Abstract
In EEG–fMRI studies, BOLD responses related to interictal epileptic discharges (IEDs) are most often the expected positive response (activation) but sometimes a surprising negative response (deactivation). The significance of deactivation in the region of IED generation is uncertain. The aim of this study was to determine if BOLD deactivation was caused by specific IED characteristics. Among focal epilepsy patients who underwent 3T EEG–fMRI from 2006 to 2011, those with negative BOLD having a maximum t-value in the IED generating region were selected. As controls, subjects with maximum activation in the IED generating region were selected. We established the relationship between the type of response (activation/deactivation) and (1) presence of slow wave in the IEDs, (2) lobe of epileptic focus, (3) occurrence as isolated events or bursts, (4) spatial extent of the EEG discharge. Fifteen patients with deactivation and 15 with activation were included. The IEDs were accompanied by a slow wave in 87 % of patients whose primary BOLD was a deactivation and only in 33 % of patients with activation. In the deactivation group, the epileptic focus was more frequently in the posterior quadrant and involved larger cortical areas, whereas in the activation group it was more frequently temporal. IEDs were more frequently of long duration in the deactivation group. The main factor responsible for focal deactivations is the presence of a slow wave, which is the likely electrographic correlate of prolonged inhibition. This adds a link to the relationship between electrophysiological and BOLD activities.
Keywords: EEG–fMRI deactivation, Focal epilepsy, Interictal epileptic discharge, Negative BOLD
Introduction
Functional magnetic resonance imaging (fMRI) uses the blood oxygen level dependent (BOLD) effect to map the neuronal activity linked to a particular event (Ogawa et al. 1992). Simultaneous electroencephalogram (EEG) and fMRI, EEG–fMRI, is a non-invasive technique that detects hemodynamic changes in the brain related to interictal epileptic discharges (IEDs) identified on scalp EEG. Combining the high temporal resolution of EEG signal with the high spatial resolution of BOLD images, EEG–fMRI has been shown to be useful to characterize various forms of focal and generalized epileptic discharges (Laufs and Duncan 2007). IED-related BOLD response usually increases in regions generating focal IEDs, but often in the context of more widespread, or even distant, responses (Fahoum et al. 2012; Thornton et al. 2011). These findings have brought to the concept of networks associated with epileptiform discharges (Gotman 2008).
IEDs related BOLD changes can be positive or negative with respect to the baseline; these two types of response are called respectively activation and deactivation. Activation has been assumed to reflect increased neuronal activity and increased synaptic activity (Attwell and Laughlin 2001), but the neurophysiologic basis of deactivation is more difficult to explain in the context of presumed increased neuronal activity (Shmuel et al. 2002, 2006). Deactivations have been reported in areas directly or indirectly involved in sensory and cognitive tasks (Hamzei et al. 2002; Czisch et al. 2004; Born et al. 2002). In epilepsy, deactivation in EEG–fMRI field was first described in patients with bursts of generalized discharges, but it has been also observed in patients with focal epilepsy and focal EEG discharges (Salek-Haddadi et al. 2006; Kobayashi et al. 2006; Jacobs et al. 2009). In some patients, this decrease is located in the presumed region of spike generation and appears to have the same localization value as the increase (Gotman and Pittau 2011).
In contrast, deactivations in the regions of default mode network were first found in generalized discharges, but then also in focal discharges (Aghakhani et al. 2004; Hamandi et al. 2006; Fahoum et al. 2012) and believed to be the result of a suspension of the baseline state of attention (Raichle et al. 2001; Gotman et al. 2005; Laufs and Duncan 2007) and do not have localization value with respect to the generator of epileptic activity.
Different mechanisms have been proposed to explain negative BOLD responses: (i) a vascular effect, with a reduction of cerebral blood flow (CBF) in the deactivated areas secondary to the increased CBF in activated regions (“steal” phenomenon) (Harel et al. 2002); (ii) an abnormal coupling between neuronal activity and regional cerebral blood flow (Suh et al. 2006); (iii) decreases in neuronal activity (Shmuel et al. 2006). Jacobs et al. (2009) have shown a preceding activation in all three of their cases that had a focal deactivation in the spike field, suggesting that the negative response may represent the undershoot of an earlier positive response occurring before the spike. These findings were confirmed but in only a minority of cases with deactivation (Rathakrishnan et al. 2010). The origin of negative BOLD changes in the focus as a result of an epileptic event therefore still remains unexplained in most of the patients in whom it occurs.
The aim of this study was to better characterize this phenomenon, investigating the correlation between BOLD deactivation and characteristics of the IEDs.
Materials and Methods
Subjects
From the pool of 184 patients with focal epilepsy who underwent EEG–fMRI (3T) from April 2006 to May 2011, we retrospectively selected from our database all patients who had a focal negative BOLD response within the spike field (remote negative responses were not investigated); the peak t-value of this deactivation had to be the maximum absolute t-value for the patient, or be at least of the same order of magnitude as the maximum positive t-value. Patients with primarily generalized discharge were excluded. If a patient had multiple types of IEDs (marked as different events), we considered only the study with the highest t value. For that study, we analyzed and described (Table 1) all the activation and deactivation responses (included those remote to the spike field). Although the t value of BOLD response may depend on different variables, including statistical method, we chose this parameter because we assume that it represents the maximal metabolic activity to the event of interest. In order to select only patients with BOLD responses with a localizing value, we excluded patients for whom the max t value corresponded to brain regions belonging to the default mode network. Among the selected patients, some could also have smaller negative responses in default mode regions.
Table 1.
