Summary
Introduction
We aim to analyze the fast oscillations in the scalp EEG of focal epilepsy patients with low-to-high rates of interictal epileptiform discharges (IEDs), in order to determine how this neurophysiological feature influences fast oscillation occurrence and their significance as markers of the seizure onset zone (SOZ).
Methods
Thirty-two patients were studied, subdivided in four categories based on IED frequency: groups A, B and C respectively with high, intermediate and low IED rate, and group D with no IED. Thirty minutes of slow-wave sleep EEG, low-pass filtered at 300 Hz and sampled at 1000 Hz, were reviewed. IEDs and fast oscillations (gamma activity, 40–80 Hz; and ripples, >80 Hz) were marked. Each channel was classified as inside or outside the irritative zone and the SOZ. We calculated the number and rates of IEDs and fast oscillation, their co-occurrence, their frequency in the irritative zone and SOZ, and the specificity, sensitivity and accuracy to determine the SOZ in the overall population and separately for each group.
Results
We analyzed 984 channels. Group A (high IED rate) showed the highest fast oscillation rate (gamma: 0.37 ± 0.73; ripples: 0.17 ± 0.26), followed by group B (gamma: 0.08 ± 0.06; ripples: 0.07 ± 0.05), group C (gamma: 0.06 ± 0.06; ripples: 0.04 ± 0.01), and finally group D, with very low values (gamma: 0.03 ± 0; ripples: 0.03 ± 0). IEDs co-occurred with gamma in 9.5% and with ripples in 3.2%; and gamma and ripples co-occurred with IEDs in 46.2% and 44.4%, respectively. The fast oscillations were more frequent inside than outside the irritative zone and the SOZ (p < 0.001). Compared to the IEDs, the fast oscillations were less sensitive (sensitivity: IEDs 78%, gamma 66% and ripples 48%) but more specific (specificity: IEDs 50%, gamma 76% and ripples 83%) and accurate (accuracy: IEDs 54%, gamma 74% and ripples 77%) in identifying the SOZ; the same results were reproduced for the different groups separately.
Conclusions
This study confirms that fast oscillations can be recorded from the scalp EEG. Gamma activity and ripples are more frequent in patients with frequent IEDs and, in general, inside the irritative zone. However, compared to IEDs, gamma and ripples are less sensitive but more specific and accurate in identifying the SOZ, and this remains in patients with low fast oscillation rates. These findings suggest that IEDs and fast oscillations could share some common neuronal network, but gamma activity and ripples are a better biomarker of epileptogenicity
Keywords: Fast oscillations, Scalp EEG, IEDs, Irritative zone, Seizure onset zone
Introduction
Over the last decade, many studies have defined the High Frequency Oscillations (HFOs) as physiological and pathological entities that can be recorded in the brain of normal rats, in animal models of mesial temporal lobe epilepsy and in humans. With intracranial stereo-EEG (SEEG) recordings in epileptic patients, HFOs were found using microelectrodes (Bragin et al., 1999a,b; Staba et al., 2002) and clinical macroelectrodes, during ictal (Jirsch et al., 2006) and interictal phase (Staba et al., 2007; Urrestarazu et al., 2007; Worrell et al., 2008); these studies suggested a possible role for HFOs as EEG biomarkers of epileptogenicity. However, the necessity of invasive techniques limited HFO analysis to a subset of patients with drug-resistant epilepsy candidate to a surgical treatment, a small percentage of the overall epileptic population.
Reports have recently demonstrated the possibility of recording HFOs non-invasively with scalp EEG. HFOs were described in patients not usually evaluated with intracranial recording, and reported ictally, at the onset of epileptic spasms (Kobayashi et al., 2004) and tonic seizures in Lennox–Gastaut Syndrome (Kobayashi et al., 2009), or interictally in children with continuous spike and wave in slow-wave sleep (Kobayashi et al., 2010), in idiopathic partial epilepsy (Kobayashi et al., 2011), and in adults with drug-resistant epilepsy and frequent focal spikes (Andrade-Valenca et al., 2011). These studies were performed in patient populations characterized by frequent interictal epileptiform discharges (IEDs), or high a rate of spiking, on scalp EEG, and so far no study analyzed if scalp HFOs are found and behave differently in patients with low IED rates or even with inactive EEG.
The aim of the present study is to evaluate how fast oscillations, namely gamma activity and ripples, correlate with the IED rate, analyzing their occurrence in a cohort of patients with focal epilepsy presenting with different degrees of spiking activity on scalp EEG. We wish to determine if fast oscillations and IEDs share a common behavior. We also want to analyze if the significance of scalp recorded fast oscillations as “marker of epileptogenicity”, as defined by a previous study from our group (Andrade-Valenca et al., 2011), is affected by IED frequency.
Methods
Subjects
Between January and September 2011, 111 epileptic patients were admitted in the EEG-telemetry Unit of the Montreal Neurological Hospital for monitoring. They all had the second night of recording with the following settings: low pass filtering at 300 Hz, sampling rate at 1000 Hz. From the overall population, 32 patients were randomly selected. The only inclusion criterion was a diagnosis of “focal epilepsy”. Each patient was placed in one of the following categories, based on the IED rate during the analyzed EEG segment: (1) group A: > 1 IED/min; (2) group B: < 1 IED/min, but > 1 IED/5 min; (3) group C: < 1 IED/5 min; and (4) group D: no IEDs. All patients gave informed consent in agreement with the Research Ethics Board of the hospital.
