Abstract
Background
Several studies demonstrate the utility of Alcohol Monitoring System's (AMS) transdermal alcohol concentration (TAC) monitor to objectively quantify drinking. AMS standard criteria (i.e., TAC > 0.02 g/dl) used for drinking detection are deliberately conservative, but consequently only detect drinking equivalent to 5 or more standard drinks. Our study sought to characterize the sensitivity of TAC measurement to detect low-level drinking defined as the consumption of 1-3 beers.
Methods
Data were pooled from three studies giving controlled doses of 1, 2, 3, 4, and 5 Corona© beers (12-oz. = 0.92 standard units) to 32 male and 29 female research volunteers wearing TAC monitors under controlled conditions. Analyses describe the sensitivity to detect drinking at various peak TAC thresholds beginning with any positive reading greater than 0 g/dl, and then by using TAC thresholds of 0.02 and 0.03 g/dl.
Results
Nearly 40% of participants drinking one beer did not have a positive TAC reading. However, positive TAC readings were observed in more than 95 and in 100% of participants drinking 2 and 3 or more beers respectively. The probability of peak TAC detection was a positive function of the number of beers consumed and a negative function of the minimum TAC threshold for detection. Drinking was somewhat more likely to be detected in females than males drinking 2-5 beers, but not after 1 beer. Use of AMS standard criteria only reliably detected the consumption of 5 beers, and 45.9% of all occasions of drinking 1-3 beers were undetected using 0.02 g/dl as a threshold.
Conclusions
Peak TAC-levels between zero and 0.02 g/dl must be considered to detect the low-level drinking of 1-3 standard drinks and such thresholds are necessary when researchers and clinicians want to detect low-level drinking.
Keywords: Alcohol Consumption, Transdermal Alcohol Monitoring, Transdermal Alcohol Concentration (TAC), Heavy Drinking, Low-Level Drinking
Introduction
Transdermal alcohol monitors have created new opportunities for the research on and treatment of problematic alcohol use. These monitors provide objective measures of transdermal alcohol concentrations (TAC) derived from alcohol excretion through the skin (Swift, 2000; 2003). The Secure Continuous Remote Alcohol Monitor (SCRAM), produced by Alcohol Monitoring Systems (AMS) Inc., is the most validated device on the market (McKnight et al., 2012). SCRAM automatically records TAC data every 30 minutes, 24-hr/day, 7 days/week in a digital format which is uploaded to an AMS Web server. AMS reviews uploaded TAC data and confirms whether or not a drinking event has occurred using proprietary methods.
Recently, the criminal justice system began using SCRAM-based TAC monitoring (Marques & McKnight, 2007; McKnight et al., 2012) to verify abstinence in driving while intoxicated (DWI) offenders who are court-ordered to remain abstinent during pretrial or probation supervision periods. Judges and probation/parole officers use TAC monitoring in conjunction with alcohol treatment services to minimize incarceration while still protecting the public from repeat offenders (Huddleston et al., 2008; Voas et al., 2011). Because of this use, AMS reports of a confirmed drinking event are deliberately conservative resulting in reliable detection only when approximately five standard drinks are consumed within two to three hours (Barnett et al., 2014; McKnight et al., 2012). One consequence of this conservative AMS standard is that lower levels of drinking (i.e., less than 5 standard drinks) will go undetected and the absence of AMS-confirmation does not mean abstinence from drinking. It also means that clinical research seeking to distinguish low-level drinking from either abstinence or heavy drinking cannot rely upon the standard of AMS confirmation of drinking.
Recently, Barnett and colleagues (2011) used SCRAM to implement contingency management procedures to decrease the frequency of drinking in a small sample of heavy drinkers. These investigators modified the AMS criteria to make them less conservative by requiring only one TAC reading above a 0.02 g/dl threshold rather than the AMS criteria of 3 or more TAC readings above 0.02 g/dl and requiring only one instead of the two defined absorption and elimination rate standards. Despite these modified criteria, the investigators acknowledged that low-levels of drinking still go undetected (Barnett et al., 2011; 2014).
The ability to detect and monitor low-levels of drinking is important to distinguish heavy or harmful drinking from drinking at lower and safer levels (NIAAA 2004, 2013). Distinguishing low-level “social drinking” from heavy drinking also could be useful for research development of clinical interventions targeting heavy and harmful drinking. We recently conducted two SCRAM-based contingency management studies targeting TAC levels above 0.03 g/dl to reduce heavy drinking (Dougherty et al 2014, 2015). We found that reductions in heavy drinking were accompanied by both an increase in apparent abstinence and an increase in low-level drinking. The impact of this continued low-level drinking only can be assessed if it can be monitored.
