Abstract
A set of specific precursor microRNAs (pre-miRNAs) are reported to localize into neuronal dendrites, where they could be processed locally to control synaptic protein synthesis and plasticity. However, it is not clear whether specific pre-miRNAs are also transported into distal axons to autonomously regulate intra-axonal protein synthesis. Here, we show that a subset of pre-miRNAs, whose mature miRNAs are enriched in axonal compartment of sympathetic neurons, are present in axons of neurons both in vivo and in vitro by quantitative PCR and by in situ hybridization. Some pre-miRNAs (let 7c-a and pre-miRs-16, 23a, 25, 125b-1, 433, and 541) showed elevated axonal levels, while others (pre-miRs-138-2, 185, and 221) were decreased in axonal levels following injury. Dicer and KSRP proteins are also present in distal axons, but Drosha is found restricted to the cell body. These findings suggest that specific pre-miRNAs are selected for localization into distal axons of sensory neurons and are presumably processed to mature miRNAs in response to extracellular stimuli. This study supports the notion that local miRNA biogenesis effectively provides another level of temporal control for local protein synthesis in axons.
Keywords: precursor miRNA, terminal loop, axonal localization, miRNA biogenesis, distal axon, sciatic nerve
Introduction
microRNAs (miRNAs) have been proposed to negatively regulate local translation in the nervous system at the translational level to precisely control morphological and physiological changes in nerve cells (Christensen & Schratt 2009, Liu & Xu 2011, Maes et al. 2009, Murashov et al. 2007, Weinberg & Wood 2009, Zhang et al. 2011, Zhou et al. 2011, Schratt et al. 2006, Siegel et al. 2009). Recent spatial profile study of miRNAs in sympathetic neurons shows that miRNAs are differentially distributed in subcellular regions in a manner similar to differential distribution of axonal mRNAs (Natera-Naranjo et al. 2010). This suggests that a subset of miRNAs may be specifically selected and transported to axons, where they modulate intra-axonal translation of localized mRNAs responding to local stimuli including injury. However, it is not clear how these miRNAs localize into axons.
miRNA biogenesis proceeds in a stepwise fashion, starting with cleavage of primary miRNA (pri-miRNA) by the nuclear Drosha RNase III in collaboration with DiGeorge syndrome critical region gene 8 (DGCR8) to generate ~70–100 nucleotide precursor miRNAs (pre-miRNAs) (Kim 2005), Filipowicz et al. 2008). The resultant pre-miRNAs translocate into the cytoplasm, where they are processed by Dicer to create miRNA duplexes, which are further processed for mature miRNAs. The primary action of miRNAs is to negatively regulate gene expression by binding to target mRNAs, typically to the 3’ untranslated region (UTR) of the target mRNA (Bartel 2009), Kosik 2006). The elucidation of control mechanisms of local protein synthesis within the axonal compartment at different levels of neuronal activity (e.g., during regeneration) could potentially lead us to develop RNA-based approaches for the treatment of neurological disorders.
Recent studies have shown that specific miRNAs localize into dendrites in a precursor form, in which the terminal loop associates with RNA-binding proteins for the dendritic localization (Bicker et al. 2013, Lugli et al. 2012, Lugli et al. 2008). These observations suggested that the localized pre-miRNAs provide a local source of mature miRNAs responding to synaptic activity. The local biogenesis of miRNAs regulating dendritic spine development is particularly interesting considering axonal regeneration following injury, because a precise regulation of intra-axonal translation from localized mRNAs is essential for successful axon regeneration (Ben-Yaakov et al. 2012, Gumy et al. 2010, Hanz & Fainzilber 2006, Perry et al. 2012, Yudin et al. 2008, Twiss et al. 2000, Hanz et al. 2003, Perlson et al. 2005). Despite our increasing knowledge about the presence of precursor miRNAs and localization mechanism in dendrites, the existence of axonal precursor miRNAs that may regulate axonal protein synthesis during regeneration has not been previously reported. Here, we used precursor miRNA-specific primer sets to interrogate localization of precursor miRNAs into distal axon. Using quantitative PCR (qPCR) and fluorescence in situ hybridization (FISH) techniques, we, for the first time, directly contrasted alterations in axonal levels of precursor miRNAs responding to nerve injury, as compared to those in uninjured nerve. Taken altogether, our results indicate that specific precursor miRNAs are selected to be transported into axons of sensory neurons and that the levels of a subset of these precursor miRNAs change in response to injury to provide another level of spatial and temporal regulation for local translation.