Recording methods and channel classification
EEGs were recorded using the Harmonie monitoring system (Stellate, Montréal, Canada), and the electrodes placed according to the international 10–20 system, with additional zygomatic and F9/F10, T9/T10, P9/P10 electrodes, CPz used as reference. Electromyogram (EMG) and electro-oculogram (EOG) were also recorded. We used the Harmonie software to compute spectral trends in the delta, alpha, and beta bands for the EEG and the power of the chin EMG with a 30-s time resolution. The EEG sections with high delta and low EMG power were visually reviewed in a bipolar montage and selected as slow-wave sleep. After analyzing all these sections, we randomly selected 30 consecutive minutes of slow-wave sleep in which no major artifacts occurred. To minimize the influence of seizures, we selected interictal samples at least 2 h before and after a seizure. All channels were defined as inside or outside the irritative zone (by definition: channels showing spiking activity, even if IEDs occurred just once during the 30 min segment) and inside or outside the SOZ (by definition: channels showing the earliest ictal EEG change from baseline prior or concomitant with clinical onset). Patients in whom the clinical onset preceded the first EEG change, or for whom the seizure onset was not lateralizing or localizing, were excluded from the SOZ analysis. We removed from the evaluation all channels showing continuous artifacts or malfunction.
IED and fast oscillation marking
The visual marking of IEDs and fast oscillations was performed by a trained neurophysiologist (F.M.), with the same methodology as described in our previous study on scalp HFOs (Andrade-Valenca et al., 2011). First, IEDs were marked using the EEG settings for clinical review (15 s/page, 30 μV/mm, LF: 0.3 Hz and HF: 70 Hz). The following transients were included in the IED marking process: spikes (sharp transient with a duration between 20 and 70 ms), sharp waves (duration between 70–200 ms), spike-and-slow-wave complexes (spike followed by a slow-wave) (Chatrian et al., 1974). The fast oscillations were subsequently analyzed, separated in gamma activity (40–80 Hz) and ripples (>80 Hz). The display was split vertically and high-pass filtered at 40 Hz (left side) and 80 Hz (right side), with a low-pass filtering at 200 Hz for both sides, using a finite impulse response (FIR) filter to minimize ringing. Timescale was stretched to 264 mm/s (1.6 s/page) and sensitivity increased to 1 μV/mm. The fast oscillations were defined as at least four consecutive oscillations with amplitude clearly standing out from the rest of the background. A gamma activity was marked if an event was visible on the left (40 Hz) and did not occur, at least with the same shape, on the right (80 Hz), and an event was regarded as a ripple if visible only on the right (Figs. 1 and 2).
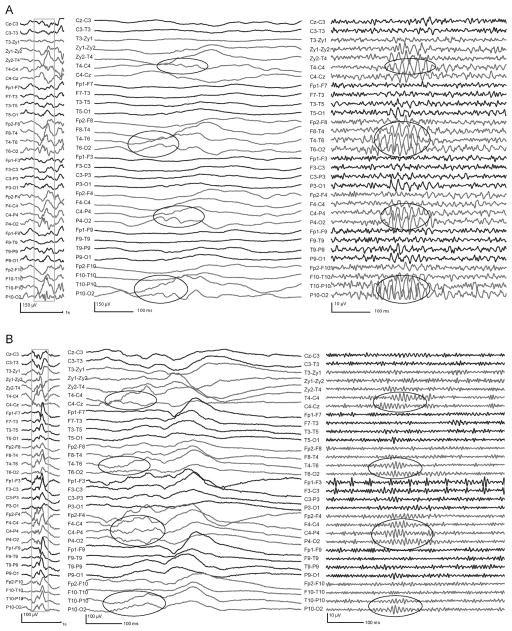
Figure 1.
Examples of gamma (A) and ripples (B), co-occurring with IEDs. In both cases, the left part shows the unfiltered EEG (LF: 0.3 Hz, HF: 70 Hz); the outlined sections are shown in the middle part unfiltered with expanded time (264 mm/s), and in the right part expanded in time and high-pass filtered at 40 Hz (A) and 80 Hz (B). Note that the fast oscillations can already be noticed before filtering, in the middle expanded section, over the ascending slope of the IEDs (highlighted in the ellipses).
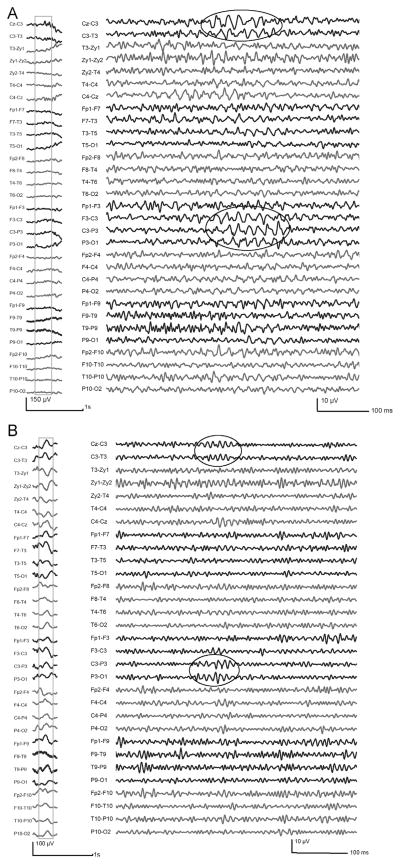
Figure 2.
Examples of gamma (A) and ripples (B), independent from IEDs. The unfiltered EEG (LF: 0.3 Hz, HF: 70 Hz) is shown on the left; the outlined sections are shown in the right part expanded in time (264 mm/s) and high-pass filtered at 40 Hz (A) and 80 Hz (B). The fast oscillations are highlighted in the ellipses.
