Abstract
Rationale
Microglial activation in autonomic brain regions is a hallmark of neuroinflammation in neurogenic hypertension (HTN). Despite evidence that an impaired sympathetic nerve activity supplying the bone marrow (BM) increases inflammatory cells and decreases angiogenic cells, little is known about the reciprocal impact of BM-derived inflammatory cells on neuroinflammation in HTN.
Objective
Test the hypothesis that pro-inflammatory BM cells from hypertensive animals contribute to neuroinflammation and HTN via a brain-BM interaction.
Methods and Results
Following BM ablation in spontaneously hypertensive rats (SHR), and reconstitution with normotensive Wistar-Kyoto (WKY) rat BM, the resultant chimeric SHR displayed significant reduction in mean arterial pressure (MAP) associated with attenuation of both central and peripheral inflammation. In contrast, an elevated MAP along with increased central and peripheral inflammation was observed in chimeric WKY rats reconstituted with SHR BM. Oral treatment with minocycline, an inhibitor of microglial activation, attenuated HTN in both the SHR and chronic angiotensin II (Ang II)-infused rats. This was accompanied by decreased sympathetic drive and inflammation. Furthermore, in chronic Ang II-infused rats, minocycline prevented extravasation of BM-derived cells to the hypothalamic paraventricular nucleus (PVN), presumably via a mechanism of decreased C-C chemokine ligand 2 levels in the cerebrospinal fluid.
Conclusions
The BM contributes to HTN by increasing peripheral inflammatory cells and their extravasation into the brain. Minocycline is an effective therapy to modify neurogenic components of HTN. These observations support the hypothesis that BM-derived cells are involved in neuroinflammation, and targeting them may be an innovative strategy for neurogenic resistant HTN therapy.
Keywords: Microglia, hypertension, bone marrow mononuclear cells, autonomic nervous system, immune system
INTRODUCTION
Hypertension (HTN) is the most modifiable risk factor for cardiovascular disease. Despite significant advancement in its control, 20–30% of all hypertensive patients remain resistant to available pharmacotherapy. This is primarily due to the involvement of a strong neurogenic component in the establishment of hypertensive state1–3. Mounting evidence implicates a key role for peripheral and neuroinflammation in the pathophysiology of HTN in both humans and animal models4–8. However, the relationship between the immune system (IS) and the central nervous system (CNS) in HTN is not well understood. An interesting relationship was revealed by the indication that increased sympathetic drive can mediate HTN by norepinephrine-mediated T-cell activation9. As a meeting point for the CNS and IS10–12, and the site of leukocyte and progenitor cell production, the bone marrow (BM) serves as an ideal link between the inflammatory system and HTN.
The BM plays an important role in cardiovascular health and disease, leading to the proposal that the autonomic pathways that control the IS may be dysregulated in HTN5, 8. Indeed, altered inflammatory responses have been associated with impaired autonomic input to the BM in the spontaneously hypertensive rats (SHR), an established animal model for human HTN10. Also, early studies have indicated that suppressing the IS by pharmacological treatment or thymectomy could blunt the development and maintenance of HTN6, 13, 14. It was later shown that T-cells are the critical immune players responsible for the genesis of HTN15. In addition to peripheral inflammatory responses, central neuroinflammation and oxidative stress have been described in several hypertensive animal models16–18. Of particular interest is the activation of microglial cells, which act as the resident immune cells of the CNS19. Recent studies have implicated the activation of microglial cell in the autonomic brain regions, particularly the hypothalamic paraventricular nucleus, plays an important role in HTN20–24. This view is further supported by clinical evidence, wherein chronic treatment of hypertensive patients with minocycline (mino), an anti-inflammatory, small molecule antibiotic that freely passes the blood brain barrier (BBB) and inhibits microglial activation, produced profound blood pressure lowering effects25. Similarly, activated microglia and neuroinflammation have been shown to be associated with cognitive impairment and Parkinson’s disease26, 27. These studies have suggested that bone marrow cells, particularly pro-inflammatory progenitors, are mobilized from BM to enter the brain parenchymal space in a C-C chemokine ligand 2 (CCL2) - and its receptor (CCR2) axis- dependent manner, thus contributing to chronic neuroinflammation28–30.
While inflammatory cells have long been described to infiltrate the vasculature and organs such as the kidney and heart in HTN31–33, the evidence supporting IC infiltration and accumulation in the brain parenchyma are fewer25, 34–36. All these observations have led us to hypothesize that the BM exhibits a pro-inflammatory state in HTN, characterized by increased inflammatory cells and cytokines. This results in increased peripheral inflammation, extravasation of inflammatory progenitors into crucial cardioregulatory brain centers, where they differentiate into microglia/macrophages and contribute to HTN. Our study was designed to address this hypothesis.
METHODS
All animal procedures were approved by the University of Florida Institute Animal Care and Use Committee. Full details of all experimental protocols are presented in the Methods section in the Online Supplemental Material.
RESULTS
Elevated pro-inflammatory markers in the bone marrow of hypertensive animals
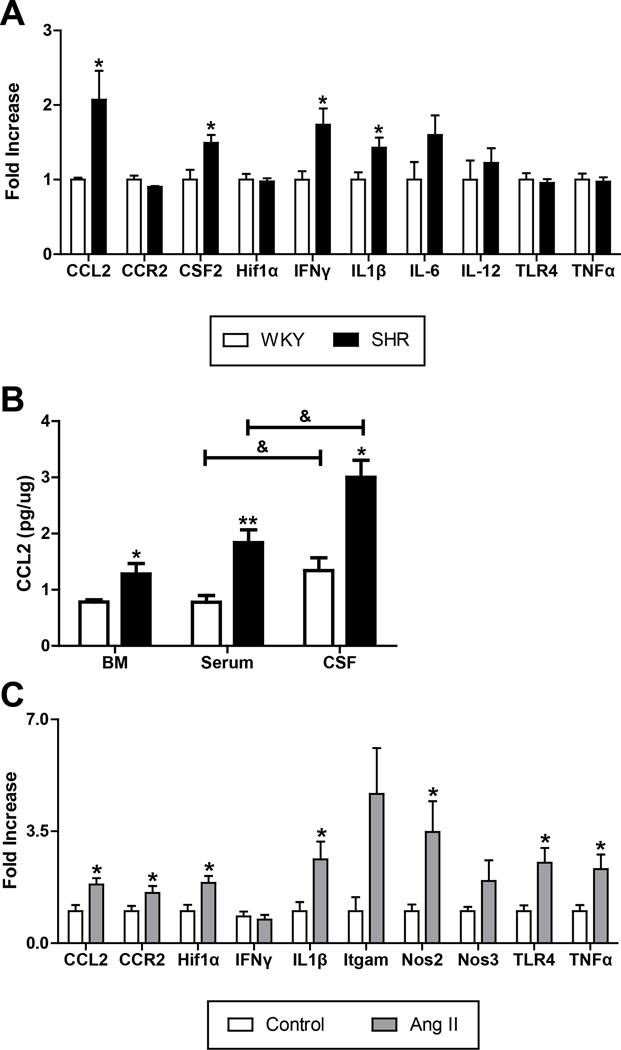
Two distinct rat models of HTN with neurogenic components [the SHR and chronic angiotensin II (Ang II)-infused rat] were used to evaluate the pro-inflammatory profile of the BM cells. We observed increased mRNA levels of interleukin-1β (IL-1β; 40%), interferon-γ (IFNγ; 80%), and colony stimulating factor 2 (CSF2; 50%) in the BM-derived mononuclear cells (MNCs) of SHR as compared with WKY (Fig. 1A). The greatest increase was observed in the CCL2 mRNA (100%). Additionally, CCL2 protein levels in the BM supernatant, serum, and cerebrospinal fluid (CSF) of the SHR were increased by 63%, 136%, and 124%, respectively (BM: 0.78±0.04 vs. 1.28±0.19 pg/ug; serum: 0.78±0.12 vs. 1.84.1±0.23 pg/ ug; CSF: 1.34±0.23 vs. 3.00±0.30 pg/ug; Fig. 1B). Importantly, there appears to be a significant gradient in CCL2 concentration from BM < Serum < CSF.
Figure 1. Pro-inflammatory markers are elevated in the bone marrow (BM) of two rat models of hypertension.
A. BM mononuclear cells (MNCs) from the SHR show increased mRNAs for CCL2, CSF2, IFNγ, and IL1β compared to WKY (n=4 per group). *p < 0.05 vs WKY. B. CCL2 levels are elevated in SHR BM supernatant, serum, and cerebrospinal fluid (CSF) (n=4 per group). *p < 0.05, **p < 0.01 vs WKY; & p < 0.05 vs serum. C. BM MNCs from chronic Ang II infusion in SD rats have increased mRNA of CCL2, CCR2, Hif1α, IL1β, Nos2, TLR4, and TNFα compared to saline-infused SD control rats (n=6 per group). *p < 0.05 vs control.
