Abstract
Background
Coffee drinking has been inversely associated with mortality as well as cancers of the endometrium, colon, skin, prostate, and liver. Improved insulin sensitivity and reduced inflammation are among the hypothesized mechanisms by which coffee drinking may affect cancer risk; however, associations between coffee drinking and systemic levels of immune and inflammatory markers have not been well characterized.
Methods
We used Luminex bead-based assays to measure serum levels of 77 immune and inflammatory markers in 1,728 older non-Hispanic Whites. Usual coffee intake was self-reported using a food frequency questionnaire. We used weighted multivariable logistic regression models to examine associations between coffee and dichotomized marker levels. We conducted statistical trend tests by modeling the median value of each coffee category and applied a 20% false discovery rate criterion to P-values.
Results
Ten of the 77 markers were nominally associated (P-value for trend<0.05) with coffee drinking. Five markers withstood correction for multiple comparisons and included aspects of the host response namely chemotaxis of monocytes/macrophages (IFNγ, CX3CL1/fractalkine, CCL4/MIP-1β), pro-inflammatory cytokines (sTNFRII) and regulators of cell growth (FGF-2). Heavy coffee drinkers had lower circulating levels of IFNγ (OR=0.35; 95% CI 0.16–0.75), CX3CL1/fractalkine (OR=0.25; 95% CI 0.10–0.64), CCL4/MIP-1β (OR=0.48; 95% CI 0.24–0.99), FGF-2 (OR=0.62; 95% CI 0.28–1.38), and sTNFRII (OR=0.34; 95% CI 0.15–0.79) than non-coffee drinkers.
Conclusions
Lower circulating levels of inflammatory markers among coffee drinkers may partially mediate previously observed associations of coffee with cancer and other chronic diseases.
Impact
Validation studies, ideally controlled feeding trials, are needed to confirm these associations.
Keywords: coffee, inflammation, serum biomarkers, cytokines, chemokines
INTRODUCTION
Coffee is a common dietary exposure that may favorably impact human health. According to a recent industry report, more than 60% of U.S. adults drink coffee daily (1). Coffee drinking appears to be inversely associated with total mortality (2–4) as well as cancers of the endometrium (5), colon (6, 7) and liver (8), fatal prostate cancer (9), type 2 diabetes (10) and chronic liver disease (11). Mechanisms underlying these observed associations are poorly understood, but improved insulin sensitivity (12–15) and reduced inflammation (16–21) are suggested pathways by which coffee consumption may alter risk of certain cancers and related chronic diseases. For example, coffee is a rich source of polyphenols, and evidence suggests that polyphenols may impact immune function and chronic inflammation (22). Prior studies of coffee drinking and systemic inflammation have considered a small number of select markers, such as C-reactive protein (CRP) and interleukin-6 (IL-6), and are marked by inconsistent results (16–21, 23). Thus, associations between coffee drinking and levels of immune and inflammatory markers in the blood have not yet been well characterized.
A broad study of immune and inflammatory markers in relation to coffee drinking may provide insight into key markers that should be measured in prospective studies that aim to identify the mechanisms mediating observed associations between coffee drinking and disease. In the current study, we used data from 1,728 individuals who participated in the screening arm of the population-based Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial to explore associations between coffee drinking and variation in the systemic levels of 77 inflammatory and immune markers.
MATERIALS AND METHODS
The PLCO Cancer Screening Trial
The PLCO Cancer Screening Trial is a multicenter randomized screening trial of prostate, lung, colorectal and ovarian cancer that enrolled approximately 155,000 adults, aged 55 to 74 years, from the general U.S. population between 1993 and 2001 (24, 25). Questionnaires were administered to study participants at baseline to collect data on demographics, diet and other general risk factors for disease (25). Annual health questionnaires and linkage to the National Death Index were used to ascertain incident cancer cases, which were subsequently confirmed by medical chart review (24). The PLCO Cancer Screening Trial was approved by the Institutional Review Board at the National Cancer Institute and at the ten participating study centers; all participants gave informed consent.