Materials and methods
Animal procedures and DRG neuronal cultures
Animal procedures were approved by Institutional Animal Care and Use Committees (IACUC), and the experiments were conducted under the IACUC at Alfred I. DuPont Hospital for Children. For injury, the sciatic nerve of 150–225 g male Sprague Dawley rats (Harlan Laboratories) was crushed at mid-thigh level. Sciatic nerves ipsilateral (injured) and contralateral (uninjured) to crush injury were collected at 7 days after injury, and processed as previously described (Merianda et al. 2013a). Dissociated DRG cultures were prepared from L2–6 DRGs as described previously (Twiss et al. 2000) and were plated on coverslips coated with poly-L-lysine (Sigma) and laminin (Millipore).
For isolation of axons from cell bodies, cells were cultured onto porous membrane culture inserts (3 µm pores; Falcon). Axonal versus cell body extracts were isolated as described previously (Zheng et al. 2001).
RNA isolation and qPCR
Total RNA was isolated from nerves using a mechanical squeezing method as described with a few modifications (Rishal et al. 2010). RNA yield was determined by fluorimetry using RiboGreen (Invitrogen). Of the total RNA, 10 ng RNA was used to reverse transcribe to cDNA using iScript RT kit (BioRad) and subsequently carried out for standard extended PCR (35 cycles). β-actin, cell body-restricted [microtubule-associated protein 2 (MAP2) and H1 histone family member 0 (H1F0)], and non-neuronal cell [glial fibrillary acidic protein (GFAP) and Receptor tyrosine-protein kinase ErbB family-3 (ErbB-3)] mRNAs were used to assess purity of RNA isolation.
For a small RNA fractionation (<200 nucleotides in length), purified RNA was further processed to an RNeasy Mini spin column followed by the RNeasy MinElute Cleanup Kit (Qiagen). For quantitative PCR (qPCR) analysis of pre-miRNAs, the purified small RNA was converted to cDNA using NCode™ VILO™ miRNA cDNA Synthesis Kit (Invitrogen) to optimize reverse transcription efficiency from the small RNAs. The resulting cDNA was then used as a template for qPCR analyses using NCode™ EXPRESS® SYBR GreenER™ miRNA qRT-PCR Kit (Invitrogen) on the ABI Prism 7900HT. We initially evaluated whether the most common reference RNAs used in qPCR experiments, such as 5S and 12S rRNAs, tRNA, and U6 snRNA, showed uniform level between two groups by plotting the raw CT values for each group. Our variance analysis in expression levels of each of the reference RNAs showed that U6 snRNA was the most consistently expressed RNA, followed by tRNA, 12S, and 5S rRNAs. Thus, the relative level of pre-miRNAs was normalized to that of U6. The final results of qPCR in relative level were expressed as the ratio of pre-miRNA in injured nerve to that in uninjured nerve using the 2−ΔΔCT method. To verify pre-miRNA-specific primer specificity, we confirmed a single peak in the melting-curve analysis as well as run the reaction products on a 2.5% agarose gel (NuSieve 3 : 1; Lonza) for all genes analyzed.
Fluorescence in situ hybridization (FISH) and Immunofluorescence (IF)
FISH/IF was performed as described previously (Merianda et al. 2013a) with minor modifications. Antisense LNA probes (Exiqon) against the terminal loops of pre-miRNAs labeled with digoxigenin (DIG) at both 5’ and 3’ ends were used. DIG-labeled, ‘scrambled’ LNA probes were used as a negative control.