Next, we investigated the effect of chronic Ang II infusion on the inflammatory profile of BM cells in this model of HTN. Similar to the SHR, we found significant increases in the mRNA levels of CCL2 (83%), and IL-1β (162%) following 8-weeks of Ang II infusion (Fig. 1C). In addition, increases of 57% in CCR2, 89% in hypoxia-inducible factor 1-α (HIF1α), 248% in inducible nitric oxide synthase (NOS2), 152% in toll-like receptor 4 (TLR4), and 132% in tumor necrosis factor-α (TNFα) mRNA levels were detected. These data indicate that although the individual cytokine profile of the BM cells is different in diverse HTN animal models, they share a common pro-inflammatory nature.
Modulation of blood pressure and hemodynamics in both SHR and WKY by bone marrow reconstitution
Since the HTN BM cells were found to have increased expression of pro-inflammatory cytokines, we tested the hypothesis that reconstitution of WKY rats with SHR BM would increase their mean arterial pressure (MAP). Details on the design of this experiment are presented in the supplemental material and Online Fig. I. The validity of reconstitution in this model was simultaneously confirmed in female rats receiving male BM cells, thereby allowing tracking of reconstitution success by Y-FISH (Online Fig. II), as previously described37. Briefly, simultaneous BM reconstitution experiments were performed in the adult female SHR, and reconstitution with male WKY and SHR whole BM cells was confirmed by detecting the Y-chromosome in BM-derived MNCs. Successful reconstitution was adjudged by >90% Y chromosome-stained MNCs isolated from the blood. In all subsequent experiments, adult age-matched male WKY and SHR were used to investigate the role of BM in HTN. Data presented from here forth is exclusively from male rats receiving male cells.
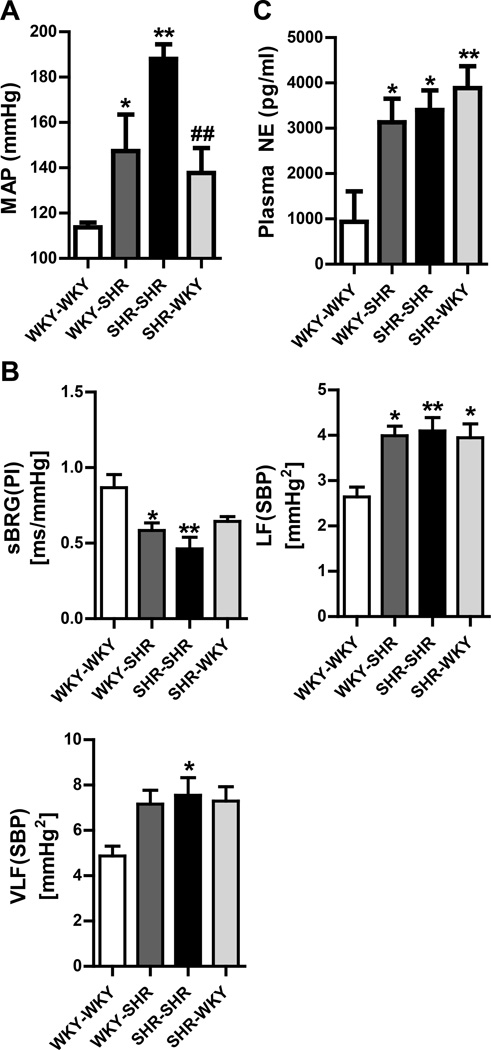
MAP was found to be elevated in WKY rats reconstituted with SHR cells as compared to those that were reconstituted with WKY cells (WKY-SHR 147±16 mmHg; WKY-WKY 114±2 mmHg; Fig. 2A). Conversely, reconstitution of SHR with WKY bone marrow resulted in lowering of MAP in comparison to those reconstituted with SHR cells (SHR-WKY 138±11 mmHg; SHR-SHR 188±6 mmHg). A parallel experiment was carried out where MAP was measured by non-invasive tail-cuff plethysmography. The data obtained from the tail-cuff method was consistent and comparable to that obtained by radiotelemetry (Online Fig. III).
Figure 2. Bone marrow (BM) reconstitution modulates blood pressure and autonomic function in SHR and WKY rats.
A. Mean arterial pressure (MAP) was measured directly by radiotelemetry (n=5 per group). Reconstitution of the WKY rat with SHR bone marrow increases MAP. Conversely, reconstitution of the SHR with WKY bone marrow lowers MAP. B. Spectral analysis of the systolic blood pressure (SBP) and pulse interval (PI) waveforms of telemetry (n=5 per group). sBRG: spontaneous baroreflex gain, LF: low frequency, VLF: very low frequency. C. Plasma norepinephrine (NE) is elevated in the WKY-SHR vs WKY-WKY. There is no change in the SHR rats with WKY bone marrow (n=5 per group). *p < 0.05, **p < 0.01 vs WKY-WKY; #p < 0.05, ##p < 0.01 vs SHR-SHR.
Next, we evaluated cardiac hypertrophy by quantifying heart weight to tibia length ratio (HW:TL). Similar trend as that seen in MAP data was observed for cardiac remodeling (WKY-WKY 27.9±1.3; WKY-SHR 32.6±1.0; SHR-SHR 36.1±1.1; SHR-WKY 30.1±2.1 mg/mm; Online Fig. IV). These findings were further confirmed by measuring the cardiomyocyte diameter from the left ventricular free wall. The results were consistent with the HW:TL findings.
Plasma norepinephrine (NE) levels were found to be elevated in the WKY-SHR as compared with the WKY-WKY controls (3131±521 vs. 937±671 pg/ml; Fig. 2C). However, no significant difference in plasma NE was observed between SHR-WKY and SHR-SHR groups (3888±484 vs. 3405±432 pg/ml). This was also confirmed by spectral analysis of the telemetry waveform of systolic blood pressure (SBP) and pulse interval, as previously described41. This analysis revealed that cardiac spontaneous baroreflex gain (sBRG) was attenuated in the WKY-SHR but not in WKY-WKY (0.58±0.05 vs. 0.87±0.09 ms/mmHg; Fig. 2B), and was further accompanied by an increase in overall vasomotor sympathetic tone in these two groups [LF(SBP); 4.0±0.2 vs 2.6±0.2 mmHg2; [VLF(SBP); 7.2±0.6 vs 4.9±0.4 mmHg2], although the latter failed to reach significance. However, none of these autonomic variables were attenuated in the SHR-WKY as compared to the SHR-SHR.
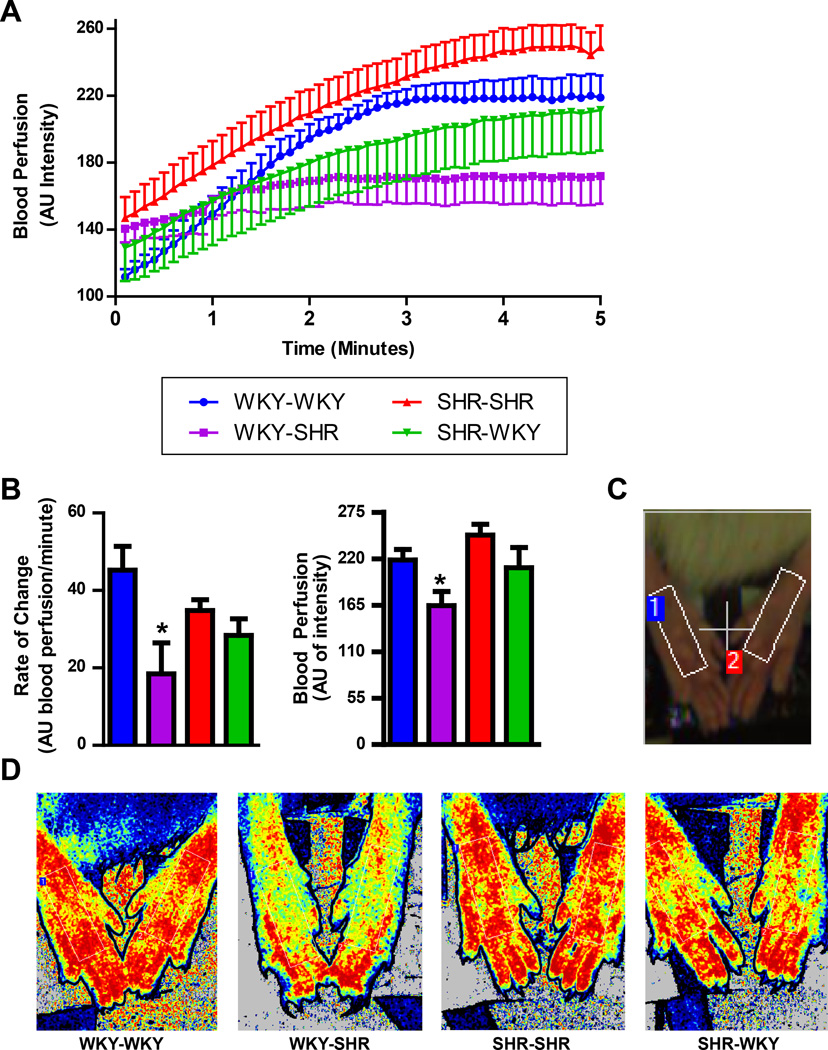
Next, we evaluated the blood perfusion to the hind-limbs of these chimeric rats utilizing a laser speckle contrast imager (Fig. 3A-D). Two analyses were performed: (1) the rate of blood flow change (slope) during the first 1.5 minutes; and (2) stabilized perfusion during the last 1.5 minutes. Isoflurane anesthesia induces a slow vasodilatory response38, which is observed in the first two minutes of recording. This rate of change in blood perfusion was decreased in the WKY-SHR when compared to the WKY-WKY (19±7 vs 45±6 AU blood perfusion/minute; Fig. 3B). Additionally, the stabilized blood perfusion was lower in the WKY-SHR when compared to the WKY-WKY (164±16 vs 219±12 AU of intensity). No differences where observed between the SHR-WKY and the SHR-SHR.
