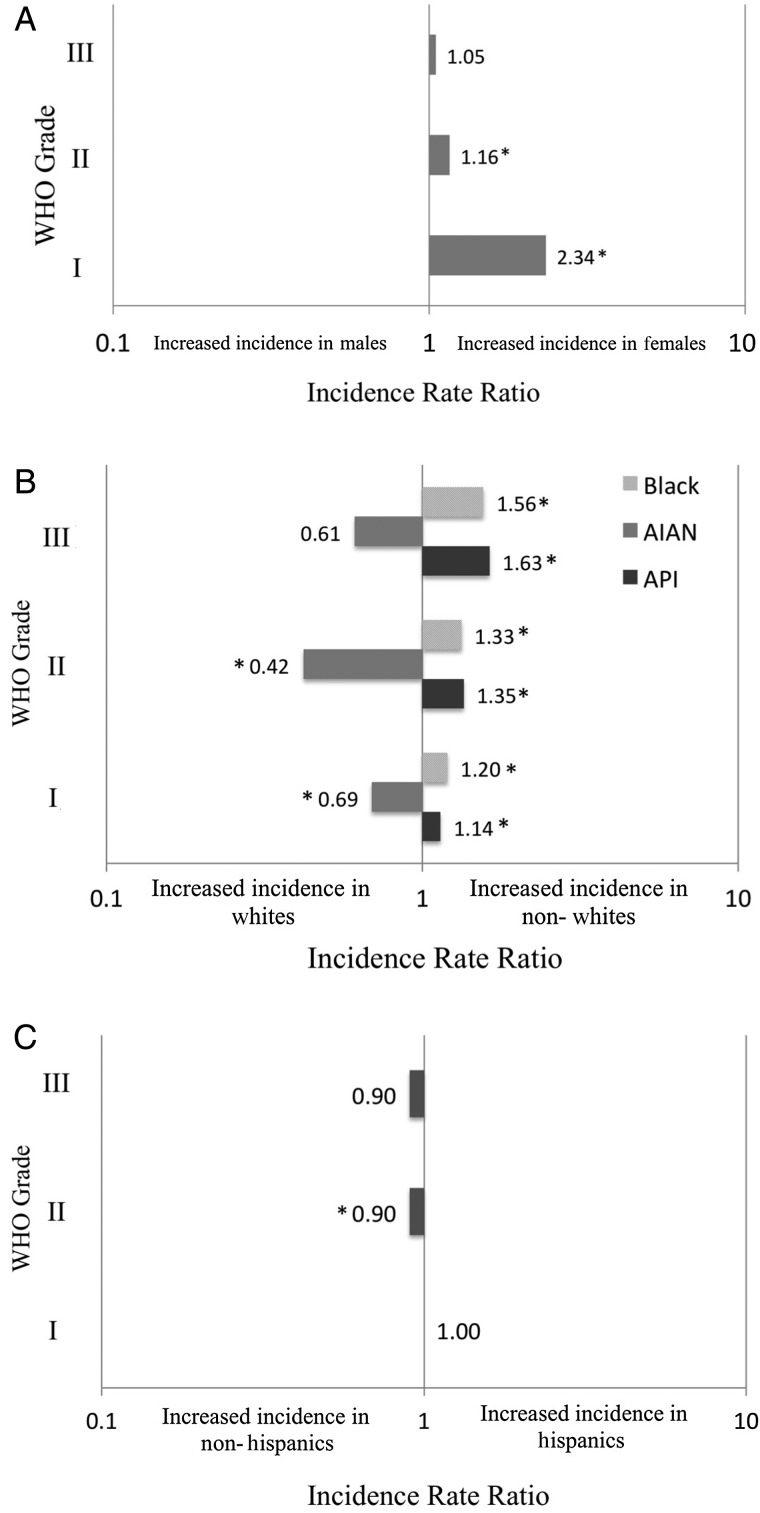
The overall incidence for 2004–2010 by WHO grade was examined. Overall, females had a statistically significant higher incidence in WHO I meningiomas than males (8.56 vs 3.68; IRR, 2.34; P < .0001) (Fig. 3A, Table 5). For WHO II meningiomas, females had a significantly higher incidence than males (0.30 vs 0.26; IRR, 1.16; P < .0001). For WHO III meningiomas, there was no significant difference in incidence between females and males (0.09 vs 0.08; IRR, 1.06; P = .354).
Fig. 3.
