Abstract
Federally qualified health centers (FQHCs) offer primary and preventive healthcare, including cancer screening, for the nation’s most vulnerable population. The purpose of this study was to explore the relationship between access to FQHCs and cancer mortality-to-incidence ratios (MIRs). One-way analysis of variance was conducted to compare the mean MIRs for breast, cervical, prostate, and colorectal cancers for each U.S. county for 2006–2010 by access to FQHCs (direct access, in-county FQHC; indirect access, adjacent-county FQHC; no access, no FQHC either in the county or in adjacent counties). ArcMap 10.1 software was used to map cancer MIRs and FQHC access levels. The mean MIRs for breast, cervical, and prostate cancer differed significantly across FQHC access levels (p < 0.05). In urban and healthcare professional shortage areas, mean MIRs decreased as FQHC access increased. A trend of lower breast and prostate cancer MIRs in direct access to FQHCs was found for all racial groups, but this trend was significant for whites only. States with a large proportion of rural and medically underserved areas had high mean MIRs, with correspondingly more direct FQHC access. Expanding FQHCs to more underserved areas and concentrations of disparity populations may have an important role in reducing cancer morbidity and mortality, as well as racial-ethnic disparities, in the United States.
Keywords: Cancer, Community health centers, Medically underserved areas, African Americans, Health care disparity
Introduction
Cancer is the second leading cause of death in the United States, with an estimated 1.67 million new cases and ≈585,720 deaths expected in 2014 [1]. Preventive health care, including screening for primary prevention as well as early detection for the down-staging of disease, is an important tool for reducing cancer-related morbidity and mortality. In addition, as advances in cancer detection and treatment are realized, cancer survivors are living longer, with many reverting back to their primary care provider for long-term survivorship care.
In the United States, breast (for women), prostate (for men), and colorectal cancers are the top three most common cancers [1]. In addition, all of these cancer types have well-established screening tests that result in either disease prevention (colorectal) [2] or early disease detection (breast and prostate) [3, 4]. These same cancer types are also the top three leading causes of cancer-related death [1]. Similar to screening for colorectal cancer, cervical cancer screening (e.g., pap smear test) is a primary prevention technique that results in the complete removal of premalignant lesions [5]. Although cervical cancer is not a leading cause of cancer incidence or mortality in the United States, there are significant proportions of minority populations (African American, Latina, and individuals from rural Appalachia) who suffer an unequal burden of disease incidence and mortality for this cancer compared to the general population [6–8]. For these reasons, it is vitally important to monitor the burdens of disease within specific geographic regions and racial groups.
Federally qualified health centers (FQHCs) are clinical providers that serve a large proportion of underserved and under-represented patients and are an important link to providers of evidence-based approaches (e.g., cancer screening) for cancer prevention and control. FQHCs, which receive funding from the Health Resources and Services Administration (HRSA), are important safety-net providers of affordable, comprehensive preventive and primary health care. FQHCs serve 20 million patients annually in the United States, and nearly two-thirds of patients served at FQHCs are ethnic minorities, low income, and uninsured [9]. FQHCs, which implement the medical home model, have tremendous potential to reduce health disparities for ethnic minorities and other underserved populations by promoting easier care transitions for patients [10]. As of 2012 in the United States, 1,198 FQHCs with 9,321 delivery sites offered preventive and primary medical care, including cancer screening, aimed at responding to disparities in healthcare access and health status [10]. FQHCs are well positioned to reduce cancer morbidity and mortality, as well as reduce health disparities, a top priority of FQHCs [11]. With the passing of the Affordable Care Act, the role of FQHCs to provide patient-centered medical homes to diverse populations, including those at risk for cancer and cancer survivors, has become even more pivotal.
Mortality-to-incidence ratios (MIRs), when compared across regions, provide a unique quantification of cancer mortality disparities that takes into account incidence while also describing mortality [5]. This measure ‘anchors’ mortality to a denominator that is limited to incident cancer cases rather than the total population, as measured by mortality rate. MIR is a valid indicator of fatality, and studies have used MIRs to compare cancer rates across populations [12, 13]. The MIR is particularly useful at describing the true burden of disease among populations and can be a useful comparison for both geographic and racial groups.
The purpose of this descriptive analysis was to explore the relationship between FQHC access and cancer MIRs at the county level across the United States. We propose that comparisons of MIRs across counties permit an assessment of the relative efficiency of the local health system in maximizing survival after cancer diagnosis given the number of incident cases diagnosed in the geographic regions surrounding FQHCs. We hypothesize that this effect may be mediated as follows: to the extent that FQHCs improve early cancer detection among medically underserved populations in the county and navigate diagnosed patients for timely care by the appropriate care providers, a county’s FQHC access level may lead to greater cancer survivorship relative to other counties, as reflected in the MIR.