Cultured neurons and nerve tissues were fixed in 4% paraformaldehyde (PFA) for 20 min and 2 h, respectively. After being cryoprotected in buffered 30% sucrose at 4 °C, the tissues were processed for cryosectioning. After hybridization, the samples were washed and processed for IF. Primary antibodies were as follows: chick anti-neurofilament (NF) H (1:1,000; Millipore) and -NFM (1:500; Aves), goat anti-Drosha (1:100; Abcam), mouse anti-Dicer (1:100; Abcam) and mouse anti-DIG (1:200; Jackson ImmunoResearch), rabbit anti-KSRP (1:50; Abcam) and Cy3-conjugated mouse anti-DIG (for tissues- 1:200; Jackson ImmunoResearch). Secondary antibodies were as follows: Alexa 488-conjugated anti-chick (1:800; Invitrogen), Cy3-conjugated anti-mouse (1:200; Jackson ImmunoResearch), Alexa 555-conjugated anti-goat (1:800; Invitrogen), and Cy3-conjugated anti-rabbit (1:200; Jackson ImmunoResearch). All FISH samples were mounted in Prolong Gold (Invitrogen) and analyzed by epifluorescent microscopy with a Leica DMRXA2 fitted with Hamamatsu ORCA-ER CCD camera. Image pairs of uninjured and injured tissues were matched for exposure, gain/offset, and post-processing. DRG cultures for IF only were imaged by laser scanning confocal microscopy with Leica TCS/SP2.
Immunoblotting was performed using goat anti-Drosha (1:2,000; Abcam) and rabbit anti-GAPDH (1:2,000; Cell Signaling). After incubation with HRP-conjugated secondary antibody (1:3,000; Abcam), signals were visualized by ECLplus per manufacturer’s protocol (GE Biosciences).
Statistical analysis
All experiments were performed in at least 3 separate experiments (n > 3) and reported as mean ± SD. Student’s t-test was used to determine significance differences between two groups; p <0.05 was considered significant. GraphPad Prism 5 software package (GraphPad) was used for all statistical analyses.
Results and Discussion
Purification of axoplasmic RNA from rat sciatic nerve
To determine whether axonally enriched miRNAs that Natera-Naranjo detected (Natera-Naranjo et al. 2010) may exist in precursor forms in axons in vivo, we isolated axoplasmic RNA from rat sciatic nerve. We used a mechanical approach to isolate axoplasm from uninjured and regenerating sciatic nerve, modifying a method originally developed by the Fainzilber group (Rishal et al. 2010, Yudin et al. 2008, Yoo et al. 2013). To minimize non-neuronal contamination in the axoplasm preparations from injured sciatic nerve, we standardized the extraction of nerve to the 10-mm segments located 3–5 mm proximal to the injury site (ipsilateral) and the corresponding uninjured nerve (contralateral) excluding the site of injury, which reduces non-neuronal contamination of the preparations. Freshly dissected proximal stumps of sciatic nerves were further cut into small pieces in a petri dish on ice and transferred into 1.5-mL Eppendorf tubes. The sciatic nerve segments were manually squeezed with a plastic pestle that fits the tube. To prevent degradation of RNA that can distort or mislead the true level of pre-miRNAs, a rapid and ribonuclease-free procedure for tissue handling was employed.