The current paper used data pooled from three laboratory studies (Dougherty et al., 2012; Hill-Kapturczak et al., 2014a, b) in which known amounts of alcohol were consumed to determine the sensitivity of various TAC-thresholds to detect and identify low-level drinking (i.e., 1-3 beers). Consistent with NIAAA guidelines (NIAAA, 2004, 2013) identifying low-level drinking as 1-2 drinks a day and identifying 4-5 drinks consumed within a 2-hour period as heavy or “risky” drinking, our study employed within-subject evaluation of a range of drinking from 1 to 5 beers in both men and women.
Methods
Study design
Data for the current analysis was pooled from three studies (Dougherty et al., 2012; Hill-Kapturczak et al., 2014a, b) conducted in our research clinic between June 2009 and October 2013. Each study administered alcohol to paid research volunteers, under controlled laboratory conditions. Each study was conducted according to ethical standards for clinical research and participants provided written informed consent to a research protocol approved by the university Institutional Review Board. Basic eligibility criteria screened participants who were physically healthy with body mass indexes (BMI) ranging from18-30 kg/m2, who did not have DSM-IV psychiatric disorders, and were able to provide urine samples free from illicit drugs. Briefly: i) participants who reported regular patterns of drinking weekly with at least 1 day per month of “heavy” drinking (defined as ≥4 standard drinks for females and ≥5 for males), were ii) invited into the laboratory to drink on five weekdays (generally Monday-Friday), iii) to drink varying number (ranging from 1 to 5) of 12 oz Corona Beers© (= 0.92 standard units each), under iv) controlled, laboratory conditions which varied slightly from one study to another.
Drinking day procedures
All participants drank designated amounts of alcohol generally beginning at 8:30 a.m. For all three studies, males drank controlled doses of 1, 2, 3, 4, and 5 Corona© beers (12-oz. = 0.92 standard units each) across five days – while females drank only 1-4 beers in Study 1 but were given 1-5 beers in Studies 2 and 3. Study 1 adjusted for known sex differences in alcohol metabolism by controlling the pace of drinking successive beers with males drinking 1 beer every 24 min while females drank at a slower rate of 1 beer every 30 min. For Study 2, both males and females drank at the same rate (i.e., 1 beer every 24 min), and for Study 3 males and females were allowed to drink at whatever rate they wished as long as all drinks for each day were consumed. All studies measured breath alcohol concentrations (BrAC) repeatedly after drinking, and participants remained in the laboratory after drinking for 7 or more hours until BrAC levels were 0.02 g/dl or less. Additionally, all participants wore SCRAM ankle bracelets in the laboratory and in Studies 2 and 3 to confirm continued abstinence after leaving the laboratory each day.
SCRAM procedures
Study 1 used the SCRAM II device while Studies 2 and 3 used SCRAMx. Both devices used the same alcohol detection technology. All monitoring devices were supplied by AMS. All data were uploaded to the AMS website by study staff and were blindly reviewed by AMS for resolution and confirmation of possible drinking events according to their standard procedures. Digital data listing all TAC readings were downloaded by study staff and were used to confirm abstinence each day before alcohol dosing and for analysis of the peak TAC readings observed on each day after drinking the specified number of beers.
AMS resolutions to confirm drinking
The exact details of AMS procedures for the resolution and confirmation of drinking events are proprietary – but in brief, AMS resolves a possible drinking event only when three or more consecutive TAC readings exceed 0.02 g/dl. At the time of resolution, AMS determines whether there is evidence of environmental contamination or tampering and confirms a drinking event if the drinking event shows absorption and elimination rates which are: i) absorption rate ≤ 0.05 g/dl//hr; and ii) the elimination rate > 0.003 g/dl/hr and ≤ 0.025 g/dl/hr if peak TAC < 0.15 g/dl or ≤ 0.035 g/dl/hr if peak TAC ≥ 0.15 g/dl. More discussion of the AMS criteria are provided elsewhere (Swift, 2003; Swift & Swette, 1992; Barnett et al., 2014).
Data Analysis
Subject demographic characteristics for the three studies are compared using t-tests or χ2 tests. The analysis of the TAC and BrAC data focused on the maximum “peak” value for each participant observed on each day of drinking. For each sex (M, F), the experimental administration of the specified number of beers provided the true positive occurrence of drinking and sensitivity for detection was analyzed as the percent of participants exceeding a specified TAC or AMS criteria at that dose. Data were: i) the frequency with which TAC levels were positive (i.e., TAC > 0 g/dl), ii) the frequency with which TAC exceeded the commonly used 0.02 g/dl threshold (Barnett et al., 2011, 2014), and iii) the frequency with which TAC exceeded the 0.03 g/dl threshold our group employed in contingency management studies (Dougherty et al, 2014, 2015). We also examined the frequencies with which AMS resolved or confirmed a drinking event on each of the experimental days. Differences among these frequencies were tested for significance using χ2 tests. Because the experimental conditions insured that there were no instances of a positive TAC reading without actual alcohol consumption, specificity analyses were not possible with these data.
