Abstract
Analysis of 327 consecutive cases at a pediatric referral hospital of Guatemala reveals that retinoblastoma accounts for 9.4% of all cancers and the estimated incidence is 7.0 cases/million children, higher than the United States or Europe. The number of familial cases is low, and there is a striking disparity in indigenous children due to late diagnosis, advanced disease, rapid progression and elevated mortality. Nine germline mutations in 18 patients were found; two known and five new mutations. Hypermethylation of RB1 was identified in 13% of the tumors. An early diagnosis program could identify cases at an earlier age and improve outcome of retinoblastoma in this diverse population.
Keywords: RB1 gene, mutations, methylation, Guatemala, ethnicity, health disparity
1. Introduction
Retinoblastoma (RB: OMIM +180200) is the most common pediatric ophthalmological cancer, and represents a significant proportion of pediatric cancers in several developing countries [5, 11, 13]. Retinoblastoma, is typically diagnosed before age five, and exists in inherited and sporadic forms. Inherited RB accounts for 40% of cases and results from dominantly inherited germline mutations in RB1, is associated with bilateral disease, and early onset. Sporadic disease presents with unilateral tumors, with somatic alterations in both RB1 gene alleles, and no family history [8]. Nevertheless, 10 to 15% of hereditary cases exhibit a unilateral pattern and cannot be distinguished from the sporadic form without molecular studies.
The high heterogeneity underlying RB1 inactivation (over 2750 known mutations) makes molecular testing of RB a challenge (http://rb1-lovd.d-lohmann.de/). And in fact, gross deletion or duplication, promoter methylation of the RB1 gene and MYCN amplification without RB1 mutation have been identified in RB tumors [2, 4, 7, 10, 14, 16, 17]. The aim of the current study was to understand the incidence of retinoblastoma in Guatemala and the nature of the RB1 mutations in patients with this intraocular tumor.
2. Materials and methods
2.1 Subjects
We examined consecutive medical records from 2000–2012 in the cancer registry of the major pediatric oncology hospital, Unidad Nacional de Oncología Pediátrica (UNOP) in Guatemala City. UNOP is the only dedicated pediatric oncology hospital in the country, care is free-of-charge, and transportation, housing and nutritional assistance are also provided. All retinoblastoma cases diagnosed in ophthalmology clinic and hospitals refer to UNOP. UNOP specialists have access to laser and cryotherapy, localized radiotherapy, imaging (RetCam) and telemedicine contact with an international team of experts (Orbis and cure4Kids.com). All patients are documented in an electronic registry supported in part by the International Outreach Program of St. Jude Children’s Research Hospital (Memphis, USA). Therefore, we estimate that over 90% of diagnosed cases of retinoblastoma in the country are entered in the registry. The Guatemala City region encompassed 20% of the pediatric population of the country, is expected to have a very low rate of undiagnosed retinoblastoma, and was therefore used for incidence estimation.
The study was conducted with the approval of the ethic and research committee of UNOP, the NIH Office of Human Research Studies and Stanford University. Patients (with parental consent for minors) were consented and enrolled by trained investigators in small groups. Nearly all indigenous parents of patients speak and understand Spanish, and Spanish-Mayan interpreters are available when required. We have documented approximately 5% of adults (including indigenous adults) refusing to participate, indicating that comprehension of the voluntary nature of the study is achieved. All identifying information remains in the cancer registry and all samples are coded to maintain privacy. Clinical and genetic counseling is provided by staff oncologists, as needed.
2.2 Patients and families used for genetic analysis
To identify the spectrum of germline RB1 mutations in patients from Guatemala we included blood or saliva DNA from 18 cases and their parents. The germline DNAs were identified as part of an ongoing collection of cases and family members initiated in 2009 [6]. For epigenetic analysis 18 formalin-fixed, paraffin-embedded (FFPE) tumor specimens were available stored samples from patients that have undergone enucleation surgery. There is no overlap between the germline and tumor DNA samples. Families were self-identified as being either indigenous or admixed and checked against cancer registry data on languages spoken by parents and grandparents and in the household.