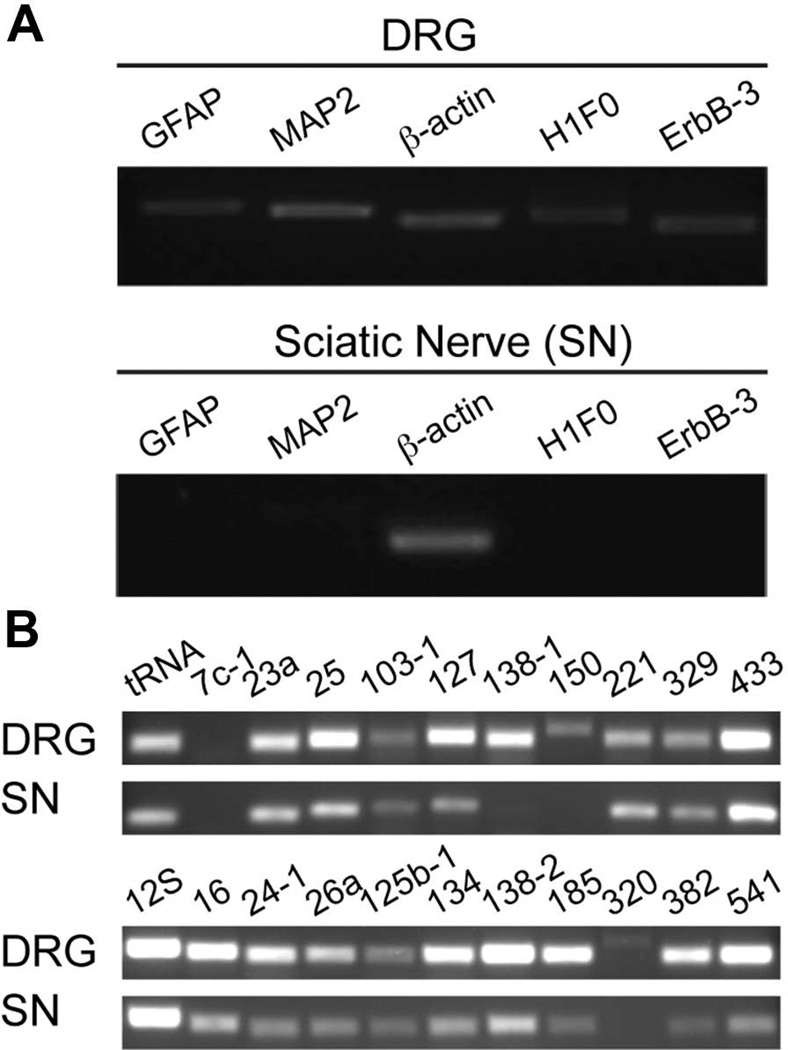
As shown in Figure 1A, the purity of the axoplasmic RNA isolated from proximal sciatic nerve was confirmed by extended cycle RT-PCR showing undetectable levels of cell body restricted (MAP2) or Schwann cell (GFAP) mRNAs, as we have previously used to show purity of axons isolated from cultured neurons (Merianda et al. 2013b, Vuppalanchi et al. 2010, Vuppalanchi et al. 2012, Willis et al. 2011, Yoo et al. 2013, Yudin et al. 2008, Willis et al. 2005). Several other mRNAs that have been shown to be present in glial cells were also not detected in the isolated axoplasm, including H1F0 and ErbB3. Given that all glial-specific and cell body-restricted transcripts were absent in the axoplasm, these levels of purity were remarkable and indicate that the axoplasmic isolates were indeed highly purified for axon-specific RNAs.
Figure 1. RNAs isolated from DRG neurons and the proximal stumps of sciatic nerve.
A. Extended PCR analysis using gene-specific primer sets for cell body restricted [microtubule-associated protein 2 (MAP2) and H1 histone family member 0 (H1F0)] and non-neuronal cells [glial fibrillary acidic protein (GFAP) and Receptor tyrosine-protein kinase ErbB family-3 (ErbB-3)] showed free of non-neuronal contamination. B. Purified pre-miRNAs were amplified from sciatic nerve cDNAs using pre-miRNA-specific primers. PCR products were resolved on 2.0% agarose gels and visualized by ethidium bromide staining.
Precursor miRNAs within axons of DRG neurons
Previously, Kaplan’s group identified a set of miRNAs that are enriched in sympathetic axons in culture (Natera-Naranjo et al. 2010). We reasoned that those miRNAs might be located in precursor forms into distal axon, where they could be locally processed to provide mature miRNAs to regulate the intra-axonal translation of relevant target mRNAs. To investigate the presence of pre-miRNAs in distal axons of DRG neurons in vivo, we first carried out extended PCR to detect precursor miRNAs using pre-miRNA-specific primers. We chose a total of 21 precursor miRNAs for the extended PCR reactions, focusing on the miRNAs that Kaplan’s group has shown to be highly enriched in distal axons in culture (Natera-Naranjo et al. 2010), as well as some that were somatodendritic miRNAs, including miRNAs-138 and -150 (Bicker et al. 2013, Siegel et al. 2009, Schratt et al. 2006, Pichardo-Casas et al. 2012). As shown in Figure 1B, the analyzed pre-miRNAs were able to be amplified from both cell bodies and axons similar to the Kaplan’s report. However, in a few cases, inconsistencies were identified. For example, pre-miRNA-7c-1 was not observed in RNA obtained from cell body and axon. In addition, amplicons for pre-miRNAs-138-1, 150 and 320 were readily visualized in RNA prepared from the cell body, but were not observed in RNA obtained from nerves, suggesting not all pre-miRNAs are localized into distal axons.