Methods
Data Sources
Age-adjusted breast (female only), cervical, colorectal, and prostate cancer mortality and incidence rates per 100,000 population for each US county for 2006–2010 were obtained from the US National Cancer Institute's (NCI) State Cancer Profile website [14]. The number of FQHCs has been growing steadily over the last decade. Therefore, the most easily accessible data from FQHCs were used. Data on FQHCs in the United States as of September 3, 2013 were downloaded from the HRSA website. The data include the addresses of FQHC delivery sites, including county information. FQHCs that (1) were located in US territories (e.g., Guam or Puerto Rico), (2) did not have address or county information, (3) provided only administrative services, or (4) were not community health centers were excluded from the analysis. FQHC delivery sites that opened after 2010 were also excluded because they are outside of the MIR time period (2006–2010). In the dataset, there were 7,240 FQHC delivery sites for analysis. From the 2011–2012 Area Resource File, county information on urban or rural designation, healthcare professional shortage areas (HPSAs) for primary care in 2007 and socioeconomic status (SES) indicators in 2005–2009, including median household income, percentage of persons below the poverty level and percentage of persons >25 years of age with a 4-year-college degree, were obtained [15]. HPSAs for primary care at the county level were measured using three categories: “none of the county is a shortage area” (no-shortage area), “entire county is a shortage area” (all-shortage area) or “part of the county designated as a shortage area” (partial-shortage area) [15]. Information on the percentage of households that do not have a vehicle available was obtained from the 2006–2010 American Community Survey [16].
Primary Variables
Cancer mortality and incidence rates were suppressed if there were less than three cases in a specific area-race category. For example, if there were less than three whites who were diagnosed with cervical cancer in a county, the incidence rate for that cancer type was suppressed. Counties with unknown, missing, or suppressed cancer mortality or incidence rates were excluded. MIRs were calculated by dividing the county-specific mortality rate by the county-specific incidence rate [12]. The number of FQHC delivery sites was summed for each county. Using ArcMap® 10.1 (Esri, Red-lands, CA), adjacent counties for each county were identified. To account for spatial correlation, we categorized counties into three levels of access to FQHCs: (1) direct access (any FQHC delivery sites in the county), (2) indirect access (no FQHC delivery site in the county but any in adjacent counties) or (3) no access (no FQHC delivery site either in the county or any in adjacent counties).
Statistical Analysis
To quantify the potential impact of FQHC access on cancer disparities, we compared mean MIRs by level of FQHC access. County-specific MIRs were calculated for strata defined by: household income distribution (above or below the median for all counties), residential area (urban; rural), HPSA designation (no-shortage area; partial-shortage area; all-shortage area), and race (white; black). Other ethnic and racial groups were excluded in the analysis because of small sample sizes. All analyses were adjusted by county-level SES indicators and the percentage of households that do not have a vehicle available. One-way analysis of variance was conducted to compare the mean MIRs in each category of FQHC access. All analyses were conducted at α = 0.05 level using SAS® 9.3 (Cary, NC).
For mapping purposes, the mean MIRs of each cancer and access to FQHCs were divided into six categories: high MIR (above the median) with direct access; high MIR with indirect access; high MIR with no access; low MIR with direct access; low MIR with indirect access; and low MIR with no access. In the figure, we used blue shading to indicate direct access, green shading for indirect access, and red shading for no access. High-MIR pairs are shown in darker tones than low-MIR pairs. This MIR–FQHC access categorization was mapped using ArcMap® 10.1 (Esri, Redlands, CA) for each cancer.
Results
There were 1,612 counties with mortality and incidence rates available for breast cancer, 234 for cervical cancer, 1,999 for colorectal cancer, and 1,383 for prostate cancer. The overall mean MIRs for breast, cervical, and prostate cancers were significantly different by FQHC access level: counties with direct access had lower cancer MIRs than those with indirect or no access (p < 0.05). Counties with indirect access had lower MIRs than counties with no access, especially for breast cancer MIRs. Counties in which the median household income is over the aggregate median had lower cancer MIRs than counties with lower median household income (Table 1).
Table 1.