2.3 Incidence Estimation
Incidence of retinoblastoma and acute lymphocytic leukemia (ALL), as a comparison group, were estimated by calculating observed cases/million children under the age of 14. There were 327 RB and 1264 ALL cases from the same time period for comparison. In total, 409 of the ALL cases were from the Guatemala capital region (estimated ALL incidence=33.5 (34.2 in admixed and 28.7 in indigenous)). Guatemalan census data (http://www.ine.gob.gt/np/poblacion/index.htm) was used for the numerator and for age-correction. To determine estimated incidences for admixed and indigenous populations by region of Guatemala, the estimated percentage of the indigenous population for each department (22 political sub-divisions) from census data was used. Age correction with US population figures was calculated as described (http://seer.cancer.gov/seerstat/tutorials/aarates/step1.html)
2.4 Occupational exposures and outcome
Father’s occupation was available in the registry for 219 of the 327 retinoblastoma cases and 912 ALL cases. Agriculture was the only occupation frequent enough for analysis and the data for the two groups were compared by Chi square statistics. Outcome was assessed by last known status of the patient, stage of disease at presentation, as well as by survival statistics. Last-known status of indigenous and admixed cases was tabulated both with and without elimination of cases lost to follow-up, abandonment of therapy or transfer to another hospital. Both comparisons showed significantly higher mortality in indigenous patients. Stage at presentation (St Jude’s Staging) was also tabulated for both groups. For survival analysis, date of diagnosis was used as a baseline and death as the outcome. Analysis was performed in STATA (StataCorp, College Station, TX).
2.5 DNA Extraction and Sequencing
Genomic DNA from saliva (DNA Genotek Inc. Ontario, Ca.) and tumor DNA (QIAamp DNA FFPE Tissue Kit, Qiagen, CA, USA) was extracted according the manufacturer’s instructions. The entire RB1 gene was sequenced using an ABI PRISM 3130XL (Applied Biosystems, Foster City, CA, USA) and sequence data was analyzed using Mutation Surveyor V9.1 software (Soft Genetics, State College, PA). Exon 15 was analyzed by using TOPO TA cloning® and sequencing of 10 clones from each patient. Mutations were named according to the genomic RB1 sequence with GenBank reference (L11910) and the RB1 mutation database LOVD2 (http://rb1-lovd.d-lohmann.de/home.php).
Molecular analysis included 18 genomic DNA and 18 tumors samples from 36 unrelated Guatemalan patients with retinoblastoma, 6 (17%) with bilateral and 32 (83%) with unilateral tumors. No bilateral and only one unilateral case had a family history of retinoblastoma. Mean age of RB onset was 33 months among all cases (bilateral: 32 and unilateral: 33 months). Notably, only one (17%) bilateral patient was diagnosed at <1 year old. To assess the DNA methylation status of the RB1 promoter, tumor DNA was analyzed and bisulfite analysis was performed [Zymo EZ ADN Methylation-Gold™ (Zymo Research; Irvine, CA)] with gene-specific primers (Supplementary Table 1). Three tumors did not yield adequate DNA quantity for analysis. Mutations were compared with data in the RB1 mutation database, and newly described missense variants analyzed by SIFT (http://sift.jcvi.org/www/SIFT_seq_submit2.html).
3. Results
3.1 Retinoblastoma in Guatemala: Clinical characteristics and estimated incidence
To determine the frequency of retinoblastoma in Guatemala, 327 consecutive RB patients diagnosed from the UNOP cancer registry from 2000–2012 were examined. Retinoblastoma accounted for 9.4% of all cancer cases during this period; and is the most common solid tumor. The estimated incidence of RB in the Guatemalan capital department is 7.0 cases/million children under the age of 14 (6.7 cases/million, age-adjusted) (Table 1).
Table 1.