Artifact identification
Careful attention was paid to reject any possible artifact during the process of marking, mostly due to the partial overlap of the frequency band between muscle activity and fast oscillations. The EMG bursts, after high pass filtering, had peculiar features distinguishing them from the fast oscillations: irregular morphology, very high amplitude compared to background, and great variations in amplitude and frequency inside the burst (Fig. 3A). The question of separating cerebral activity from EMG artifact represents a technical challenge, as already pointed in previous studies (Otsubo et al., 2008; Andrade-Valenca et al., 2011; Zijlmans et al., 2012).
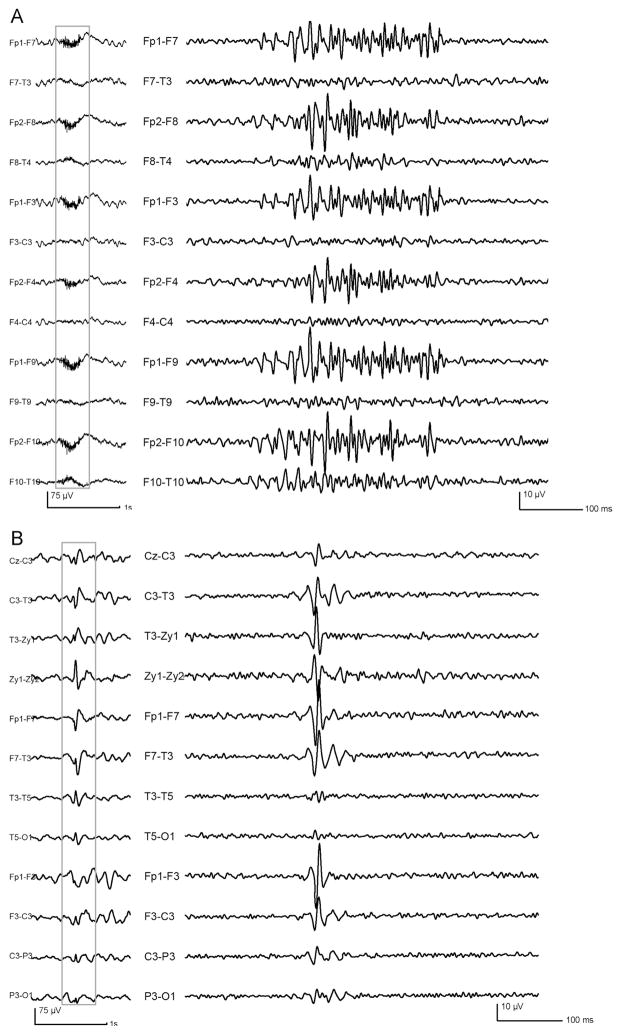
Figure 3.
Examples of possible artifact sources. (A) EMG bursts, presenting with irregular morphology and variations in amplitude and frequency inside the burst. (B) Oscillations resulting from spike filtering: note that, compared to the genuine fast oscillations, they are brief in duration, with a single sharp central component with higher amplitude. Left part: unfiltered EEG (LF: 0.3 Hz, HF:70 Hz); right part: outlined sections, expanded in time and high-pass filtered at 40 Hz.
Another possible artifact source was the oscillations resulting from the process of epileptic spike filtering. The shape was more regular in frequency than muscle activity and resembled the morphology of the non-EMG oscillations. However, the artifact due to the filtering was usually brief, with only two-three consecutive oscillations, and a high amplitude sharp central component preceded and followed by smaller oscillations (Fig. 3B). These features allowed to identify and to reject those artifacts during the marking process.
The EEGs were reviewed a second time after marking all the events, with standard display parameters, to remove all marks corresponding to oscillations associated with artifacts (muscle activity and patient movements) as defined in the standard EEG.
Statistical analysis
After marking all events, a MATLAB program (The Math-works Inc., Natick, Massachusetts, USA) calculated in the 30 min segment for each channel the total number of events and rates (events/min) of IED, gamma and ripple. The co-occurrence between IEDs and fast oscillations was defined as an occurrence of gamma or ripple marker within 50 ms before or after an IED marker. The rates of occurrence were reported as means with standard deviation. The Kruskal–Wallis one-way ANOVA was used to compare the IED/gamma/ripple median rates in the four patient groups, and the Mann–Whitney U analysis was used to compare the rates of fast oscillations between the channels inside/outside the irritative zone and the SOZ. We calculated the sensitivity, specificity and accuracy of IEDs, gamma and ripples to identify the SOZ. Sensitivity was defined as [SOZ channels with fast oscillations/(SOZ channels with fast oscillations + SOZ channels without fast oscillations)] × 100; specificity was [non-SOZ channels without fast oscillations/(non-SOZ channels without fast oscillations + non-SOZ channels with fast oscillations)] × 100; accuracy was [(SOZ channels with fast oscillations + non-SOZ channels without fast oscillations)/total channels] × 100. The level of significance was set at 0.05.