miRNAs negatively regulate their target transcripts that are transported into distal axons. Both the transport and translation of axonally localizing transcripts are specifically regulated in response to extracellular stimuli including injury (Willis et al. 2007, Vuppalanchi et al. 2009, Wu et al. 2005, Zhang et al. 1999). We speculated that regulation of the transcripts whose axonal levels were altered in response to injury is accompanied by corresponding changes at the levels of individual pre-miRNAs. To test the possibility that a set of pre-miRNAs that are localized to distal axons shows changes in axonal levels in response to injury, we compared levels of pre-miRNAs present in injured nerve to those in naïve nerve by qPCR (Fig. 2). We were able to detect that a number of pre-miRNAs were significantly increased in injured nerve compared with those in uninjured, while others were significantly decreased in injured nerve. For example, pre-miRNAs-16, 23a, 25, 125b-1, 433, and 541 showed a significant increase, while pre-miRNAs-138-2 and 185 were significantly decreased. Together, these results indicate that a subset of pre-miRNAs is transported into axons of sensory neurons and that the levels of these pre-miRNA change in response to injury.
Figure 2. Alteration of pre-miRNA levels in sciatic nerve with injury was quantified using qPCR.
The graph showed the relative level of pre-miRNAs in injured axons compared with those measured in naïve nerve. Note that the reduced level of pre-miRNA-185 was actually less than 40% of that in naïve. Relative expression was calculated using the 2−ΔΔCT value after normalizing to the levels of U6 snRNA. Error bars represent standard deviation (n = 3).
However, a recent study has shown that pri-miRNAs are detected in isolated post-synaptic densities, suggesting their dendritic localization (Lugli et al. 2012). Although an RNA fractionation method was utilized to isolate small RNAs (<200 nucleotides in length) from large RNAs including pri-miRNAs, we cannot rule out the possibility that pri-miRNAs might also be present locally in axons.
Although our axoplasmic isolates were pure by our standard extended PCR analysis, we wanted to be certain that these were actually from the axons. Thus, we used fluorescence in situ hybridization (FISH) with locked nucleic acid (LNA) probes specific for the terminal loops of pre-miRNAs-25, -433 and -150 (Fig. 3). In cultured DRG neurons, pre-miRNA-25 was visible by FISH in cell body and axons (arrows). miRNAs-150 and -138 have been shown to be localize to dendrites (Siegel et al. 2009, Pichardo-Casas et al. 2012), but the pre-miRNAs-150 and -138 were restricted to cell body and excluded from the axons (Fig. 3A and data not shown, respectively), consistent with the PCR analysis (Fig. 2). Further, consistent with increased axonal pre-miRNA-433 level upon injury (Fig. 2), we were able to detect pre-miRNA-433 in the axons of cultured DRG neurons, and sciatic nerve FISH samples showed clear increase in pre-miRNA-433 in axons in vivo after sciatic nerve crush (Fig. 3D vs. C).
Figure 3. Representative fluorescence in situ hybridization images.
FISH was performed on cultured DRG neuron (top panels) and sciatic nerve (bottom panels) using LNA probes (red) specific for the terminal loops of pre-miRNAs-150 (A), -25 (B), and -433 (C, D). Arrows indicate pre-miRNA signals in the axons of DRG neuron. Exposure matched images for scrambled probe are shown in the inset panels.
Dicer and KSRP proteins in distal axons of DRG neuron
The qPCR and FISH analyses indicated that some pre-miRNAs were localized into distal axons and showed alterations in axonal level in response to injury. Given that miRNA biogenesis starts with cleavage of pri-miRNA into pre-miRNA by Drosha in the nucleus, we asked if Drosha localizes to axons. Although we easily detected Drosha immunofluorescent signal in the cell body, no signals were seen in the DRG axons (Fig. 4A). Dicer required for processing pre-miRNA to mature miRNA was, however, clearly seen in axons and growth cones as previously shown (Fig. 4B, green) (Hengst et al. 2006, Lugli et al. 2005, Murashov et al. 2007, Wu et al. 2012, Aschrafi et al. 2008). The KH-type splicing regulatory protein (KSRP) is a multifunctional RNA- and single-stranded DNA-binding protein (Min et al. 1997, Rehbein et al. 2002, Gherzi et al. 2004) that has recently been shown to be a component of Dicer complex for biogenesis of a subset of miRNAs (Trabucchi et al. 2011, Trabucchi et al. 2009, Ruggiero et al. 2009). KSRP has been detected in neurites of a differentiating mouse neuroblastoma cell line, but its presence in primary DRG neurons has not been reported. Interestingly, co-labeling DRG cultures for Dicer and KSRP showed overlapping signals in granular foci in axons and growth cones of DRG neurons (Fig. 4B).