Estimated Incidence of retinoblastoma and ALL in Guatemala from 2000–2012
| Department | Cases | Admixed | Indigenous | Population <14 | Estimated Incidence | Adm. Inc. | Ind. Inc. |
|---|---|---|---|---|---|---|---|
| ALTA VERAPAZ | 14 | 2 | 12 | 496,634 | 2.4 | - | 2.3 |
| BAJA VERAPAZ | 10 | 6 | 4 | 118,154 | 7.1 | 9.6 | - |
| CHIMALTENANGO | 19 | 8 | 11 | 263,098 | 6.0 | 11.2 | 4.5 |
| CHIQUIMULA | 7 | 6 | 1 | 157,963 | 3.7 | 4.5 | - |
| EL PROGRESO | 4 | 4 | 0 | 60,615 | - | - | - |
| El QUICHE | 41 | 13 | 28 | 443,546 | 7.7 | 14.4 | 6.3 |
| ESCUINTLA | 6 | 5 | 1 | 254,221 | 2.0 | 1.8 | - |
| GUATEMALA | 85 | 75 | 10 | 1,016,936 | 7.0 | 7.0 | 6.8 |
| HUEHUETENANGO | 26 | 18 | 8 | 493,222 | 4.4 | 8.5 | 2.1 |
| IZABAL | 9 | 7 | 2 | 170,073 | 4.4 | 4.5 | - |
| JALAPA | 10 | 10 | 0 | 141,725 | 5.3 | 10.8 | - |
| JUTIAPA | 11 | 11 | 0 | 184,761 | 4.5 | 4.8 | - |
| PETEN | 9 | 9 | 0 | 273,323 | 2.7 | 3.7 | - |
| QUETZALTENANGO | 18 | 10 | 8 | 311,118 | 4.8 | 6.7 | 3.6 |
| RETALHULEU | 7 | 4 | 3 | 118,895 | 4.9 | - | - |
| SACATEPEQUEZ | 6 | 4 | 2 | 117,327 | 4.3 | - | - |
| SAN MARCOS | 14 | 12 | 2 | 422,176 | 2.8 | 4.2 | - |
| SANTA ROSA | 8 | 8 | 0 | 139,814 | 4.8 | 4.9 | - |
| SOLOLA | 6 | 4 | 2 | 187,357 | 2.7 | - | - |
| SUCHITEPEQUEZ | 10 | 7 | 3 | 210,668 | 4.0 | 6.4 | - |
| TOTONICAPAN | 8 | 3 | 5 | 208,296 | 3.2 | - | 2.1 |
| ZACAPA | 1 | 1 | 0 | 86,737 | - | - | - |
| Total RB | 327 | 225 | 102 | 5,876,659 | 4.6 | 5.7 | 3.3 |
| Total ALL | 1264 | 914 | 350 | 5,876,659 | 17.9 | 23.1 | 11.3 |
Adm. Inc. = Estimated admixed incidence; Ind. Inc.=Estimated indigenous incidence, Values only calculated for cells with 5 or more entries.
Approximately 40% of Guatemalans are indigenous from one of 22 different, mostly Mayan, ethnic groups [3], while the remainder of the population is admixed (European, Amerindian and to a lesser extent, African). In the Department of Guatemala, the crude incidence of retinoblastoma in admixed and indigenous populations is similar, 7.0 and 6.8, respectively. However, in departments where there were enough cases of each ethnicity to compare, the apparent incidence in indigenous children was consistently lower (Table 1), particularly in regions far from the capital, probably reflecting lack of diagnosis or referral. We performed the same analysis with 741 consecutive cases of acute lymphoblastic leukemia (ALL), the most common cancer. The incidence of ALL under age 14 is 34/million in the Guatemala capital region, similar to Caucasian children in the US and UK (36–38/million) [http://info.cancerresearchuk.org/cancerstats/types/leukaemia/incidence/#trends] [9]. However, both RB and ALL show an apparent incidence much lower in the rest of the country, consistent with a significant under-ascertainment of rural and/or indigenous cases.
The cases studied were 51% female, 31% indigenous, and 24% had bilateral disease (Table 2). As expected, bilateral retinoblastoma cases are diagnosed at an earlier age and unilateral cases show an older age distribution. Indigenous unilateral cases show the oldest age of onset, and only 17% of indigenous unilateral cases are diagnosed by the age of 2 compared to 35% for admixed children and 63% in the US (Table 2, data not shown).