MIRs for breast, cervical, colorectal, and prostate cancers
| Cancer | Total |
Under median household incomea |
Over median household income |
||||||
|---|---|---|---|---|---|---|---|---|---|
| # of counties | Mean ± SE | p | # of counties | Mean ± SE | p | # of counties | Mean ± SE | p | |
| Breast | |||||||||
| No accessb | 59 | 0.224 ± 0.007 | 0.021 | 26 | 0.247 ± 0.012 | 0.099 | 33 | 0.203 ± 0.008 | 0.082 |
| Indirect accessc | 564 | 0.206 ± 0.002 | 297 | 0.220 ± 0.004 | 267 | 0.193 ± 0.003 | |||
| Direct accessd | 989 | 0.204 ± 0.002 | 482 | 0.221 ± 0.003 | 507 | 0.187 ± 0.002 | |||
| Cervix | |||||||||
| No access | 0 | NA | 0 | NA | 0 | NA | |||
| Indirect access | 13 | 0.423 ± 0.027 | <0.001 | 6 | 0.507 ± 0.042 | <0.001 | 7 | 0.337 ± 0.032 | 0.230 |
| Direct access | 221 | 0.318 ± 0.006 | 111 | 0.340 ± 0.010 | 110 | 0.297 ± 0.008 | |||
| Colorectal | |||||||||
| No access | 102 | 0.389 ± 0.008 | 0.407 | 46 | 0.401 ± 0.013 | 0.702 | 56 | 0.379 ± 0.010 | 0.559 |
| Indirect access | 744 | 0.395 ± 0.003 | 387 | 0.408 ± 0.005 | 357 | 0.382 ± 0.004 | |||
| Direct access | 1,153 | 0.390 ± 0.002 | 566 | 0.404 ± 0.004 | 587 | 0.376 ± 0.003 | |||
| Prostate | |||||||||
| No access | 35 | 0.188 ± 0.011 | 0.026 | 15 | 0.236 ± 0.018 | 0.120 | 20 | 0.150 ± 0.012 | <0.001 |
| Indirect access | 458 | 0.193 ± 0.003 | 241 | 0.206 ± 0.005 | 217 | 0.181 ± 0.004 | |||
| Direct access | 890 | 0.183 ± 0.002 | 435 | 0.200 ± 0.003 | 455 | 0.165 ± 0.002 | |||
Bold values indicate statistical significant (p < 0.05)
MIR Mortality-to-Incidence Ratio, SE Standard Error, NA Data not available
Median household income: breast cancer $43,317; cervical cancer $51,645; colorectal cancer: $42,397; prostate cancer: $44,172
No FQHC delivery site both in the county and adjacent counties
No FQHC delivery site in the county, but any in adjacent counties
Any FQHC delivery sites in the county
In general, the mean MIRs for breast, colorectal, and prostate cancers were higher in rural areas than in urban areas. In urban areas, the mean MIRs for the four cancers decreased significantly as the level of FQHC access increased (p < 0.05); there were no significant differences in rural areas, except for in breast cancer MIRs (Table 2).
Table 2.
MIRs for breast, cervical, colorectal, and prostate cancers, by urban/rural
| Urban |
Rural |
|||||
|---|---|---|---|---|---|---|
| # of counties | Mean ± SE | p | # of counties | Mean ± SE | p | |
| Breast | ||||||
| No accessa | 9 | 0.253 ± 0.015 | <0.001 | 50 | 0.231 ± 0.009 | 0.160 |
| Indirect accessb | 228 | 0.201 ± 0.003 | 336 | 0.214 ± 0.003 | ||
| Direct accessc | 590 | 0.191 ± 0.002 | 399 | 0.218 ± 0.003 | ||
| Cervix | ||||||
| No access | 0 | NA | 0 | NA | ||
| Indirect access | 11 | 0.416 ± 0.028 | <0.001 | 2 | 0.583 ± 0.068 | 0.235 |
| Direct access | 216 | 0.314 ± 0.006 | 5 | 0.438 ± 0.038 | ||
| Colorectal | ||||||
| No access | 13 | 0.413 ± 0.020 | 0.028 | 89 | 0.394 ± 0.010 | 0.821 |
| Indirect access | 256 | 0.390 ± 0.005 | 488 | 0.401 ± 0.004 | ||
| Direct access | 622 | 0.377 ± 0.003 | 531 | 0.401 ± 0.004 | ||
| Prostate | ||||||
| No access | 5 | 0.225 ± 0.025 | <0.001 | 30 | 0.202 ± 0.013 | 0.919 |
| Indirect access | 196 | 0.187 ± 0.004 | 262 | 0.202 ± 0.004 | ||
| Direct access | 562 | 0.169 ± 0.002 | 328 | 0.200 ± 0.004 | ||
Bold values indicate statistical significant (p < 0.05)
MIR Mortality-to-Incidence Ratio, SE Standard Error, NA Data not available
No FQHC delivery site both in the county and adjacent counties
No FQHC delivery site in the county, but any in adjacent counties
Any FQHC delivery sites in the county
When examined by HPSA designations, the mean MIRs for breast, cervical, and prostate cancers significantly decreased as the level of FQHC access increased among counties identified as HPSAs (all or in part), although the interaction between FQHC access level and HPSA designation was not statistically significant. However, there was no significant association of cancer MIRs and access to FQHC in the no-shortage areas (Table 3).