Characteristics of the marked IEDs and corresponding EEG–fMRI results
| Patients | EEG–fMRI IED types (number) | Single/ burst | Average and max duration of the events (s) | Slow wave presence | Focal/ diffuse | Maximum IED distribution | fMRI max deactivation (max t-value) | fMRI max activation (max t-value) |
|---|---|---|---|---|---|---|---|---|
| Deactivation | ||||||||
| 1 | Runs of spike and wave complexes in the BiO regions, max at O2 (160) | Burst | 2.3; 7.5 | Yes | Diffuse | O2 > O1 | R (−20) > L occipital | Bil T (+10) |
| 2 | Rhythmic slow waves over the R hemisphere max at O2–P10–T6 (38) | Burst | 1.5; 6.6 | Yes | Focal | T6–P10–O2 | R occipital (−7.86) and DMN | Bil thal (+6.17) |
| 3 | Burst of bilateral and diffuse spike and slow wave complexes prevalent in the two occipital regions (sometimes more in the R; sometimes in the L) (157) | Burst | 14; 47.2 | Yes | Diffuse | O2–O1 | Bil occipital (−17.9) | Mid cing (+11.8) |
| 4 | Spike and wave L frontocentral (F3–C3) (130) | Single | _ | Yes | Focal | F7–C3 | Post part second F gyrus (−4.43) | L pars triangularis inf F gyrus (+5.27) |
| 5 | Rhythmic sharp slow waves over the L hemisphere predominant over TPO region (24) | Burst | 2.28; 6.4 | Yes | Diffuse | T5–P9–O1 | Post T2 (−8.26) | Cereb (+4.55) |
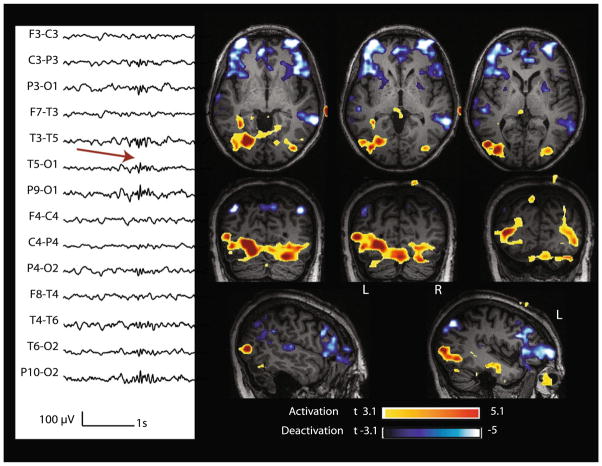
| 6 | 2 Hz runs of spike and waves, diffuse, max at T4–T6 (89) | Burst | 2.5; 4.2 | Yes | Diffuse | T4–T6 | Mid and Post T (−8.25) | Mesial part of lesion (+5) |
| 7 | Burst of diffuse spike and slow wave complexes prevalent in the 2 occipital regions (R > L) (157) | Burst | 6.9; 28.9 | Yes | Diffuse | O2–O1 | R (−17.26) > L occipital | Cing cortex (+11), bil insulae |
| 8 | Rhythmic slow wave with some intermingled spikes max at F7 (42) | Burst | 5.4; 15.15 | Yes | Focal | F7 | L FC (−9) | Small patches (+6.71) |
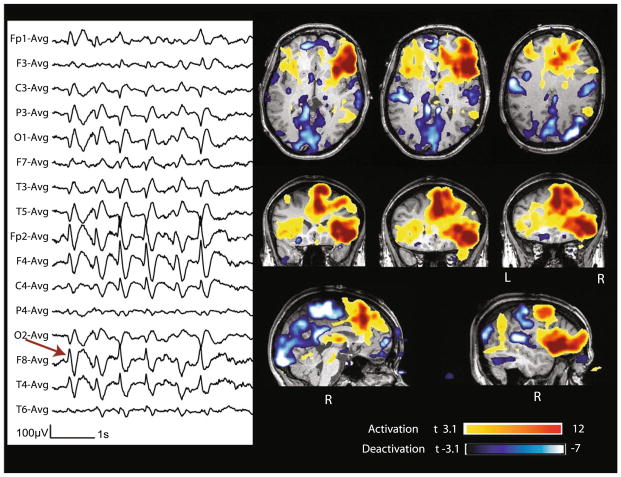
| 9 | Bursts of spike and wave max at P10–T6–O2 (115) | Burst | 3.8; 10.4 | Yes | Diffuse | T6–P10–O2 | R TPO (−6.7) | R mid cing (+6) |
| 10 | Fp2–F4 > Fp1–F3 spike and wave (53) | Single | _ | Yes | Diffuse | Fp2– F4 > Fp1– F3 | R F (−5.64)-bil head of caudate | None |
| 11 | Burst of diffuse spike and waves-more evident in the R O region (24) | Burst | 8.2; 34.1 | Yes | Diffuse | O2 with BDD | R occipital (−22) and DMN | Mid cing (+2) |
| 12 | Spike and wave phase reversal at T3 and equipotential at F9–T9 (12) | Burst | 2.3; 6.1 | Yes | Focal | T3–F9–T9 | L lateral OF (−6.55) | Ventricles (+5.26) |
| 13 | Spike or polyspike and slow wave over F8–T4–F10–T10 (17) | Single | _ | Yes | Focal | F8–T4 | R Hippocampus (−5.4). Midline cereb-pons-bil thal | Bil F (+4) |
| 14 | F4–C4 spike (436) | Single | _ | No | Focal | F4–C4 | Along post part of the lesion (−7.71)-DMN | Along ant part of the lesion (+7.31)-bil thal |
| 15 | Low amplitude spikes at F3–F7 (459) | Single | _ | No | Focal | F3–F7 | L second F gyrus (−4.7)-L > R paramedian first F gyrus | L second F gyrus (+4)-mid L cing gyrus |
| Activation | ||||||||
| 16 | Spike with phase reversal at F8 (Fp2–F8–F10–T10) (31) | Single | _ | No | Focal | Fp2–F8– F10–T10 | None | Lateral R OF (+3.9) |
| 17 | Spike phase reversal at F7–T3 (16) | Single | _ | No | Focal | F7–T3 | 3rd and lateral ventricles | Ant second LF (+5.2)-L thal |
| 18 | Spike with phase reversal at F8 or T4 (3) | Single | _ | No | Focal | F8–T4 | None | R amygdala (+4.71) |
| 19 | Runs of spikes with phase reversal at T5 (4) | Burst | 36.5; 56.2 | No | Focal | T5 | R OF mesial (−8.17) | L supramarginal gyrus (+11.72) |
| 20 | Spike at F8-equipotential at F10– T10 (6) | Single | _ | No | Focal | F10–T10 | Ant R F lobe (−3.7) | Body of the R hippocampus (+6.13) |
| 21 | Spikes C3–P3 (34) | Single | _ | No | Focal | C3–P3 | None | L (+5.1) > R mid postcentral cortex |
| 22 | Spike and slow wave complexes-diffuse-but more prevalent in the R FT region (46) | Burst | 5.5; 61.6 | Yes | Diffuse | F8–T10 with BDD | Paracentral lobules (−11) | Pars triangularis inf F gyrus (+18.7) and cing cortex |
| 23 | Spike and slow waves max at T3– T5 (12) | Burst | 4.8; 35.33 | Yes | Focal | T3–T5 | R angular gyrus (−5.12) | First T gyrus (+5.83) |
| 24 | F8–T4 spike (192) | Single | _ | No | Focal | F8 T4 | Ventricles | R lat OF (+14.8)-ant cing |
| 25 | Spike and slow wave max at F7 (8) | Single | _ | Yes | Focal | F7 | Bil P R (−4) > L | L hipp (+6.42)-ant L T1 |
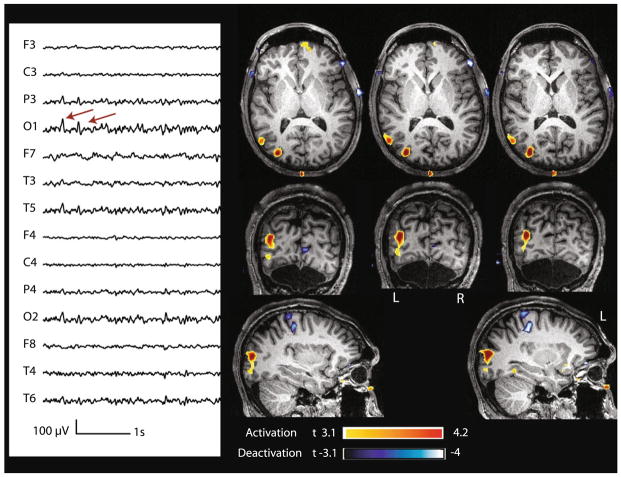
| 26 | Frequent spikes max at P3-O1 (300) | Single | _ | No | Focal | P3–O1 | Small patches | Lateral L O (+5.35) |
| 27 | Sharp and slow wave with phase reversal at T3 (170) | Single | _ | Yes | Focal | T3 | Bil P-bil F (−4.9) | Ant second F gyrus (+7.6)-ant cing-L thal |
| 28 | Bursts of spike and slow waves complexes on the L hemisphere-max at T5(6) | Burst | 0.5; 4.9 | Yes | Diffuse | T5 | Posterior cing (−7) | L ins TPO (+16.8) (heterotopia) |