Table 2.
Demographics and survival data of Study Subjects
| All subjects N (%) | Indigenous N (%) | Admixed N (%) | P value | ||
|---|---|---|---|---|---|
| Total | 356 (100) | 102 (31) | 225 (69) | ||
| Laterality | Unilateral | 239 (76) | 74 (73) | 165 (78) | 0.54 |
| Bilateral | 75 (24) | 28 (27) | 47 (22) | ||
| Gender | Feminine | 168 (51) | 57 (56) | 111 (49) | 0.27 |
| Masculine | 159 (49) | 45 (44) | 114 (51) | ||
| Age of Diagnosis (yrs) | 2.8 | 3.2 | 2.3 | ||
| Unilateral | 3.1 | 3.5 | 3.1 | 0.85 | |
| Bilateral | 1.8 | 2.3 | 1.6 | ||
| RB cases | ALL cases | ||||
| Occupation of Father | Agriculture | 110 (50) | 335 (37) | 0.02 | |
| Data on occupation | 219 | 912 | |||
| Survival Dataa | Alive | Deceased | % Deceased | ||
| Indigenous | 29 | 55 | 65% | 0.00011 | |
| Admixed | 103 | 68 | 40% | ||
| Unilateral | 101 | 84 | 45% | ||
| Bilateral | 29 | 29 | 50% | ||
| Indigenous Unilateral | 25 | 36 | 59% | 0.0091 | |
| Admixed Unilateral | 76 | 48 | 39% | ||
| Indigenous Bilateral | 4 | 18 | 82% | 0.00015 | |
| Admixed Bilateral | 25 | 11 | 31% | ||
| Stage Global SJ | Admixed | Indigenous | |||
| I | 66 | 17 | |||
| II | 16 | 15 | |||
| III | 14 | 13 | |||
| IV | 4 | 3 | |||
| I | II, III, IV | ||||
| Admixed | 66 | 34 | 0.00045 | ||
| Indigenous | 17 | 31 |
Last known status, after elimination of cases that have abandoned therapy
An elevated incidence of retinoblastoma could be due to 1) an increased number of familial cases due to a common founder mutation(s); 2) a unique environmental factor(s) contributing to disease; or 3) an epigenetic or unique molecular mechanism of disease such as MYCN amplification reported in some non-RB1 mutated tumors [17]. We determined that only 8 of the cases derived from familial retinoblastoma out of the 327 cases, a frequency lower than typically reported, ruling out explanation 1 above.
To begin to understand environmental factors that may play a role in retinoblastoma we examined the father’s occupation (Table 2). There is an association with agriculture in the children with retinoblastoma; 50% of retinoblastoma cases have a father engaged in farming compared to 37% of the ALL cases (p= 0.019). Detailed analysis of the environment of cases and controls would be needed to further explore an environmental etiology
3.2 Molecular characteristics of selected tumors
To determine if the molecular etiology of the disease is similar to other countries, the RB1 gene was sequenced and nine germline oncogenic variations were detected in eight out of 18 patients (44%; Table 3). Bilateral patients (1/2, 50%) and unilateral patients (7/16, 44%) both displayed mutations, and one of the unilateral cases with an identified mutation has familial disease. Three mutations were insertion/deletions (p.Ala17ProfsX3, p.Glu19ThrfsX10 and p.Phe198PhefsX4 and four were single base substitutions (p.Ser648X, c.607+1G>C, c.2326-2A>G). The only bilateral mutation carrier harbored a nonsense mutation (p.Ser648X) and was diagnosed at 2.6 years of age and the mutation was confirmed in the patient’s parent, who had RB diagnosed at 23 years of age. Because RB1 can be affected by larger in/dels MLPA was used on a total of 18 patients to identify germline copy number variants. However, no gains and losses were detected (data not shown).
Table 3.