Table 3.
MIRs for breast, cervical, colorectal, and prostate cancers, by HPSA designation
| No HPSA |
Partial HPSA |
All HPSA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| # of counties | Mean ± SE | p | # of counties | Mean ± SE | p | # of counties | Mean ± SE | p | |
| Breast | |||||||||
| No accessa | 30 | 0.212 ± 0.009 | 0.278 | 11 | 0.238 ± 0.014 | 0.049 | 18 | 0.236 ± 0.015 | 0.099 |
| Indirect accessb | 251 | 0.199 ± 0.003 | 163 | 0.204 ± 0.004 | 150 | 0.216 ± 0.005 | |||
| Direct accessc | 155 | 0.205 ± 0.004 | 448 | 0.202 ± 0.002 | 386 | 0.208 ± 0.003 | |||
| Cervix | |||||||||
| No access | 0 | NA | 0 | NA | 0 | NA | |||
| Indirect access | 9 | 0.342 ± 0.042 | 0.906 | 2 | 0.427 ± 0.072 | 0.148 | 2 | 0.601 ± 0.049 | <0.001 |
| Direct access | 9 | 0.349 ± 0.031 | 115 | 0.321 ± 0.009 | 90 | 0.312 ± 0.007 | |||
| Colorectal | |||||||||
| No access | 47 | 0.387 ± 0.011 | 0.959 | 27 | 0.376 ± 0.016 | 0.258 | 28 | 0.407 ± 0.016 | 0.376 |
| Indirect access | 304 | 0.383 ± 0.004 | 215 | 0.399 ± 0.006 | 225 | 0.404 ± 0.006 | |||
| Direct access | 172 | 0.384 ± 0.006 | 504 | 0.389 ± 0.004 | 477 | 0.395 ± 0.004 | |||
| Prostate | |||||||||
| No access | 17 | 0.168 ± 0.014 | 0.558 | 8 | 0.201 ± 0.022 | 0.678 | 10 | 0.209 ± 0.021 | 0.007 |
| Indirect access | 210 | 0.184 ± 0.004 | 135 | 0.184 ± 0.005 | 113 | 0.209 ± 0.006 | |||
| Direct access | 130 | 0.182 ± 0.005 | 411 | 0.182 ± 0.003 | 349 | 0.186 ± 0.004 | |||
Bold values indicate statistical significant (p < 0.05)
MIR Mortality-to-Incidence Ratio, SE Standard Error, NA Data not available, HPSA Healthcare Professional Shortage Area
No FQHC delivery site both in the county and adjacent counties
No FQHC delivery site in the county, but any in adjacent counties
Any FQHC delivery sites in the county
Blacks had higher mean MIRs for breast, cervical, and prostate cancers than whites. A higher level of access to FQHCs corresponded to lower MIRs for breast (p = 0.004) and prostate (p = 0.019) cancers for whites only (Table 4).
Table 4.
MIRs for breast, cervical, colorectal, and prostate cancers, by race
| Cancer | Whites |
Blacks |
||||
|---|---|---|---|---|---|---|
| # of counties | Mean ± SE | p | # of counties | Mean ± SE | p | |
| Breast | ||||||
| No accessa | 55 | 0.221 ± 0.007 | 0.004 | 0 | 0.390 | |
| Indirect accessb | 522 | 0.201 ± 0.002 | 38 | 0.274 ± 0.012 | ||
| Direct accessc | 889 | 0.196 ± 0.002 | 272 | 0.264 ± 0.004 | ||
| Cervix | ||||||
| No access | 0 | NA | 0 | NA | ||
| Indirect access | 5 | 0.368 ± 0.035 | 0.061 | 0 | NA | |
| Direct access | 172 | 0.301 ± 0.006 | 46 | 0.436 ± 0.013 | ||
| Colorectal | ||||||