| 29 | Burst of polyspikes max at T5–P3– O1 (12) | Single | _ | No | Focal | T5–P3–O1 | Ant ins-ant (−6) and post cing-precuneus-bil P | L (+6.7) > R temporo-occipital region |
| 30 | C3–P3–O1–Pz spikes (100) | Single | _ | No | Focal | C3–P3–O1 | R (−4.2) > L pre-central gyri | L P lobule (+6.2) |
Ant anterior, Bil bilateral, C central, Cereb cerebellum, Cing cingulate gyrus, F frontal, IED interictal epileptic discharge, Inf inferior, Ins insula, L left, OF orbito-frontal, P parietal, Post posterior, R right, Sup superior, SW spike and wave complexes, T temporal, Thal thalamus, TPO temporo-parieto-occipital region
As a control group we retrospectively selected from our database (starting from the most recent patient and going backward) patients who underwent 3T EEG–fMRI and had a focal positive BOLD response with a maximum absolute t-value within the spike field. Patients were selected consecutively and recruitment stopped when we reached the same number of patients as in the negative BOLD group. The nature of the experimental procedures was explained and written informed consent in accordance with the Research Ethics Committee of the Montreal Neurological Institute and Hospital was obtained from each subject.
EEG–fMRI Acquisition
EEG was continuously recorded inside a 3T MRI scanner (Siemens, Trio, Germany). No sedation was given. The EEG acquisition was performed with 25 MR compatible electrodes (Ag/AgCl) placed on the scalp using the 10–20 (reference at FCz) and the 10–10 (F9, T9, P9, F10, T10, and P10) placement systems. Two electrodes were placed on the back to record the electrocardiogram. The head of the patient was immobilized with a pillow filled with foam microspheres (Siemens, Germany) to minimize movement artifacts and for patient’s comfort. Data were transmitted from a BrainAmp amplifier (Brain Products, Munich, Germany, 5 kHz sampling rate) to the EEG monitor located outside the scanner room via an optic fiber cable.
A T1-weighted anatomic (MP-RAGE) acquisition was first done (1 mm slice thickness, 256′256 matrix; echo time [TE] = 7.4 ms and repetition time [TR] = 23 ms; flip angle 30°) and used to superimpose the functional images. The functional data were acquired in runs of 6 min each with the patient in the resting state using a T2*-weighted EPI sequence (64′64 matrix; either 25 slices, 5′5′5 mm, TE = 30 ms, TR = 1.7 s, or 33 slices, 3.7′3.7′3.7 mm, TE = 25 ms, TR = 1.9 s; flip angle 90°).
EEG–fMRI Processing
EEG
Brain Vision Analyser software (Brain Products, Munich, Germany) was used for off-line correction of the gradient artifact (Allen et al. 2000). A 50-Hz low-pass filter was also applied to remove the remaining artifact. The ballistocardiogram artifact was removed by independent component analysis (Bénar et al. 2003). A neurologist reviewed the EEG recording and marked the interictal epileptic discharges, according to those observed during clinical monitoring (outside the scanner). In the figures the montage of the EEG is displayed in the most illustrative way, as used in clinic practice (bipolar, referential at FCz or average).
fMRI
The EPI images were motion corrected and smoothed (6 mm full width at half maximum) using the software package from the Brain Imaging Center of the Montreal Neurological Institute (http://www.bic.mni.mcgill.ca/software/). Data were then analyzed as an event-related design using fMRIstat software (Worsley et al. 2002).
The EPI frames were realigned using a linear 6-parameter rigid-body transformation (3 translations and 3 rotations) to correct for movement effects. To account for residual movement artifacts, the 6 parameters used for the realignment were also integrated in the analysis as confound regressors in the general linear model. A regressor for each type of interictal epileptic discharge was built using the timing and duration of each event and convolved with 4 hemodynamic response functions (HFRs) with peaks at 3, 5, 7, and 9 s (Bagshaw et al. 2004). All these regressors were included in the same general linear model. A statistic t map was obtained for each regressor using the other regressors as confounds (a study was performed for each type of interictal event) in the fMRI analysis (fMRI-stat) (Worsley et al. 2002). At each voxel, the maximum t value was taken from the 4 individual t maps created with the 4 HRFs. To be significant, a response needed to have a spatial extent threshold of 5 contiguous voxels having a t-value > 3.1 corresponding to p < 0.05, corrected for multiple comparisons (family wise error rate = FWE) resulting from the number of voxels in the brain and the use of 4 HRFs. The t map results were represented using red–yellow scale corresponding to positive BOLD changes (activation) and blue–white scale to negative BOLD changes (deactivation). Responses outside the brain parenchyma were ignored.
