Abstract
Objectives
In patients with intractable epilepsy, predicting seizures above chance and with clinically acceptable performance has yet to be demonstrated. In this study, an intracranial EEG-based seizure prediction method using measures of similarity with a reference state is proposed.
Methods
1565 h of continuous intracranial EEG data from 17 patients with mesial temporal lobe epilepsy were investigated. The recordings included 175 seizures. In each patient the data was split into a training set and a testing set. EEG segments were analyzed using continuous wavelet transform. During training, a reference state was defined in the immediate preictal data and used to derive three features quantifying the discrimination between preictal and interictal states. A classifier was then trained in the feature space. Its performance was assessed using testing set and compared with a random predictor for statistical validation.
Results
Better than random prediction performance was achieved in 7 patients. The sensitivity was higher than 85%, the warning rate was less than 0.35/h and the proportion of time under warning was less than 30%.
Conclusion
Seizures are predicted above chance in 41% of patients using measures of state similarity.
Significance
Sensitivity and specificity levels are potentially interesting for closed-loop seizure control applications.
Keywords: Seizure prediction, Temporal lobe epilepsy, Discriminant analysis, Classification, Wavelet energy and entropy, Continuous wavelet transform, Lines of local maxima, Statistical validation
1. Introduction
Research on epileptic seizure prediction has been driven by the need of an alternative therapeutic solution for patients who fail antiepileptic drugs and for whom surgical treatment is not possible or did not have a satisfactory outcome. A system capable of alerting patients to approaching seizures could make a considerable contribution to improving their well-being (Schulze-Bonhage and Kühn, 2008). Such a system could be an implantable device that ‘silently’ abates seizures by altering their generation mechanism in response to warnings. The ability to control seizures using other modalities than anti-epileptic drugs has been demonstrated experimentally and through clinical trials. Focal cooling of the cortex and optical activation of inhibitory neurotransmitters have shown promising results in suppressing experimental seizures (Rothman, 2008). Stimulating the vagus nerve (Fisher and Handforth, 1999; Thompson et al., 2012) and the trigeminal nerve (DeGiorgio et al., 2009) proved to be effective in supressing seizures. Stimulation targeting brain structures, mainly the anterior nucleus of the thalamus and seizure foci, has been investigated through randomized controlled trials and showed efficacy in reducing seizure frequency (Fisher et al., 2010; Morrell, 2011).
The majority of the aforementioned modalities have been investigated in an open-loop protocol. However, responsively controlling seizures is appealing: a closed-loop system has the advantage of requiring less power than open-loop systems (Krieger and Litt, 2008). Also, dose-dependent side effects of antiepileptic drugs (Gomer et al., 2007) are expected to be alleviated in systems using closed-loop drug delivery, as treatment becomes only interventional rather than continuous.
A seizure prediction method driving a closed-loop seizure control device has to demonstrate a clinically acceptable performance. The levels of acceptable sensitivity and specificity along with the required intervention time are generally patient and application dependant and they are unknown during development of the seizure prediction method. It was therefore recommended that a seizure prediction method be assessed for a range of intervention periods (Maiwald et al., 2004). As a minimum requirement, prediction performance needs to be above chance (Andrzejak et al., 2009).
For a long time, statistical validation was overlooked in seizure prediction. Most early studies did not investigate whether the performance was statistically significant. It is only in recent studies that rigorous statistical validation were included (Mormann et al., 2007). Such a validation is generally based on Monte Carlo simulations (Andrzejak et al., 2003; Kreuz et al., 2004) or naïve prediction schemes (Winterhalder et al., 2003; Schelter et al., 2006). Translating statistical evaluation into clinical utility for seizure warning or seizure control devices has been a subject of debate. Snyder et al. (2008) proposed a statistical approach to practically evaluate seizure prediction algorithms. Addressing the question of variability in temporal relationship between algorithm warnings and seizure onset, their approach is based on a new seizure warning protocol and a model for chance predictor with new performance metrics and methods for hypothesis testing.
An EEG based prediction method performs above chance when (1) there exists a preictal change in cortical dynamics, (2) the EEG measure is sensitive to this change and (3) electrode contacts are placed in areas where the preictal change is detectable. If the mechanisms underlying the preictal state in focal seizures engender spatially localized activity in the brain, then electrode location becomes of crucial importance. With ictogenesis yet to be fully understood, defining cortical areas where best prediction performance could be achieved remains hypothetic. In a recent study (Gadhoumi et al., 2012), we demonstrated that preictal and interictal states could be distinguished in EEG recordings from depth electrode contacts in the seizure onset zone. Other studies claimed that sites remote to the seizure onset zone also carried predictive power (Mormann et al., 2003; D’Alessandro et al., 2005; Kuhlmann et al., 2010).
In this study, we present and evaluate an intracerebral EEG based seizure prediction method for patients with mesial temporal lobe epilepsy. We use measures of similarity between the brain state underlying an EEG epoch and a reference state to identify EEG changes leading to seizures in a classification based approach. The premise of the method relies on our study of the discrimination between preictal and interictal epochs using high frequency content of intracerebral EEG (Gadhoumi et al., 2012). Because of the variability across patients in preictal and interictal EEG patterns, the method is patient-specific: in-sample optimization is carried out during training for each patient. Special care was taken not to use test data set during training. We assume that seizures are stereotypical within patients. Such an assumption is essential for a good generalization of the training performance over test data.
The method performance and its superiority to chance are evaluated using the statistical framework proposed by Snyder et al. (2008). The ultimate goal is to design a reliable seizure prediction method that proves utility in clinical applications. For this, we test the method in quasi-prospective setting using long-lasting multi-day raw EEG recordings and report the results of sensitivity and specificity suggested in the statistical framework.
2. Materials and methods
2.1. Materials
Seventeen consecutive patients admitted in the Montreal Neurological Institute between 2004 and 2011 for presurgical intracerebral depth electrodes investigation, were evaluated. The patients responded to two inclusion criteria: a diagnosis of mesial temporal lobe epilepsy and a minimum number of 5 seizures (2 for training and 3 for testing) recorded at 2000 Hz. In total, 1565 h of intracerebral EEG recorded using a 128-channel Harmonie monitoring system (Stellate Systems Inc.) filtered at 500 Hz and sampled at 2000 Hz were analyzed. Of this data, 1446 h were continuous long-lasting EEG recordings that were used in testing. The remaining 119 h were used for training. Up to three preictal epochs, lasting up to 22 min each, were selected for each patient in the training procedure. The 22 min maximum duration of a training preictal epoch was chosen based on our earlier study (Gadhoumi et al., 2012). The actual training preictal epoch duration varied depending on the availability of continuous uninterrupted preictal EEG recordings. It ranged between 6.3 and 22 min. Five interictal epochs lasting approximately 1 h each and separated by at least 1 h were selected for each patient for training. These epochs were at least 4 h from any seizure.