Incidence rate ratios of intracranial meningiomas by WHO grade and (A) sex, (B) race, and (C) ethnicity. * = statistically significant different incidence rate between groups; API = Asian Pacific Islander; AIAN = American Indian/Alaskan Native.
Table 5.
Age-adjusted incidence rate (per 100 000 population) of intracranial meningiomas by World Health Organization grade and sex (2004–2010)
| Rate (95% CI) |
||
|---|---|---|
| WHO Grade | Male | Female |
| I | 3.68 (3.64–3.71) | 8.56 (8.51–8.61) |
| II | 0.26 (0.25–0.27) | 0.30 (0.29–0.31) |
| III | 0.08 (0.08–0.09) | 0.09 (0.08–0.09) |
Abbreviations: CI, confidence interval; WHO, World Health Organization.
There was a similar trend in incidence by race across the different WHO grades. Overall, black and Asian Pacific Islander races were associated with the highest incidence of meningiomas of all WHO grades (Table 6). Incident rate ratios (compared to with white race) associated with black race increased with WHO grade, from 1.20 (P < .0001) for WHO I to 1.56 (P < .0001) for WHO III meningiomas (Fig. 3b). IRR associated with Asian Pacific Islander race also increased from 1.14 (P < .0001) for WHO I to 1.63 (P < .0001) for WHO III meningiomas. American Indian/Alaskan Native race was associated with the lowest incidence of meningiomas of all WHO grades. Lastly, there were no significant differences in incidence rates between Hispanic and non-Hispanic ethnic groups except for WHO II meningiomas, in which a slightly higher incidence was associated with non-Hispanic ethnicity (IRR 0.90; P = .024) (Table 7, Fig. 3c). Data regarding age, sex, and ethnicity were present for all cases. Race information was missing in 28 (0.5%) cases for WHO II and 13 (0.4%) cases for WHO III meningiomas.
Table 6.
Age-adjusted incidence rate (per 100 000 population) of intracranial meningiomas by World Health Organization grade and race (2004–2010)
| WHO Grade | Rate (95% CI) |
|||
|---|---|---|---|---|
| White | Black | AIAN | API | |
| I | 6.14 (6.10–6.18) | 7.36 (7.25–7.48) | 4.24 (3.90–4.60) | 6.99 (6.81–7.17) |
| II | 0.27 (0.26–0.28) | 0.36 (0.33–0.38) | 0.11 (0.06–0.18) | 0.36 (0.32–0.40) |
| III | 0.08 (0.07–0.08) | 0.12 (0.10–0.13) | 0.05 (0.02–0.09) | 0.12 (0.10–0.15) |
Abbreviations: AIAN, American Indian/Alaskan Native; API, Asian Pacific Islander; CI, confidence interval.
Table 7.
Age-adjusted incidence rate (per 100 000 population) of intracranial meningiomas by World Health Organization grade and race (2004–2010)
| WHO Grade | Rate (95% CI) |
|
|---|---|---|
| Non-Hispanic | Hispanic | |
| I | 6.33 (6.29–6.36) | 6.36 (6.24–6.49) |
| II | 0.29 (0.28–0.29) | 0.26 (0.23 ,0.28) |
| III | 0.08 (0.08–0.09) | 0.07 (0.06–0.09) |
Abbreviations: CI, confidence interval; WHO, World Health Organization.
Discussion
Interpretation
During the study period, WHO II and III meningiomas accounted for 4.2% and 1.2% of all newly diagnosed meningiomas, respectively. Hospital-based incidence studies, particularly from large tertiary facilities, have previously reported a higher proportion of WHO II/III meningiomas.8,13,14,17 Interestingly, a hospital-based study of 936 meningioma cases from the Department of Neurosurgery of Helsinki, Helsinki University Central Hospital, Finland, which captures all neurosurgical cases from Finland, found a very similar distribution of meningiomas when graded by histological anaplasia: benign 94.3%, atypical 4.7%, and anaplastic 1.0%.18 One population-based Australian study from 2000–2008 found a slightly higher proportion of atypical meningiomas.19 No prior studies have reported an age-adjusted incidence rate for WHO II/III meningiomas. One older study from 1989 reported a crude incidence rate of malignant meningiomas of 0.17 per 100 000 in Manitoba, Canada, which was lower than that found in our study.9
Our study demonstrates that the incidence of WHO II meningiomas is increasing, while the incidence of WHO III meningiomas is decreasing. No prior studies have evaluated temporal trends in WHO II and III meningiomas for comparison. The temporal trends possibly represent progressive adoption of the 2000 WHO guidelines, which would shift the subset of cases featuring brain invasion without histological anaplasia from WHO III to II or I. It is worth noting that a small but important modification was made in the WHO 2007 histological classification system. Meningiomas with otherwise grade I histological features, but with microscopic brain invasion, were moved into the WHO II category. It is possible that this modification contributed in part to the continued increase in grade II tumors between 2007 and 2010 in our study.20,21 Our study also demonstrates that the incidence of WHO I meningiomas is increasing as well (APC +3.4%), a finding confirmed by other studies.22,23 This may be due, in part, to increased utilization of imaging modalities as incidental meningiomas have been detected in 0.29%–0.9% of asymptomatic volunteers.24,25 However, if this is not entirely due to detection bias, one might then expect an increase in atypical histological cases as well.