EEG-Correspondence Between EEG and BOLD Localizations
The spike field (the region thought to generate the spike) was estimated at the sub-lobar level by visual inspection of the scalp EEG (for example, F7–T3–T5 spikes generated in the anterior aspect of left temporal lobe, or Fp1–F3–F7 generated in the anterior aspect of left frontal lobe) by two different neurologists. Concordance between each BOLD response (positive and negative) and each type of IED was established if the max t-value corresponded to the localization of the spike field by EEG.
We established the relationship between the type of response (activation or deactivation) and:
IED morphology. The presence of slow wave in the IEDs was considered (spikes or polyspikes vs. spikes or polyspikes and slow wave). A slow wave was defined as a waveform lasting at least 300 ms and having the same polarity as the preceding spike.
IED duration, i.e. single events or bursts. When the events were characterized by bursts, they were marked with “event-start” and “event-end”. For these cases, we reported the average and the maximum burst length (Table 1). The length of the bursts was convolved with the HRF to include length in the analysis. Because of their short duration (<500 ms), bursts of polyspike were considered as single event (examples of burst are shown in the figures).
IED extent. The discharge was considered diffuse when it involved bilateral homologous regions or more than four channels of one hemisphere. If an event started in only one channel but became diffuse in less than 200 ms it was considered diffuse. If an event was bilateral and diffuse but the amplitude of the spike was higher in one or two channels this event was still considered diffuse. If all these conditions were not present, the discharge was considered focal.
The maximum IEDs localization at the lobar level.
Chi Square Test was used to compare deactivation/ activation patterns with the presence of slow wave, duration of events and the extent. The level of significance was set at 0.05.
Results
Subjects
From 184 patients of our database, 15 patients with a deactivation pattern as described above were included (8 %; 9 females). The mean age at seizure onset was 10 years (range 1–21) and the mean age at evaluation was 29 years (range 18–48). Clinical details of patients are reported in Table S1. In seven patients structural MRI was normal or unspecific, in one it showed the surgical bed of anterior callosotomy, in seven it showed a lesion (nodular heterotopia in 1 patient, perysilvian polymicrogyria in 1, focal cortical dysplasia in 2, hemimegalencephaly in 1, mesial temporal lobe sclerosis in 1, an occipital cyst in 1).
Fifteen patients with activation were included as control group (10 females). The mean age at seizure onset was 12.5 years (range 1–25) and the mean age at evaluation was 30 years (range 19–50). In three patients structural MRI was normal or unspecific, in one it showed the surgical bed of left temporal lobectomy, in 11 it showed a lesion (nodular heterotopia in 1 patient, perysilvian polymicrogyria in 1, focal cortical dysplasia in 5, mesial temporal lobe sclerosis in 4).
BOLD Response
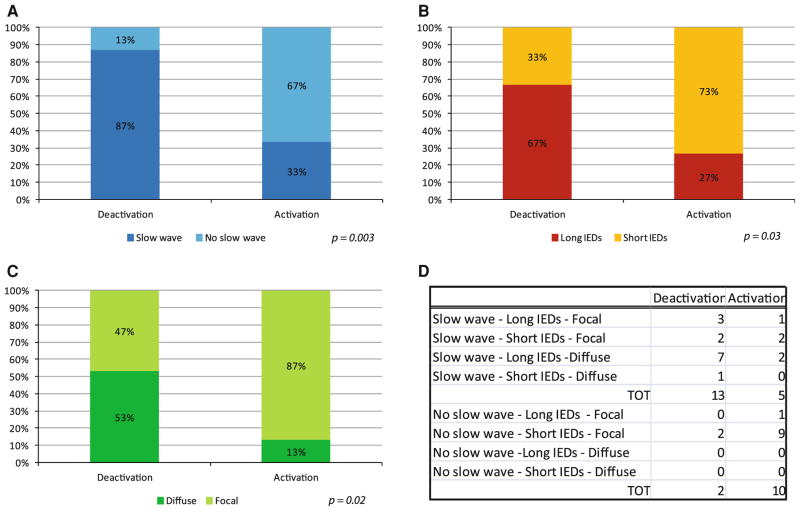
Table 1 and Fig. 1 report the characteristics of the marked IEDs end of the corresponding BOLD responses, and the relationship between the type of response (activation or deactivation) and the studied parameters. In the deactivation group, the number of IEDs recorded during the fMRI session ranged from 12 to 459 (average 127.5, median 89). All patients but one presented also a cluster of activation with a lower t-value remote form the spike field. IEDs were accompanied by a slow wave in 13/15 cases (87 %; Figs. 2, 3, 4). Regarding the localization of the epileptic focus, it was in the occipital or posterior quadrant region in 7/15 patients (47 %) (Figs. 2, 3), frontal in 6 (40 %) and temporal in 2 (13 %). With respect to duration, in 10/15 (67 %), IEDs were characterized by bursts (Fig. 4). Eight of 15 (53 %) patients had diffuse discharges (in 5 the discharges involved bilateral homologous regions, in 3 they were bilateral and diffuse).
Fig. 1.
Deactivation/activation variables versus the three analyzed parameters of IEDs (presence of slow wave, duration, extent). a Deactivation is significantly related with the presence of slow wave in IEDs (p < 0.01), b with IEDs of long duration (p = 0.03) and c diffuse p = 0.02). d In the deactivation group IEDs had more often slow wave, were long and extended (13 deactivation vs. 5 activation), whereas in the activation group, IEDs more often did not have a slow wave, were short and more focal (10 activation vs. 2 deactivation)
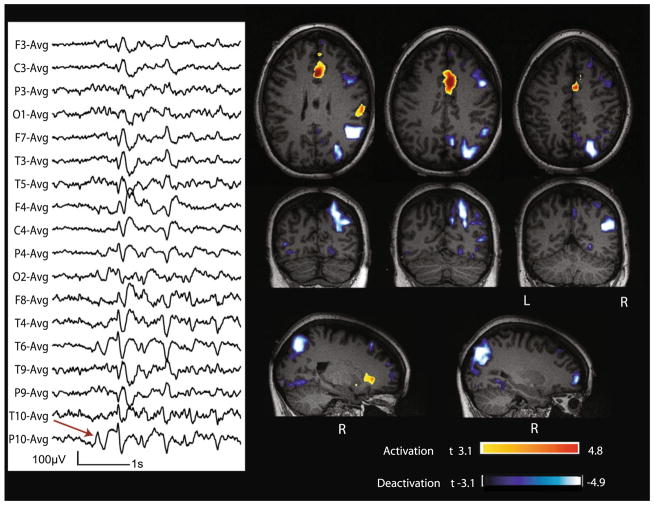
Fig. 2.