Results
Participant Characteristics
Table 1 displays participant demographics in the three studies used for this analysis. Consistent with San Antonio demographics, the sample was predominately white of Hispanic origin (63.9%) with a good balance of males and females across the studies. Participants' ages ranged from 21-47 years of age. The only significant difference (p < 0.001) between studies was the greater number of drinks per week observed in Study 2.
Table 1.
Participant Demographics across 3 Studies.
| Characteristics | Study 1 | Study 2 | Study 3 | Total | p-value |
|---|---|---|---|---|---|
| N | 21 | 21 | 19 | 61 | |
| % Males | 47.6% | 52.4% | 57.9% | 52.5% | ns |
| Age | 27.4 | 28.8 | 26.9 | 27.7 | ns |
| Years Education | 14.3 | 13.3 | 13.3 | 13.7 | ns |
| BMI | 24.5 | 25.2 | 24.8 | 24.8 | ns |
| % White | 61.9% | 57.1% | 73.7% | 63.9% | ns |
| % Hispanic | 66.7% | 57.1% | 47.4% | 63.9% | ns |
| # Drinks per Week | 12.7 | 26.8* | 17.5 | 19.1 | 0.001 |
Note.
Study 2 significantly (p < 0.05) different than Study 1 and 3.
Ns = not significant. BMI= body mass index.
Relationship of BrAC and TAC
Figure 1 shows scatter plots of the relationship between peak BrAC and peak TAC. Detectable (non-zero) BrAC levels were observed for all participants at all doses of beer consumption. However, TAC levels were zero (i.e. note points lying on the abscissa) for some participants after the consumption of one or two beers. Positive and significant (p < 0.008) regression slopes were obtained for each sex and the slopes of the two sexes were not different from each other.
Figure 1. Relationship between Peak Breath Alcohol and peak TAC.
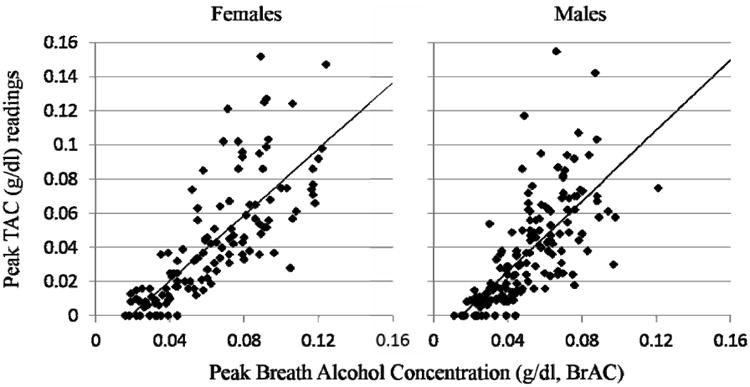
Scatter-plots of individual observations of peak breath alcohol concentrations (pkBrAC, in % blood alcohol concentration) and transdermal alcohol concentration (pkTAC) readings (in g/dl) for Female and Male Participants. Data include all observations of drinking 1-5 beers for each of 29 Female and 32 Male participants. The Diagonal line represents unity, and the vertical boxes highlight those observations occurring with pkBrAC ≤ 0.06 %.
TAC relationship to the number of drinks consumed
In Figure 2, the same distribution of TAC readings is shown as a function of the number of beers consumed. Notably, TAC levels were zero for 39% of participants after the consumption of one beer, only 7% of participants after two beers, and were always positive (not zero) after three or more beers. After drinking only one beer, all participants, and after two beers, the majority of participants exhibited peak TAC levels below 0.02 g/dl. In contrast, the TAC threshold of 0.02 g/dl was usually exceeded with 3 beers, and universally when 5 beers were consumed. The TAC threshold of 0.03 g/dl, was commonly exceeded by the consumption of 3 or 4 beers and universally exceeded by the consumption of 5 beers.
Figure 2. Peak TAC Levels as a Function of the Number of Beers Consumed.
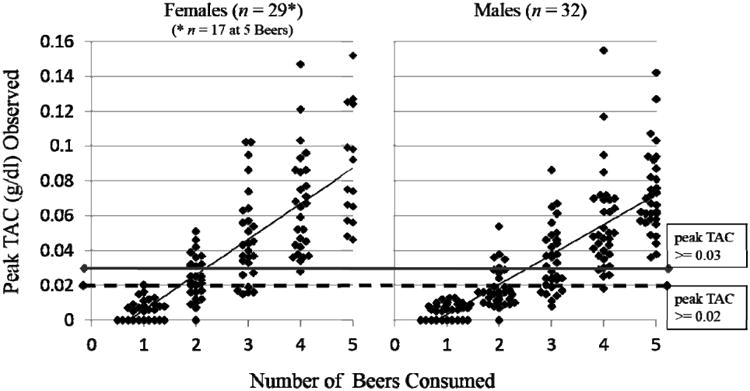
Individual observations of peak TAC readings (in g/dl) expressed as a function of the number of beers consumed. Horizontal lines are drawn at the 0.03 and 0.02 g/dl TAC levels to highlight those threshold criteria. The linear trend line is also plotted to highlight the effect of number of beers consumed. Other details are the same as Figure 1.