Figure 3. Bone marrow (BM) reconstitution with SHR cells alters blood perfusion of the hind limbs in the WKY.
A. Hind paw perfusion in arbitrary units (AU) over five minutes of recording time. B. Rate of blood perfusion change over the first 1.5 minutes and stabilized blood perfusion of the hind paw are both decreased in WKY-SHR vs WKY-WKY. C. Photograph indicating region of interest on both hind paws. D. Representative blood flow intensity maps during the last minute of the sample period. The color scale is from blue to red, lower to higher flow. (n=3–4 per group) *p < 0.05 vs WKY-WKY.
Decreased peripheral inflammation and activated microglia in the PVN of SHR reconstituted with WKY bone marrow
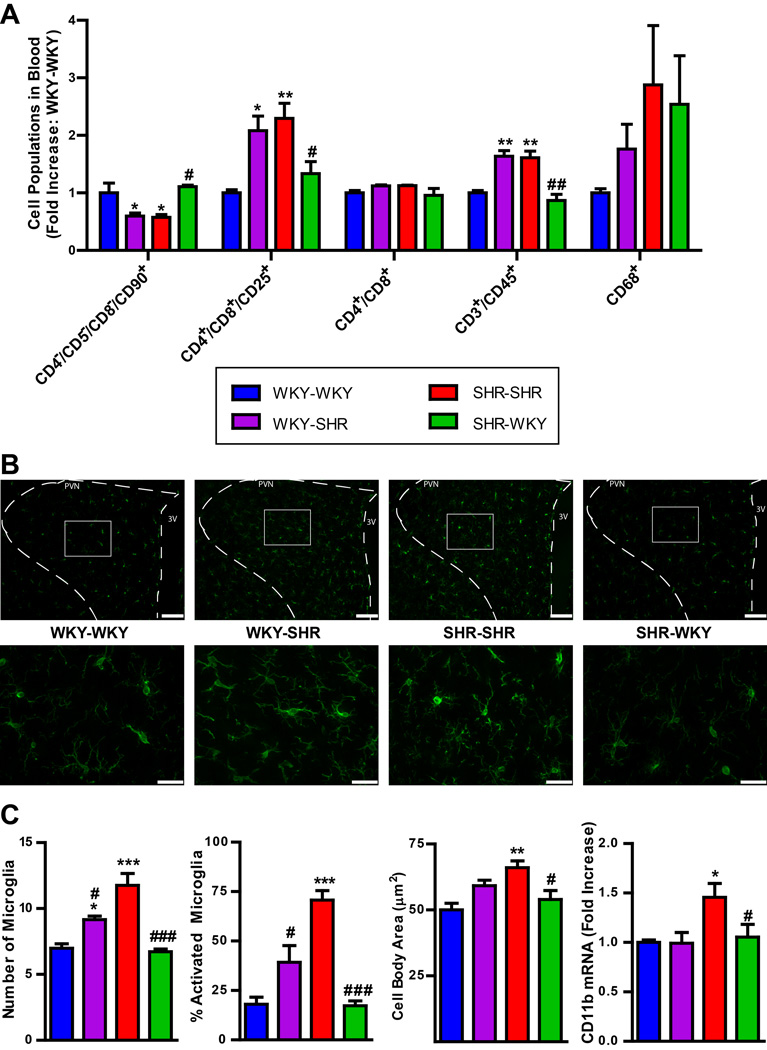
Our previous studies have established that HTN is associated with increases in pro-inflammatory cells and decreases in angiogenic progenitor cells (APCs)10, 11, 42. Thus, we decided to investigate the circulatory levels of these cells in chimeric rats. We measured circulating CD4−/CD5−/CD8−/CD90+ (representing APCs), and CD4+/CD8+/CD25+ and CD3+/CD45+ cells (representing subpopulations of T-cells previously shown to be elevated in HTN10, 11, 42, 43) in WKY-SHR animals, to determine if the increase in MAP in these animals is associated with changes in these cell populations. We observed a 43% reduction in circulating CD4−/CD5−/CD8−/CD90+ cells, a 61% increase in circulating CD4+/CD8+/CD25+ cells, and a 40% increase in CD3+/CD45+ cells in the WKY-SHR as compared with WKY-WKY (Fig. 4A). In contrast, the SHR-WKY group presented with a 92% increase in APCs, a 45% reduction in CD4+/CD8+/CD25+ cells and a 37% reduction in CD3+/CD45+ cells as compared to SHR-SHR. No changes were observed in the CD4+/CD8+ or CD68+ populations.
Figure 4. Peripheral inflammatory cells and activated microglia in the hypothalamic paraventricular nucleus (PVN) are decreased in the SHR following reconstitution with WKY bone marrow.
A. Specific inflammatory cell populations are increased in the circulation of WKY-SHR, including CD4+/CD8+/CD25+ and CD3+/CD45+ T-cells; these were decreased in the SHR-WKY. Additionally, CD4−/CD5−/CD8−/CD90+ were decreased in the WKY-SHR, and increased in the SHR-WKY (n=5–8 per group). B. Representative images at 10x and 40x magnification of Iba1+ microglia in the PVN. Scale bar is 100µm in 10x, and 30µm in 40x. C. Quantification of activated microglia in the PVN: number of microglia and % of activated microglia per 40,000µm2, cell body area, are increased in WKY-SHR; these values are decreased in SHR-WKY (n=5 per group). CD11b mRNA was higher in the SHR-SHR compared to WKY-WKY control, and restored in the SHR-WKY (n=5 per group). However, no changes were detected in WKY-SHR vs WKY-WKY. *p < 0.05, **p < 0.01 vs WKY-WKY; #p < 0.05, ##p < 0.01 vs SHR-SHR.
Next, we compared the levels of activated microglia in different experimental groups to determine if the change in MAP was associated with changes in microglial activation in the PVN. We found a significant decrease in activated microglia in SHR-WKY rats vs. SHR-SHR rats when measured as total number of microglia per 40,000µm2 (43%), percent activated microglia (76%), and cell body area (18%, Fig. 4B and C). This was confirmed by a 27% decrease in the mRNA levels of CD11b in the SHR-WKY group (Fig. 4C). On the other hand, total microglia per 40,000 µm2 was increased by 31% in the PVN of WKY-SHR vs WKY-WKY; however, changes in the percent activated microglia and cell body area did not reach significance.
These observations demonstrate transplantation of SHR BM into the WKY rats increases MAP, elevates circulating pro-inflammatory cells, and promotes microglial cells in the PVN. On the contrary, transplantation of WKY cells into SHR decreases MAP that is associated with increased APCs, decreased pro-inflammatory cells and reduced PVN microglial activation.