Out of 214 seizures, 39 were rejected from the analysis as they were not separated by at least 2 h. This criterion was used to minimize the impact of postictal dynamics on the EEG analysis. Seizure electrographic onsets were determined by an experienced neurologist. Only bipolar channels from the 4 deepest contacts of bilaterally implanted electrodes in the amygdala, hippocampus and parahippocampus were analyzed. The total number of analyzed channels per patient ranged between 9 and 18 depending on the number of electrodes implanted in the mesial structures. Tables 1 and 2 summarize the details of seizures and EEG data.
Table 1.
Summary of EEG dataset and seizure onset.
| Patient | Sex/age | Seizure onset | Number of seizures recorded | Number of seizures analyzed | Total duration of analyzed EEG (h) |
|---|---|---|---|---|---|
| 1 | M/29 | Bil., R > L | 8 | 7 | 56.8 |
| 2 | F/42 | R | 9 | 9 | 132.5 |
| 3 | F/44 | Bil.,L > R | 6 | 6 | 47.7 |
| 4 | M/46 | R | 9 | 8 | 110.7 |
| 5 | F/40 | L | 30 | 10 | 57.7 |
| 6 | F/53 | Bil.,L > R | 7 | 6 | 84.4 |
| 7 | M/24 | R | 8 | 8 | 109.6 |
| 8 | M/25 | Bil., R > L | 6 | 7 | 147.7 |
| 9 | M/44 | L | 6 | 6 | 140.8 |
| 10 | F/30 | Bil., R > L | 18 | 17 | 52.8 |
| 11 | F/47 | Bil.,L > R | 31 | 23 | 55.6 |
| 12 | M/28 | Bil., R > L | 27 | 27 | 39.6 |
| 13 | M/23 | Unclear | 9 | 9 | 17.4 |
| 14 | M/38 | R | 6 | 6 | 89 |
| 15 | M/33 | Bil. | 7 | 17 | 169.5 |
| 16 | M/21 | R | 13 | 13 | 143.1 |
| 17 | F/28 | Bil., R > L | 6 | 6 | 110.4 |
R: right. L: left, A: amygdala, H: hippocampus, P: parahippocampus.
Bil.: bilateral, >/< designate preponderance (based on 70% or more of number of seizures originating from one side).
Table 2.
Number and duration of training EEG epochs.
| Patient | Number of training preictal epochs | Number of training interictal epochs | Total duration of training preictal epochs (h) | Total duration of training interictal epochs (h) | Number of testing seizures | Total duration of testing (contiguous) epoch (h) |
|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 0.7 | 5 | 5 | 49.8 |
| 2 | 3 | 5 | 1.1 | 4.9 | 6 | 125.9 |
| 3 | 2 | 5 | 0.7 | 6.3 | 4 | 39.8 |
| 4 | 3 | 5 | 1.1 | 5.3 | 6 | 102.8 |
| 5 | 2 | 5 | 0.7 | 6 | 8 | 50.4 |
| 6 | 3 | 5 | 1 | 4.9 | 3 | 76.3 |
| 7 | 3 | 5 | 1.1 | 5 | 5 | 102.9 |
| 8 | 3 | 5 | 1.1 | 5 | 4 | 139.6 |
| 9 | 3 | 5 | 1.1 | 5 | 3 | 133.5 |
| 10 | 3 | 5 | 1.1 | 4.8 | 14 | 45.7 |
| 11 | 3 | 5 | 1 | 4.9 | 20 | 48.6 |
| 12 | 3 | 5 | 1.1 | 4.3 | 24 | 32.6 |
| 13 | 3 | 5 | 0.9 | 4 | 6 | 12.5 |
| 14 | 3 | 5 | 1.1 | 5 | 3 | 82.2 |
| 15 | 3 | 5 | 0.9 | 5 | 4 | 163 |
| 16 | 3 | 5 | 0.9 | 5.4 | 10 | 135.3 |
| 17 | 2 | 5 | 0.4 | 5 | 3 | 104.9 |
| Total | 47 | 85 | 16 | 85.8 | 128 | 1445.8 |
2.2. Concepts
The seizure prediction method is based on the study by Gadhoumi et al. (2012). In the following sections we first summarize the concepts and the methodology of that study: we briefly review the feature definitions and how preictal and interictal epochs are discriminated using those features. We then describe in detail the processing blocks of the proposed method.
2.2.1. The reference state and similarity measure features
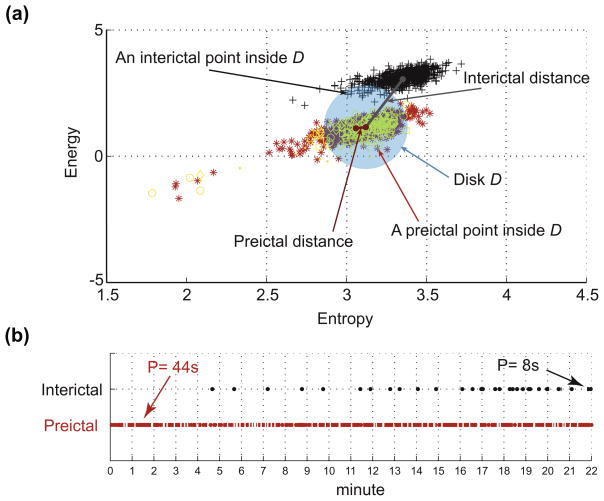
Preictal and interictal epochs are analyzed using continuous wavelet transform by calculating in different frequency bands the wavelet energy and entropy in a 2 s non-overlapping sliding window. These two measures are extracted from lines of local maxima in the wavelet domain to characterize singularities (Mallat and Hwang, 1992). Each epoch is represented by its distribution of (energy, entropy) points in a 2-dimentional space (this distribution is hereafter referred to as energy and entropy profile). The state underlying an epoch is characterized by its relative similarity with a reference state defined from preictal epochs of a training subset. To quantify this similarity, the reference state is represented in the 2-dimentional space with a disk in which the center and the radius are learned from the training preictal epochs subset. Then three features are introduced (see Fig. A1): (1) the distance of an epoch energy and entropy profile to the center of the disk, (2) the percentage of points included in the disk in an epoch energy and entropy profile and (3) the duration corresponding to the maximum number of points in an epoch energy and entropy profile remaining consecutively (in time) confined in the disk. Similarity features are computed for an epoch in a 1 min sliding window with 75% overlap between windows.