| No access | 99 | 0.389 ± 0.009 | 0.269 | 0 | 0.674 | |
| Indirect access | 706 | 0.394 ± 0.003 | 12 | 0.377 ± 0.028 | ||
| Direct access | 1,067 | 0.387 ± 0.003 | 177 | 0.389 ± 0.007 | ||
| Prostate | ||||||
| No access | 30 | 0.184 ± 0.011 | 0.019 | 0 | 0.932 | |
| Indirect access | 397 | 0.187 ± 0.003 | 26 | 0.271 ± 0.018 | ||
| Direct access | 772 | 0.175 ± 0.002 | 246 | 0.270 ± 0.006 | ||
Bold values indicate statistical significant (p < 0.05)
MIR Mortality-to-Incidence Ratio, SE Standard Error, NA Data not available
No FQHC delivery site both in the county and adjacent counties
No FQHC delivery site in the county, but any in adjacent counties
Any FQHC delivery sites in the county
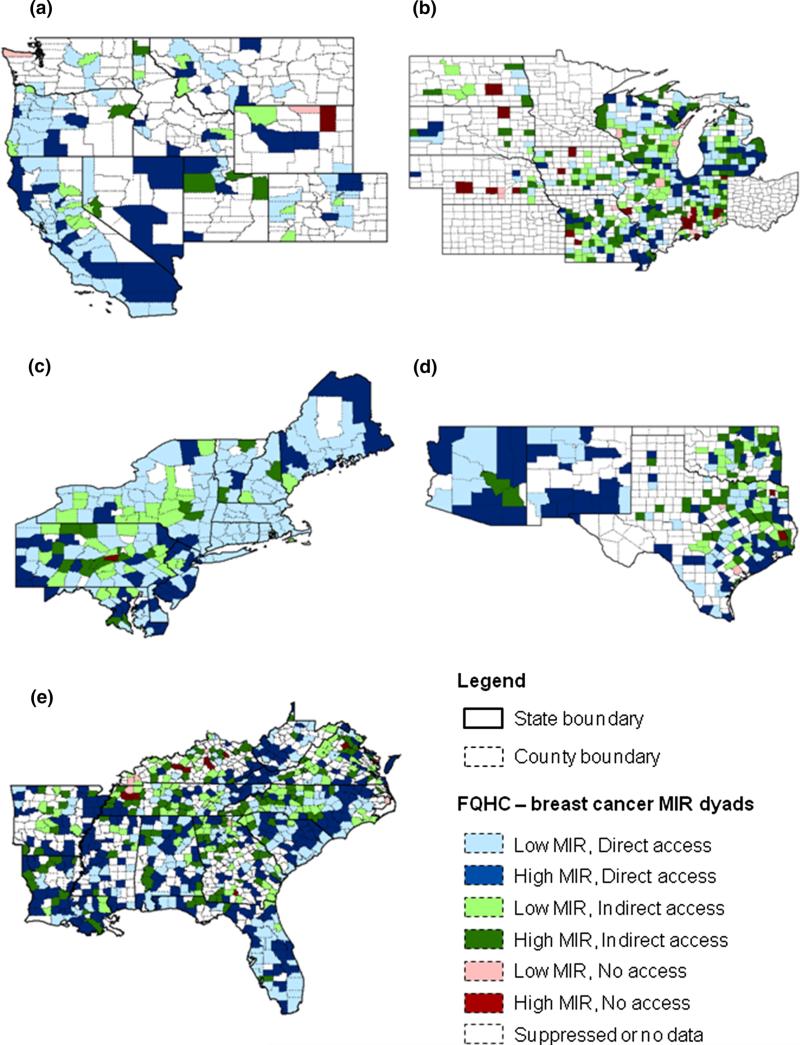
Figure 1 shows US regional maps depicting access to FQHC–MIR dyads for breast cancer. Additional maps for colorectal and prostate cancers (Figure S1 & S2) are provided in the Appendix. Because of the large amount of suppressed or missing cervical cancer MIR data, the map for cervical cancer is not shown. In general, California, Florida, and many Northeastern states had a high proportion of counties with low cancer MIRs and direct access to FQHCs. Conversely, Arizona, New Mexico, North Carolina, and South Carolina (states with a large proportion of rural and medically underserved areas) had a high proportion of counties with high cancer MIRs and direct access to FQHCs.
Fig. 1.
Access to FQHCs and breast cancer MIRs by region. a West, b Midwest, c Northeast, d Southwest, e Southeast
Discussion
Results from this study are consistent with the hypothesized mitigating effect of FQHCs in reducing mortality among people diagnosed with cancer. The results were robust across breast, cervical, and prostate cancers. FQHCs provide a safety net for individuals who cannot afford healthcare and disproportionately serve racial/ethnic minorities and low-income populations who are more likely to be uninsured [9]. Because private primary care practitioners often limit or refuse to take Medicaid and uninsured patients [17], FQHCs are left as the key providers for these populations [18]. Furthermore, uninsured patients attending FQHC clinics receive more preventive services than the average uninsured person nationally, particularly pap tests and mammograms [19]. Finally, it is acknowledged that FQHCs provide high-quality care comparable to, or exceeding, that of other primary health care providers [20]. This study extends the findings of earlier studies and may represent indirect evidence that FQHCs have a far-reaching role in reducing cancer mortality through providing cancer screening.