Mutations identified in RB patients
| Patient | Exon | DNA position (GRCh37) | Mutation | Consequence | Reference# |
|---|---|---|---|---|---|
| AP701348 | Exon 1 | g. 48878093 | c.45_79 del TGCCGCCGCGGAACCCCC GGCACCGCCGCCGC/- |
p.A15X p.A16fs*3 |
COSM29200 |
| AP700763 | Exon 1 | g. 48878103 | C.55_57delGA | p.E19X* | |
| AP700069 | Exon 6 | g. 48923151 | c.600_601insT TTA >TTT |
p.L200fs | |
| AP700815 | Intron 6 | g. 48923159 | c.607+1 G>T | Splicing | |
| AP700815 | Exon 15 | g. 48954199 | c.1420 A>G AGC>GGC |
p.S474G | |
| AP700826 | Exon 17 | g.48955529 | c.1646 A>T CAT>CTT |
p. H549L | |
| AP700603 | Exon 19 | g. 49030468* | c.1942 C>A TCA>TAA |
p. S648X | |
| AP700759 | Exon 19 | g. 49030457 | C.1931 C>A TCT>TAT |
P.S644Y | |
| AP700480 | Exon 25 | g.49050889 | c.2272 G/A GTG> GAG |
p.V858M |
de novo mutation;
Catalogue of somatic mutations in cancer (COSMIC)
We assessed the somatic methylation of the RB1 promoter by using methylation-specific PCR in 15 RB tissue samples; and identified two hypermethylation events (2/15; 13%). Both patients with methylated RB1 were of admixed ethnicity and diagnosed as unilateral RB. Thirteen tumor samples (13/15; 87%) did not exhibit gene hypermethylation (Table 4).
Table 4.
Methylation changes in RB Tumors.
| Samples Id | Ethnicity | Phenotype | % Methylation |
|---|---|---|---|
| EO-07-2584 | Admixed | Unilateral | 0.04 |
| EO-10-2778 | Admixed | Unilateral | 0.43 |
| 2475 | Admixed | Bilateral | 0.05 |
| 3304 | Admixed | Bilateral | 0.06 |
| EO-07-1624 | Admixed | Unilateral | 0.01 |
| EO-07-3700 | Indigenous | Unilateral | 0.03 |
| EO-08-2510 | Admixed | Unilateral | 0.02 |
| EO-10-2108 | Admixed | Unilateral | 81.51 |
| EO-08-447 | Admixed | Unilateral | 0.3 |
| 1042 | Admixed | Unilateral | 0.4 |
| EO-07-1518 | Admixed | Bilateral | 0.2 |
| EO-08-576 | Admixed | Unilateral | Undetermined |
| EO-08-1413 | Admixed | Unilateral | 37.7 |
| EO-07-2550 | Indigenous | Unilateral | 0.5 |
| 3340 | Admixed | Bilateral | Undetermined |
| EO-07-2769 | Admixed | Unilateral | 0.3 |
| EO-07-3639 | Admixed | Unilateral | 0.1 |
| EO-10-2917 | Admixed | Unilateral | Undetermined |
Bold character: Hypermethylation > 25%
3.3 Disease stage and progression by ethnicity
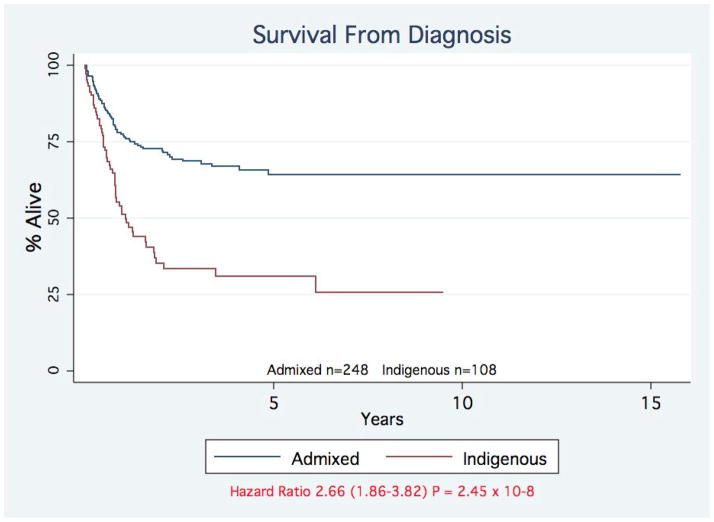
To support our hypothesis that the lower apparent incidence of rural indigenous retinoblastoma cases is due to late or absent diagnosis, we compared the mortality and stage of diagnosis of indigenous and admixed cases. We found a significantly higher mortality of indigenous cases (65 vs. 40%, P= 0.00011; Table 2). Survival analysis from diagnosis demonstrated a highly significant difference [Hazard Ratio 2.66 (1.86–3.82) P = 2.45×10-8] for indigenous cases (Figure 1). Both indigenous unilateral and bilateral cases show a significantly increased mortality (Table 2). This disparity is reflected in the stage of diagnosis with significantly more indigenous cases being diagnosed at stage II, III, and IV (P=0.00045). Survival of indigenous and admixed cases, adjusted for stage of diagnosis was not significantly different, demonstrating that care after diagnosis is equivalent (Supplemental Figure 1).