Fig. A1.
Illustration of the distance, inclusion and persistence features in the energy and entropy space. (a) Energy and entropy profiles of a 22 min window of an interictal epoch (black symbols) and a preictal epoch (red symbols) are at different distances from the center (mean point of 90 s immediate preictal energy and entropy profile shown in yellow symbols) of the disk D, calculated from a separate set of preictal epochs. Any point inside the disk D counts for the inclusion rate of the distribution. (b) Temporal distribution of the amount of time spent inside the disk D in the same preictal (red dots) and interictal (black dots) energy and entropy profiles shown in (a). The dots indicate points inside the disk (each point represents 2 s duration). The persistence is the period of time corresponding to the maximum number of temporally contiguous points in the disk D. In this example preictal persistence is 22 pts. × 2 s = 44 s and interictal persistence is 4 pts. × 2 s = 8 s. Reproduced with Permission from: Gadhoumi K, Lina JM, Gotman J. Discriminating preictal and interictal states in patients with temporal lobe epilepsy using wavelet analysis of intracerebral EEG. Clin Neurophysiol 2012;123:1906–1916.
2.2.2. State discrimination
Epochs are split into training and testing subsets. Using discriminant analysis and in-sample cross validation applied to the data set of features calculated, the training subset is used to learn a classifier and to preselect the frequency band and the set of channels that best discriminate preictal and interictal epochs. The testing subset is then used to assess the performance of the classifier in discriminating preictal and interictal states by measuring the sensitivity and specificity of preictal and interictal epochs classification, analyzing only the pre-selected channels and preselected frequency band.
2.3. The seizure prediction method
The method has training and testing parts. In each part, the analysis is performed independently on each channel. Main procedures in each part are illustrated in Fig. 1.
Fig. 1.
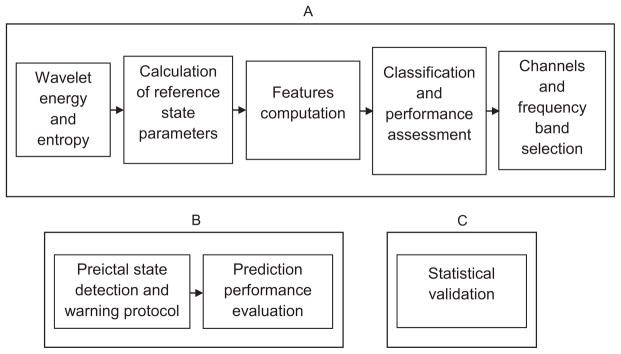
Block diagram of the method training (A), testing (B) and validation (C).
2.3.1. Training
Training is based on the study by (Gadhoumi et al., 2012). A detailed summary of its main procedures is presented in Appendix A.
2.3.2. Testing
The method is tested on continuous EEG data independent from the training data. Recording interruptions present in data are not analyzed. Using the disk DN, features are calculated in a sliding window for the selected channels and the frequency band as described in the training procedure (see Appendix A). The window length is set to 1 min and the overlap between windows is set to 75% as in training. For all selected channels, features data calculated in each window are classified into a ‘preictal’ or ‘interictal’ class using the final classifier. Majority voting rule is then applied to window classification results of each channel and the class of the window is determined.
Consecutive preictal classifications are interpreted as seizure warnings. We set the consecutive number of preictal classifications needed to raise a warning to 5. This means that a warning is raised whenever 2 min of EEG is continuously classified as ‘preictal’. Warnings are raised according to persistence of warning lights protocol (Snyder et al., 2008) whereby a warning remains active (illuminated light) for as long as five new consecutive preictal classifications are detected within a given time horizon, in which condition, the original warning light is extended for another duration τ. Uninterrupted illumination of the warning light is considered a single warning, regardless of its duration. The period τ, originally referred to as persistence parameter in the study by Snyder et al. (2008), is referred to as the persistence-τ to avoid confusion with the terminology used in our study. The persistence-τ corresponds to sum of the seizure occurrence period (the period during which the seizure is to be expected) and the seizure prediction horizon (a minimum window of time between warning and the beginning of the seizure occurrence period) as defined by Winterhalder et al. (2003) and Schelter et al. (2006).
Since the information on the state of patients (ictal or interictal) during interruption of EEG recording is missing, warnings are discarded if they are followed, within the period of the light illumination, by an interruption that lasts more than the duration of a seizure. In temporal lobe epilepsy, a typical seizure lasts up to 2 min. We discarded warnings followed by interruptions of 3 min or more. The epoch between the light illumination and the beginning of interruption is removed from the analysis.
To assess the performance of the prediction method, we evaluate its sensitivity and specificity. The sensitivity is defined as the probability of correctly predicting a seizure within a time horizon. We measure the sensitivity by calculating the proportion of seizures within the light illumination. One measure of specificity is the rate of false predictions per hour. In the chosen warning protocol, this measure is not suitable as it does not provide information on the amount of time spent in warning therefore it could potentially lead to misinterpreted results. We adopt instead the proportion of time under warning ρ and the warning rate r as specificity-related measures. These measures provide more practical information from patient and closed-loop intervention system perspectives since they assess the frequency and duration of the inconvenience caused by warnings.