Case 9 with non-lesional right posterior quadrant epilepsy. Marked events (red arrow): bursts of diffuse spike and slow wave complexes starting at T6–P10–O2. BOLD response: max is deactivation in the right temporo-pariato-occipital junction; activation in mid cingulate cortex
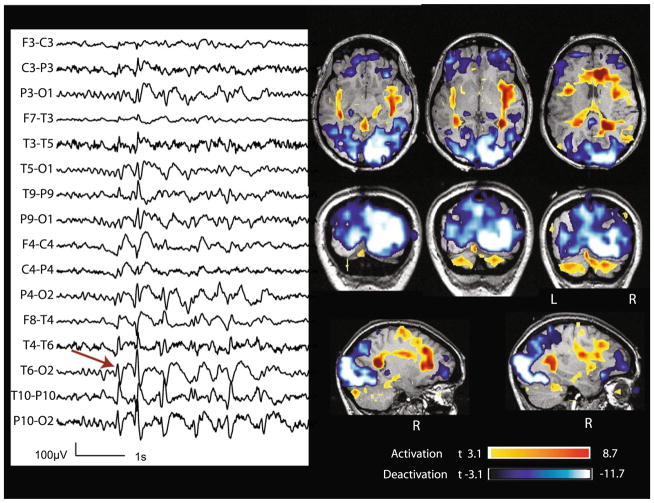
Fig. 3.
Case 7 with non-lesional right occipital epilepsy. Marked events: bursts of spike and slow wave complexes with max amplitude at O2 (red arrow). BOLD response: max is deactivation in the right occipital cortex; activation in the cingulate cortex and bilateral insulae
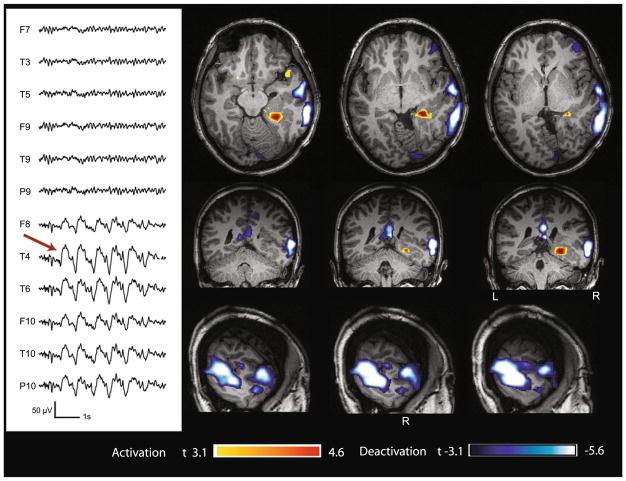
Fig. 4.
Case 6 with lesional right temporal epilepsy (nodular heterotopia). Marked events: bursts of spike and slow wave complexes with max amplitude at T4–T6. BOLD response: max is deactivation in the cortex overlying the lesion (mid and posterior temporal); activation in the mesial part of the lesion
In the activation group, the number of IEDs recorded during the fMRI session ranged from 3 to 300 (average 62.5, median 16). The difference in IED rate was significantly lower than in the deactivation group (p = 0.042; Mann–Whitney U-test). All patients but three presented also a cluster of deactivation with a lower t-value remote form the spike field. IEDs were accompanied by a slow wave in 5/15 cases (33 %, Fig. 5). In 2/15 (13.3 %) the focus was in the posterior quadrant (Figs. 6, 7), frontal in 2 (13.3 %), temporal in 9 (60 %) and parietal in 2 (13.3 %). In 4/15 (27 %), IEDs were characterized by bursts. Two of 15 patients (13 %) had diffuse discharges (both bilateral). The Chi square test indicated that the type of BOLD response (activation/deactivation) was significantly related to the presence of slow wave in IEDs (χ2 (1, N = 30) = 8.89 p < 0.01), to whether IEDs were characterized by bursts or not (χ2 (1, N = 30) = 3.33 p = 0.0281), and to whether the discharge was diffuse or focal (χ2 (1, N = 30) = 5.4 p = 0.020). Deactivation was mainly associated to slow waves, IED bursts, and diffuse discharges.
Fig. 5.
Case 22 with lesional right frontal epilepsy (FCD). Marked events: bursts of spike and slow wave complexes with max amplitude at Fp2–F8. BOLD response: max is activation in the right frontal operculum (lesion) and cingulate cortex. Other clusters of activations and deactivations were present, but not investigated because of the lower t-value and because they were remote from the spike field
Fig. 6.
Case 26 with non-lesional left occipital epilepsy. Marked events: spikes with max amplitude at O1 (montage: ref. at Fz–Cz). BOLD response: max is activation in the left lateral occipital region. Other clusters of deactivations were present, but not investigated because of the lower t-value and because they were remote from the spike field
Fig. 7.
Case 29 with non-lesional left posterior quadrant epilepsy. Marked events: Burst of polyspikes with max amplitude at T5 P3 O1. BOLD response: max is activation in left temporo-occipital region
Discussion
In our population of patients with focal epilepsy with heterogeneous etiology, the incidence of negative BOLD response concordant with the spike field is almost 8 %. This is in agreement with previous EEG–fMRI studies in focal epilepsy (Rathakrishnan et al. 2010; Al-Asmi et al. 2003; Bagshaw et al. 2004; Kobayashi et al. 2006). The incidence is higher in the pediatric population, as it varies from 27.2 to 36 %, (Jacobs et al. 2009, Jacobs et al. 2007). The reason for this higher percentage in children is not clear; a possible role of sedation has been proposed (Jacobs et al. 2007).