Results
Patient and EEG channel distribution
We studied 32 patients with focal epilepsy (18 males, mean age at evaluation: 40 ± 13 years; mean age at seizure onset: 18 ± 15 years; and mean duration of epilepsy: 21 ± 14 years), and analyzed 984 channels (31 patients with 31 channels, and one with 23 channels). Forty-four channels were excluded due to artifacts (8 channels in 3 patients of group A; 11 channels in 3 patients of group B; 21 channels in 4 patients of group C; 4 channels in one patient of group D); the excluded channels were equally distributed over the zygomatic, frontopolar, parietal and temporal regions. Brain MRI was normal in 14 patients, and the most common pathological finding consisted of hippocampal atrophy or mesial temporal sclerosis (other significant electro-clinical characteristics are reported in Table 1). The distribution among the four categories was as follows: group A (high IED rate): 12 patients (364 channels); group B (mid-IED rate): 8 patients (248 channels); group C (low IED rate): 7 patients (217 channels); and group D (no IEDs): 5 patients (155 channels).
Table 1.
Electroclinical data of all 32 patients.
| Patient # | Gender/age (years) | Group | Irritative zone | Seizure onset zone | Seizure semiology | MRI |
|---|---|---|---|---|---|---|
| 1 | M/42 | A | Bifrontal R > L (Fp2-F10, Fp2-F4; Fp1-F7) | Bifrontal changes, no localizing features | Asymmetric tonic posturing, secondary generalization | Mild L Hc atrophy |
| 2 | M/24 | A | R anterior sylvian (F8-T4) | R FCT (Zy2-T4/T4-C4/P4-O2/T4-T6/F4-C4) | Epigastric aura, secondary generalization | Agenesia of CC, R schizencephaly, R F PMG, R NH |
| 3 | M/36 | A | L anterior sylvian (T3-Zy1, F9-T9) | L FT (F7-T3/Fp1-F3/Fp1-F9/F9-T9) | Hypermotor | Normal |
| 4 | F/26 | A | Bifrontal (Fp2-F10, Fp2-F4; Fp1-F3) | Bifrontal changes, no localizing features | Focal motor, secondary generalization | Normal |
| 5 | F/56 | A | L anterior sylvian (Zy1, F7, F9-T9, T3) | L hemispheric (Fp1-F9/F9-T9/T9-P9/P9-O1/T3-T5/F7-T3/T3-Zy1) | LOC, hand automatisms, secondary generalization | L MTS |
| 6 | F/23 | A | R posterior T (T10-P10) | R TPO (F8-T4/T4-T6/T10-P10/P10-O2) | Head turning, LOC | Normal |
| 7 | M/57 | A | L FT anterior sylvian (F9, F3-C3, T3-Zy1, F7-T3) | Muscle artifacts, no localizing features | Asymmetric tonic posturing | Porencephalic cyst L TO |
| 8 | F/29 | A | Bitemporal R > L (Zy2-T4, T4-T6; T3-T5) | 1) R FT (Fp2-F8/F8-T4/Zy2-T4); 2) L FT (Fp1-F7/F7-T3/T3-Zy1) | Hypertonic | Normal |
| 9 | F/42 | A | L CT (C3-P3-T5) | L CTP (C3-P3/P3-O1/T5-O1/T3-T5) | Asymmetric tonic posturing, secondary generalization | Normal |
| 10 | M/29 | A | L F (Fp1-F3) | Bifrontal changes, no localizing features | Hypertonic | L F deep gyrus FCD between F1 and F2 (partly resected in 2009) |
| 11 | M/26 | A | L PO (P5-O1) | L TPO (T3-T5/T5-O1/C3-P3/P9-O1/P3-O1) | Focal motor, secondary generalization | Normal |
| 12 | F/47 | A | Bitemporal L > R (F8-T4; F7-T3, F9-T9) | Bitemporal changes, no localizing features | Generalized tonic-clonic | Normal |
| 13 | M/53 | B | Bitemporal (T3-Zy1, F7-T3; F8-T4, T4-T6) | 1) R FCT (Zy2-T4/T4-C4/Fp2-F10/F2-F8/T4-T6/F8-T4); 2) L FT (Fp1-F7/F7-T3) | Head turning, oral automatisms | Bilateral MTS |
| 14 | F/27 | B | Bitemporal R > L (Zy2-T4, F10-T10, T4-T6; F9-T9) | 1) R T (Zy2-T4/F10-T10); 2) L T (F7-T3/F9-T9) | Epigastric aura, LOC | R MTS |
| 15 | M/53 | B | Bitemporal (T3-Zy1, F7-T3; Zy2-T4, F10-T10) | R FT (T10-P10/T4-C4 Zy2-T4/Fp2-F10/F10-T10/F8-T4) | LOC, dystonic posturing, head turning | R MTS |
| 16 | M/41 | B | Bitemporal (T4, T10; F7, F9-T9) | Muscle artifacts, no localizing features | LOC, hand automatisms | Bilateral P-O encephalomalacia |
| 17 | M/22 | B | L anterior T (T3, F7-T3, F9-T9) | L FCT (C3-T3/T3-Zy1/F7-T3/T3-T5/Fp1-F9/F9-T9) | LOC, hand automatisms, dystonic posture | Normal |
| 18 | M/57 | B | R anterior T (T4-Zy2, F8-T4, F10-T10) | R T (T4-Zy2/F8-T4/F10-T10) | Epigastric aura, hand automatisms, dystonic posture | R MTS |
| 19 | F/27 | B | L T-P (T3-T5, P3, P9) | L TPO (F7-T3/T3-T5/P3-O1) | Autonomic signs, LOC, visual hallucinations | L MTS |