The inverse association between cancer MIRs and FQHC access was observed more strongly in counties with a high median household income. In our analyses, counties with higher median household income generally had a greater number of FQHC delivery sites than counties with a lower median household income. This may have been partially responsible for the findings upon stratification by median household income.
Urban counties showed a stronger inverse association between cancer MIRs and FQHC access. However, this association was not apparent in rural counties. Although rural households are more likely to possess private cars, rural residents must travel longer distances to seek and receive health care than their urban counterparts [21, 22]. Because rural residents are accustomed to traveling longer distances, they might use health care services regardless of FQHC existence in the surrounding area.
The inverse relationship between FQHC access and MIR was strongest for breast, prostate, and cervical cancers and was weaker for colorectal cancer. Cervical cancer screening is a standard procedure in most FQHCs. Drawing of blood needed to conduct prostate-specific antigen (PSA) screening is relatively simple in the FQHC environment. In addition, many FQHC sites are beginning to either offer mammography services within their centers or have initiatives to streamline referrals to mammography centers. By contrast, colorectal cancer screening (namely colonoscopy) requires referral arrangements for highly specialized services involving specialists and technology-intensive infrastructure. Sustaining such referral arrangements for uninsured patients may be challenging for FQHCs because of a lack of payer source. There is also a significant burden on patients in preparations for colonoscopy procedures (bowel prep and pre-surgical consults). Another hypothesis may be that colonoscopy is a primary prevention measure (i.e., removing precancerous lesions), and so potential cancer is identified before they can progress to cancer. Hence the MIR statistic would not reflect this premalignancy preventive measure. Additionally, with the advent of PSA screening in the United States, prostate cancer has tended to be diagnosed at early stages, and therefore has been associated with generally longer survival [4, 23]. This trend should be tempered with the recently updated recommendations by the US Preventive Services Task Force, but may have unique implications for high-risk minority groups such as blacks [24]. In contrast to colorectal cancer, both cervical and prostate cancers manifest the largest racial and socioeconomic disparitiesamong all common cancers [12, 25]. Colorectal cancer tends to be diagnosed at later stages, when prognosis is poorer despite a greater effort made at preventing death [26]. The fact that these results were strongest in HPSAs suggests that FQHCs may be doing a particularly effective job among individuals who would otherwise have poorer outcomes after receiving a cancer diagnosis.
The analyses by race reveal interesting findings. Although the MIRs for blacks most often showed the greatest reductions with higher health care access, these reductions were not significant in this study. The number of counties included in the analyses of cancer MIRs for blacks is relatively small, which may account for the insignificant findings. Nevertheless, breast, cervical, and prostate cancer MIRs, regardless of FQHC access, were higher among blacks than whites. The race-specific findings and HPSA results, both, suggest the value of FQHCs at reducing cancer morality while making the case for expansions of services and programs to further reduce such disparities.
There are limitations that should be considered when interpreting these findings. First, this is a descriptive, ecological study, therefore, other unmeasured factors that influence cancer incidence and mortality at the individual level cannot be controlled. Consequently, although our work certainly details areas for further research, our conclusion must be framed from a perspective of hypothesis generation and should not be interpreted as causal. However, we should underscore that this ecologic investigation is an important descriptive analysis that begins to explore the larger implications of FQHCs on cancer statistics. Second, because of the county-level cancer incidence and mortality data used, the data cannot account for those individuals who may move after their cancer diagnosis. We would expect this type of population drift to be similar across most states, thereby lessening its potential impact on the study findings. Additionally, most state cancer registries have reciprocal data-sharing agreements with neighboring states, thereby minimizing this potential for bias further.
Despite the limitations, this investigation highlights the potential impact of FQHCs further ‘downstream’ in terms of cancer mortality, as well as their impact on more proximal factors such as cancer surveillance and detection. Other more expensive and much less practicable methodologies would be needed to conduct more detailed levels of analysis. Another important consideration when using the MIR statistic is the time lag between cancer mortality and incidence. For this analysis, overlapping periods of incidence and mortality were used; however, non-virulent cancers (particularly breast and prostate) developing within the later years of the incidence period would not be reflected in the mortality period. This research team previously conducted a sensitivity analysis utilizing non-overlapping periods of time to account for the impact of extended survival times. The results remained unchanged from what was found previously with overlapping periods [12]. After some debate concerning the most appropriate time point to measure FQHC access relative to cancer MIRs, current FQHC data were chosen for this study. Sensitivity analyses using alternative time points for FQHC access were conducted, with no change in the original findings.