Figure 4. Immunolocalization of Drosha, Dicer, and KSRP in adult DRG neuron.
A. Drosha (red) was exclusively located in the nucleus but no signals were detected in axons labeled with neurofilament (NF, blue). B. Dicer (green) and KSRP (red) proteins were colocalized (arrows) along the axonal shaft and growth cone and observed as punctate deposits. C. Representative immunoblot for cell body and axoplasm extracts from DRG neurons cultured on porous membrane culture inserts was shown. A GAPDH immunoblot was included as loading control. Although Drosha signal showed consistently a prominent band of about 160 kDa in the cell body lysates (arrow), the axonal lysates did not show Drosha signals.
To further test whether the absence of Drosha immunofluorescence in the DRG axons was due to the lack of sensitivity detecting the Drosha in axons, we performed immunoblotting with anti-Drosha antibody (Fig. 4C). For this, we cultured dissociated DRG neurons on a porous membrane insert for separation of axonal versus cell body lysates (Zheng et al. 2001). Consistent with the IF analysis (Fig. 4A), we were able to detect Drosha protein in the cell body extract, but the axonal extract showed no Drosha signals.
Taken together, our studies indicate that selected pre-miRNAs specifically localize into the axonal compartment. Although it is completely unknown how pre-miRNAs could be selectively transported into distal axons of neurons, recent studies demonstrate that pre-miR-134 is localized into dendrites with an association of unique targeting cis-signals within the terminal loop and trans-acting RNA-binding protein DHX-36 and subsequent local processing into mature miRNAs (Bicker et al. 2013). Similar mechanisms that are utilized to spatially and temporally target mRNAs into distal axons are likely to be used in pre-miRNA localization into dendrites and axons. Further, the locally processed mature miRNAs from precursors could only target mRNAs at or near the site of their maturation. The localized mRNAs could be immediately recruited to the translational machinery in response to signals such as axonal injury, and down regulated upon subsequent pre-miRNA processing. Despite the molecular mechanisms for regulation of local miRNA biogenesis in dendrites and axons remain to be elusive, we concluded that axons of sensory neurons contain a heterogeneous population of pre-miRNAs and have at least some of the machinery needed to generate mature functional miRNAs locally. Future studies are needed to determine molecular mechanisms of pre-miRNA localization and local miRNA biogenesis in distal axons, and the significance for regeneration.
Acknowledgements
This work was funded by awards from the National Institutes of Health (P20-GM103464 and R21-NS085691 to SY).
Abbreviations
- miRNA
microRNA
- pre-miRNA
precursor microRNA
- pri-miRNA
primary microRNA
- KSRP
KH-type splicing regulatory protein
- DGCR8
DeGeorge syndrome critical region gene 8
- UTR
untranslated region
- FISH
fluorescence in situ hybridization
- DRG
dorsal root ganglion
- qPCR
quantitative PCR
- LNA
locked nucleic acid
- MAP2
microtubule-associated protein2
- GFAP
glial fibrillary acidic protein
- H1F0
H1 histone family member 0
- ErbB-3
Receptor tyrosine-protein kinase ErbB family-3
Footnotes
The authors declare no competing financial interests.