2.3.3. Comparison of classifier performance in training and testing
To compare the performance of the final classifier in separating preictal and interictal epochs of training dataset and its performance in testing dataset, we compare the average score of the three selected channels in training with a score Ptest that combines the sensitivity and the specificity of the method as measured by predicting seizures of testing dataset using selected channels. We define the score Ptest by using the sensitivity S and the proportion of time under warning ρ:
| (1) |
2.4. Statistical validation
The performance of the proposed method is tested for it superiority to chance level. A naïve prediction scheme based on a Poisson process is chosen as the chance predictor. A Poisson process uses no information from the EEG signal and generates preictal classification according to uniformly distributed probability. One way to measure the improvement-over-chance is to evaluate the difference between the sensitivity of the proposed method and that of the chance predictor (Snyder et al., 2008). In fact, it is demonstrated that the sensitivity of the chance predictor is approximately equal to the proportion of time under warning ρ. The predictive ability of the proposed method is therefore measured by the difference between sensitivity and the proportion of time under warning. Only if the sensitivity significantly exceeds the proportion of time under warning can the method claim to demonstrate predictive power above chance.
Given that n of N seizures are correctly predicted by the proposed method and Snc is the sensitivity of the chance predictor, the one-sided p-value estimation of the significance of the improvement over chance is:
| (2) |
using Eq. (2), we assess the statistical significance of the improvement over chance in the proposed method at the 5% level.
2.5. Predictors of method performance
Assuming seizures are predictable above chance level in a subgroup of patients, we statistically analyze a set of patient characteristics for a possible association with seizures predictability. Patient characteristics included age, sex and history of neurobiological (duration of epilepsy, generalized tonic clonic seizures, status epilepticus, febrile seizures at childhood, bilateral independent epileptic foci) and neuroimaging investigations (mesial temporal atrophy detected at MRI).
3. Results
3.1. Performance
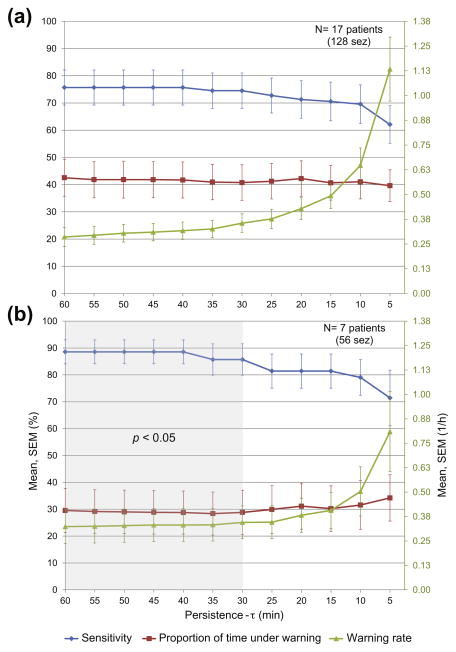
We evaluated the sensitivity, the proportion of time under warning and the warning rate for a range of persistence-τ values between 5 and 60 min. For each value of persistence-τ, we assessed the significance of the improvement over chance. Fig. 2a shows the mean across patients of the performance metrics in the 17 patients.
Fig. 2.
Mean values (with standard errors of the mean) of sensitivity, proportion of time under warning and warning rate for a range of persistence-τ across all patients (a) and across the 7 patients in whom seizures are predicted above chance for a sub-range of persistence-τ (greyed area) (b).
In 7 patients (group A, 56 test seizures), sensitivity was significantly higher than the proportion of time under warning (p < 0.05) for persistence-τ values between 30 and 60 min. In remaining patients (group B), sensitivity values were not significantly higher than proportion of time under warning for any of persistence-τ values. Fig. 2b shows mean values of performance metrics across patients of group A. For persistence-τ values above 30 min, the mean sensitivity was higher than 85%, the mean proportion of time under warning was less than 30% and the mean warning rate was less than 0.35/h.
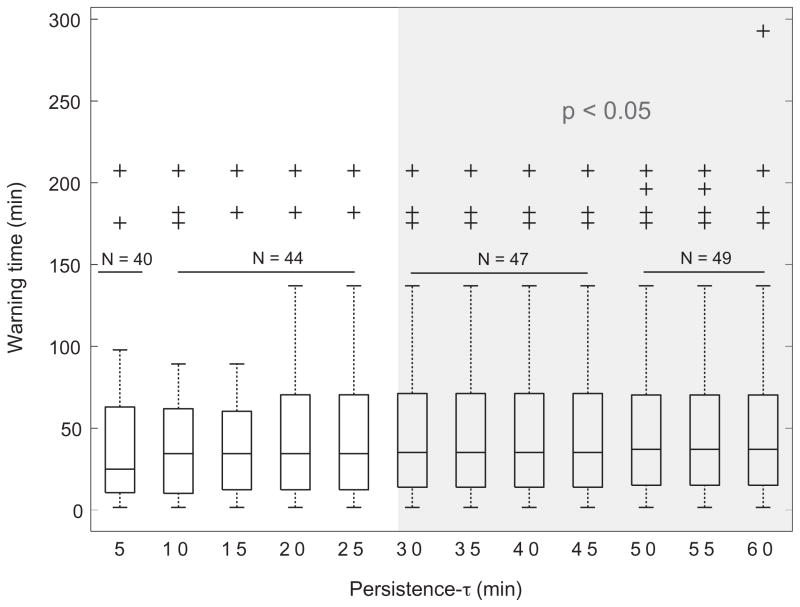
Fig. 3 shows the median across correctly predicted seizures in patients of group A of the warning time for the evaluated range of persistence-τ. The warning time is the duration between the rise of a warning and the occurrence of the correctly predicted seizure. For persistence-τ values above 30 min, the median warning time was around 36 min.
Fig. 3.
Median warning time across patients in whom seizures are predicted above chance level for persistence-τ values above 30 min (greyed area). N is the number of predicted seizures. Crosses represent outliers.
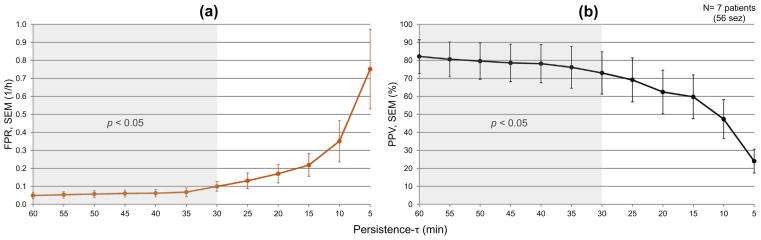
The average false prediction rate across patients of group A was below 0.10/h for persistence-τ values above 30 min (Fig. 4a). For the same group, the positive predictive value was higher than 73% for persistence-τ values above 30 min (Fig. 4b).