Study Population
The present cross-sectional investigation includes participants from the screening arm of PLCO who were selected as either cases or controls for one of three previously conducted nested case-control studies of lung cancer (526 cases and 592 controls) (26), ovarian cancer (150 cases and 149 controls) (27) or non-Hodgkin lymphoma (NHL) (301 cases and 301 controls) (28). Participants in the three case-control studies were weighted to the screening-arm of PLCO using weights discussed in the Statistical Analysis section. Detailed descriptions of the exclusion criteria, matching factors and inflammatory markers measured in these case-control studies have been reported elsewhere (29). Six individuals were included in two or more studies but were counted only once, for the case-control study to which they were first selected, in this analysis. The combined dataset excluded individuals who reported a history of cancer prior to baseline (n=31), incomplete smoking data (n=11), or a race other than non-Hispanic White (n=149). An insufficient number of individuals in other racial/ethnic groups precluded the inclusion of race in the model that was used to calculate study weights; consequently, this analysis only weights up to the non-Hispanic White population in the PLCO screening arm. For the present analysis, we additionally excluded individuals who lacked information on coffee consumption (n=90) resulting in an analytic sample size of 1,728. We included incident cancer cases as part of the main analysis since their blood was obtained prior to cancer diagnosis. Post-weighting, cancer cases accounted for a small fraction (2.8%) of the data, and the main findings were not meaningfully altered when we excluded cases from the primary analysis (Supplementary Table S1).
Laboratory Analysis
Serum samples from the lung cancer and NHL studies were collected at baseline; serum samples from the ovarian cancer study were collected at baseline or at a follow-up visit to ensure a relatively equal distribution of specimens between 2 and 14 years prior to diagnosis (27). All samples were centrifuged at 1,200×g for 15 minutes, frozen within two hours of collection and stored at −70°C. These samples were later used to measure circulating levels of 86 inflammatory and immune markers (Supplementary Table S2) that were selected based on the results of a methodological study that assessed the performance and reproducibility of the multiplexed assays (30). The lung cancer study, NHL study and ovarian cancer study measured 77, 83 and 60 markers, respectively (Millipore Inc., Billerica, MA) (Supplementary Table S3). Detailed descriptions of laboratory methods and assay reproducibility have been previously reported (26–28, 30). In brief, marker concentrations were calculated using either a four- or five-parameter standard curve. Serum samples were assayed in duplicate and averaged. To evaluate assay reproducibility coefficients-of-variation (CVs) and intraclass correlation coefficients (ICCs) of log-transformed marker values were calculated from blinded duplicates in the lung and NHL studies and from duplicate measurements on study participants in the ovarian cancer study. Log-transformed ICCs were greater than 0.8 in 91%, 91% and 78% of evaluable markers in the lung, NHL and ovary studies, respectively (29). ICCs below 0.70 were reported for one marker (IL-2) in the lung study (26) and five markers (IFNγ, IL-1RA, PYY, sIL-4R, and sVEGF-2) in the ovarian cancer study (27). Additional information on quality control measurements can be found elsewhere (26–28). Eight markers with >90% of concentrations below the lowest limit of detection (LLOD) and one marker with >70% of concentrations above the upper limit of detection were excluded from the study leaving 77 evaluable markers in our primary analysis.
Statistical Analysis
Propensity-score adjusted sampling weights were developed to ensure that analyses accounted for the study-specific sampling strategy and inclusion/exclusion criteria (Supplementary Table S3). These sampling weights, which have been described in detail elsewhere, allowed us to include all participants with biomarker data, including cancer cases who were cancer-free at the time of blood draw, and made our analysis as representative as possible of the non-Hispanic White PLCO screening arm population. Details on the development of sampling weights have been described in detail elsewhere (29). To summarize, study-, gender- and case/control status- specific logistic regression models adjusted for age, detailed smoking history, and vital status on December 31, 2009 were used to estimate the probability that an eligible screening arm participant would be selected into any given case-control study. Combinations of study-specific weights were created for markers that were measured in multiple case-control studies (e.g. all three studies, lung and NHL, lung and ovary, and NHL and ovary) (29).