Figure 1. Survival of Indigenous and admixed patients with retinoblastoma.
The survival of patients with retinoblastoma is shown by ethnicity. Admixed children (blue line); indigenous children (red line). Hazard Ratio 2.66 (1.86–3.82) P = 2.45×10−8.
4. Discussion
This work presents the first comprehensive study of retinoblastoma in Guatemala. As in several low and middle income countries, such as Mexico (22%), Nigeria (20%) and India (13%); retinoblastoma accounts for a large percentage of pediatric cancer cases. In Guatemala retinoblastoma is 9.4% of all cancers, more than double that in high income countries (3–5%) [5, 9, 15, 18]. Because only 8 (2.4%) of the 327 cases had a sibling with RB, familial cases do not account for the elevated incidence in Guatemala. We also did not see gross epigenetic changes (only 13% of 15 tumors have a methylated RB1 gene), and find typical germline mutations, indicating that the molecular basis of the disease is not grossly different. But we do find that a significantly larger percentage of retinoblastoma cases have a father employed in agriculture, suggesting that there may be environmental/dietary factors for the disease. There is evidence for a larger percentage of undiagnosed ALL cases than RB, contributing to the higher relative fraction of patients diagnosed with RB.
Access to healthcare screening may confound our case ascertainment. Moreover, low income level, education status and limited accessibility to medical services in Guatemala likely influence our findings. While we believe that the majority of diagnosed retinoblastoma cases in the Guatemala capital region are included in the hospital’s cancer registry, there is a need for a nationwide cancer registry to improve studies of cancer incidence.
Economic disadvantage, a rural location and membership in minority and/or indigenous populations are all associated with under-diagnosis, and poor treatment outcomes for pediatric cancers [13]; and our data from Guatemala support this. There is a clear trend of earlier ascertainment of urban and non-indigenous cases as compared to rural and isolated regions. This disparity is supported by a highly significant increase in death as an outcome for indigenous RB cases, that holds for both unilateral and bilateral disease. In addition, indigenous children with RB present at a much higher rate with stage II or greater disease, and more often require palliative care. Undoubtedly language barriers, access to health care, transportation and communication barriers contribute to this disparity. However, once diagnosed and treated at UNOP, indigenous children have similar stage-corrected survival (Supplemental Figure 1). To begin to address late diagnosis, a pilot program with posters in Spanish and three major Mayan languages has been initiated and resulted in earlier diagnosis of at least two cases (data not shown, Supplemental Figure 2).
The pattern and the nature of mutations are very similar to those reported in other populations. The main mutation type are frameshifts (43%), followed by splicing and non-sense (29%) alterations, and 67% of all of them were newly described mutations [1, 12]. Typically, nonsense and frameshift mutations lead to bilateral tumors because of the complete absence of the RB protein, some splicing mutations are associated with incomplete penetrance and milder expressivity due to residual function of RB1.
By using the SIFT program, the rare exonic variants we described were not predicted to be pathogenic. However, the role of the synonymous variants in the etiology of RB require further evaluation because there is evidence showing that certain synonymous SNPs involving frequent-to-rare codon substitutions may result in ribosome stalling, due to either a lower concentration of cognate tRNAs or an alteration of the RNA structure [19].