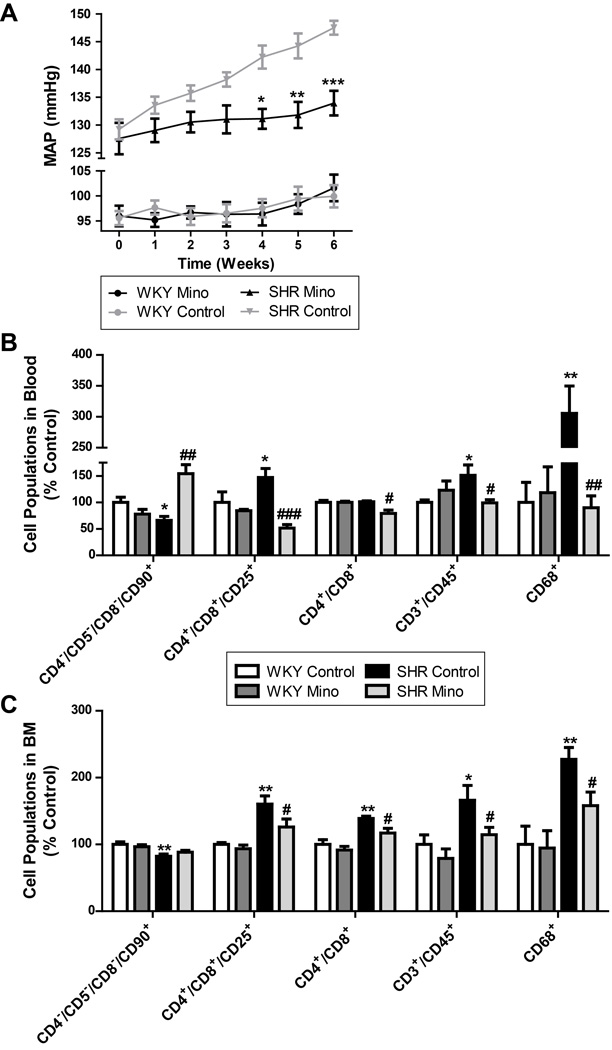
Oral delivery of minocycline attenuates mean arterial pressure, decreases inflammation, and restores autonomic balance in SHR
Next, we examined the effects of oral minocyline (mino) treatment on HTN and associated pathophysiology, including the BM. Our previous studies have shown that intracerebroventricular delivery (ICV) of mino prevents the development of HTN in the chronic Ang II infusion model22. Mino is an anti-inflammatory, tetracycline-derived antibiotic, which crosses the blood brain barrier44 and inhibits activation of microglial cells in the CNS45. Mino is currently being evaluated for many clinical applications, including stroke, due to its inhibitory effects on microglial cell activation46.
Oral delivery of mino to adult SHR rats attenuated the increase in MAP over 6 weeks of treatment (Fig. 5A). The HW:TL ratio, an index of cardiac hypertrophy, increased in vehicle treated SHRs as compared to WKY rats, but was lowered by mino treatment (Online Fig. V) These effects were associated with reduction in the levels of circulating CD4+/CD8+ (~22%), CD4+/CD8+/CD25+ (~65%), CD3+/CD45+ (~35%) and CD68+ (~70%) cells in mino-treated SHR as compared to SHR control. Additionally, CD4−/CD5−/CD8−/CD90+ cells were increased by ~134%. This was coupled with similar reductions in CD4+/CD8+ (~12%), CD4+/CD8+/CD25+ (~21%), CD3+/CD45+ (~31%) and CD68+ (~31%) cells in mino-treated SHR. No significant effect of mino treatment was observed on the BM or blood cell populations of WKY rats.
Figure 5. Oral minocycline (mino) attenuates mean arterial pressure (MAP) and peripheral inflammation in SHRs.
A. MAP measure by telemetry indicates that mino attenuates the development of HTN in the SHR (n=5-6 per group). B. Specific inflammatory cell populations were increased in the blood in SHRs, including CD4+/CD8+/CD25+, CD4+/CD8+, and CD68+. Mino treatment lowers these ratios back to control (n=5-6 per group). C. Following a similar trend, CD4+/CD8+/CD25+ and CD4+/CD8+ cells were increased in the bone marrow (BM) and decreased by mino treatment. CD4−/CD5−/CD8−/CD90+ cells (APCs) were lower in SHRs compared to WKY, and restored by oral mino (n=4-8 per group). *p < 0.05, **p < 0.01, ***p < 0.001 vs WKY control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs SHR control.
Spectral analysis of the SBP signal from transmitter-implanted rats revealed dampening of the spontaneous baroreflex gain (ΔsBRG) in SHR versus WKY rats (0.06±0.01 vs 0.24±0.05 ms/mmHg; Online Fig. VI). However, this effect was attenuated by mino treatment (−0.24±0.04 ms/mmHg). Additionally, LF[SBP], VLF[SBP], and vasovagal balance (ΔLF[SBP]:HF[PI]) values were increased in SHRs compared to WKY (0.5±0.3 mmHg2; 1.4±0.4 mmHg2; 0.1±0.03 mmHg2/ms2, respectively), which were normalized by mino treatment (−1.1±0.2 mmHg2; −1.1±0.4 mmHg2; −0.7±0.03 mmHg2/ms2, respectively). No significant changes were observed in WKY rats treated with mino.
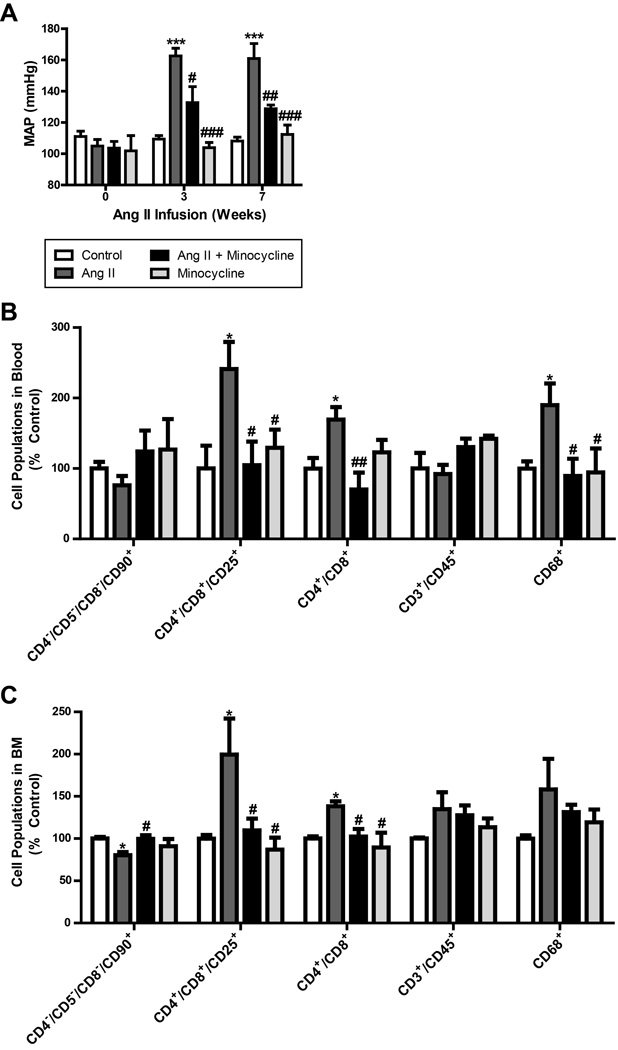
Oral delivery of minocycline attenuates mean arterial pressure, decreases inflammation, and restores autonomic balance in the chronic Ang II infusion rat model of hypertension
Oral delivery of mino to SD rats attenuated the increase in MAP induced by chronic Ang II infusion (129±3 vs. 161±10 mmHg; Fig. 6A). The telemetry data on MAP were comparable with the tail cuff plethysmography measurements (Online Fig. VII). The HW:TL ratio was found to be increased by chronic Ang II infusion (Online Fig. VII), which was significantly decreased by oral mino treatment (Control 28.5±1.5; Ang II 37.2±1.8; Ang II + Mino 31.4±1.0; Mino 30.4±1.5 mg/mm). Additionally, chronic Ang II infusion was associated with increased levels of circulating CD4+/CD8+ (30%), CD4+/CD8+/CD25+ (130%) and CD68+ (100%) cells in comparison to the control group (Fig. 6B). This was coupled with similar increases in CD4+/CD8+ and CD4+/CD8+/CD25+ cells in the BM, and a 20% decrease in CD4−/CD5−/CD8−/CD90+ APCs (Fig. 6C). However, these cell populations were restored back to control levels upon treatment with mino. Small differences are present between the blood and BM cell populations; however, they follow the same trends in both and support the same conclusions.
Figure 6. Oral minocycline (mino) attenuates mean arterial pressure (MAP) and peripheral inflammation in chronic Ang II infusion.
A. Mino attenuates the development of HTN in the chronic Ang II infusion model (n=4 per group). This effect was consistent over 7 weeks of treatment. B. Specific inflammatory cell populations were increased in the blood in chronic Ang II infusion, including CD4+/CD8+/CD25+, CD4+/CD8+, and CD68+. Mino treatment lowers these ratios back to control (n=4-8 per group). C. Following a similar trend, CD4+/CD8+/CD25+ and CD4+/CD8+ cells were increased in the bone marrow (BM) and decreased by mino treatment. CD4−/CD5−/CD8−/CD90+ cells (APCs) were lower in chronic Ang II infusion, and restored by oral mino (n=4-8 per group). *p < 0.05, **p < 0.01, ***p < 0.001 vs control; #p < 0.05, ## p < 0.01, ###p < 0.001 vs Ang II.