Sensitivity of detection at various TAC criteria
Table 2 shows the number and percentage of participants exceeding various TAC-level criteria. Generally, the percentage of participants exceeding each criterion level was an increasing function of the number of beers consumed. Also, the percentage of participant drinking detected at each level of beer consumption decreased as a function of increasing the TAC criterion threshold. After drinking one beer, TAC levels of 0.0 g/dl were exceeded by only 62.5% of males and 58.6% of females, but all of these were below the TAC level criterion of 0.02 g/dl. After drinking two beers, all but two males and one female had non-zero TAC readings but only 25.0% of males and 58.6% of females had peak TAC > 0.02 g/dl. The majority (75% of males and 79.3% of females) exceeded TAC > 0.02 g/dl after the consumption of three or more beers and the sensitivity for detection was less when TAC > 0.03 g/dl was the threshold. Typically, it required the consumption of 4 or more beers to get TAC levels above either the 0.02 g/dl or the 0.03 g/dl threshold in more than 90% of participants. In general, females were more likely than males to exceed various TAC thresholds at higher levels of consumption, but these effects were significant (p < 0.05) only for 2 or 3 beers using the 0.03 g/dl criteria and for 2 beers using the 0.02 g/dl criteria.
Table 2. TAC-based and AMS Criteria for the Detection of Drinking Events.
| Males | Females | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beers (Units) Consumed | 1 (0.92) | 2 (1.84) | 3 (2.76) | 4 (3.68) | 5 (4.60) | 1 (0.92) | 2 (1.84) | 3 (2.76) | 4 (3.68) | 5 (4.60) | Total |
|
| |||||||||||
| Total n | 32 | 32 | 32 | 32 | 32 | 29 | 29 | 29 | 29 | 17 | 293 |
|
| |||||||||||
| Exceed 0 n (%) | 20 (62.5) | 30 (93.8) | 32 (100) | 32 (100) | 32 (100) | 17 (58.6) | 28 (96.6) | 29 (100) | 29 (100) | 17 (100) | 266 (90.8) |
| Exceed 0.02 n (%) | 0 (0) | 8 (25.0) | 24 (75.0) | 31 (96.9) | 32 (100) | 0 (0) | 17 (58.6) | 23 (79.3) | 29 (100) | 17 (100) | 181 (61.8) |
| Exceed 0.03 n (%) | 0 (0) | 4 (12.5) | 15 (46.9) | 29 (90.6) | 32 (100) | 0 (0) | 11 (37.9) | 21 (72.4) | 28 (96.6) | 17 (100) | 157 (53.6) |
| AMS Resolved n (%) | 0 (0) | 8 (25.0) | 19 (59.4) | 30 (93.8) | 32 (100) | 0 (0) | 13 (44.8) | 19 (65.5) | 29 (100) | 17 (100) | 167 (56.9) |
| AMS Confirmed n (%) | 0 (0) | 8 (25.0) | 16 (50.0) | 27 (84.4) | 31 (96.9) | 0 (0) | 11 (37.9) | 18 (62.1) | 28 (96.6) | 17 (100) | 156 (53.2) |
Note. Displayed are the number of subjects and the percentage (%) of 32 males and 29 females whose TAC levels were greater than (exceed) various criterion for detection after drinking the designated number of beers (# beers). Shown also are the number of standard drinks (# units) contained in each # beers.
Sensitivity of AMS detection
The bottom two rows of the Table 2 show how many participants had TAC levels high enough to be resolved by AMS as a possible drinking event, and how many of those were actually “confirmed” by AMS as a drinking event. Notably, AMS criteria did not identify any participants after drinking 1 beer and only a minority of participants after drinking 2 beers. After drinking 3 beers, AMS confirmed drinking for only 50% of males and 62.1% of females. AMS resolution and confirmation exceeded 80% of participants only after drinking 4 or more beers, and exceeded 95% only after 5 beers were consumed. After drinking five beers, AMS confirmation of drinking achieved 100% in females but was less than 100% in males because the drinking of one male was not confirmed by AMS. There was a general tendency for a greater likelihood of AMS confirmation in females than with males but that difference was not significant (p > 0.10).