Fig. 4.
Average false prediction rates (a) and positive predictive value (b) in patients of group A, as a function of persistence-τ. Seizures are predicted above chance for persistence-τ values above 30 min (greyed area). SEM: standard error of the mean.
3.2. Best performing channels and frequency band
Performance scores of selected channels in training ranged between 0.49 and 1 for all patients. Table 3 shows score values of selected channels and the selected frequency band in groups A and B. There was no significant difference between channel score distributions in both groups using an unpaired two-sample comparison t-test with no assumption of variance equality (p = 0.09).
Table 3.
Selected frequency band and scores of selected channels. Patients in whom seizures are predictable above chance level (group A) are indicated with *.
| Patient | Selected frequency band (Hz) | Selected channels scores | ||
|---|---|---|---|---|
| 1* | 50–150 | 1 | 0.99 | 0.93 |
| 2* | 250–350 | 0.62 | 0.58 | 0.53 |
| 3 | 150–250 | 0.73 | 0.68 | 0.67 |
| 4* | 250–350 | 0.99 | 0.99 | 0.99 |
| 5 | 150–250 | 0.57 | 0.56 | 0.56 |
| 6 | 150–250 | 0.67 | 0.63 | 0.62 |
| 7 | 250–350 | 0.54 | 0.51 | 0.49 |
| 8 | 350–450 | 0.63 | 0.63 | 0.63 |
| 9 | 150–250 | 0.55 | 0.5 | 0.5 |
| 10 | 50–150 | 0.65 | 0.57 | 0.49 |
| 11* | 50–150 | 0.76 | 0.68 | 0.61 |
| 12 | 350–450 | 0.86 | 0.86 | 0.86 |
| 13* | 50–150 | 0.66 | 0.66 | 0.66 |
| 14 | 50–150 | 0.68 | 0.66 | 0.61 |
| 15 | 50–150 | 0.93 | 0.86 | 0.85 |
| 16* | 250–350 | 0.85 | 0.84 | 0.78 |
| 17* | 250–350 | 0.66 | 0.62 | 0.58 |
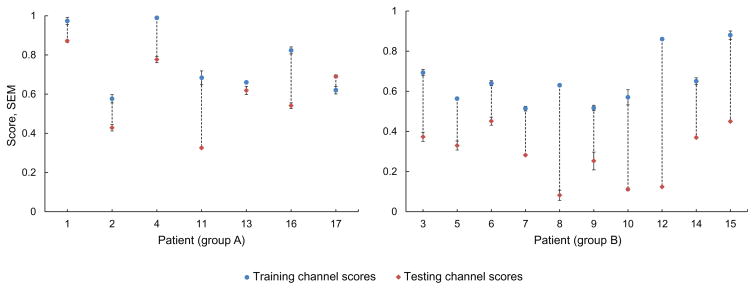
We compared the average performance score of selected channels in training and in testing P̄test (we averaged performance scores across persistence-τ values) for both groups of patients. The difference between scores was significantly smaller in group A than in group B (p < 0.05, unpaired t-test with unequal variances). Fig. 5 shows average scores in both groups.
Fig. 5.
Average performance score of selected channels in training and in testing (scores across persistence-τ values were averaged) for patients of groups A and B. Difference between scores (in dotted lines) is significantly smaller in group A than in group B (p < 0.05). SEM: standard error of the mean.
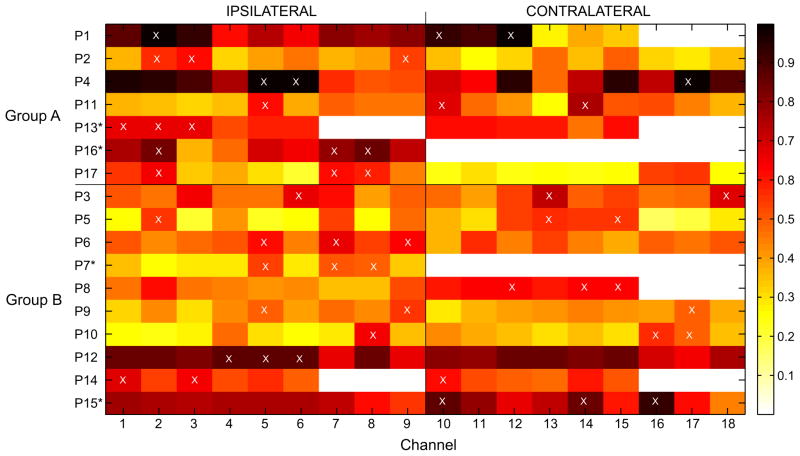
Excluding 2 patients with unilateral implantation, bilateral independent foci or unclear focus in each group, selected channels were relatively more preponderant in the ipsilateral side of seizure onset zone (SOZ) in group A (10 ipsilateral and 5 contralateral channels) (Fig. 6). This preponderance was less significant in group B (13 ipsilateral and 11 contralateral channels). A chi-square test however, showed no relationship between the number of channels selected ipsilaterally or contralaterally and seizure predictability above chance (p = 0.44).
Fig. 6.
Average channel scores across 10 partitions of training datasets of group A and B. With reference to SOZ, the left side of the figure shows scores of ipsilateral channels and the right side shows scores of contralateral channels. On each side, the 3 deepest channels (sorted by depth, with deepest channel indicated first) of the hippocampus, the amygdala and the parahippocampus are indicated by numbers on the x-axis of the figure in the respective order. White boxes indicate channels not available. The 3 best average channel scores (corresponding to selected channels) are indicated by white crosses. Patients in whom SOZ cannot be lateralized (either bilateral foci, channels not available or unclear focus) are indicated with *.
Of 10 patients in whom selected channels were partially or totally in the contralateral side of the SOZ, only 4 have seizures origins exclusively from one hemisphere. The remaining 6 patients have seizures preponderantly originating from one hemisphere.
Using Fisher’s exact test to compare selected frequency bands in groups A and B of patients, we found no association between the selected frequency band and seizure predictability above chance (p = 0.08).
3.3. Preictal changes and seizure onset
In patients of group A, we analyzed preictal changes that triggered true seizure warnings in test epochs using a persistence-τ value of 30 min in order to determine the laterality of detected changes with reference to SOZ. For each seizure correctly predicted, we determined whether channels with ‘preictal’ classifications that led to warning were ipsilateral, contralateral or bilateral to SOZ.