This study has several strengths. By grouping counties according to FQHC access in neighboring counties, we were able to account for spatial correlation. Additionally, the variability in race-specific cancer MIRs that we found may be higher than that in centers or studies serving predominantly white populations, such as university teaching hospitals or established cohorts. The NCI's State Cancer Profile data provided a very robust, objective source for estimating MIRs. Not only did it provide data from a large sample of counties, but it also incorporated data over multiple years (2006–2010). The selection of this database resulted in rates that are more stable and robust than those obtained from data covering fewer years and from a smaller geographical sampling frame. Using these readily available data, it revealed that FQHCs may have a moderate impact on cancer mortality by influencing outcomes among individuals with a cancer diagnosis.
In conclusion, this work highlights the potential impact of FQHCs on such ‘downstream’ outcomes as cancer and cancer disparities. To reduce disparities in healthcare access and improve health outcomes to poor and underserved populations, the role of FQHCs in providing quality and comprehensive preventive and primary healthcare services needs to be further supported across several levels, including policy and environmental facilitation. Continued partnerships between academic institutions, FQHCs, and other affiliated organizations (e.g., regional and national membership associations) are one of the avenues in support of this goal. In these partnerships, FQHC administration and healthcare providers bring a ‘real life/in the trenches’ perspective and pose research questions to traditional academic research, enabling greater translation and benefit of research findings into practice. Alternatively, academic institutions provide expertise in research, which enables them to enhance the quality and scope of services they provide.
To our knowledge, this is the first linkage of these geographic data to investigate the relationship between FQHC access and MIRs. Such ecologic analyses of geographic data are an important way to evaluate health care reform issues, and offer great potential to understand the implications of health policy, health care investments, and natural experiments. Similar research should be conducted to determine whether these findings generalize to other health issues.
Supplementary Material
Acknowledgments
This publication was supported by Cooperative Agreement Number U48/DP001936 from the Centers for Disease Control and Prevention (Prevention Research Centers) and the National Cancer Institute (PIs: Dr. Hébert, Dr. Friedman). This work also was partially supported by: an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the NCI to Dr. Hébert (K05 CA136975), U54 CA153461-01 from the National Cancer Institute, Center to Reduce Cancer Health Disparities (Community Networks Program) to the South Carolina Cancer Disparities Community Network-II (SCCDCN-II), and an NCI K01 Career Development Grant to Dr. Tucker-Seeley (K01 CA169041). Dr. Yip was partially supported by the National Cancer Institute (R01 CA 124397; PI: S-P Tu).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10900-014-9978-8) contains supplementary material, which is available to authorized users.
Conflict of interest No financial disclosures were reported by the authors of this paper.
Contributor Information
Swann Arp Adams, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA; Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA; College of Nursing, University of South Carolina, 1601 Greene street, Columbia, SC 29208, USA.
Seul Ki Choi, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA; Department of Health Promotion, Education, and Behavior, Arnold School of Public Health, University of South Carolina, 915 Greene street, Columbia, SC 29208, USA.
Leepao Khang, Department of Public Health, California State University, Fresno, 2345 E. San Ramona Avenue M/S MH30, Fresno, CA 93740, USA.
Dayna A. Campbell, South Carolina Primary Health Care Association, 3 Technology Circle, Columbia, SC 29203, USA
Daniela B. Friedman, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA Department of Health Promotion, Education, and Behavior, Arnold School of Public Health, University of South Carolina, 915 Greene street, Columbia, SC 29208, USA.
Jan M. Eberth, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA.
Russell E. Glasgow, School of Medicine and Colorado Health Outcomes Research Program, University of Colorado at Denver, 13199 E Montview Blvd, Suite 300, Aurora, CO 80045, USA
Reginald Tucker-Seeley, Department of Social and Behavioral Sciences, Harvard School of Public Health, 677 Huntington Avenue, Boston, MA 02115, USA; Center for Community Based Research, Dana-Farber Cancer Institute, 450 Brookline Avenue, LW703, Boston, MA 02215, USA.
Sudha Xirasagar, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA; Department of Health Services Policy and Management, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA.
Mei Po Yip, Division of General Internal Medicine, University of Washington, 325 Ninth Avenue, Seattle, WA 98104, USA.