Taken together, the high incidence, late onset, high percentage of sporadic retinoblastoma and low mutation detection rate in the RB1 gene of Guatemalan retinoblastoma patients supports the hypothesis that other genes or environmental factors may contribute to the disease. Although amplification of MYCN gene has been described in some tumors, at least in North American and European patients non-RB1 mechanisms account for less than 3% of retinoblastoma [17].
In conclusion, we document an elevated incidence of retinoblastoma in Guatemala, but provide evidence of under-diagnosis in rural and indigenous groups suggesting that the actual incidence may be higher. We identified sequence alterations, but no gross insertions or deletions of the RB1 gene, as mechanisms of germline inactivation. Because of the apparent elevated RB incidence, the involvement of other molecular mechanisms, viruses or lifestyle in retinoblastoma etiology in Guatemala needs to be further evaluated in larger cohorts.
Supplementary Material
Acknowledgments
The authors thank the patients and their families who participated in the present study, as well as the cancer registry and staff of UNOP, Patricia Zaid and Martha Balsells de Sechel for assistance in sample collection and shipping, and Bert Gold, Peggy Tucker, Carlos Rodriguez-Galindo and Federico Antillon-Klussmann for comments on the manuscript. Supported in part by the Intermural Research Program, National Institutes of Health; the St. Judes International Outreach Program, and the Department of Pediatrics, Stanford University.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest regarding this manuscript to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abidi O, Knari S, Sefri H, Charif M, Senechal A, Hamel C, Rouba H, Zaghloul K, El Kettani A, Lenaers G, Barakat A. Mutational analysis of the RB1 gene in Moroccan patients with retinoblastoma. Molecular vision. 2011;17:3541–3547. [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht P, Ansperger-Rescher B, Schuler A, Zeschnigk M, Gallie B, Lohmann DR. Spectrum of gross deletions and insertions in the RB1 gene in patients with retinoblastoma and association with phenotypic expression. Human mutation. 2005;26:437–445. doi: 10.1002/humu.20234. [DOI] [PubMed] [Google Scholar]
- 3.M.d.E.G. The Road of the future-Shared challenges. Informe Nacional república de Guatemala, Ministerio de Educación-MINEDUC; Guatemala: 2008. CA, Inclusion Educativa: El camino del futuro un desafio para compartir; p. 57. [Google Scholar]
- 4.Cohen Y, Merhavi-Shoham E, Avraham RB, Frenkel S, Pe’er J, Goldenberg-Cohen N. Hypermethylation of CpG island loci of multiple tumor suppressor genes in retinoblastoma. Experimental eye research. 2008;86:201–206. doi: 10.1016/j.exer.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Fajardo-Gutierrez A, Juarez-Ocana S, Gonzalez-Miranda G, Palma-Padilla V, Carreon-Cruz R, Ortega-Alvarez MC, Mejia-Arangure JM. Incidence of cancer in children residing in ten jurisdictions of the Mexican Republic: importance of the Cancer registry (a population-based study) BMC cancer. 2007;7:68. doi: 10.1186/1471-2407-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido C, Santizo VG, Mullers P, Soriano DR, Avila GB, Dean M, Jimenez-Morales S. Frequency of thiopurine S-methyltransferase mutant alleles in indigenous and admixed Guatemalan patients with acute lymphoblastic leukemia. Medical oncology. 2013;30:474. doi: 10.1007/s12032-013-0474-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph B, Mamatha G, Raman G, Shanmugam MP, Kumaramanickavel G. Methylation status of RB1 promoter in Indian retinoblastoma patients. Cancer biology & therapy. 2004;3:184–187. doi: 10.4161/cbt.3.2.620. [DOI] [PubMed] [Google Scholar]