Table 4 depicts the results of this analysis. Seizures of 2 patients for whom SOZ was reported unclear were excluded from this analysis. In the 37 correctly predicted seizures of the remaining 5 patients, preictal changes were either bilateral (in 23 seizures) or ipsilateral (in 13 seizures) to SOZ. The only exception was one patient, in whom contralateral preictal changes were found solely in one of his seizures.
Table 4.
Relative laterality to SOZ of preictal changes.
| Patient | Number of predicted seizures | Relative side of observed preictal change |
|---|---|---|
| 1 | 5 | 5 × Bil. |
| 2 | 6 | 6 × Ips. |
| 4 | 5 | 5 × Ips. |
| 11 | 18 | 18 × Bil. |
| 13* | 4 | N/A |
| 16* | 6 | N/A |
| 17 | 3 | 2 × Ips., 1 × Con. |
Patients with *had no clear SOZ.
Bil.: bilateral, Ips.: ispilateral, Con.: contralateral.
3.4. Predictors of performance outcome
Two-tailed Mann–Whitney test showed no significant difference in the age (p = 0.69) and duration of epilepsy (p = 0.94) between groups A and B. Using Fisher’s exact test, no significant difference was found in foci laterality (p = 1), history of febrile seizures (p = 1), history of generalized tonic clonic seizures (p = 0.61), and presence of mesial temporal lobe atrophy (p = 0.64). However, history of status epilepticus showed a significant difference between the 2 groups (p < 0.05) with patients of group A having 4 patients with history of status epilepticus versus none in group B.
4. Discussion
Gadhoumi et al. (2012) demonstrated that selected preictal and interictal epochs of intracerebral EEG could be distinguished using measures of wavelet entropy and energy. We have applied the framework of this study to seizure prediction and proposed a new method capable of anticipating seizure occurrence in patients with intractable mesial temporal lobe epilepsy. Above chance prediction performance was obtained in a subgroup of 7 patients which could not be pre-identified, making the use of the method depend on trial, with a chance of obtaining successful results in around 40% of patients.
Choosing a seizure occurrence period above 30 min, the method performed above chance with sensitivities higher than 85%, proportions of time under warning less than 30%, warning rates below 0.35/h and false prediction rates below 0.1/h. These results cannot be judged independently from a clinical application context where the method is applied. With warning raised around 48 min before seizure occurrence, the method cannot evidently be used in a patient advisory seizure prediction system; in fact patients do not require more than 3 to 5 min warning time of an impending seizure (Arthurs et al., 2010). Rather, the method could be used in a closed loop system where the action time of therapeutic intervention is in the range of the warning time, providing the system’s required levels of sensitivity and specificity are also within method’s range.
One feature of this study is that EEG datasets were analysed without preprocessing to remove noise and artefacts. Electrode disconnections and reconnection fluctuations and out-of-range signal amplitudes are inherently filtered out by the wavelet analysis. In fact, the local modulus maxima coefficients from which energy and entropy were calculated would only carry relevant information about the EEG at the frequency range we analysed (Mallat and Hwang, 1992). We did not separately assess the robustness of denoising the EEG by means of wavelet analysis but pre-identification and removal of noise and artifacts may enhance the method performance particularity by reducing false predictions. Whether this enhancement would be significant enough to justify the implementation a separate preprocessing method is to be explored.
Channels preselected in training did not always have optimal score values (close to 1). This was equally true for all patients studied. Although high performance scores of preselected channels in training were not associated with prediction performance in testing, it was interesting that the average performance score of preselected channels measured in test data was closer to the average performance score in training in patients with seizures predicted above chance than in the remaining patients. This suggests that in cases where seizures are predictable above chance, improving the classification performance in training may lead to improving the prediction performance of the method. One possible improvement to classification performance is to use a classifier based on Support Vector Machines (SVMs). SVMs have been shown to outperform LDA on a variety of datasets (Gokcen and Peng, 2002). The use of SVMs based classifier may potentially enhance the prediction performance for seizures predicted above chance but may also provide superiority to the chance level in predicting other seizures. This can be investigated in a future study.
Compared to what has been found in our previous study (Gadhoumi et al., 2012), preselected channels were not all ipsilateral to seizure onset zone, though they were 67% more preponderant ipsilaterally in patients with seizures predictable above chance, compared to 54% in remaining patients. This discrepancy may be attributed to data sampling. In fact selected channels are sensitive to a selection criterion where only the 3 top channel scorers are kept. If the 4th best channel has a score almost as high as the third it will still be rejected. A different sampling of training data may lead to selecting a new set of channels. This sensitivity was minimized in the previous study by using a bootstrapping approach, which could not be implemented in this study because of the quasiprospective design. Reducing the variability in training results can always be addressed in a seizure prediction method if more data can be allocated to training and computational cost can be adequately handled.
Frequency bands preselected in training varied between patients. This variability suggests that the predictive information is patient-specific and could be spectrally limited. In order to detect a preictal state with high sensitivity and specificity using EEG, an analysis of a broad spectrum and the identification of a frequency band with the highest discrimination between preictal and interictal states may be important.
A noteworthy finding is that preictal changes were almost never detected exclusively contralateral to seizure onset. Preictal change appears always to be detectable in the area where upcoming seizure will occur and in some cases also contralaterally. EEG studies on seizure prediction reported in general discordant findings about sites of first preictal change; These were found ipsilaterally (Martinerie et al., 1998; Le Van Quyen et al., 2001) and contralaterally (Mormann et al., 2003; D’Alessandro et al., 2005; Kuhlmann et al., 2010). Recent studies using microelectrodes (Bower et al., 2012) have reported preictal changes in temporal lobe structures located within and outside the seizure onset zone, although the specificity of these changes could not be assessed in the context of seizure prediction. A recommendation on areas where to place sensing electrodes of a seizure prediction system however, is yet to be made. Based on our studies, we believe that preictal changes can be better detected in sites of preponderant seizure onset. A further support to this claim is the rather consistency of preictal changes laterality within patient’s seizures.
In an attempt to unravel predictors of seizures predictability, retrospective analysis of demographic, neurobiological and neuroimaging characteristics revealed in general no association with predictability of seizures above chance. A history of status epilepticus could however be linked to patients in whom seizures were predicted above chance. As important as this finding might be, it must be interpreted with caution. Its statistical significance is limited by the size of patient’s sample and needs to be confirmed on a larger sample.