Spectral analysis of the SBP signal from transmitter-implanted rats revealed that sBRG was dampened in Ang II-infused rats versus control (−0.52±0.07 vs. 0.21±0.23 ms/mmHg; Online Fig. VIII). However, this effect was attenuated by mino treatment (−0.11±0.06 ms/mmHg). LF[SBP], VLF[SBP] and ΔLF[SBP]:HF[PI] were all increased in Ang II infused rats (1.08±0.34 mmHg2; 3.5±0.3 mmHg2; 0.12±0.05 mmHg2/ms2, respectively), but were normalized by mino treatment (−0.66±0.28 mmHg2; 0.6±0.8 mmHg2; −0.11±0.05 mmHg2/ms2, respectively). In contrast, no significant changes were observed in cardiac parasympathetic (ΔHF[PI]) and sympathetic tone (ΔLF[PI]:HF[PI]; data not shown).
Additionally, plasma NE levels were increased by chronic Ang II infusion (885±62 vs. 1610±165 pg/ml; Online Fig. VIII), while mino treatment resulted in a significant decrease (733±92 pg/ml). These findings are consistent with BM supernatant NE contents (control 54±17; Ang II 280±66; Ang II + Mino 120±22; Mino 165±22 pg/ug).
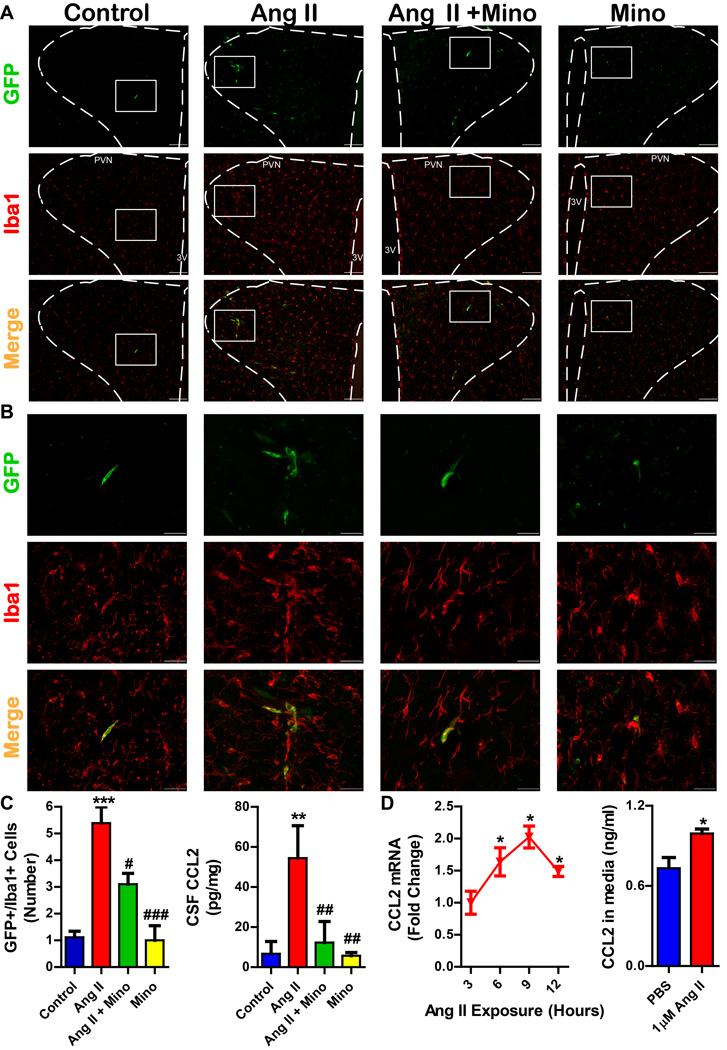
Chronic Ang II infusion increases BM-derived microglia/macrophages in the PVN
Finally, we investigated the link between the BM and brain microglial activation. Chronic Ang II infusion in the eGFP chimeric SD rats was used to track mobilization of the BM-derived cells. Online Figure IX shows that the Ang II-induced increase in MAP is comparable in chimeric eGFP and naïve SD rats (149±3 mmHg in chimera vs. 151±8 mmHg in naïve). We observed a 4.3 fold increase in GFP+/Iba1+ cells in the PVN of Ang II-infused chimeric animals (Fig 7. A-C). Mino treatment was associated with a 37% decrease in GFP+/Iba1+ cells in the PVN. No distinct changes were observed in other autonomic brain centers, including the subfornical organ and the solitary tract nucleus (Online Fig. X).
Figure 7. Chronic Ang II infusion increases bone marrow derived microglia/macrophages in the hypothalamic paraventricular nucleus (PVN).
A. Representative images at 10x magnification from the PVN of experimental groups. GFP+ cells are bone marrow derived, and Iba1+ cells indicate microglia/macrophages. Scale bar is 100µm; images taken at Bregma −1.80mm; PVN and third ventricle (3V) are labeled for orientation. B. Higher magnification (40x) images of GFP+/Iba1+ cells in the PVN. Scale bar is 30µm. C. Quantification of GFP+/Iba1+ cells in the PVN reveals an increase in the number of cells in chronic Ang II infusion group, which is decreased by minocycline (mino) treatment. CCL2 content in the CSF was also attenuated by mino (n=5-8 per group). D. Ang II treatment (1µM) of primary hypothalamic neurons induces an increase in CCL2 mRNA and CCL2 protein in the cell culture media. *p < 0.05, **p < 0.01, ***p < 0.001 vs control; #p < 0.05, ###p < 0.001 vs Ang II.
Since the mechanism of BM-cell extravasation into the CNS has been suggested to be associated with the CCL2/CCR2 chemokine axis 28–30, we further investigated this possibility. We observed that chronic Ang II infusion increased the protein concentration of CCL2 in the CSF (Fig. 7C; 47.4±10.6 vs. 6.7±3.5 pg/ug protein). This increase was blocked with mino treatment (12.3±6.1 pg/ug). Additionally, incubation of primary neuronal cultures with Ang II (1µM) significantly increased the mRNA expression of CCL2 (1.6, 2.0, and 1.5 fold increase at 6, 9, and 12 hours respectively), while increased levels of CCL2 protein were observed in the culture media after 9 hours of Ang II exposure (~35% increase; Fig. 7D).
DISCUSSION
The major findings of this study are as follows: (1) the SHR BM is characterized by increased inflammatory cells and cytokines in HTN, and plays a key role in blood pressure regulation; (2) pro-inflammatory BM cells migrate to the PVN and enhance neuroinflammation, and (3) oral mino produces antihypertensive effects by attenuating both peripheral- and neuroinflammation. These findings greatly enhance our understanding of the communication that exists between the autonomic nervous system (ANS) and IS, which contributes to the development and maintenance of HTN. Furthermore, our findings suggest that mino could be a potential therapeutic approach for combating drug-resistant hypertension in patients exhibiting high levels of inflammation.
Evidence supporting the pro-inflammatory status of BM in HTN is presented not only in this study, but also in published literature: (1) Our previous data have demonstrated that changes in the direct BM innervation through the femoral sympathetic nerve are associated with changes in the BM activity10, 12; (2) the SHR BM is characterized by elevated inflammatory cell (IC) counts compared to WKY rats10; (3) similar increases in BM ICs have also been demonstrated in the chronic Ang II infusion model of HTN11; and (4) neurohormonal modulation of the SHR IS is pro-inflammatory39. Thus, accumulating data from experimental models of HTN and other pathophysiological conditions describe a neural control of the IS5, 7, 8. The novelty of the present study lies in the finding that the pro-hypertensive, pro-inflammatory BM may also affect the brain, i.e. that the overactive IS may reciprocally modulate the ANS, and further contribute to its dysfunction by exacerbating neuroinflammation.