Our study suggests that deactivation is significantly related with the presence of a slow wave: indeed, the IEDs were followed by a slow wave in almost all patients whose primary BOLD was a deactivation and only in 33 % of patients with activation. Kobayashi et al. (2006) investigated, in patients with focal or generalized epilepsies, the characteristics of EEG–fMRI studies that produced robust deactivation, not specifically into the spike field, as compared to studies that had only robust activation or both types of responses. They found that spike-and-slow waves were always associated with deactivation, which was not observed with spikes not followed by a slow wave. Our results are in agreement with this study. We defined a slow wave as a waveform lasting at least 300 ms and having the same polarity as the preceding spike (in one patient IEDs consisted only of bursts of slow waves). Experimental studies have shown that slow wave is caused by a robust hyperpolarization of the membrane of the pyramidal cells of the third and fifth cortical layers (Pollen 1964; Neckelmann et al. 2000). There are different opinions about the fact that the hyperpolarization associated with the slow wave is a process that requires energy or not. On one hand it has been demonstrated with multiple intracellular recordings that virtually all neurons (local inhibitory interneurons included) are silent during this phase (Fisher and Prince 1977; Amzica 2009), suggesting that the neuron membrane is in a “refractory mode”. This hypothesis would imply that for this process no energy is required. On the other hand it has been shown that this hyperpolarization is due to the role of GABA inhibitory interneurons. This active process should implicate an increased metabolism, as suggested by Bénar et al. (2006), who, comparing the concordance between EEG–fMRI results and stereotaxic EEG (SEEG) recordings, observed a higher proportion of energy in the low frequencies for the SEEG recorded in regions with fMRI signal increase compared to the regions with fMRI signal decrease. Nevertheless it has been shown that, due to their peculiar branched shape, their fast connections and the position of their synapses along the soma of the neurons, inhibitory interneurons are very efficient in inhibiting a high number of neurons despite a low consumption of energy (Chatton et al. 2003; Koos and Tepper 1999). Therefore, even if the hyperpolarization is an active process, it could cause a decrease in neuronal activity with respect to the baseline. The relationship between the GABAergic system and negative BOLD has been further demonstrated in a spectroscopy study by Northoff et al. (2007), who observed that the concentration of GABA in the anterior cingulate cortex specifically correlates with the amount of negative BOLD responses in that region.
Different studies (Shmuel et al. 2002, 2006), have shown that BOLD deactivations are generally related to decreasing neuronal activity. Shmuel et al. (2006) obtained electrical recordings simultaneously with fMRI in anesthetized monkeys and found negative BOLD response beyond the stimulated regions of visual cerebral area V1. This response was associated with decreases in neuronal activity below spontaneous baseline activity, suggesting that a substantial component of the negative BOLD response can be attributed to decreased neuronal activity. We cannot infer a direct link between stimulus-induced deactivations in healthy animal and events-deactivations in pathological states like epilepsy, as the preservation of the neurovascular coupling in non-healthy states is still uncertain (Laufs 2012).
We did not investigate neuro-vascular coupling in our patients with epilepsy, relying on the assumed coupling between neuronal activity, cerebral blood flow (CBF) and oxygenation. Indeed it has been demonstrated (Stefanovic et al. 2005) that patients with generalized spike and wave do not have, during baseline and during bursts of spike and waves, a significant difference between ΔCBF/ΔBOLD within areas of negative BOLD, suggesting that cerebral energy consumption and neurovascular coupling to BOLD signal are generally maintained between states and in brain regions exhibiting deactivations. Nevertheless, it has been demonstrated in non-epileptic patients (Rother et al. 2002; Hamzei et al. 2003), that extra- or intracranial artery diseases influence cerebrovascular reserve capacity (CVRC) and consequently motor tasks are related to a negative BOLD response, whereas patients with intact CVRC (without artery disease) do not show this phenomenon. These findings suggest that CVCR influences the BOLD signal and that our results may be important for the understanding of neurovascular coupling in epilepsy. It is possible that vascular abnormalities cause epileptic discharges, but we have no direct evidence of this.
In our population, most patients with normal or near-normal MRIs were in the negative BOLD group, whereas patients with lesional epilepsy were more represented in the positive BOLD group, suggesting that the lesions found in our patients do not interfere with neurovascular coupling.
The fact that several patients with negative BOLD had occipital non-lesional epilepsy with secondarily bilateral diffuse spike and waves (thalamic pathway) can find a parallel in the findings of Logothetis (2012). He showed that thalamus (particularly lateral geniculate nucleus, connected monosynaptically to primary visual areas) stimulation inhibits neuronal activity (resulting in negative BOLD) within visual areas and that this inhibition depends on the frequency of stimulation: visual brain regions connected by polysynaptic tracts (like secondary visual areas) are inhibited at low stimulation frequency and are progressively more inhibited by increasing frequency. Visual brain regions connected by monosynaptic tract (like primary visual areas) are inhibited only for low frequency stimulation (<12 Hz) and each stimulus is followed by a refractory period of 300–400 ms, during which the neurons are not excitable. This neuronal inhibition in primary visual cortex is abolished by injection of GABA antagonist, strengthening the concept that the slow wave inhibition period is mediated by GABA. Another finding that corroborates the relationship between slow waves and negative BOLD is the demonstration that spontaneous fluctuations in BOLD signals correlate with locally measured changes in neuronal activity in a frequency-band dependent manner (Naaman et al. 2011). These authors obtained neurophysiological recordings simultaneously with optical imaging of intrinsic signals in anesthetized rats and found a positive correlation with the low (30–50 Hz) and the high-gamma band (50–100 Hz). This positive correlation occurred simultaneously with negative correlation between BOLD signal and the delta (1–4 Hz) theta (5–8 Hz) and alpha (9–14 Hz) bands. These findings suggest that one important factor responsible for focal deactivations is the presence of slow waves, which can be the EEG correlate of prolonged inhibition (Gloor 1978). BOLD positive responses related to slow waves in epileptic patients have been described (Manganotti et al. 2008; Laufs et al. 2006), but it is likely that the mechanisms generating slow waves occurring independently of spikes are different from those generating the slow wave following a spike. Because in our population with deactivation almost all events were characterized by a spike preceding the slow wave, an important question could be: why is the BOLD deactivation not co-localized with an activation? The BOLD response has too slow a temporal resolution to separate the response related to the slow wave from that related to the spike. We record the compound response to both components and in some cases the positive response dominates whereas the negative dominates in others.