While disease characteristics do not seem to help identify a priori patients in whom seizures would be predicated above chance, other hypotheses need to be verified as they may provide more insight on seizure predictability. In particular, if ictogenesis is a rather spatially local mechanism in the seizure onset zone, electrode contacts need to be abundant enough to cover a large volume (covering the seizure onset zone) and able to record from small enough volume in order to pick up any preictal change. Such electrode contacts do exist in the form of micro-contacts (Kelly et al., 2007; Stead et al., 2010) and studies on seizure prediction analysing data recorded with these electrodes are expected to provide answers to important questions on seizure predictability.
Finally, the use of the proposed method in patients with other forms of focal epilepsy (neocortical, extra-temporal) can be envisaged. Its performance has to be revalidated, as different types of epilepsy may exhibit different underlying mechanisms, which may lead to alteration of predictive performance. Preictal changes may appear exclusively in areas remote from the seizure onset zone and therefore it is recommended that large numbers of channels be analysed for an initial investigation.
HIGHLIGHTS.
Seizures are predictable above chance level in a subset of patients.
Preictal changes are detected on average 36 min before seizure onset.
Preictal changes are ipsilateral and bilateral but rarely contralateral to seizure onset zone.
Seizure predictability may be linked to history of status epilepticus.
Acknowledgments
We would like to thank Dr. Piero Perucca and Dr. Federico Melani for their help on reviewing EEG data, and Ms Natalja Zazubovits for her technical assistance. This work was supported by the Canadian Institutes of Health Research (CIHR) grants MOP-10189/102710 and by the Royal Society of Canada and the Natural Sciences and Engineering Research Council of Canada (NSERC) grant CHRPJ 323490-06 and by the joint NSERC/CIHR grant CHRP-CPG-80098.
Appendix A. Training
The training procedure consists of determining: (1) a classifier, (2) a set of three channels and (3) a frequency band by which separation of preictal and interictal training epochs in the inclusion, persistence and distance features plane is possible and optimal. Based on the earlier study by Gadhoumi et al. (2012), the best discrimination results were obtained with diagonal linear discriminant analysis (dLDA). We therefore adopted dLDA approach to determine the parameters of the classifier.
Training is performed in 6 main stages: (1) Calculation of energy and entropy measures, (2) Calculation of the reference state parameters, (3) Feature computation, (4) Channel and frequency band selection, and (5) Construction of the final classifier. We hereafter describe the data set and the analysis used in each stage.
Appendix A1. Calculation of energy and entropy measures
For all training preictal and interictal epochs, continuous wavelet transform using Morse wavelet (Lilly and Olhede, 2010) is performed in a 2 s sliding window with 2 s gap between windows. Wavelet energy and entropy are calculated from lines of local maxima in 4 frequency bands: 50–150 Hz, 150–250 Hz, 250–350 Hz and 350–450 Hz.
Appendix A2. Calculation of the reference state parameters
Using N training preictal epochs with time interval [0 ti] each, (i = 1:N), the center CN of a disk DN representing the reference state is defined as the mean point of the N 90 s immediate preictal energy and entropy profiles. The radius RN of the disk DN is such 85% of the average of energy and entropy profiles of the N preictal epochs [0 ti–90s], is included in the disk.
Appendix A3. Features computation
Distance, inclusion and persistence features are calculated for the entire duration of each training interictal epoch and for time interval [0 ti–90s] of each training preictal epoch Fig. A1. The features are calculated in a 1 min sliding window with 75% overlap between windows. The distance is computed by measuring the Euclidian distance between the center CN and the average of all points of the energy and entropy profile of the epoch. The inclusion is the percentage of points of the energy and entropy profile confined within the disk DN. Finally the persistence is the total duration corresponding to the maximum number of consecutive 2 s points of the energy and entropy profile that remain confined inside the disk DN.
Appendix A4. Channels and frequency band selection
The training set is partitioned into subsets A and B for each patient. Subset A contains N−1 preictal epochs and 5−1 = 4 interictal epochs. Features are calculated for all epochs of both subsets using a disk DN−1, the parameters of which are calculated from the N−1 preictal epochs of subset A. Supervised dLDA is then carried out on subset A features data in order to separate preictal and interictal features data groups. The performance of the resulting classifier is assessed on subset B which contains one preictal and one interictal epoch. This performance is quantified for each of the channels and the frequency bands analyzed using a score combing the sensitivity and the specificity of the classification (Chaovalitwongse et al., 2005). For a given frequency band f and a channel ch, is given by:
| (3) |
where S is the sensitivity and F is the false positive rate. In a given frequency band f, the 3 channels with the highest scores are considered the best ‘discriminating’ channels. We define the best ‘discriminating’ frequency band the one for which the sum of the three highest scores is the largest.
To reduce variability, 10 rounds of the above described process are performed using 10 different partitions of the original training data set. The scores are averaged across rounds for each channel and for each frequency band. The best discriminating channels and frequency band determined as described above using averaged scores are retained for subsequent test analysis.
Appendix A5. Construction of final classifier
For each of the selected channels one final classifier is calculated. The parameters of each classifier are derived from a discriminant analysis of the features data calculated from the N preictal and 5 interictal channel epochs of the original training set. The features are calculated for the selected frequency band using the disk DN.