Our study is the first to demonstrate the extravasation of BM cells into the autonomic CNS areas in HTN. More specifically, increased infiltration of the pro-inflammatory BM cells into the PVN of hypertensive rats was associated with increased CCL2 levels in the CSF, which was considerably decreased by oral mino treatment. These observations, although novel in the field of HTN, align with those reported in chronic psychological stress28, amyotrophic lateral sclerosis40, 41, experimental autoimmune encephalomyelitis42, 43, and Alzheimer’s disease44, 45. This concept has been further validated by in vivo imaging of turnover of microglia and infiltration of BM-derived cells into the murine retina46. Although the total number of GFP+/Iba1+ macrophage/microglia cells in the PVN may appear low, it is nevertheless significant, and can have a drastic effect on perpetuation of neuroinflammation due to release of reactive oxygen species and pro-inflammatory cytokines, especially as the 5-fold increase in GFP+/Iba1+ microglia/macrophages observed in the present study is similar to that previously reported in the PVN of chronically stressed mice28. Our study, showing an increase in CCL2 in the CSF and a significant gradient from BM<Serum<CSF, suggests the involvement of CCL2/CCR2 signaling system in extravasation of BM cells in HTN and neuroinflammation. The ability of mino to attenuate MAP and to decrease CCL2 levels in the CSF further supports this contention. It is pertinent to note that this chemokine axis is shown to be an important player in the migration of T-lymphocytes, monocytes, and natural killer cells in type 2-diabetes and rheumatoid arthritis47. There is evidence that Ang II directly stimulates the production of CCL2 in monocytes48, and vascular smooth muscle cells49 in an AT1 receptor mediated pathway50–52. In addition to these sources of CCL2, we have presented data that indicates that Ang II can also directly stimulate the production and release of CCL2 in hypothalamic neurons. Thus, we believe that the beneficial effects of mino involve multiple mechanisms that include inhibiting the activation of microglia, and lowering CCL2 concentration in the CSF.
As a therapeutic agent, mino was also able to decrease total sympathetic nerve activity, as measured by plasma NE concentration and spectral analysis in two different models of HTN. Therefore, mino may provide a novel avenue to target the neurogenic component of resistant HTN. The neural effects of oral mino stem from its ability to cross the blood brain barrier, where once inside the brain parenchyma, mino can act as an anti-inflammatory inhibitor of microglial activation, as supported by our data. Our previous study has indicated that decreased microglia activation either by ICV mino or ICV MitoTEMPO is associated with decreased MAP11, 20. Thus, the present study further supports the involvement of microglial activation in neurogenic HTN. However, we incorporated an additional novel aspect to this hypothesis, as we report an increase in BM-derived microglia/macrophages in the brain parenchyma of the PVN in HTN. This extravasation of the pro-inflammatory BM cells to the PVN could also be blocked by oral mino treatment, suggesting a dual role for mino: as a central and peripheral anti-inflammatory agent. Considering the present evidence for reciprocal ANS-IS communication in HTN, this apparent characteristic of mino to act peripherally and centrally may be vital in breaking the vicious cycle of drug-resistant HTN.
It has been previously shown that transplanting the thymus from a normotensive WKY can decrease HTN in the SHR6, 53. Here, we generated SHR and WKY chimeric animals to establish a direct relationship between the BM and blood pressure regulation. We did not observe any acute or chronic graft rejection or adverse graft-vs-host effects because: (1) all groups had a greater than 80% survival and did not display sickness behaviors, (2) the results from WKY to SHR reconstitution and SHR to WKY reconstitution are different and opposite, (3) a set of control animals survived over six months without complications. Therefore, our results represent true effects of BM cells and long-term compatibility of WKY and SHR tissues is not an issue.
The levels of inflammatory cells were dependent on the type of BM cells in that particular animal; that is, rats receiving SHR BM cells consistently presented with higher percentages of the T-cell subpopulations CD4+/CD8+/CD25+ and CD3+/CD45+ in the blood than those receiving WKY BM cells. This finding is particularly important as HTN development has been shown to be heavily dependent on T-cells15. Additionally, the SHR BM cells contributed to an elevation in sympathetic drive in the WKY recipients, which was associated with increased activated microglia, suggesting that the extravasation of the pro-inflammatory BM cells to the PVN contributed to neuroinflammation and HTN in WKY animals. However, the WKY BM cells were unable to lower the overactive sympathetic drive in the SHR, despite somewhat reversing the neuroinflammation. This is supported by the hemodynamic measurements that indicate a difference in blood perfusion between the WKY-SHR and WKY-WKY, but no change between the SHR-WKY and SHR-SHR groups. It appears that reducing both systemic and neuroinflammation by transplanting the WKY BM in the SHR is not sufficient to correct/reverse the autonomic dysfunction, despite significantly lowering the blood pressure. Therefore, this may indicate that BM inflammatory cells, under already established HTN in the SHR, may not play a major role in neurogenic regulation of blood pressure, and that the effect may predominantly be peripheral, at least under these specific experimental conditions. Considering that by transplanting the WKY BM in the SHR we also see a significant improvement in the BM APCs, previously shown to have both angiogenic and reparative abilities, it is possible that the WKY BM transplant has also improved the vascular endothelial repair in the SHR, thereby contributing to blood pressure lowering in these rats.
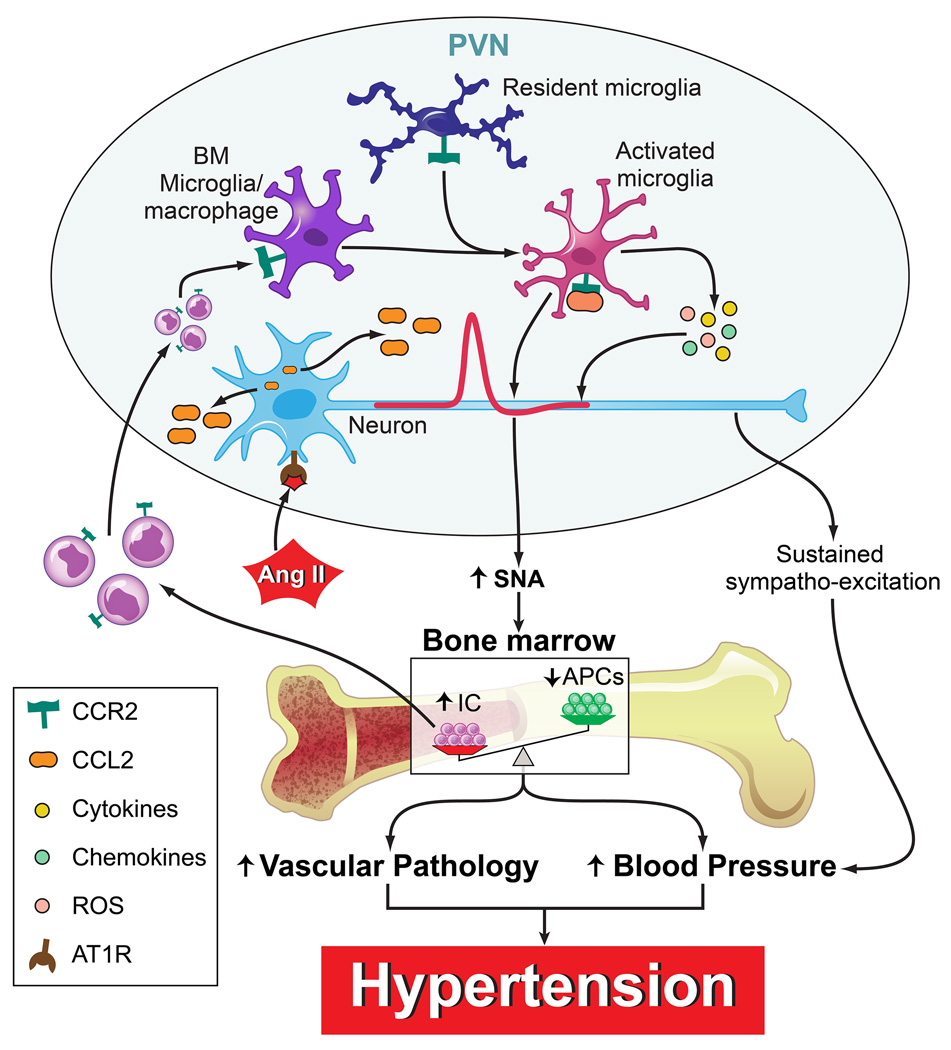
In summary, we propose the following hypothesis (Fig. 8). Pro-hypertensive signals such as Ang II activate PVN pre-autonomic neurons to increase in sympathetic nerve activity (SNA) and cause release of CCL2. The increased SNA impacts the BM resulting in an increase in inflammatory cells (IC) and decrease in APCs. This imbalance is associated with vascular pathology and increase in blood pressure. Therefore, the BM of hypertensive animals (both SHR and chronic Ang II infusion model) is characterized by an increase in inflammatory cells and factors, and is in turn able to contribute to the blood pressure regulation. Additionally, some of these inflammatory progenitors migrate to the PVN as a result of an increased neuronal release of CCL2 where they differentiate into BM-derived microglia/macrophages and exacerbate neuroinflammation, thereby perpetuating the CNS-BM dysfunctional pathway in the establishment of resistant HTN. Both resting microglia and BM-derived microglia/macrophages are activated to release an array of cytokines, chemokines, and reactive oxygen species (ROS) which will further increase pre-autonomic neuronal activity. This leads to a state of sustained sympatho-excitation which will result in a perpetuation of high blood pressure and ultimately established hypertension. Extensive evidence exists for the role of neuroinflammation in HTN, which are thoroughly outlined in our previous review54. Increased renin-angiotensin system activity in hypertensive models has a role in driving pro-inflammatory responses in the brain55, 56. Neuroinflammation in key cardiovascular centers of the brain is associated with increased sympathetic activity and HTN, and inhibition of inflammation in these brain regions attenuates the HTN11, 16–18, 20. Additionally, these cardioregulatory brain centers appear to control the peripheral immune system through autonomic output9, 10.