| 20 | M/53 | B | Bitemporal L > R (T3-Zy1, T9-P9, F9-T9, F7-T3; Zy2-T4, F8-T4) | no seizures recorded | LOC, hand/oral automatisms | Normal |
| 21 | M/40 | C | R CP (C4-P4) | R FCP (F4-C4/C4-P4) | Hemiclonic, secondary generalization | Suspected for R F FCD |
| 22 | M/66 | C | L anterior sylvian (T3, T3-Zy1, F7-T3, F9-T9) | L T (T3-Zg1/F7-T3) | Oral automatisms, head deviation | L Hc atrophy |
| 23 | F/40 | C | L anterior sylvian (F7-T3, T3-T5) | no seizures recorded | Eye deviation, secondary generalization | Normal |
| 24 | F/55 | C | Bitemporal (T3-T5; Zy2-T4, T4-T6, F8-T4) | 1) R TPO (Zy2-T4/T4-C4/T6-O2/T10-P10); 2) L TPO (F7-T3/T9-P9/P9-O1) | Oral automatisms, hypertonic posture | Previous L SeAH (1994), no other changes |
| 25 | F/34 | C | Bitemporal R > L (Zy2-T4, F8-T4, T4-T6; T9-P9, F9-T9) | 1) L FT (Fp1-F7/F7-T3/T3-T5/T5-O1/Fp1-F9/T9-P9); 2) RT (F8-T4/T4-T6) | LOC, oral automatisms, head deviation | L Hc atrophy |
| 26 | F/43 | C | L anterior sylvian (Zy1, F7, T3) | Muscle artifacts, no localizing features | Sensory aura, oral automatisms, secondary generalization | L Hc atrophy |
| 27 | M/22 | C | L F (F3, Fp1) | L FCP (F3-C3/C3-P3) | LOC, fencing posture | Normal |
| 28 | F/23 | D | No spike | Muscle artifacts, no localizing features | Epigastric aura, LOC | Previous R SeAH, L Hc atrophy |
| 29 | M/35 | D | No spike | Muscle artifacts, no localizing features | Epigastric aura, LOC, oral/hand automatisms | R T and inferior F malacia and R MTS |
| 30 | F/68 | D | No spike | no seizures recorded | Olfactory aura, oral automatisms | Normal |
| 31 | M/50 | D | No spike | Muscle artifacts, no localizing features | Hypermotor | Normal |
| 32 | M/36 | D | No spike | Bifrontal changes, no localizing features | Hypermotor | Normal |
C, central; CC, corpus callosum; F, frontal; FCD, focal cortical dysplasia; Hc, hippocampus; L, left; LOC, loss of consciousness; MTS, mesial temporal sclerosis; NH, nodular heterotopia; O, occipital; P, parietal; PMG, polymicrogyria; R, right; SeAH, selective amygdalohippocampectomy; T, temporal.
Fast oscillation and IED rates
For the overall population, the IEDs were the most common events marked, followed by gamma activity and then by ripples (number of events reported in Table 2). The rates of occurrence in the channels where the events were recorded were as follows (mean ± SD): IEDs (0.72 ± 1.31), gamma (0.25 ± 0.58), and ripples (0.12 ± 0.21). Performing a separate analysis for each group, the fast oscillations were recorded in all patients of groups A, B and C and only in one out of five patients of group D (Table 2); gamma and ripples were found in all regions (frontal, parietal, central, temporal, occipital), with no preferential regional distribution. The rates of the fast oscillations reflected the IED frequency in each group, with group A showing the highest frequency values (gamma rate: 0.37 ± 0.73 per min; ripple rate: 0.17 ± 0.26 per min), followed by group B (gamma: 0.08 ± 0.06; ripple: 0.07 ± 0.05), group C (gamma: 0.06 ± 0.06; ripple: 0.04 ± 0.01), and then by group D with very low values (gamma: 0.03 ± 0; ripple: 0.03 ± 0). The fast oscillation rates were statistically different between all four groups (gamma: p < 0.001; ripples: p < 0.001, Kruskal–Wallis ANOVA) (Fig. 4). IEDs and fast oscillations occurred most frequently independently of each other: the IEDs co-occurred with gamma and ripples in 9.5% and 3.2% of cases, respectively; the co-occurrence with IEDs was 46.2% for gamma and 44.4% for ripples.
Table 2.
Number of event marked.
| Spikes | Gamma | Ripples | |
|---|---|---|---|
| (A) Overall population | 10103 | 2098 | 731 |
| (B) Different groups | |||
| Group A (12/12 pts) | 9370 | 1882 | 588 |
| Group B (8/8 pts) | 551 | 146 | 116 |
| Group C (7/7 pts) | 181 | 69 | 23 |
| Group D (1/5 pt) | 0 | 1 | 4 |
Figure 4.
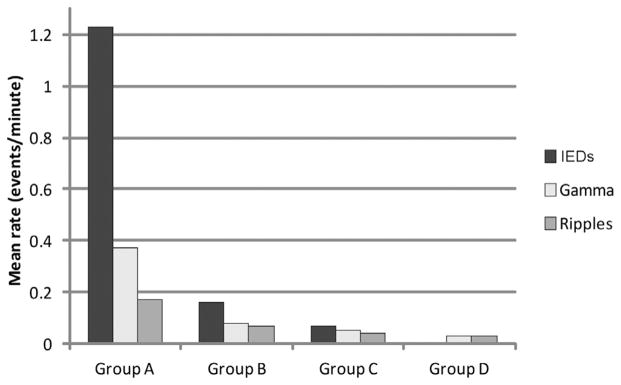
Graphs showing the rates of IEDs, gamma and ripples in the four groups separately. Group A, with frequent IEDs (>1/min), also shows higher rates of fast oscillations (p < 0.001). Mean rates across channels are reported.