In the deactivation group, the epileptic focus was more frequently in the posterior quadrant and involving larger cortical areas, whereas in the activation group it was more frequently temporal and focal. Moreover it was more frequently of long duration in the deactivation group than in the activation group. The distribution of the localization of the focus in the activation group is representative of the distribution of epilepsy in adults (Engel et al. 1997), temporal lobe epilepsy being the most common form of partial epilepsy in adults. Only two patients had an activation in the occipital areas: in the first one the marked events were bursts of focal polyspikes, and in the second focal spikes. In the deactivation group, in all the seven patients with a focus in the posterior quadrant region, the IEDs had slow wave as component of the marked events. Moreover they were very frequently marked as bursts and were diffuse. This could be related to the fact that the classical interictal pattern of the idiopathic, and more rarely cryptogenic, occipital epilepsies, comprises runs of nearly continuous high amplitude, rhythmic 2–3 Hz, unilateral or bilateral posterior sharp and slow wave complexes (Taylor et al. 2003). It could therefore be argued that posterior quadrant IEDs are more frequently diffuse, have long duration, and correlate with the presence of deactivation only because they had slow waves. A multivariate analysis could solve this issue, but the relative rarity of negative BOLD responses end the consequent small size of our sample did not allow us to perform this analysis.
We found that IEDs were more frequent in the activation group than in the deactivation group. We do not think this can have an influence on the nature of our results since the number of IEDs is not a factor in determining the direction of the response (activation or deactivation).
To conclude, the IEDs were accompanied by a slow wave in almost all patients whose primary BOLD was a deactivation and only in 33 % of patients with activation. In the deactivation group, the epileptic focus was more frequently than expected in the posterior quadrant and involving larger cortical areas but this may not be a determining factor in the presence of a deactivation because IEDs in posterior regions all included slow waves. One important factor responsible for focal deactivations therefore appears to be the presence of a slow wave, which can be the EEG correlate of prolonged inhibition.
Supplementary Material
Acknowledgments
The authors thank Natalja Zazubovits for helping to collect and analyze the data. Pittau F. thanks Dr. Gaetano Cantalupo for the thoughtful comments during the manuscript preparation. This work was supported by the Canadian Institutes of Health Research (CIHR) grant MOP-38079. Pittau F. was supported by the Savoy Foundation for epilepsy.
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s10548-013-0302-1) contains supplementary material, which is available to authorized users.
Contributor Information
Francesca Pittau, Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, QC H3A3B4, Canada. IRCCS Istituto delle Scienze Neurologiche, Bologna, Italy.
Firas Fahoum, Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, QC H3A3B4, Canada.
Rina Zelmann, Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, QC H3A3B4, Canada.
François Dubeau, Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, QC H3A3B4, Canada.
Jean Gotman, Montreal Neurological Institute and Hospital, McGill University, 3801 University Street, Montreal, QC H3A3B4, Canada.
References
- Aghakhani Y, Bagshaw AP, Benar CG, Hawco C, Andermann F, Dubeau F, Gotman J. fMRI activation during spike and wave discharges in idiopathic generalized epilepsy. Brain. 2004;127 (Pt 5):1127–1144. doi: 10.1093/brain/awh136. [DOI] [PubMed] [Google Scholar]
- Al-Asmi A, Benar CG, Gross DW, Khani YA, Andermann F, Pike B, Dubeau F, Gotman J. fMRI activation in continuous and spike-triggered EEG–fMRI studies of epileptic spikes. Epilepsia. 2003;44(10):1328–1339. doi: 10.1046/j.1528-1157.2003.01003.x. 01003. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12(2):230–239. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- Amzica F. Seizure initiation: the transition from sleep-related oscillations to epileptiform discharge. In: Schwartzkroin PA, editor. Encyclopedia of basic epilepsy research. Vol. 3. Academic Press; Oxford: 2009. pp. 1343–1350. [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21(10):1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bagshaw AP, Aghakhani Y, Benar CG, Kobayashi E, Hawco C, Dubeau F, Pike GB, Gotman J. EEG–fMRI of focal epileptic spikes: analysis with multiple haemodynamic functions and comparison with gadolinium-enhanced MR angiograms. Hum Brain Mapp. 2004;22(3):179–192. doi: 10.1002/hbm.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénar C, Aghakhani Y, Wang Y, Izenberg A, Al-Asmi A, Dubeau F, Gotman J. Quality of EEG in simultaneous EEG–fMRI for epilepsy. Clin Neurophysiol. 2003;114(3):569–580. doi: 10.1016/s1388-2457(02)00383-8. [DOI] [PubMed] [Google Scholar]
- Bénar CG, Grova C, Kobayashi E, Bagshaw AP, Aghakhani Y, Dubeau F, Gotman J. EEG–fMRI of epileptic spikes: concordance with EEG source localization and intracranial EEG. Neuroimage. 2006;30(4):1161–1170. doi: 10.1016/j.neuroimage.2005.11.008. S1053-8119(05)02452-3. [DOI] [PubMed] [Google Scholar]
- Born AP, Law I, Lund TE, Rostrup E, Hanson LG, Wildschiodtz G, Lou HC, Paulson OB. Cortical deactivation induced by visual stimulation in human slow-wave sleep. Neuroimage. 2002;17(3):1325–1335. doi: 10.1006/nimg.2002.1249. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci USA. 2003;100(21):12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czisch M, Wehrle R, Kaufmann C, Wetter TC, Holsboer F, Pollmacher T, Auer DP. Functional MRI during sleep: BOLD signal decreases and their electrophysiological correlates. Eur J Neurosci. 2004;20(2):566–574. doi: 10.1111/j.1460-9568.2004.03518.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Williamson PD, Wieser HG. Mesial temporale lobe epilepsy. In: Engel J, Pedley TA, editors. Epilepsy: a comprehensive textbook. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 2417–2426. [Google Scholar]
- Fahoum F, Lopes R, Pittau F, Dubeau F, Gotman J. Widespread epileptic networks in focal epilepsies: EEG–fMRI study. Epilepsia. 2012;53(9):1618–1627. doi: 10.1111/j.1528-1167.2012.03533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Prince DA. Spike-wave rhythms in cat cortex induced by parenteral penicillin. I. Electroencephalographic features. Electroencephalogr Clin Neurophysiol. 1977;42(5):608–624. doi: 10.1016/0013-4694(77)90279-6. [DOI] [PubMed] [Google Scholar]