Summing across the 1-5 beers for both sexes (data excerpted from Table 2), 293 doses of alcohol were administered in the study and 266 (90.8%) of those drinking events resulted in positive TAC readings. However, only 56.9% of the drinking events were resolved by AMS and only 53.2% of those were “confirmed” by AMS. Therefore, AMS reviewed for consideration only 62.8% of all non-zero positive TAC readings, and confirmed 93.4% of the TAC readings they reviewed. Of all participants drinking of 1-3 beers, there were 84 cases (representing 45.9% of total cases) where non-zero TAC readings were below the 0.02 g/dl TAC level and therefore never reviewed by AMS for resolution. Across the entire study, there were 110 cases of non-zero TAC readings that were not confirmed by AMS which represents 41.3% of all cases of non-zero TAC readings.
Drinking events not confirmed by AMS
Table 3 lists the 11 TAC events that were reviewed by AMS but not confirmed as a drinking event. The table also lists the AMS-reported reason that these events were resolved as “Unable to Confirm” with possible environmental contamination being the most common reason. Peak BrAC levels observed for these drinking events ranged from 0.034-0.074 g/dl indicating that pharmacologically-relevant BrAC readings occurred with these unconfirmed drinking events.
Table 3. Positive TAC Events Resolved as “Unable To Confirm”.
| Subject | Beers | Peak TAC | Peak BrAC | AMS Resolution = “Unable To Confirm” |
|---|---|---|---|---|
| M-1 | 3 | 0.033 | 0.034 | TAC detection does not meet AMS strict criteria for confirmation. |
| M-2 | 3 | 0.025 | 0.06 | Does not meet AMS strict criteria for confirmation due to environment contributing to detection. |
| M-3 | 3 | 0.023 | 0.063 | Auto Resolved - Unable to Confirm, Environmental Alcohol Detected. |
| M-3 | 4 | 0.025 | 0.07 | Auto Resolved - Unable to Confirm, Environmental Alcohol Detected. |
| M-4 | 4 | 0.072 | 0.051 | TAC detection does not meet AMS strict criteria for confirmation. |
| M-5 | 4 | 0.047 | 0.052 | Does not meet AMS strict criteria for confirmation due to environment contributing to detection. |
| M-5 | 5 | 0.036 | 0.064 | TAC detection is suspect, however is outside of AMS criteria due to environment contributing to this detection. |
| F-1 | 2 | 0.032 | 0.053 | Probable Consumption. Unable to confirm due to environment contributing to this event. |
| F-2 | 2 | 0.037 | 0.036 | Does not meet AMS strict criteria for confirmation due to environment contributing to detection. |
| F-2 | 3 | 0.056 | 0.055 | TAC detection is suspect, however is outside of AMS criteria due to environment contributing to this detection. |
| F-3 | 4 | 0.042 | 0.074 | TAC detection seen as suspect. However, unable to confirm, due to environment contributing during this alert. |
Note. Displayed are individual participant cases for males (M) and females (F) where AMS resolution was “Unable to Confirm” the possible drinking event. Also shown are the number of beers consumed and their associated peak TAC and BrAC values. Other details are as described for Table 2.
Drinking events exceeding absorption or elimination criteria
Importantly, all of the unconfirmed events listed in Table 3 were within the AMS-established TAC absorption or elimination rate criteria. However, Table 4 shows eight cases where the absorption or elimination rate criteria established by AMS were exceeded. The maximum absorption rate of 0.05 g/dl/hr was exceeded in three participants drinking 3-5 beers but all of those cases were still resolved as “Confirmed” by AMS. The maximum elimination rate of 0.025 g/dl/hr was exceeded in three participants drinking 4-5 beers but AMS still “Confirmed” two of those drinking events. We also observed two participants drinking 2-3 beers who were below the minimum elimination of 0.003 g/dl/hr, but these occurred with low peak TAC readings below the criteria for AMS resolution. In the entire study, there were no cases where both the absorption rate and elimination rate parameters were exceeded at the same time. These 8 cases represent only 3% of all 266 positive TAC events, but importantly show that the AMS absorption and elimination rate criteria, if strictly applied, could exclude as many as 3% of actual cases of drinking in the range of 2-5 beers.