References
- Andrzejak RG, Mormann F, Kreuz T, Rieke C, Kraskov A, Elger CE, et al. Testing the null hypothesis of the nonexistence of a preseizure state. Phys Rev E. 2003;67:010901. doi: 10.1103/PhysRevE.67.010901. [DOI] [PubMed] [Google Scholar]
- Andrzejak RG, Chicharro D, Elger CE, Mormann F. Seizure prediction: any better than chance? Clin Neurophysiol. 2009;120:1465–78. doi: 10.1016/j.clinph.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Arthurs S, Zaveri HP, Frei MG, Osorio I. Patient and caregiver perspectives on seizure prediction. Epilepsy Behav. 2010;19:474–7. doi: 10.1016/j.yebeh.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Bower MR, Stead M, Meyer FB, Marsh WR, Worrell GA. Spatiotemporal neuronal correlates of seizure generation in focal epilepsy. Epilepsia. 2012;53:807–16. doi: 10.1111/j.1528-1167.2012.03417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaovalitwongse W, Iasemidis LD, Pardalos PM, Carney PR, Shiau DS, Sackellares JC. Performance of a seizure warning algorithm based on the dynamics of intracranial EEG. Epilepsy Res. 2005;64:93–113. doi: 10.1016/j.eplepsyres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- D’Alessandro M, Vachtsevanos G, Esteller R, Echauz J, Cranstoun S, Worrell G, et al. A multi-feature and multi-channel univariate selection process for seizure prediction. Clin Neurophysiol. 2005;116:506–16. doi: 10.1016/j.clinph.2004.11.014. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Murray D, Markovic D, Whitehurst T. Trigeminal nerve stimulation for epilepsy: long-term feasibility and efficacy. Neurology. 2009;72:936–8. doi: 10.1212/01.wnl.0000344181.97126.b4. [DOI] [PubMed] [Google Scholar]
- Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Handforth A. Reassessment: vagus nerve stimulation for epilepsy: a report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology. 1999;53:666–9. doi: 10.1212/wnl.53.4.666. [DOI] [PubMed] [Google Scholar]
- Gadhoumi K, Lina JM, Gotman J. Discriminating preictal and interictal states in patients with temporal lobe epilepsy using wavelet analysis of intracerebral EEG. Clin Neurophysiol. 2012;123:1906–16. doi: 10.1016/j.clinph.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokcen I, Peng J. Comparing Linear Discriminant Analysis and Support Vector Machines. In: Yakhno T, editor. Lecture Notes in Computer Science. Springer; Berlin/Heidelberg: 2002. pp. 104–13. [Google Scholar]
- Gomer B, Wagner K, Frings L, Saar J, Carius A, Harle M, et al. The influence of antiepileptic drugs on cognition: a comparison of levetiracetam with topiramate. Epilepsy Behav. 2007;10:486–94. doi: 10.1016/j.yebeh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Kelly RC, Smith MA, Samonds JM, Kohn A, Bonds AB, Movshon JA, et al. Comparison of recordings from microelectrode arrays and single electrodes in the visual cortex. J Neurosci. 2007;27:261–4. doi: 10.1523/JNEUROSCI.4906-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuz T, Andrzejak RG, Mormann F, Kraskov A, Stogbauer H, Elger CE, et al. Measure profile surrogates: a method to validate the performance of epileptic seizure prediction algorithms. Phys Rev E. 2004;69:061915. doi: 10.1103/PhysRevE.69.061915. [DOI] [PubMed] [Google Scholar]
- Krieger D, Litt B. Seizure Prediction: Its Evolution and Therapeutic Potential. In: Shorvon S, Pedely T, editors. Blue Book of Neurology: the epilepsies 3. Philadelphia: Saunders; 2008. [Google Scholar]
- Kuhlmann L, Freestone D, Lai A, Burkitt AN, Fuller K, Grayden DB, et al. Patient-specific bivariate-synchrony-based seizure prediction for short prediction horizons. Epilepsy Res. 2010;91:214–31. doi: 10.1016/j.eplepsyres.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Martinerie J, Navarro V, Boon P, D’Have M, Adam C. Anticipation of epileptic seizures from standard EEG recordings. Lancet. 2001;357:183–8. doi: 10.1016/S0140-6736(00)03591-1. [DOI] [PubMed] [Google Scholar]
- Lilly JM, Olhede SC. On the analytic wavelet transform. IEEE T Inform Theory. 2010;57:4135–56. [Google Scholar]
- Maiwald T, Winterhalder M, Aschenbrenner-Scheibe R, Voss HU, Schulze-Bonhage A, Timmer J. Comparison of three nonlinear seizure prediction methods by means of the seizure prediction characteristic. Physica D. 2004;194:357–68. [Google Scholar]
- Mallat S, Hwang WL. Singularity detection and processing with wavelets. IEEE T Inform Theory. 1992;38:617–43. [Google Scholar]
- Martinerie J, Adam C, Le Van Quyen M, Baulac M, Clemenceau S, Renault B, et al. Epileptic seizures can be anticipated by non-linear analysis. Nat Med. 1998;4:1173–6. doi: 10.1038/2667. [DOI] [PubMed] [Google Scholar]
- Mormann F, Kreuz T, Andrzejak RG, David P, Lehnertz K, Elger CE. Epileptic seizures are preceded by a decrease in synchronization. Epilepsy Res. 2003;53:173–85. doi: 10.1016/s0920-1211(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: the long and winding road. Brain. 2007;130:314–33. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Rothman SM. Seizure Prediction in Epilepsy. Wiley-VCH Verlag GmbH & Co. KGaA; 2008. Beyond Prediction – Focal Cooling and Optical Activation to Terminate Focal Seizures; pp. 269–282. [Google Scholar]
- Schelter B, Winterhalder M, Maiwald T, Brandt A, Schad A, Schulze-Bonhage A, et al. Testing statistical significance of multivariate time series analysis techniques for epileptic seizure prediction. Chaos. 2006;16:013108. doi: 10.1063/1.2137623. [DOI] [PubMed] [Google Scholar]
- Schulze-Bonhage A, Kühn A. Seizure Prediction in Epilepsy. Wiley-VCH Verlag GmbH & Co. KGaA; 2008. Unpredictability of Seizures and the Burden of Epilepsy; pp. 1–10. [Google Scholar]
- Snyder DE, Echauz J, Grimes DB, Litt B. The statistics of a practical seizure warning system. J Neural Eng. 2008;5:392–401. doi: 10.1088/1741-2560/5/4/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–97. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EM, Wozniak SE, Roberts CM, Kao A, Anderson VC, Selden NR. Vagus nerve stimulation for partial and generalized epilepsy from infancy to adolescence. J Neurosurg Pediatr. 2012;10:200–5. doi: 10.3171/2012.5.PEDS11489. [DOI] [PubMed] [Google Scholar]
- Winterhalder M, Maiwald T, Voss HU, Aschenbrenner-Scheibe R, Timmer J, Schulze-Bonhage A. The seizure prediction characteristic: a general framework to assess and compare seizure prediction methods. Epilepsy Behav. 2003;4:318–25. doi: 10.1016/s1525-5050(03)00105-7. [DOI] [PubMed] [Google Scholar]