Correlations between fast oscillations and the irritative zone
We studied 27 patients from groups A, B and C; group D was excluded from this analysis because, by definition, no irritative zone in this group could be identified. A total of 829 channels were analyzed, 453 belonging to the irritative zone. Considering the overall population, the fast oscillations had much higher rates in the irritative zone (gamma: 0.14 ± 0.48; ripple: 0.04 ± 0.15) compared to the non-spiking channels (gamma: 0.01 ± 0.04; ripple: 0.01 ± 0.03) (p < 0.001, Mann–Whitney U analysis) (Fig. 5A). Performing the same analysis for each group, the rates (mean ± SD) in the irritative zone versus non spiking channels were as follows: for group A, gamma (0.24 ± 0.62 vs 0.02 ± 0.06; p < 0.001) and ripples (0.07 ± 0.20 vs 0.02 ± 0.04; p < 0.01); for group B, gamma (0.03 ± 0.06 vs 0.01 ± 0.03; p < 0.001) and ripples (0.02 ± 0.04 vs 0.01 ± 0.04; p < 0.05); and for group C, gamma (0.02 ± 0.06 vs 0.004 ± 0.01; p < 0.001) and ripples (0.006 ± 0.01 vs 0.002 ± 0.01; p = 0.11). Therefore, except for ripples in group C, the fast oscillations in the other two groups were statistically more frequent in the irritative zone.
Figure 5.
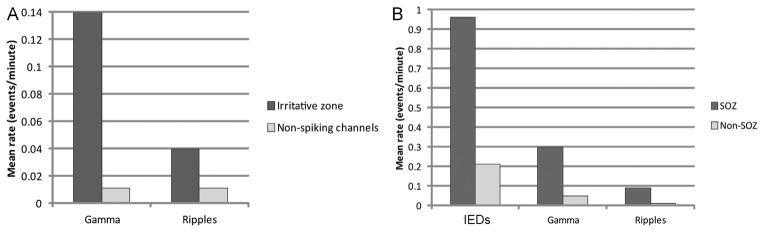
Graphs showing in the overall population higher rates of events in the channels inside the irritative zone (A) and SOZ (B) (p < 0.001). Mean rates across channels are reported.
Correlations between fast oscillations and the seizure onset zone
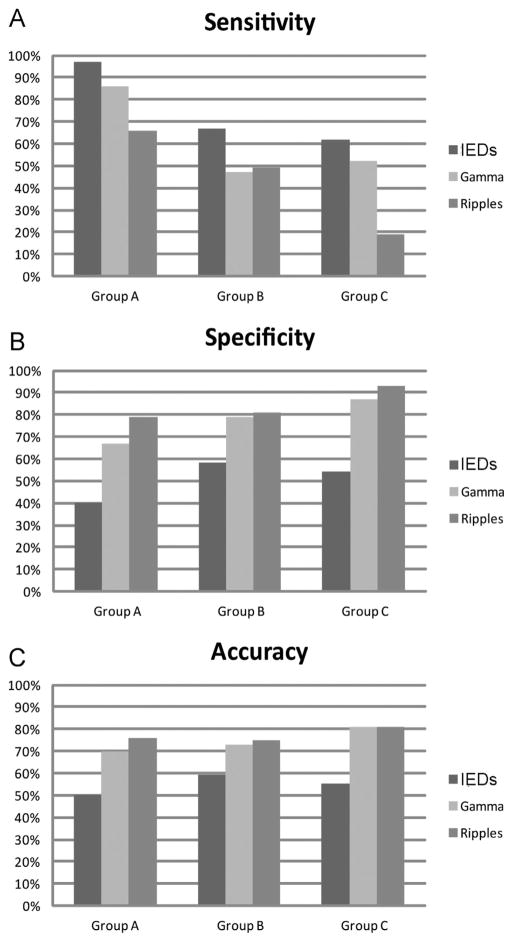
The SOZ was identified in 18 patients (group A, 7 patients; group B, 6 patients; group C, 5 patients; and group D, none). A total of 558 channels were analyzed, 86 were inside the SOZ. The rates, for all the events marked, were much higher in the SOZ channels (spike: 0.96 ± 1.75; gamma: 0.30 ± 0.83; ripple: 0.09 ± 0.27) than in non-SOZ channels (spike: 0.21 ± 0.65; gamma: 0.05 ± 0.25; ripple: 0.02 ± 0.06) (p < 0.001, Mann–Whitney U analysis) (Fig. 5B). The IEDs were more sensitive to identify the SOZ (sensitivity: IEDs 78%, gamma 66%, and ripples 48%), but gamma activity and ripples were more specific (specificity: IEDs 50%, gamma 76%, and ripples 83%) and accurate (accuracy: IEDs 54%, gamma 74%, ripples 77%). This result was confirmed also performing the same analysis specifically evaluating the fast oscillations occurring without spikes: specificity and accuracy were still higher for gamma and ripples outside the spikes than for the IEDs (specificity: IEDs 50%, gamma outside spikes 81%, ripples outside spikes 86%; accuracy: IEDs 54%, gamma outside spikes 76%, ripples outside spikes 79%). The same results were obtained when analyzing separately each group (Fig. 6).
Figure 6.
Values of sensitivity (A), specificity (B) and accuracy (C) to identify the SOZ, calculated separately in group A, B, and C (group D excluded because no patient had a defined SOZ). For each group the IEDs were more sensitive, but gamma and ripples were more specific and accurate.