References
- Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci. 2008;28:12581–12590. doi: 10.1523/JNEUROSCI.3338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yaakov K, Dagan SY, Segal-Ruder Y, et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. Embo J. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker S, Khudayberdiev S, Weiss K, Zocher K, Baumeister S, Schratt G. The DEAH-box helicase DHX36 mediates dendritic localization of the neuronal precursor-microRNA-134. Genes Dev. 2013;27:991–996. doi: 10.1101/gad.211243.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M, Schratt GM. microRNA involvement in developmental and functional aspects of the nervous system and in neurological diseases. Neurosci Lett. 2009;466:55–62. doi: 10.1016/j.neulet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Gherzi R, Lee KY, Briata P, Wegmuller D, Moroni C, Karin M, Chen CY. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Molecular cell. 2004;14:571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Gumy LF, Tan CL, Fawcett JW. The role of local protein synthesis and degradation in axon regeneration. Exp Neurol. 2010;223:28–37. doi: 10.1016/j.expneurol.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S, Fainzilber M. Retrograde signaling in injured nerve--the axon reaction revisited. J Neurochem. 2006;99:13–19. doi: 10.1111/j.1471-4159.2006.04089.x. [DOI] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, et al. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. J Neurosci. 2006;26:5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–920. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Liu NK, Xu XM. MicroRNA in central nervous system trauma and degenerative disorders. Physiol Genomics. 2011;43:571–580. doi: 10.1152/physiolgenomics.00168.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli G, Larson J, Demars MP, Smalheiser NR. Primary microRNA precursor transcripts are localized at post-synaptic densities in adult mouse forebrain. J Neurochem. 2012;123:459–466. doi: 10.1111/j.1471-4159.2012.07921.x. [DOI] [PubMed] [Google Scholar]
- Lugli G, Larson J, Martone ME, Jones Y, Smalheiser NR. Dicer and eIF2c are enriched at postsynaptic densities in adult mouse brain and are modified by neuronal activity in a calpain-dependent manner. J Neurochem. 2005;94:896–905. doi: 10.1111/j.1471-4159.2005.03224.x. [DOI] [PubMed] [Google Scholar]
- Lugli G, Torvik VI, Larson J, Smalheiser NR. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J Neurochem. 2008;106:650–661. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: Implications for Alzheimer Disease and other Human CNS Disorders. Curr Genomics. 2009;10:154–168. doi: 10.2174/138920209788185252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, Gomes C, Yoo S, Vuppalanchi D, Twiss JL. Axonal localization of neuritin/CPG15 mRNA in neuronal populations through distinct 5' and 3' UTR elements. J Neurosci. 2013a;33:13735–13742. doi: 10.1523/JNEUROSCI.0962-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, Vuppalanchi D, Yoo S, Blesch A, Twiss JL. Axonal Transport of Neural Membrane Protein 35 mRNA Increases Axon Growth. J Cell Sci. 2013b;126:90–102. doi: 10.1242/jcs.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H, Turck CW, Nikolic JM, Black DL. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 1997;11:1023–1036. doi: 10.1101/gad.11.8.1023. [DOI] [PubMed] [Google Scholar]
- Murashov AK, Chintalgattu V, Islamov RR, Lever TE, Pak ES, Sierpinski PL, Katwa LC, Van Scott MR. RNAi pathway is functional in peripheral nerve axons. Faseb J. 2007;21:656–670. doi: 10.1096/fj.06-6155com. [DOI] [PubMed] [Google Scholar]
- Natera-Naranjo O, Aschrafi A, Gioio AE, Kaplan BB. Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA. 2010;16:1516–1529. doi: 10.1261/rna.1833310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Perry RB, Doron-Mandel E, Iavnilovitch E, et al. Subcellular Knockout of Importin beta1 Perturbs Axonal Retrograde Signaling. Neuron. 2012;75:294–305. doi: 10.1016/j.neuron.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichardo-Casas I, Goff LA, Swerdel MR, et al. Expression profiling of synaptic microRNAs from the adult rat brain identifies regional differences and seizure-induced dynamic modulation. Brain Res. 2012;1436:20–33. doi: 10.1016/j.brainres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehbein M, Wege K, Buck F, Schweizer M, Richter D, Kindler S. Molecular characterization of MARTA1, a protein interacting with the dendritic targeting element of MAP2 mRNAs. J Neurochem. 