Table 4. Drinking Events Exceeding Absorption or Elimination Criteria.
| Subject | Beers | Peak TAC | Peak BrAC | Absorption Rate | Elimination Rate | AMS Resolution | |
|---|---|---|---|---|---|---|---|
| Absorption Rate Too High | F-1 | 3 | 0.102 | 0.069 | 0.050 | 0.0140 | Confirmed |
| M-6 | 4 | 0.117 | 0.049 | 0.077 | 0.0150 | Confirmed | |
| M-7 | 5 | 0.142 | 0.087 | 0.069 | 0.0170 | Confirmed | |
| Elimination Rate Too High | M-4 | 4 | 0.072 | 0.051 | 0.018 | 0.0470 | Unable To Confirm |
| F-4 | 4 | 0.147 | 0.124 | 0.048 | 0.0260 | Confirmed | |
| F-3 | 5 | 0.142 | 0.106 | 0.015 | 0.0550 | Confirmed | |
| Elimination Rate Too Low | F-5 | 2 | 0.007 | 0.038 | 0.007 | 0.0027 | Unresolved |
| M-8 | 3 | 0.017 | 0.032 | 0.007 | 0.0025 | Unresolved |
Note. Displayed are individual participant cases for males (M) and females (F) where TAC curves were outside of the normal the AMS standard criteria for TAC absorption and elimination rates. Absorption and elimination rates are expressed as g/dl/hr. Also shown are the AMS Resolution and the number of beers consumed and their associated peak TAC and BrAC values.
Discussion
The current study examined the sensitivity of SCRAM transdermal alcohol monitors to detect drinking under experimental conditions where male and female volunteers received controlled doses of 1, 2, 3, 4, and 5 Corona© beers (12-oz. = 0.92 standard units each). A sensitivity analysis quantified drinking detection using different TAC thresholds and the standard criterion applied by the manufacturer (AMS). We found that non-zero TAC readings occurred in 60.7% of participants after drinking only one beer (0.92 standard units), but increased to 95.1% of participants after two beers (1.84 standard units) and reached 100% after drinking three (2.76 standard units) or more beers. As the minimum TAC threshold increased to 0.02, and 0.03g/dl, the sensitivity for detection declined, but for each threshold, the probability of detection was still an increasing function of the number of beers consumed. Using a minimum TAC criterion of 0.02 g/dl did not detect anyone drinking one beer, missed more than 68% of people drinking 2 beers, and even 23% of persons drinking 3 beers. Thus most of the low-level drinking of 1-3 beers will go undetected when using the 0.02 g/dl TAC threshold used by AMS and others (Barnett et al., 2014; Marques and McKnight, 2009). Therefore TAC thresholds below the 0.02 g/dl cutoff are required when it is desirable to detect the drinking of three or fewer standard drinks.
After drinking 3 or more beers in our study, females were more likely than males to be detected by either the 0.02 or 0.03 g/dl TAC criteria or by AMS confirmation of drinking. The greater TAC detection of females than males at higher levels of drinking is likely due to the known sex differences in alcohol distribution resulting in higher blood alcohol levels in females at equivalent levels of consumption (e.g., Baraona et al. 2001; Breslin et al. 1997; Dettling et al. 2007; Jones & Jones 1976). However, the fact that a sex difference was not observed after drinking only 1-2 beers suggests other possible sex differences in the transdermal migration of low blood alcohol concentrations into the SCRAM device (Hawthorne & Wojcik, 2006).
Importantly, for participants drinking 1-3 beers, AMS confirmed only 33.9% of the 156 non-zero TAC events observed – most of these (62% of measureable TAC readings) were never reviewed by AMS because the TAC levels were below the minimum AMS criteria for resolution. In the present study, AMS confirmed 93.4% of the cases they reviewed. However, there were 11 cases of subjects drinking 2-5 beers that were not confirmed by AMS – mostly due to presumed environmental contamination. AMS confirmed drinking in only 55.7% of participants drinking 3 beers, 90.2% of participants drinking 4 beers, and failed to achieve 100% detection even when 5 beers were consumed. Clearly, AMS criteria for resolving measureable TAC readings are too conservative to detect drinking at the 1-3 beer level. These data demonstrate that the AMS criteria reliably detect only the consumption of about 5 drinks (4.6 standard drinks in the current study) even when these are consumed in the relatively brief time-periods (i.e., less than three hours) of the laboratory study.
Understandably, AMS established conservative criteria to avoid false positive detections when using SCRAM monitoring for court-adjudicated offenders (Marques & McKnight, 2007; McKnight et al., 2012). Barnett and colleagues (2014) used SCRAM to monitor the outpatient drinking of heavy drinkers and compared it to self-reported drinking. In order to make the detection of alcohol drinking less conservative than the AMS standard, they modified the criteria for alcohol detection by requiring only one TAC reading above the 0.02 g/dl threshold and requiring only one of the AMS absorption or elimination criteria to be met. Though these authors noted that their modified TAC criteria improved the sensitivity for alcohol detection above the standard AMS criteria, they still only detected 72.8% of the self-reported drinking days indicating a substantial amount of drinking is going undetected even with these modified criteria. Because the rate of TAC-detection was higher (i.e., 93%) when self-reports indicated heavy drinking of five or more drinks, the authors concluded that SCRAM could reliably detect only 5 or more drinks in males and females – a finding that our laboratory study would confirm if we applied the same criteria. However, the current laboratory data revealed that 46.1% of all occasions of drinking 1-3 beers resulted in measureable TAC readings below 0.02 g/dl which means that the false negative detections would be relatively high if the 0.02 g/dl threshold was employed to monitor drinking in a population who was drinking moderately. We also found that either the maximal absorption or elimination rates listed by the AMS criterion were exceeded in six cases of participants drinking 3-5 beers, but both criteria were never exceeded and AMS still confirmed 5 of those 6 cases indicating that strict adherence to AMS-stipulated rate criteria is not required (Barnett et al., 2014).