Figure 8. Proposed hypothesis for the extravasation of bone marrow cells to the hypothalamic paraventricular nucleus (PVN) and the involvement of neuroinflammation in hypertension (HTN).
Pro-hypertensive signals such as angiotensin II (Ang II) activate PVN preautonomic neurons to increase in sympathetic nerve activity (SNA) and cause release of C-C chemokine ligand 2 (CCL2). The increased SNA impacts the bone marrow (BM) resulting in an increase in inflammatory cells (IC) and decrease in angiogenic progenitor cells (APCs). This imbalance is associated with vascular pathology and increase in blood pressure. Additionally, some of these inflammatory progenitors migrate to the PVN as a result of an increased neuronal release of CCL2 where they differentiate into BM-derived microglia/macrophages. Both resting microglia and BM-derived microglia/macrophages are activated to release an array of cytokines, chemokines, and reactive oxygen species (ROS) which will further increase preautonomic neuronal activity. This leads to a state of sustained sympatho-excitation which will result in a perpetuation of high blood pressure and ultimately established hypertension.
This proposal in supported by a preliminary report where mino was able to produce impressive antihypertensive effects in obese, drug resistant hypertensive patients25. However, considering its antibiotic nature and peripheral anti-inflammatory role, the contribution of potential changes in gut microbiota in the overall beneficial effects of mino cannot be ruled out at the present time. This is particularly relevant in view of the evidence that mino has been shown to influence inflammatory responses in depression, protect the gut mucosal damage induced by chemotherapy, enhance morphine’s effectiveness in diabetic neuropathy, and improve intestinal damage and prevent reactivation of colitis57, 58. Clinical trials are underway (ClinicalTrials.gov Identifiers: NCT02133872 and NCT02188381) to evaluate the role of neuroinflammation and gut microbiota in the treatment of resistant HTN with mino.
Perspectives
In this study, we present direct evidence for the involvement of whole BM cells in HTN. This is evidenced by the following: (1) whole BM reconstitution between SHR and WKY animals is able to modulate blood pressure in the parent rat, (2) BM status contributes to peripheral inflammation, and (3) central inflammation by modulation of microglia activation in the PVN. We found evidence that BM cells extravasated into the brain and contributed to central inflammation via the CCL2/CCR2 chemokine axis. Of clinical relevance, we found that oral delivery of mino was able to attenuate HTN in two different rat models of HTN, lower central and peripheral inflammation, as well as decrease total sympathetic activity. This study supports further investigation of mino in resistant HTN patients with neurogenic components.
Supplementary Material
Novelty and Significance.
What Is Known?
Increased sympathetic nerve activity and inflammation are hallmarks of human HTN, particularly drug-resistant HTN.
The central nervous system to immune system communication appears to be altered in HTN and cardiovascular disease in humans, although exact mechanisms remain to be elucidated.
What New Information Does This Article Contribute?
Oral minocycline delivery is a novel therapy for drug resistant hypertension with neurogenic components, and may be utilized as a therapy for HTN can also target neurogenic and inflammatory components of HTN.
Generation of chimeric rats from normotensive and hypertensive BM demonstrates direct involvement of BM cells on blood pressure regulation.
BM cells migrate and extravasate into the PVN, where they differentiate into microglia/macrophages and contribute to neuroinflammation in HTN.
HTN is associated with increased sympathetic drive to the BM that leads to dysfunctional BM activity, characterized by elevated inflammatory cells and decreased reparative APCs. However, the reciprocal effect of the BM on the CNS is unknown. In this study, the SHR BM is characterized by increased inflammatory cells and factors, and is in turn able to contribute to the blood pressure regulation. Some of these progenitor cells migrate to the central nervous system via the CCL2/CCR2 chemokine axis where they exacerbate neuroinflammation, thereby perpetuating the CNS-BM dysfunctional pathway in the establishment of resistant HTN. Finally, minocycline proved to have impressive anti-hypertensive and anti-inflammatory effects in two rat models of HTN. Therefore, further investigation into the therapeutic potential of minocycline is necessary.
ACKNOWLEDGMENTS
We would like to thank UF Molecular Pathology Core for histology services, and the UF Interdisciplinary Center for Biotechnology Research for flow cytometry services.
SOURCES OF FUNDING
This study was supported by National Institutes of Health grant HL33610 (M.K.R.), American Heart Association pre-doctoral fellowship 18590018 (M.M.S.), and AHA scientist development grant 00109813 (J.Z.).
Nonstandard Abbreviations and Acronyms
- ANS
autonomic nervous system
- APC
angiogenic progenitor cell
- BBB
blood brain barrier
- BM
bone marrow
- CNS
central nervous system
- CSF
cerebrospinal fluid
- HTN
hypertension
- HW:TL
heart weight to tibia length ratio
- ICV
intracerebroventricular
- IS
immune system
- MAP
mean arterial pressure
- Mino
minocycline
- MNC
mononuclear cell
- SBP
systolic blood pressure
- sBRG
spontaneous baroreflex gain
- SD
Sprague Dawley
- SHR
spontaneously hypertensive rat
- WKY
Wistar Kyoto
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Anderson EA, Sinkey CA, Lawton WJ, Mark AL. Elevated sympathetic nerve activity in borderline hypertensive humans. Evidence from direct intraneural recordings. Hypertension. 1989;14:177–183. doi: 10.1161/01.hyp.14.2.177. [DOI] [PubMed] [Google Scholar]
- 2.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Dibona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. doi: 10.1161/HYPERTENSIONAHA.111.00633. [DOI] [PubMed] [Google Scholar]
- 4.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (c-reactive protein, interleukin-6, and tnf-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 5.Zubcevic J, Santisteban MM, Pitts T, Baekey DM, Perez PD, Bolser DC, Febo M, Raizada MK. Functional neural-bone marrow pathways: Implications in hypertension and cardiovascular disease. Hypertension. 2014;63:e129–e139. doi: 10.1161/HYPERTENSIONAHA.114.02440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130–140. [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc. 2014;125:130–138. discussion 138-140. [PMC free article] [PubMed] [Google Scholar]
- 8.Singh MV, Chapleau MW, Harwani SC, Abboud FM. The immune system and hypertension. Immunol Res. 2014;59:243–253. doi: 10.1007/s12026-014-8548-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of t-lymphocyte activation and vascular inflammation produced by angiotensin ii-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, Li W, Santisteban MM, Yuan W, Febo M, Mocco J, Feng Y, Scott E, Baekey DM, Raizada MK. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63:542–550. doi: 10.1161/HYPERTENSIONAHA.113.02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun JY, Zubcevic J, Qi Y, Afzal A, Carvajal JM, Thinschmidt JS, Grant MB, Mocco J, Raizada MK. Brain-mediated dysregulation of the bone marrow activity in angiotensin ii-induced hypertension. Hypertension. 2012;60:1316–1323. doi: 10.1161/HYPERTENSIONAHA.112.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–329. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25:257–264. [PubMed] [Google Scholar]
- 14.White FN, Grollman A. Autoimmune factors associated with infarction of the kidney. Nephron. 1964;1:93–102. doi: 10.1159/000179322. [DOI] [PubMed] [Google Scholar]
- 15.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waki H, Hendy EB, Hindmarch CC, Gouraud S, Toward M, Kasparov S, Murphy D, Paton JF. Excessive leukotriene b4 in nucleus tractus solitarii is prohypertensive in spontaneously hypertensive rats. Hypertension. 2013;61:194–201. doi: 10.1161/HYPERTENSIONAHA.112.192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG. Role of the nadph oxidases in the subfornical organ in angiotensin ii-induced hypertension. Hypertension. 2013;61:382–387. doi: 10.1161/HYPERTENSIONAHA.111.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dange RB, Agarwal D, Masson GS, Vila J, Wilson B, Nair A, Francis J. Central blockade of tlr4 improves cardiac function and attenuates myocardial inflammation in angiotensin ii-induced hypertension. Cardiovasc Res. 2014;103:17–27. doi: 10.1093/cvr/cvu067. [DOI] [PubMed] [Google Scholar]