The overall male-to-female ratio in WHO II/III meningiomas is similar to that reported in prior studies.6,8,9 When stratified by age, females had the highest incidence rate ratios for meningiomas of all WHO grades during middle ages (35–64 y), whereas males had a higher incidence in older ages (≥75 y). An increase in risk excess in middle-aged women has been demonstrated with WHO I meningiomas in prior studies as well.26 It is possible that the same etiology for this trend is present across meningiomas of all grades. The association between the exposure to exogenous and endogenous estrogen and an increased risk of meningioma has been previously reported27–30; both estrogen and progesterone receptors are often present on meningiomas of all histological subtypes,4,31 but no causal link has been made.1
Our study demonstrates an increasing incidence of WHO II and III meningiomas with age. However, unlike WHO I meningiomas, WHO II/III meningiomas have a peak age incidence in the 75–84 year age group followed by a drop-off in incidence in the ≥85 year older group. This relationship between age and sex in the incidence of WHO II and III meningiomas has not been evaluated in prior studies.
Our data suggest that black and Asian Pacific Islander (API) races are associated with the highest incidence of both WHO II and III meningiomas. Conversely, there was no difference in incidence between Hispanic and non-Hispanic ethnicity. No prior studies have evaluated the incidence of WHO II and III meningiomas by race. Of note, sample size was not large enough to compare white and black race stratified by ethnicity (e.g., black Hispanic vs black non-Hispanic).
Limitations
There are several limitations to the study. First, the Benign Brain Tumor Act went into effect on January 1, 2004. As a result, increases in incidence may be related to improvement in case ascertainment and reporting over time. However, a recent CBTRUS analysis examined the incidence of all nonmalignant primary brain tumors during the beginning of nonmalignant tumor registration in the United States and found no significant increase in incidence after 2005.32 Two recent studies from Australia (2000–2008) and Europe (1998–2003), primarily of WHO I meningiomas, also demonstrated increasing incidence over a time frame similar to that found in the CBTRUS database, but these may be subject to similar trends in case ascertainment and reporting.19,22 If the incidence of WHO I meningiomas is truly increasing, the increased use of ionizing radiation for diagnostic and/or therapeutic purposes and increasing life expectancy may be 2 contributing factors.
Second, only the initial diagnosis of a tumor is reported to central cancer registries. For example, if a patient was initially diagnosed with a WHO I meningioma but later had a recurrence that was atypical or anaplastic, only the first occurrence would be recorded in the database. Prior studies have demonstrated that ∼14%–29% of recurrent benign tumors will be atypical or anaplastic.5,33–35 It is also possible that some of the WHO I meningiomas that were diagnosed by imaging alone may actually have been higher grade. We elected to include imaging-based WHO I meningiomas because excluding them would result in a greater inaccuracy in the estimated incidence. Third, we cannot differentiate in the database between de novo, transformed, or radiation-induced WHO II/III meningiomas. It has been demonstrated that de novo and transformed atypical and anaplastic tumors have different cytogenetic, hormone receptor, proliferative index, and clinical profiles.4 Fourth, the database does not provide detailed information on the anatomical location of the meningioma. Prior studies have demonstrated an increased risk of higher grade meningiomas in the convexity location.5,8,9,36,37 Fifth, the current study is limited to the population of the United States, which limits the ability to generalize the results to other countries that may have significantly different genetic and environmental factors. Sixth, we must acknowledge limitations in the collection of race and ethnicity data. In the CBTRUS database, race and ethnicity are obtained from the patient's medical records and categorized to US census race and ethnicity groups. In addition, Hispanic ethnicity is assessed in cancer registry data using the North American Association of Central Cancer Registries (NAACCR) Hispanic/Latino identification algorithm, which uses patient name to assess ethnicity. As such, there may be inaccuracies in the assignment of race and ethnicity.