In our contingency studies (Dougherty et al., 2014, 2015), we achieved reductions in “heavy” drinking by compensating participants for maintaining TAC below 0.03 g/dl all week. In those studies, participants increased the frequency of complete abstinence defined as days of TAC = 0, but also increased their frequency of days of “low-level” drinking defined as measurable TAC levels below the 0.03 g/dl threshold. The current study supports this contention by showing that 88% of subjects consuming 1-2 beers and 41% of subjects consuming 3 beers produced TAC levels below the 0.03 g/dl criterion while this criterion was exceeded for more than 96% of the participants drinking 4-5 beers.
A major limitation of our sensitivity analysis is that the laboratory design prevents an estimation of the specificity or the false-positive rate of using TAC readings below 0.02 g/dl to indicate that low-level drinking has occurred. Even if we had included a blinded placebo alcohol beverage (which we did not use), this still would not have estimated the false positive rate that might occur in the “real-world” because the controlled laboratory conditions minimized the possibility of environmental contamination or other factors that might engender low TAC readings. When Barnett and colleagues (2014) compared TAC readings to self-reported drinking in outpatients, they identified 22 cases of TAC levels > 0.02 g/dl in which outpatients denied drinking which allowed them to estimate a false detection rate of 4.2%. Though we assert that quantitative TAC thresholds below 0.02 g/dl would be necessary to detect low-level drinking, it is not known how much doing this will increase the false detection rate in outpatient environments. Further research will be required to develop methods to minimize or control for the likelihood of false-positive TAC detection using lower TAC thresholds. In any case, it depends upon the purposes of TAC monitoring to determine whether the risks of a false positive report out-weighs the need to know if low level drinking has occurred. For example, it is for society or the criminal justice system to decide whether or not it is important that offenders who are supposed to remain abstinent are actually doing so. There certainly are research applications of TAC monitoring where being able to distinguish heavy vs. lower-level drinking is important. Also, treatment research may seek to selectively reduce heavy drinking or to objectively verify abstinence. Certainly, the current data demonstrate unequivocally, that keeping TAC levels below the commonly accepted AMS criteria or the 0.02g/dl threshold criteria modifications of Barnett and colleagues (2014) does not indicate abstinence, per se.
Beyond the insensitivity of the TAC 0.02 g/dl threshold to detect low-level drinking, false negatives also do occur during the AMS resolution process. We found 11 occasions where participants drinking 2-5 beers exhibited TAC readings above the 0.02 g/dl criteria which were resolved by AMS as “Unable to Confirm.” For most of these TAC readings, AMS could not rule out “environmental” causes. Because our controlled laboratory conditions made environmental contamination unlikely, these data allow an estimation of a false negative rejection rate of 6.7% of all cases of AMS resolution.
In summary, we conclude that the AMS criteria for the confirmation of drinking by SCRAM will reliably detect only heavy drinking levels of approximately 4 standard drinks for females and 5 for males (4.6 in our study) when these are consumed in less than three hours. We also conclude that low-level drinking of 1-3 drinks can be detected with reasonable sensitivity, only when TAC levels below the 0.02 g/dl criterion employed. Thus, any use of TAC-based technology to identify low-level drinking and distinguish it from heavy drinking must consider the use of measureable TAC levels below the 0.02 g/dl threshold. Current work in our research clinic seeks ways to validate low-level TAC readings indicative of low-level drinking in outpatient environments, while still controlling for false-positive TAC readings that are not indicative of drinking. Furthermore, we suggest that TAC detection offers tremendous promise as an objective measure for interventional approaches to reduce heavy drinking, so long as one recognizes that lower level TAC readings below 0.02 g/dl are common with low-level alcohol consumption and do not indicate abstinence.
Acknowledgments
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism [R01AA14988] and the National Institute of Drug Abuse [T32DA031115] of the National Institutes of Health.