- 19.Aguzzi A, Barres BA, Bennett ML. Microglia: Scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56:297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi P, Grobe JL, Desland FA, Zhou G, Shen XZ, Shan Z, Liu M, Raizada MK, Sumners C. Direct pro-inflammatory effects of prorenin on microglia. PLoS One. 2014;9:e92937. doi: 10.1371/journal.pone.0092937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Igf-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab. 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation. 2012;9:212. doi: 10.1186/1742-2094-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin ii gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension. 2014;63:572–579. doi: 10.1161/HYPERTENSIONAHA.113.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu P, Thinschmidt JS, Yan Y, Hazra S, Bhatwadekar A, Caballero S, Salazar T, Miyan JA, Li W, Derbenev A, Zsombok A, Tikhonenko M, Dominguez JM, 2nd, McGorray SP, Saban DR, Boulton ME, Busik JV, Raizada MK, Chan-Ling T, Grant MB. Cns inflammation and bone marrow neuropathy in type 1 diabetes. Am J Pathol. 2013;183:1608–1620. doi: 10.1016/j.ajpath.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blandini F. Neural and immune mechanisms in the pathogenesis of parkinson's disease. J Neuroimmune Pharmacol. 2013;8:189–201. doi: 10.1007/s11481-013-9435-y. [DOI] [PubMed] [Google Scholar]
- 27.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 28.Ataka K, Asakawa A, Nagaishi K, Kaimoto K, Sawada A, Hayakawa Y, Tatezawa R, Inui A, Fujimiya M. Bone marrow-derived microglia infiltrate into the paraventricular nucleus of chronic psychological stress-loaded mice. PLoS One. 2013;8:e81744. doi: 10.1371/journal.pone.0081744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sawada A, Niiyama Y, Ataka K, Nagaishi K, Yamakage M, Fujimiya M. Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain. 2014;155:1762–1772. doi: 10.1016/j.pain.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 30.Lampron A, Pimentel-Coelho PM, Rivest S. Migration of bone marrow-derived cells into the central nervous system in models of neurodegeneration. J Comp Neurol. 2013;521:3863–3876. doi: 10.1002/cne.23363. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: All for one and one for all. Am J Physiol Renal Physiol. 2004;286:F606–F616. doi: 10.1152/ajprenal.00269.2003. [DOI] [PubMed] [Google Scholar]
- 32.Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez GE, Rhaleb NE, D'Ambrosio MA, Nakagawa P, Liu Y, Leung P, Dai X, Yang XP, Peterson EL, Carretero OA. Deletion of interleukin-6 prevents cardiac inflammation, fibrosis and dysfunction without affecting blood pressure in angiotensin ii-high salt-induced hypertension. J Hypertens. 2015;33:144–152. doi: 10.1097/HJH.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waki H, Gouraud SS, Maeda M, Paton JF. Evidence of specific inflammatory condition in nucleus tractus solitarii of spontaneously hypertensive rats. Exp Physiol. 2010;95:595–600. doi: 10.1113/expphysiol.2009.047324. [DOI] [PubMed] [Google Scholar]
- 35.Vital SA, Terao S, Nagai M, Granger DN. Mechanisms underlying the cerebral microvascular responses to angiotensin ii-induced hypertension. Microcirculation. 2010;17:641–649. doi: 10.1111/j.1549-8719.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigues SF, Almeida-Paula LD, Granger DN. Synergistic effects of high blood cholesterol and hypertension on leukocyte and platelet recruitment in the cerebral microcirculation. Hypertension. 2014;63:747–752. doi: 10.1161/HYPERTENSIONAHA.113.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slayton WB, Li XM, Butler J, Guthrie SM, Jorgensen ML, Wingard JR, Scott EW. The role of the donor in the repair of the marrow vascular niche following hematopoietic stem cell transplant. Stem Cells. 2007;25:2945–2955. doi: 10.1634/stemcells.2007-0158. [DOI] [PubMed] [Google Scholar]
- 38.Schwinn DA, McIntyre RW, Reves JG. Isoflurane-induced vasodilation: Role of the alpha-adrenergic nervous system. Anesth Analg. 1990;71:451–459. doi: 10.1213/00000539-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res. 2012;111:1190–1197. doi: 10.1161/CIRCRESAHA.112.277475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in pu. 1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JC, Seong J, Kim SH, Lee SJ, Cho YJ, An J, Nam DH, Joo KM, Cha CI. Replacement of microglial cells using clodronate liposome and bone marrow transplantation in the central nervous system of sod1(g93a) transgenic mice as an in vivo model of amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2012;418:359–365. doi: 10.1016/j.bbrc.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 42.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger eae progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 43.Davoust N, Vuaillat C, Cavillon G, Domenget C, Hatterer E, Bernard A, Dumontel C, Jurdic P, Malcus C, Confavreux C, Belin MF, Nataf S. Bone marrow cd34+/b220+ progenitors target the inflamed brain and display in vitro differentiation potential toward microglia. Faseb j. 2006;20:2081–2092. doi: 10.1096/fj.05-5593com. [DOI] [PubMed] [Google Scholar]
- 44.Zhang K, Tian L, Liu L, Feng Y, Dong YB, Li B, Shang DS, Fang WG, Cao YP, Chen YH. Cxcl1 contributes to beta-amyloid-induced transendothelial migration of monocytes in alzheimer's disease. PLoS One. 2013;8:e72744. doi: 10.1371/journal.pone.0072744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of cns myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 46.Alt C, Runnels JM, Mortensen LJ, Zaher W, Lin CP. In vivo imaging of microglia turnover in the mouse retina after ionizing radiation and dexamethasone treatment. Invest Ophthalmol Vis Sci. 2014;55:5314–5319. doi: 10.1167/iovs.14-14254. [DOI] [PubMed] [Google Scholar]
- 47.Madrigal JL, Caso JR. The chemokine (c-c motif) ligand 2 in neuroinflammation and neurodegeneration. Adv Exp Med Biol. 2014;824:209–219. doi: 10.1007/978-3-319-07320-0_15. [DOI] [PubMed] [Google Scholar]
- 48.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for ccr2 and mcp-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin ii induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 1998;83:952–959. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- 50.Dai Q, Xu M, Yao M, Sun B. Angiotensin at1 receptor antagonists exert anti-inflammatory effects in spontaneously hypertensive rats. Br J Pharmacol. 2007;152:1042–1048. doi: 10.1038/sj.bjp.0707454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koh KK, Quon MJ, Han SH, Chung WJ, Ahn JY, Seo YH, Kang MH, Ahn TH, Choi IS, Shin EK. Additive beneficial effects of losartan combined with simvastatin in the treatment of hypercholesterolemic, hypertensive patients. Circulation. 2004;110:3687–3692. doi: 10.1161/01.CIR.0000143085.86697.13. [DOI] [PubMed] [Google Scholar]
- 52.Marketou ME, Kontaraki JE, Tsakountakis NA, Zacharis EA, Kochiadakis GE, Arfanakis DA, Parthenakis F, Chlouverakis G, Vardas PE. Differential effect of telmisartan and amlodipine on monocyte chemoattractant protein-1 and peroxisome proliferator-activated receptor-gamma gene expression in peripheral monocytes in patients with essential hypertension. Am J Cardiol. 2011;107:59–63. doi: 10.1016/j.amjcard.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 53.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of t cell depression and suppression of blood pressure in spontaneously hypertensive rats (shr) by thymus grafts or thymus extracts. J Immunol. 1982;128:1211–1216. [PubMed] [Google Scholar]
- 54.Santisteban MM, Zubcevic J, Baekey DM, Raizada MK. Dysfunctional brain-bone marrow communication: A paradigm shift in the pathophysiology of hypertension. Curr Hypertens Rep. 2013;15:377–389. doi: 10.1007/s11906-013-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cardinale JP, Sriramula S, Mariappan N, Agarwal D, Francis J. Angiotensin ii-induced hypertension is modulated by nuclear factor-kappabin the paraventricular nucleus. Hypertension. 2012;59:113–121. doi: 10.1161/HYPERTENSIONAHA.111.182154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwal D, Dange RB, Raizada MK, Francis J. Angiotensin ii causes imbalance between pro- and anti-inflammatory cytokines by modulating gsk-3beta in neuronal culture. Br J Pharmacol. 2013;169:860–874. doi: 10.1111/bph.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garrido-Mesa N, Utrilla P, Comalada M, Zorrilla P, Garrido-Mesa J, Zarzuelo A, Rodriguez-Cabezas ME, Galvez J. The association of minocycline and the probiotic escherichia coli nissle 1917 results in an additive beneficial effect in a dss model of reactivated colitis in mice. Biochem Pharmacol. 2011;82:1891–1900. doi: 10.1016/j.bcp.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Garrido-Mesa N, Camuesco D, Arribas B, Comalada M, Bailon E, Cueto-Sola M, Utrilla P, Nieto A, Zarzuelo A, Rodriguez-Cabezas ME, Galvez J. The intestinal anti-inflammatory effect of minocycline in experimental colitis involves both its immunomodulatory and antimicrobial properties. Pharmacol Res. 2011;63:308–319. doi: 10.1016/j.phrs.2010.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.