Usual coffee consumption in the previous 12 months was assessed at baseline in the screening arm using a semi-quantitative 137-item food frequency questionnaire, which was based on two previously validated food frequency questionnaires (31, 32). A single coffee question queried about frequency (never to 6 or more times/day) and portion size of total, caffeinated or decaffeinated, coffee intake. Previous longitudinal studies have demonstrated that semi-quantitative food frequency questionnaires provide a reproducible and valid measure of coffee intake (33, 34), and in subgroup of PLCO participants who completed two food frequency questionnaires, approximately three years apart, the Spearman correlation coefficient was 0.76. Among coffee drinkers, the reported frequency of total coffee intake (less than once/month to 6 or more times/day) was multiplied by a gram amount, which was dependent on the gender of the subject and their response to serving size (small, medium, or large cup); gram amounts came from the USDA’s 1994–1996 Continuing Survey of Food Intakes by Individuals (CSFII) database (35). Gram amounts were converted to number of medium (i.e. 12 oz.) cups for ease of interpretation. We then categorized individuals as either non-, moderate (below median coffee intake; <2.5 cups/day) or heavy (above median; ≥2.5 cups/day) coffee drinkers.
A considerable fraction of marker values below the LLOD (Supplementary Table S4) precluded analysis of all markers as continuous outcomes; therefore, markers were dichotomized as above or below the median value, or as detectable or undetectable if more than 50% of the values were below the LLOD. Models categorizing marker levels into quartiles, for markers with <25% below LLOD, or tertiles, for markers with 25–50% below LLOD, produced similar results (data not shown).
First we assessed the associations between coffee drinking and each of the 77 dichotomized markers using weighted logistic regression models adjusted for the following potential confounders: age, sex, detailed smoking history, alcohol drinking, education, NSAID use, multivitamin use, body mass index (BMI), case-control study of origin, year of serum collection, estimated daily energy intake, and nutrient density adjusted daily intakes of fruit, vegetables, red meat, and white meat (e.g. poultry and fish). Less than 2% of the cohort lacked information on BMI; nonetheless, we included an indicator variable for missing BMI data in the regression models. All a priori selected covariates, except NSAID use, multivitamin use and food intake variables, altered risk estimates by 10% or more for some of the nominally significant markers. P-values for trend across categories of coffee drinking were calculated by assigning each individual the midpoint value for the category and modeling this as a continuous variable. We applied a 20% false discovery rate (FDR) criterion (36) to the P-values for trend from these models, and retained the markers that remained statistically significant for secondary analyses. We also considered a more stringent 5% FDR criterion. Additionally, we conducted a sensitivity analysis that excluded markers with <25% detectability in any of the three nested case-control studies.
We used Pearson’s correlation coefficients to estimate the weighted correlations between the markers that met nominal criteria for significance (P-value for trend<0.05) in the primary analysis for the lung study as this was the only study to include all 10 nominally significant markers. We also ran multivariable logistic regression models for each of the five inflammatory markers that met our 20% FDR criterion adjusted for the other four significant markers in order to evaluate the whether these associations were independent of each other.