Discussion
The role for HFOs as potential EEG biomarkers of epileptogenicity has emerged during the last decade from the evaluation of patients with invasive SEEG recordings, and recently, a possible similar role has emerged also for the HFOs recorded form scalp EEG. They have a relationship with the period of active seizure occurrence in idiopathic partial epilepsy (Kobayashi et al., 2011); they are associated with the lesional zone in patients with symptomatic West syndrome (Iwatani et al., 2012); they co-localize with the ictal onset sites in a pediatric cohort of patients (Wu et al., 2008); and they are a marker of the SOZ in adults with focal epilepsy (Andrade-Valenca et al., 2011).
In the present study, we confirmed in a new group of patients compared to Andrade-Valenca et al. (2011) that fast oscillations can be recorded non-invasively using scalp EEG in patients with focal epilepsy. An important and new finding is the demonstration that the occurrence of the fast oscillations directly reflects the degree of epileptic activity of the EEG: patients with high IED frequency also show high rates of gamma and ripples while patients with low IED rates show the lowest fast oscillation rates and patients without IEDs have most often no recordable fast oscillations.
Moreover both gamma activity and ripples are significantly more frequent in the channels belonging to the irritative zone, although about half of the events occurred independently of IEDs. This spatial overlap between the brain areas generating spikes and fast oscillations suggests that the generators involved in those two entities share neuronal networks, but they differ in their spatial extent. Differences in the size of generators may explain the higher rates for IEDs compared to the fast oscillations, the latter being issued from a volume of tissue most often not sufficiently extended to be visible on the scalp. Interestingly, in a previous study on SEEG patients, we found similar results (higher rates of ripples and fast ripples in spiking than in non-spiking channels), suggesting that IEDs and HFOs are partially independent hypersynchronous events (Jacobs et al., 2008). Not only the generator size but also different cellular mechanisms may be involved in the origin of spikes and fast oscillations: IEDs are considered to reflect large excitatory postsynaptic potentials (de Curtis & Avanzini, 2001) from abnormally hypersynchronous neurons; intracranial HFOs most probably reflect synchronized co-firing of small clusters of principal cells, pathologically interconnected within local areas (Bragin et al., 2002). These different mechanisms could involve similar “hyperactive” neuronal networks; studies with simultaneous surface and intracranial EEG recordings should be able to provide answers to these issues.
IEDs and fast oscillations differ in their ability to define the SOZ. In our first study looking at gamma activity and ripples in scalp EEGs (Andrade-Valenca et al., 2011), we demonstrated that spikes are more sensitive, but gamma activity and ripples more specific and accurate to determine the regions of the brain participating in seizure generation. In this study similar results confirmed these original findings, but this time across the spectrum of different fast oscillation rates: patients with high (group A), as well as with intermediate and low gamma and ripples frequency (group B and C), showed lower fast oscillation sensitivity but higher specificity and accuracy, compared to IEDs, thus indicating again a strong correlation between fast oscillations and the SOZ. These results were confirmed also performing a separate analysis for the gamma activity and ripples outside the spikes, showing that they were not potentially biased by the co-occurrence of IEDs.
Similar findings could not be replicated for group D, as none of the patients had a clear SOZ, due to the fact that no seizures were recorded or to the EEGs having no localizing features (sudden bilateral changes or muscle artifacts covering the traces at seizure onset). Moreover, only one of the five patients showed gamma activity and ripples, with a number of events that was too low for statistical analysis. Future studies specifically addressing large populations of patients with no IEDs on scalp EEG could add clinical knowledge on the behavior of gamma activity and ripples in this group, clarifying their potential role in patients with no irritative zone.
The detection of fast oscillations with scalp EEG carries multiple technical challenges due to many sources of artifact. A major problem is the muscle activity recorded at frequencies that overlap with the HFO frequency band. In order to identify the morphologic characteristics differentiating EMG bursts from genuine oscillations, the visual marking of the scalp EEGs requires even more careful evaluation than for invasive records. Selecting the EEG segments during slow-wave sleep reduced the occurrence of muscle artifacts. Moreover, we routinely reviewed a second time the traces and removed all the oscillations associated with clear artifacts as identified in the standard recording. Another source of artifacts is the process of spike filtering. Sharp transients can be constituted of a broad frequency spectrum, and when band pass-filtered result in a short-duration oscillation close to the impulse response of the filter leading to “false” fast oscillations (Bénar et al., 2010). This effect can be reduced but not completely eliminated using FIR filters. In addition, we required a minimum number of cycles for the “genuine” fast oscillations (Jacobs et al., 2009; Urrestarazu et al., 2007). Considering all the above-mentioned technical precautions, we were quite confident that our results were not influenced by artifacts.
Conclusions
This study confirms that fast oscillations can be recorded with scalp EEG, routinely performed in epileptic patients. In contrast to intracranial EEG recordings, more technical awareness is required due to several artifact sources. High rates of gamma activity and ripples are more likely to be found in patients with frequent IEDs, indicating that it is better to focus on such patients when performing studies on fast oscillations. In patients without IEDs, the likelihood of recording gamma activity and ripples is very low. Both fast oscillations, even at low rates, are more specific and accurate to identify the SOZ compared to IEDs.
This suggests a possible future role for the scalp-recorded fast oscillations in clinical practice as a diagnostic tool to delineate the epileptogenic areas, of potential use in presurgical evaluation as an additional method to guide and better define the area to remove.
Acknowledgments
This work was supported by grant MOP-102710 of the Canadian Institutes of Health Research and Preston Robb fellowship.