- Gloor P. Generalized epilepsy with bilateral synchronous spike and wave discharge. New findings concerning its physiological mechanisms. Electroencephalogr Clin Neurophysiol Suppl. 1978;34:245–249. [PubMed] [Google Scholar]
- Gotman J. Epileptic networks studied with EEG–fMRI. Epilepsia. 2008;49(Suppl 3):42–51. doi: 10.1111/j.1528-1167.2008.01509.x. EPI1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Pittau F. Combining EEG and fMRI in the study of epileptic discharges. Epilepsia. 2011;52(Suppl 4):38–42. doi: 10.1111/j.1528-1167.2011.03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci USA. 2005;102(42):15236–15240. doi: 10.1073/pnas.0504935102. 0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamandi K, Salek-Haddadi A, Laufs H, Liston A, Friston K, Fish DR, Duncan JS, Lemieux L. EEG–fMRI of idiopathic and secondarily generalized epilepsies. Neuroimage. 2006;31(4):1700–1710. doi: 10.1016/j.neuroimage.2006.02.016. S1053-8119(06)00110-8. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Dettmers C, Rzanny R, Liepert J, Buchel C, Weiller C. Reduction of excitability (“inhibition”) in the ipsilateral primary motor cortex is mirrored by fMRI signal decreases. Neuroimage. 2002;17(1):490–496. doi: 10.1006/nimg.2002.1077. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Knab R, Weiller C, Rother J. The influence of extra-and intracranial artery disease on the BOLD signal in FMRI. Neuroimage. 2003;20(2):1393–1399. doi: 10.1016/S1053-8119(03)00384-7. [DOI] [PubMed] [Google Scholar]
- Harel N, Lee SP, Nagaoka T, Kim DS, Kim SG. Origin of negative blood oxygenation level-dependent fMRI signals. J Cereb Blood Flow Metab. 2002;22(8):908–917. doi: 10.1097/00004647-200208000-00002. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kobayashi E, Boor R, Muhle H, Stephan W, Hawco C, Dubeau F, Jansen O, Stephani U, Gotman J, Siniatchkin M. Hemodynamic responses to interictal epileptiform discharges in children with symptomatic epilepsy. Epilepsia. 2007;48(11):2068–2078. doi: 10.1111/j.1528-1167.2007.01192.x. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Moeller F, Boor R, Stephani U, Gotman J, Siniatchkin M. Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG–fMRI. Neuroimage. 2009;45(4):1220–1231. doi: 10.1016/j.neuroimage.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J. Negative BOLD responses to epileptic spikes. Hum Brain Mapp. 2006;27(6):488–497. doi: 10.1002/hbm.20193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2(5):467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Laufs H. A personalized history of EEG–fMRI integration. Neuroimage. 2012;62(2):1056–1067. doi: 10.1016/j.neuroimage.2012.01.039. [DOI] [PubMed] [Google Scholar]
- Laufs H, Duncan JS. Electroencephalography/functional MRI in human epilepsy: what it currently can and cannot do. Curr Opin Neurol. 2007;20(4):417–423. doi: 10.1097/WCO.0b013e3282202b9200019052-200708000-00008. [DOI] [PubMed] [Google Scholar]
- Laufs H, Hamandi K, Walker MC, Scott C, Smith S, Duncan JS, Lemieux L. EEG–fMRI mapping of asymmetrical delta activity in a patient with refractory epilepsy is concordant with the epileptogenic region determined by intracranial EEG. Magn Reson Imaging. 2006;24(4):367–371. doi: 10.1016/j.mri.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. Intracortical recordings and fMRI: an attempt to study operational modules and networks simultaneously. Neuroimage. 2012;62(2):962–969. doi: 10.1016/j.neuroimage.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Formaggio E, Gasparini A, Cerini R, Bongiovanni LG, Storti SF, Mucelli RP, Fiaschi A, Avesani M. Continuous EEG–fMRI in patients with partial epilepsy and focal interictal slow-wave discharges on EEG. Magn Reson Imaging. 2008;26(8):1089–1100. doi: 10.1016/j.mri.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Naaman S, Bortel A, Mocanu V, Shmuel A. Neuroscience Meeting Planner. Society for Neuroscience; Washington, DC: 2011. Neurophysiological mechanisms of spontaneous fluctuations in blood-oxygenation signals. [Online] [Google Scholar]
- Neckelmann D, Amzica F, Steriade M. Changes in neuronal conductance during different components of cortically generated spike-wave seizures. Neuroscience. 2000;96(3):475–485. doi: 10.1016/s0306-4522(99)00571-0. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10(12):1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89(13):5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen DA. Intracellular studies of cortical neurons during thalamic induced wave and spike. Electroencephalogr Clin Neurophysiol. 1964;17:398–404. doi: 10.1016/0013-4694(64)90163-4. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.67698/2/676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathakrishnan R, Moeller F, Levan P, Dubeau F, Gotman J. BOLD signal changes preceding negative responses in EEG–fMRI in patients with focal epilepsy. Epilepsia. 2010;51(9):1837–1845. doi: 10.1111/j.1528-1167.2010.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother J, Knab R, Hamzei F, Fiehler J, Reichenbach JR, Buchel C, Weiller C. Negative dip in BOLD fMRI is caused by blood flow-oxygen consumption uncoupling in humans. Neuroimage. 2002;15(1):98–102. doi: 10.1006/nimg.2001.0965. [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Diehl B, Hamandi K, Merschhemke M, Liston A, Friston K, Duncan JS, Fish DR, Lemieux L. Hemodynamic correlates of epileptiform discharges: an EEG–fMRI study of 63 patients with focal epilepsy. Brain Res. 2006;1:148–166. doi: 10.1016/j.brainres.2006.02.098. S0006-8993(06)00524-5. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron. 2002;36(6):1195–1210. doi: 10.1016/s0896-6273(02)01061-9. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006;9(4):569–577. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Stefanovic B, Warnking JM, Kobayashi E, Bagshaw AP, Hawco C, Dubeau F, Gotman J, Pike GB. Hemodynamic and metabolic responses to activation, deactivation and epileptic discharges. Neuroimage. 2005;28(1):205–215. doi: 10.1016/j.neuroimage.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Suh M, Ma H, Zhao M, Sharif S, Schwartz TH. Neurovascular coupling and oximetry during epileptic events. Mol Neurobiol. 2006;33(3):181–197. doi: 10.1385/MN:33:3:181. [DOI] [PubMed] [Google Scholar]
- Taylor I, Scheffer IE, Berkovic SF. Occipital epilepsies: identification of specific and newly recognized syndromes. Brain. 2003;126(Pt 4):753–769. doi: 10.1093/brain/awg080. [DOI] [PubMed] [Google Scholar]
- Thornton R, Vulliemoz S, Rodionov R, Carmichael DW, Chaudhary UJ, Diehl B, Laufs H, Vollmar C, McEvoy AW, Walker MC, Bartolomei F, Guye M, Chauvel P, Duncan JS, Lemieux L. Epileptic networks in focal cortical dysplasia revealed using electroencephalography-functional magnetic resonance imaging. Ann Neurol. 2011;70(5):822–837. doi: 10.1002/ana.22535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15(1):1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.