References
- Barnett NP, Meade T, Glynn R. Predictors of Detection of Alcohol Use Episodes Using a Transdermal Alcohol Sensor. Exp Clin Psychopharm. 2014;22:86–96. doi: 10.1037/a0034821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R, Colby SM. Contingency management for alcohol use reduction: a pilot study using a transdermal alcohol sensor. Drug Alcohol Depend. 2011;118:391–399. doi: 10.1016/j.drugalcdep.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25:502–507. [PubMed] [Google Scholar]
- Breslin FC, Kapur BM, Sobell MB, Cappell H. Gender and alcohol dosing: a procedure for producing comparable breath alcohol curves for men and women. Alcohol Clin Exp Res. 1997;21:928–930. doi: 10.1111/j.1530-0277.1997.tb03860.x. [DOI] [PubMed] [Google Scholar]
- Dettling A, Fischer F, Bohler S, et al. Ethanol elimination rates in men and women in consideration of the calculated liver weight. Alcohol. 2007;41:415–420. doi: 10.1016/j.alcohol.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Charles NE, Acheson A, John S, Furr MR, Hill-Kapturczak N. Comparing the detection of transdermal and breath alcohol concentrations during periods of alcohol consumption ranging from moderate drinking to binge drinking. Exp Clin Psychopharm. 2012;20:373–81. doi: 10.1037/a0029021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill-Kapturczak N, Liang Y, Karns TE, Cates SE, Lake SL, Mullen J, Roache JD. Use of Continuous Transdermal Alcohol Monitoring during a Contingency Management Procedure to Reduce Excessive Alcohol Use. Drug Alc Depend. 2014;142:301–306. doi: 10.1016/j.drugalcdep.2014.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Karns TE, Mullen J, Liang Y, Lake SL, Roache JD, Hill-Kapturczak N. Transdermal Alcohol Concentration Data Collected During a Contingency Management Program to Reduce At-Risk Drinking. Drug Alc Depend. 2015;148:77–84. doi: 10.1016/j.drugalcdep.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne JS, Wojcik MH. Transdermal alcohol measurement: A review of the literature. Can Soc Forensic J. 2006;39:65–71. [Google Scholar]
- Hill-Kapturczak N, Lake SL, Roache JD, Cates SE, Liang Y, Dougherty DM. Do Variable Rates of Alcohol Drinking Alter the Ability to Use Transdermal Alcohol Monitors to Estimate Peak Breath Alcohol and Total Number of Drinks? Alc Clin Exp Res. 2014a;38:2517–2522. doi: 10.1111/acer.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Roache JD, Liang Y, Karns TE, Cates SE, Dougherty DM. Accounting for Sex-Related Differences in the Estimation of Breath Alcohol Levels using Transdermal Alcohol Monitoring. Psychopharmacology. 2014b doi: 10.1007/s00213-014-3644-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddleston CW, Marlowe DB, Casebolt R. Painting the current picture: A national report card on drug courts and other problem solving court programs in the United States 1 II. Alexandria, VA: National Drug Court Institute; 2008. May, [Google Scholar]
- Jones BM, Jones MK. Alcohol effects in women during the menstrual cycle. Ann N Y Acad Sci. 1976;273:576–587. doi: 10.1111/j.1749-6632.1976.tb52931.x. [DOI] [PubMed] [Google Scholar]
- Marques PR, McKnight AS. National Highway Traffic Safety Administration Evaluating transdermal alcohol measuring devices. Washington, DC: U.S. Government; 2007. Report No. DOT HS 810 875. [Google Scholar]
- McKnight AS, Fell JC, Auld-Owens A. Transdermal alcohol monitoring: Case studies. Washington, DC: National Highway Traffic Safety Administration; 2012. Report No. DOT HS 811 603. [Google Scholar]
- National Institute of Alcohol Abuse and Alcoholism (NIAAA) [Accessed June 3, 2013];National Institute of Alcohol Abuse and Alcoholism Council Approves Definition of Binge Drinking. 2004 NIAAA Newsletter, No.3 http://pubs.niaaa.nih.gov/publications/newsletter/winter2004/newsletter_number3.pdf.
- National Institute of Alcohol Abuse and Alcoholism (NIAAA) Moderate and Binge Drinking. [Accessed on May 1, 2013];2013 at http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Swift R. Transdermal alcohol measurement for estimation of blood alcohol concentration. Alcohol Clin Exp Res. 2000;24:422–423. [PubMed] [Google Scholar]
- Swift R. Direct measurement of alcohol and its metabolites. Addiction. 2003;98:73–80. doi: 10.1046/j.1359-6357.2003.00605.x. [DOI] [PubMed] [Google Scholar]
- Swift RM, Swette L. In: Assessment of ethanol consumption with a wearable, electronic ethanol sensor/recorder in Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Litten RZ, Allen JP, editors. Humana Press; New Jersey: 1992. pp. 189–202. [Google Scholar]
- Voas RB, DuPont RL, Talpins SK, Shea CL. Towards a national model for managing impaired driving offenders. Addiction. 2011;106:1221–1227. doi: 10.1111/j.1360-0443.2010.03339.x. [DOI] [PubMed] [Google Scholar]