Finally, WHO grade is not consistently listed in the database, and WHO classification has been reconstructed from ICD-O-3 histology and behavior codes. There is no central pathology review in this study, and categories are based on the diagnosis made by individual pathologists at the institution of initial diagnosis. We excluded any WHO II and III meningiomas diagnosed by radiographic evidence alone to strengthen the reliability of recorded histology included in the study.
Conclusion
During the study period (in the era after the WHO 2000 meningioma grading revisions), the incidence of WHO II meningiomaswas increasing, whereas that of WHO III meningiomas was decreasing. Women are at higher risk than men for WHO II and III meningiomas during middle age (35–64 years), whereas men are at higher risk than women during advanced age (≥75 years). Black and Asian Pacific Islander races are associated with the highest incidence of WHO II and III meningiomas. This study represents the largest population-based descriptive epidemiology study of WHO II and III meningiomas in the United States.
Funding
The following organizations contributed to the maintenance of the CBTRUS database in 2014: the Centers for Disease Control and Prevention (CDC) under Agreement 5U58DP00381-03, The Sontag Foundation (www.songtagfoundation.org), the Pediatric Brain Tumor Foundation (www.curethekids.org) along with the Musella Foundation (www.virtualtrials.com), Novocure, Inc. (www.novocure.com), Voices Against Brain Cancer (www.voicesagainstbraincancer.org) as well as private and in-kind donations. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Conflict of interest statement. The authors of this manuscript report no conflicts of interest in relation to the content of this manuscript.
References
- 1.Claus EB, Bondy ML, Schildkraut JM, et al. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088–1095. discussion 1088–1095. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combs SE, Schulz-Ertner D, Debus J, et al. Improved correlation of the neuropathologic classification according to adapted world health organization classification and outcome after radiotherapy in patients with atypical and anaplastic meningiomas. Int J Radiat Oncol Biol Phys. 2011;81(5):1415–1421. [DOI] [PubMed] [Google Scholar]
- 4.Krayenbuhl N, Pravdenkova S, Al-Mefty O. De novo versus transformed atypical and anaplastic meningiomas: comparisons of clinical course, cytogenetics, cytokinetics, and outcome. Neurosurgery. 2007;61(3):495–503, discussion 503–494. [DOI] [PubMed] [Google Scholar]
- 5.Modha A, Gutin PH. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57(3):538–550. discussion 538–550. [DOI] [PubMed] [Google Scholar]
- 6.Pasquier D, Bijmolt S, Veninga T, et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71(5):1388–1393. [DOI] [PubMed] [Google Scholar]
- 7.Perry A, Scheithauer BW, Stafford SL, et al. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer. 1999;85(9):2046–2056. [DOI] [PubMed] [Google Scholar]
- 8.Mahmood A, Caccamo DV, Tomecek FJ, et al. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33(6):955–963. [DOI] [PubMed] [Google Scholar]
- 9.Rohringer M, Sutherland GR, Louw DF, et al. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989;71(5 Pt 1):665–672. [DOI] [PubMed] [Google Scholar]
- 10.Kleihues P, Burger PC, Scheithauer BW. Histological Typing of Tumours of the Central Nervous System. 2nd ed Berlin: Springer-Verlag; 1993. [Google Scholar]
- 11.Kleihues P, Cavenee WK. Pathology and Genetics of Tumours of the Central Nervous System. 2nd ed Lyon: IARC Press; 2000. [Google Scholar]
- 12.McLean CA, Jolley D, Cukier E, et al. Atypical and malignant meningiomas: importance of micronecrosis as a prognostic indicator. Histopathology. 1993;23(4):349–353. [DOI] [PubMed] [Google Scholar]
- 13.Perry A, Stafford SL, Scheithauer BW, et al. Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol. 1997;21(12):1455–1465. [DOI] [PubMed] [Google Scholar]
- 14.Yang SY, Park CK, Park SH, et al. Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79(5):574–580. [DOI] [PubMed] [Google Scholar]
- 15.Percy C, Fritz A, Jack A, et al. International Classification of Diseases for Oncology (ICD-O). 3rd ed. Geneva: World Health Organization; 2000.