In secondary analyses, we stratified by sex and contributing study (lung, NHL, ovary) to explore possible effect modification. Since smoking, diabetes and BMI may be associated with both coffee drinking and chronic inflammation, we also performed sensitivity analyses in which we limited the analytic sample to never smokers (n=520), those not reporting diabetes (n=1,613), or participants with normal BMI (18.5 ≥ BMI > 25 kg/m2) (n=606). We formally tested for effect modification by sex, contributing study (lung, NHL, ovary), smoking status (never, former, current), diabetes status and BMI (<18.5, 18.5–24.9, 25–29.9, ≥30 kg/m2) using the Wald chi-square statistic to test the significance of the cross-product term for the single variable coffee and the variable of interest (e.g. sex) or to test the global significance of the cross-product terms for the single coffee variable and each level of the stratifying variable (e.g. smoking). All reported P-values are based on two-sided tests. Finally, as a sensitivity analysis to our previously described trend analysis, we considered the natural log-transformed values of coffee intake plus a small constant (i.e., ln(coffee intake + c) where c = 0.01 cups) in order to assess the trend analysis for continuous coffee consumption. For secondary analyses, a P-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using SAS (version 9.3; SAS Institute Inc, Cary, NC); weighted analyses were conducted using SURVEY procedures in SAS.
RESULTS
Individuals with available immune and inflammatory markers were more likely to be current smokers and heavy coffee drinkers than the overall screening arm from which they were drawn (Table 1). This reflects the overrepresentation of smokers in the lung cancer study, as coffee drinking was associated with cigarette smoking in our study. Approximately, 62% and 50% of current and former smokers, respectively, were heavy coffee drinkers compared to 34% of never smokers. However, our weighted analytic sample was very similar to the eligible PLCO screening arm population with regards to smoking status and other exposures indicating that these weights help account for the non-representative sampling.
Table 1.
Participant characteristics in 1) subjects with DQX and measured inflammatory marker data (N=1,728), 2) weighted sample with DQX, and 3) eligible PLCO Cancer Screening Trial sample
| Characteristic | Ne | %e | Weighted %e | PLCO Screening Arm, %e |
|---|---|---|---|---|
| Male | 953 | 55.2 | 51.5 | 51.9 |
| Age group (y) | ||||
| ≤59 | 368 | 21.3 | 31.8 | 33.4 |
| 60–64 | 505 | 29.2 | 34.4 | 31.3 |
| 65–69 | 518 | 30.0 | 19.5 | 22.4 |
| ≥70 | 337 | 19.5 | 14.3 | 12.9 |
| Education | ||||
| 11 years or less | 142 | 8.2 | 6.5 | 6.1 |
| 12 years or completed high school | 440 | 25.5 | 24.9 | 23.4 |
| Some post high school education | 586 | 33.9 | 33.9 | 34.0 |
| College graduate or postgraduate | 560 | 32.4 | 34.7 | 36.3 |
| BMI category (kg/m2) | ||||
| <25 | 615 | 35.6 | 31.7 | 32.2 |
| 25–30 | 747 | 43.2 | 45.7 | 43.0 |
| ≥30 | 345 | 20.0 | 21.1 | 23.9 |
| Missing | 21 | 1.2 | 1.5 | 0.9 |
| Coffee drinking status (cups/day) | ||||
| None | 144 | 8.3 | 10.2 | 12.5 |
| <2.5 | 719 | 41.6 | 46.3 | 45.9 |
| ≥2.5 | 865 | 50.1 | 43.6 | 41.6 |