2002;82:1039–1046. doi: 10.1046/j.1471-4159.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- Rishal I, Michaelevski I, Rozenbaum M, Shinder V, Medzihradszky KF, Burlingame AL, Fainzilber M. Axoplasm isolation from peripheral nerve. Dev Neurobiol. 2010;70:126–133. doi: 10.1002/dneu.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero T, Trabucchi M, De Santa F, Zupo S, Harfe BD, McManus MT, Rosenfeld MG, Briata P, Gherzi R. LPS induces KH-type splicing regulatory protein-dependent processing of microRNA-155 precursors in macrophages. Faseb J. 2009;23:2898–2908. doi: 10.1096/fj.09-131342. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Siegel G, Obernosterer G, Fiore R, et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol. 2009;11:705–716. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucchi M, Briata P, Filipowicz W, Ramos A, Gherzi R, Rosenfeld MG. KSRP Promotes the Maturation of a Group of miRNA Precuresors. Adv Exp Med Biol. 2011;700:36–42. doi: 10.1007/978-1-4419-7823-3_4. [DOI] [PubMed] [Google Scholar]
- Trabucchi M, Briata P, Garcia-Mayoral M, Haase AD, Filipowicz W, Ramos A, Gherzi R, Rosenfeld MG. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459:1010–1014. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiss JL, Smith DS, Chang B, Shooter EM. Translational control of ribosomal protein L4 mRNA is required for rapid neurite regeneration. Neurobiol Dis. 2000;7:416–428. doi: 10.1006/nbdi.2000.0293. [DOI] [PubMed] [Google Scholar]
- Vuppalanchi D, Coleman J, Yoo S, Merianda TT, Yadhati AG, Hossain J, Blesch A, Willis DE, Twiss JL. Conserved 3'-untranslated region sequences direct subcellular localization of chaperone protein mRNAs in neurons. J Biol Chem. 2010;285:18025–18038. doi: 10.1074/jbc.M109.061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalanchi D, Merianda TT, Donnelly C, Pacheco A, Williams G, Yoo S, Ratan RR, Willis DE, Twiss JL. Lysophosphatidic acid differentially regulates axonal mRNA translation through 5'UTR elements. Mol Cell Neurosci. 2012;50:136–146. doi: 10.1016/j.mcn.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuppalanchi D, Willis DE, Twiss JL. Regulation of mRNA transport and translation in axons. Results Probl Cell Differ. 2009;48:193–224. doi: 10.1007/400_2009_16. [DOI] [PubMed] [Google Scholar]
- Weinberg MS, Wood MJ. Short non-coding RNA biology and neurodegenerative disorders: novel disease targets and therapeutics. Hum Mol Genet. 2009;18:R27–R39. doi: 10.1093/hmg/ddp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, et al. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, van Niekerk EA, Sasaki Y, et al. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, Xu M, Donnelly CJ, et al. Axonal Localization of transgene mRNA in mature PNS and CNS neurons. J Neurosci. 2011;31:14481–14487. doi: 10.1523/JNEUROSCI.2950-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Raafat A, Pak E, Clemens S, Murashov AK. Dicer-microRNA pathway is critical for peripheral nerve regeneration and functional recovery in vivo and regenerative axonogenesis in vitro. Exp Neurol. 2012;233:555–565. doi: 10.1016/j.expneurol.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S, Kim HH, Kim P, et al. A HuD-ZBP1 ribonucleoprotein complex localizes GAP-43 mRNA into axons through its 3' untranslated region AU-rich regulatory element. J Neurochem. 2013;126:792–804. doi: 10.1111/jnc.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D, Hanz S, Yoo S, et al. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 2008;59:241–252. doi: 10.1016/j.neuron.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HL, Singer RH, Bassell GJ. Neurotrophin regulation of beta-actin mRNA and protein localization within growth cones. J Cell Biol. 1999;147:59–70. doi: 10.1083/jcb.147.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Zheng SJ, Zhao JH, et al. MicroRNAs 144, 145, and 214 are down-regulated in primary neurons responding to sciatic nerve transection. Brain Res. 2011;1383:62–70. doi: 10.1016/j.brainres.2011.01.067. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Yu B, Qian T, Yao D, Wang Y, Ding F, Gu X. Early changes of microRNAs expression in the dorsal root ganglia following rat sciatic nerve transection. Neurosci Lett. 2011;494:89–93. doi: 10.1016/j.neulet.2011.02.064. [DOI] [PubMed] [Google Scholar]