- 16.Surawicz TS, McCarthy BJ, Jukich PJ, et al. The accuracy and completeness of primary brain and central nervous system tumor data: results from the Central Brain Tumor Registry of the United States. J Registry Manage. 2000;27(2):51–56. [Google Scholar]
- 17.Goyal LK, Suh JH, Mohan DS, et al. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46(1):57–61. [DOI] [PubMed] [Google Scholar]
- 18.Kallio M, Sankila R, Hakulinen T, et al. Factors affecting operative and excess long-term mortality in 935 patients with intracranial meningioma. Neurosurgery. 1992;31(1):2–12. [DOI] [PubMed] [Google Scholar]
- 19.Dobes M, Khurana VG, Shadbolt B, et al. Increasing incidence of glioblastoma multiforme and meningioma, and decreasing incidence of Schwannoma (2000–2008): Findings of a multicenter Australian study. Surg Neurol Int. 2011;2:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backer-Grondahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012;5(3):231–242. [PMC free article] [PubMed] [Google Scholar]
- 21.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deltour I, Johansen C, Auvinen A, et al. Time trends in brain tumor incidence rates in Denmark, Finland, Norway, and Sweden, 1974–2003. J Natl Cancer Inst. 2009;101(24):1721–1724. [DOI] [PubMed] [Google Scholar]
- 23.Klaeboe L, Lonn S, Scheie D, et al. Incidence of intracranial meningiomas in Denmark, Finland, Norway and Sweden, 1968–1997. Int J Cancer. 2005;117(6):996–1001. [DOI] [PubMed] [Google Scholar]
- 24.Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. [DOI] [PubMed] [Google Scholar]
- 26.Preston-Martin S, Staples M, Farrugia H, et al. Primary tumors of the brain, cranial nerves and cranial meninges in Victoria, Australia, 1982–1990: patterns of incidence and survival. Neuroepidemiology. 1993;12(5):270–279. [DOI] [PubMed] [Google Scholar]
- 27.Blitshteyn S, Crook JE, Jaeckle KA. Is there an association between meningioma and hormone replacement therapy? J Clin Oncol. 2008;26(2):279–282. [DOI] [PubMed] [Google Scholar]
- 28.Jhawar BS, Fuchs CS, Colditz GA, et al. Sex steroid hormone exposures and risk for meningioma. J Neurosurg. 2003;99(5):848–853. [DOI] [PubMed] [Google Scholar]
- 29.Korhonen K, Auvinen A, Lyytinen H, et al. A nationwide cohort study on the incidence of meningioma in women using postmenopausal hormone therapy in Finland. Am J Epidemiol. 2012;175(4):309–314. [DOI] [PubMed] [Google Scholar]
- 30.Schlehofer B, Blettner M, Wahrendorf J. Association between brain tumors and menopausal status. J Natl Cancer Inst. 1992;84(17):1346–1349. [DOI] [PubMed] [Google Scholar]
- 31.Carroll RS, Zhang J, Black PM. Expression of estrogen receptors alpha and beta in human meningiomas. J Neurooncol. 1999;42(2):109–116. [DOI] [PubMed] [Google Scholar]
- 32.McCarthy BJ, Kruchko C, Dolecek TA. The impact of the Benign Brain Tumor Cancer Registries Amendment Act (Public Law 107–260) on non-malignant brain and central nervous system tumor incidence trends. J Reg Manage. 2013;40(1):32–35. [PubMed] [Google Scholar]
- 33.Adegbite AB, Khan MI, Paine KW, et al. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58(1):51–56. [DOI] [PubMed] [Google Scholar]
- 34.Al-Mefty O, Kadri PA, Pravdenkova S, et al. Malignant progression in meningioma: documentation of a series and analysis of cytogenetic findings. J Neurosurg. 2004;101(2):210–218. [DOI] [PubMed] [Google Scholar]
- 35.Jaaskelainen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25(3):233–242. [DOI] [PubMed] [Google Scholar]
- 36.Sanai N, Sughrue ME, Shangari G, et al. Risk profile associated with convexity meningioma resection in the modern neurosurgical era. J Neurosurg. 2010;112(5):913–919. [DOI] [PubMed] [Google Scholar]
- 37.Wong G, Harper C. Atypical meningiomas: clinical pathological correlation. Aust N Z J Surg. 1984;54(4):331–336. [DOI] [PubMed] [Google Scholar]