Vicki M. Young, South Carolina Primary Health Care Association, 3 Technology Circle, Columbia, SC 29203, USA
James R. Hébert, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. New England Journal of Medicine. 2012;366(25):2345–2357. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: Impact of mammographic screening on breast cancer mortality during 3 decades. Radiology. 2011;260(3):658–663. doi: 10.1148/radiol.11110469. [DOI] [PubMed] [Google Scholar]
- 4.Tangen CM, Hussain MH, Higano CS, et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346). Journal of Urology. 2012;188(4):1164–1169. doi: 10.1016/j.juro.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA: A Cancer Journal for Clinicians. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simard EP, Naishadham D, Saslow D, Jermal A. Age-specific trends in black–white disparities in cervical cancer incidence in the United States: 1975–2009. Gynecologic Oncology. 2012;127(3):611–615. doi: 10.1016/j.ygyno.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics for hispanics/latinos, 2012. CA: A Cancer Journal for Clinicians. 2012;62(5):283–298. doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 8.Hopenhayn C, Bush H, Christian A, Shelton BJ. Comparative analysis of invasive cervical cancer incidence rates in three Appalachian states. Preventive Medicine. 2005;41(5):859–864. doi: 10.1016/j.ypmed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Shin P, Rosenbaum S, Paradise J. Community health centers: The challenge of growing to meet the need for primary care in medically underserved communities. The Henry J. Kaiser FamilyFoundation; Washington, DC: 2012. Available at: https://publichealth.gwu.edu/departments/healthpolicy/DHP_Publications/pub_uploads/dhp Publication_3B043800-5056-9D20-3D5DCAA18AC4BD43.pdf. [Google Scholar]
- 10.Bureau of Primary Health Care, US Department of Health and Human Services, Health Resources and Services Administration HRSA geospatial data warehouse. http://datawarehouse.hrsa.gov/HRSAActivityStatus.aspx. Accessed September 3, 2013.
- 11.Human Resources and Service Administration, US Department of Health and Human Services Health centers and the affordable care act fact sheet. http://bphc.hrsa.gov/about/healthcenterfactsheet.pdf. Accessed May 2, 2014.
- 12.Hébert JR, Daguise VG, Hurley DM, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115(11):2539–2552. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner SE, Hurley DM, Hébert JR, McNamara C, Bayakly AR, Vena JE. Cancer mortality-to-incidence ratios in Georgia. Cancer. 2012;118(16):4032–4045. doi: 10.1002/cncr.26728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute [September 3, 2013];State Cancer Profiles: Dynamic views of cancer statistics for prioritizing caner control efforts in the nation, states, and counties. http://statecancerprofiles.cancer.gov/index.html.
- 15.US Department of Health and Human Services, Health Resources and Services Administration [September 2, 2012];Area Resource File (ARF) http://www.arfsys.com.
- 16.U.S. Census Bureau [October 25, 2013];American Community Survey. http://www.census.gov/acs/www/data_documentation/data_main/.
- 17.Bodenheimer T, Pham HH. Primary care: Current problems and proposed solutions. Health Affairs. 2010;29(5):799–805. doi: 10.1377/hlthaff.2010.0026. [DOI] [PubMed] [Google Scholar]
- 18.National Association of Community Health Centers [October 22, 2013];A sketch of community health centers: Chart book. 2013 http://www.nachc.com/client/Chartbook2013.pdf.
- 19.Shi L, Stevens GD. The role of community health centers in delivering primary care to the underserved: experiences of the uninsured and Medicaid insured. The Journal of Ambulatory Care Management. 2007;30(2):159–170. doi: 10.1097/01.JAC.0000264606.50123.6d. [DOI] [PubMed] [Google Scholar]
- 20.Starfield B, Powe NR, Weiner JR, et al. Costs vs quality in different types of primary care settings. JAMA. 1994;272(24):1903–1908. [PubMed] [Google Scholar]
- 21.Probst JC, Laditka SB, Wang JY, Johnson AO. Effects of residence and race on burden of travel for care: Cross sectional analysis of the 2001 US National Household Travel Survey. BMC Health Services Research. 2007;7(1):40. doi: 10.1186/1472-6963-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pucher J, Renne JL. Rural mobility and mode choice: Evidence from the 2001 National Household Travel Survey. Transportation. 2005;32(2):165–186. [Google Scholar]
- 23.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 24.Moyer VA. Screening for prostate cancer: US preventive services task force recommendation statement. Annals of Internal Medicine. 2012;157(2):120–134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 25.Adams SA, Fleming A, Brandt HM, et al. Racial disparities in cervical cancer mortality in an African American and European American cohort in South Carolina. Journal-South Carolina Medical Association. 2009;105(7):237–244. [PMC free article] [PubMed] [Google Scholar]
- 26.Moyer A, Sohl SJ, Knapp-Oliver SK, Schneider S. Characteristics and methodological quality of 25 years of research investigating psychosocial interventions for cancer patients. Cancer Treatment Reviews. 2009;35(5):475–484. doi: 10.1016/j.ctrv.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.