- 8.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004) Cancer. 2008;112:416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 10.Livide G, Epistolato MC, Amenduni M, Disciglio V, Marozza A, Mencarelli MA, Toti P, Lazzi S, Hadjistilianou T, De Francesco S, D’Ambrosio A, Renieri A, Ariani F. Epigenetic and copy number variation analysis in retinoblastoma by MS-MLPA. Pathology oncology research: POR. 2012;18:703–712. doi: 10.1007/s12253-012-9498-8. [DOI] [PubMed] [Google Scholar]
- 11.Luna-Fineman S, Barnoya M, Bonilla M, Fu L, Baez F, Rodriguez-Galindo C. Retinoblastoma in Central America: report from the Central American Association of Pediatric Hematology Oncology (AHOPCA) Pediatric blood & cancer. 2012;58:545–550. doi: 10.1002/pbc.23307. [DOI] [PubMed] [Google Scholar]
- 12.Macias M, Dean M, Atkinson A, Jimenez-Morales S, Garcia-Vazquez FJ, Saldana-Alvarez Y, Ramirez-Bello J, Chavez M, Orozco L. Spectrum of RB1 gene mutations and loss of heterozygosity in Mexican patients with retinoblastoma: identification of six novel mutations. Cancer biomarkers: section A of Disease markers. 2008;4:93–99. doi: 10.3233/cbm-2008-4205. [DOI] [PubMed] [Google Scholar]
- 13.Magrath I, Steliarova-Foucher E, Epelman S, Ribeiro RC, Harif M, Li CK, Kebudi R, Macfarlane SD, Howard SC. Paediatric cancer in low-income and middle-income countries. The lancet oncology. 2013;14:e104–116. doi: 10.1016/S1470-2045(13)70008-1. [DOI] [PubMed] [Google Scholar]
- 14.Mairal A, Pinglier E, Gilbert E, Peter M, Validire P, Desjardins L, Doz F, Aurias A, Couturier J. Detection of chromosome imbalances in retinoblastoma by parallel karyotype and CGH analyses. Genes, chromosomes & cancer. 2000;28:370–379. [PubMed] [Google Scholar]
- 15.Mohammed AZ, Edino ST, Ochicha O, Gwarzo AK, Samaila AA. Cancer in Nigeria: a 10-year analysis of the Kano cancer registry. Nigerian journal of medicine: journal of the National Association of Resident Doctors of Nigeria. 2008;17:280–284. doi: 10.4314/njm.v17i3.37396. [DOI] [PubMed] [Google Scholar]
- 16.Ohtani-Fujita N, Dryja TP, Rapaport JM, Fujita T, Matsumura S, Ozasa K, Watanabe Y, Hayashi K, Maeda K, Kinoshita S, Matsumura T, Ohnishi Y, Hotta Y, Takahashi R, Kato MV, Ishizaki K, Sasaki MS, Horsthemke B, Minoda K, Sakai T. Hypermethylation in the retinoblastoma gene is associated with unilateral, sporadic retinoblastoma. Cancer genetics and cytogenetics. 1997;98:43–49. doi: 10.1016/s0165-4608(96)00395-0. [DOI] [PubMed] [Google Scholar]
- 17.Rushlow DE, Mol BM, Kennett JY, Yee S, Pajovic S, Theriault BL, Prigoda-Lee NL, Spencer C, Dimaras H, Corson TW, Pang R, Massey C, Godbout R, Jiang Z, Zacksenhaus E, Paton K, Moll AC, Houdayer C, Raizis A, Halliday W, Lam WL, Boutros PC, Lohmann D, Dorsman JC, Gallie BL. Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. The lancet oncology. 2013;14:327–334. doi: 10.1016/S1470-2045(13)70045-7. [DOI] [PubMed] [Google Scholar]
- 18.Schultz KR, Ranade S, Neglia JP, Ravindranath Y. An increased relative frequency of retinoblastoma at a rural regional referral hospital in Miraj, Maharashtra, India. Cancer. 1993;72:282–286. doi: 10.1002/1097-0142(19930701)72:1<282::aid-cncr2820720149>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 19.Tsai CJ, Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM, Nussinov R. Synonymous mutations and ribosome stalling can lead to altered folding pathways and distinct minima. Journal of molecular biology. 2008;383:281–291. doi: 10.1016/j.jmb.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.