Abbreviations
- HFOs
high frequency oscillations
- IEDs
interictal discharges
- SOZ
seizure onset zone
Biographies
Federico Melani is a neurologist at the Pediatric Neurology Unit of the Children’s Hospital A. Meyer. His expertise includes electromyography, evoked potentials and intraoperative neurophysiological monitoring. He has been interested in management of patients with drug-resistance epilepsy including presurgical evaluation, invasive EEG techniques (subdural and depth electrodes), postsurgical management. He spent part of his training at the EEG department of the Montreal Neurological Institute, under the supervision of prof. Gotman, where he studied the role of High Frequency Oscillations as a biomarker of epileptogenicity, and published papers about this topic.
Rina Zelmann received her degree in Electronics Engineering from University of Buenos Aires (Argentina) in 2002 and her Masters in Engineering (Biomedical) from McGill University in 2007. She has graduated as PhD at McGill University (Biomedical Engineering). Her current research focuses on the characterization and automatic detection of high-frequency oscillations (80–500 Hz) in intracranial recordings in humans with intractable epilepsy; she published various papers about this topics.
François Dubeau is a neurologist and Head of the EEG laboratory and Epilepsy Monitoring Unit at the Montreal Neurological Hospital. He is Associate Professor of clinical neurology in the department of Neurology and Neurosurgery at McGill University. He is involved in the evaluation and management of patients with difficult-to-treat epilepsy disorders, including invasive intracranial EEG monitoring for patients with refractory focal epilepsies. He collaborates to clinical research centered on epileptic discharges related-EEG/fMRI responses and on the role of High Frequency Oscillations as a biomarker of epileptic activity. He is the author or co-author of 203 peer-reviewed papers.
Jean Gotman is Professor in the departments of Neurology and Biomedical Engineering at McGill University and carries out his research at the Montreal Neurological Institute. He has been interested in elecrophysiological and functional imaging aspects of epilepsy. His recent interests have centered on the combination of EEG and fMRI to study the source of epileptic discharges and on the role of High Frequency Oscillations as a biomarker of epileptic activity. He has published over 200 peer-reviewed papers.
Footnotes
Conflicts of interests
None of the authors has any conflict of interest to disclose.
References
- Andrade-Valenca LP, Dubeau F, Mari F, Zelmann R, Gotman J. Interictal scalp fast oscillations as a marker of the seizure onset zone. Neurology. 2011;77:524–531. doi: 10.1212/WNL.0b013e318228bee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar CG, Chauvière L, Bartolomei F, Wendling F. Pitfalls of high-pass filtering for detecting epileptic oscillations: a technical note on “false” ripples. Clin Neurophysiol. 2010;12:301–310. doi: 10.1016/j.clinph.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999a;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Vizentin E, Mathern GW. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal KA injection. Epilepsia. 1999b;40:1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrian GE, Bergamini L, Dondey M, Klass DW, Lennox-Buchthal M, Petersén I. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr Clin Neurophysiol. 1974;37:538–548. doi: 10.1016/0013-4694(74)90099-6. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Iwatani Y, Kagitani-Shimono K, Tominaga K, Okinaga T, Kishima H, Kato A, Nagai T, Ozono K. Ictal high-frequency oscillations on scalp EEG recordings in symptomatic West syndrome. Epilepsy Res. 2012;102:60–70. doi: 10.1016/j.eplepsyres.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Châtillon CE, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Oka M, Akiyama T, Inoue T, Abiru K, Ogino T, Yoshinaga H, Ohtsuka Y, Oka E. Very fast rhythmic activity on scalp EEG associated with epileptic spasms. Epilepsia. 2004;45:488–496. doi: 10.1111/j.0013-9580.2004.45703.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Inoue T, Watanabe Y, Oka M, Endoh F, Yoshinaga H, Ohtsuka Y. Spectral analysis of EEG gamma rhythms associated with tonic seizures in Lennox–Gastaut syndrome. Epilepsy Res. 2009;86:15–22. doi: 10.1016/j.eplepsyres.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Watanabe Y, Inoue T, Oka M, Yoshinaga H, Ohtsuka Y. Scalp-recorded high-frequency oscillations in childhood sleep-induced electrical status epilepticus. Epilepsia. 2010;51:2190–2194. doi: 10.1111/j.1528-1167.2010.02565.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Yoshinaga H, Toda Y, Inoue T, Oka M, Ohtsuka Y. High-frequency oscillations in idiopathic partial epilepsy of childhood. Epilepsia. 2011;52:1812–1819. doi: 10.1111/j.1528-1167.2011.03169.x. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Ochi A, Imai K, Akiyama T, Fujimoto A, Go C, Dirks P, Donner EJ. High-frequency oscillations of ictal muscle activity and epileptogenic discharges on intracranial EEG in a temporal lobe epilepsy patient. Clin Neurophysiol. 2008;119:862–868. doi: 10.1016/j.clinph.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, Ogren J, Fried I, Wilson CL, Engel J., Jr Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, Meyer FB, Marsh R, Litt B. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Koh S, Sankar R, Mathern GW. Paroxysmal fast activity: an interictal scalp EEG marker of epileptogenesis in children. Epilepsy Res. 2008;82:99–106. doi: 10.1016/j.eplepsyres.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jiruska P, Zelmann R, Leijten FS, Jefferys JG, Gotman J. High-frequency oscillations as a new biomarker in epilepsy. Ann Neurol. 2012;71:169–178. doi: 10.1002/ana.22548. [DOI] [PMC free article] [PubMed] [Google Scholar]