| Smoking status | ||||
| Never | 520 | 30.1 | 46.0 | 47.3 |
| Former | 820 | 47.5 | 44.3 | 43.1 |
| Current | 388 | 22.5 | 9.6 | 9.6 |
| Alcohol drinking status (drinks/day) | ||||
| None | 260 | 15.1 | 17.0 | 17.5 |
| <1 | 1008 | 58.3 | 60.8 | 58.5 |
| 1–3 | 281 | 16.3 | 13.2 | 15.4 |
| ≥3 | 179 | 10.4 | 9.0 | 8.6 |
| Multivitamin usea | 855 | 49.5 | 52.4 | 51.1 |
| NSAID useb | 1110 | 64.2 | 64.5 | 62.3 |
| Original case-control study | ||||
| Lung cancer study | 947 | 54.8 | 42.5 | --- |
| NHL study | 550 | 31.8 | 41.9 | --- |
| Ovarian cancer study | 231 | 13.4 | 15.6 | --- |
| Red meat intake (g/1000 kcal/day)c | 33.9 (21.7–48.5) | 31.4 (20.9–45.9) | 32.8 (20.9–48.2) | |
| White meat intake (g/1000 kcal/day)d | 19.2 (12.1–30.4) | 19.6 (12.3–30.5) | 20.7 (12.8–32.4) | |
| Fruit intake (cups/1000 kcal/day) | 1.0 (0.6–1.5) | 1.1 (0.7–1.6) | 1.0 (0.7–1.5) | |
| Vegetable intake (cups/1000 kcal/day) | 1.3 (0.9–1.6) | 1.3 (1.0–1.7) | 1.3 (1.0–1.6) | |
Includes current and recent (within previous 2 years) multivitamin use
Includes aspirin and ibuprofen use
Includes red and processed meat
Includes poultry and fish
Median and Interquartile range (IQR) for continuous variables
Heavy coffee drinking appeared to be associated with male gender, heavier alcohol use, higher red meat intake as well as lower fruit and vegetable intake (Table 2). Coffee consumption did not differ meaningfully by age group, NSAID use, multivitamin use, BMI category or white meat intake (Table 2).
Table 2.
Participant characteristicsa by level of coffee intake among non-Hispanic Whites in the screening arm of the PLCO Cancer Screening Trial
| Coffee Consumption | ||||
|---|---|---|---|---|
| Characteristic | None (n=144) |
<2.5 cups/day (n=719) |
≥2.5 cups/day (n=865) |
P-valueg |
| Sex | <0.0001 | |||
| Female | 70.3 (97) | 67.6 (470) | 23.1 (208) | |
| Male | 29.7 (47) | 32.2 (249) | 76.9 (657) | |
| Age group (y) | 0.51 | |||
| ≤59 | 31.2 (38) | 31.2 (139) | 32.6 (191) | |
| 60–64 | 44.6 (46) | 34.9 (219) | 31.5 (240) | |
| 65–69 | 11.4 (35) | 19.3 (213) | 21.6 (270) | |
| ≥70 | 12.8 (25) | 14.6 (148) | 14.3 (164) | |
| Education | 0.02 | |||
| 11 years or less | 4.2 (8) | 5.1 (54) | 8.6 (80) | |
| 12 years or completed high school | 42.5 (51) | 25.0 (181) | 20.6 (208) | |
| Some post high school education | 27.0 (44) | 36.2 (254) | 33.0 (288) | |
| College graduate or postgraduate | 26.3 (41) | 33.7 (230) | 37.8 (289) | |
| BMI category (kg/m2)b | 0.11 | |||
| <25 | 35.3 (55) | 36.3 (274) | 27.1 (286) | |
| 25–30 | 45.7 (59) | 40.3 (289) | 53.0 (399) | |
| ≥30 | 19.0 (29) | 23.4 (146) | 19.9 (170) | |
| Smoking status | <0.0001 | |||
| Never | 80.2 (92) | 48.4 (276) | 35.6 (152) | |
| Former | 17.6 (40) | 44.1 (332) | 50.8 (448) | |
| Current | 2.2 (12) | 7.5 (111) | 13.6 (265) | |
| Alcohol drinking status (drinks/day) | <0.0001 | |||
| None | 56.1 (64) | 14.9 (86) | 10.2 (110) | |
| <1 | 41.2 (64) | 65.5 (467) | 60.4 (477) | |
| 1–3 | 2.2 (10) | 12.9 (120) | 16.1 (151) | |
| ≥3 | 0.5 (6) | 6.7 (46) | 13.3 (127) | |
| Multivitamin usec | 46.2 (71) | 56.8 (391) | 49.2 (393) | 0.19 |
| NSAID used | 57.8 (82) | 62.9 (446) | 67.9 (582) | 0.33 |
| Original case-control study | <0.0001 | |||
| Lung cancer study | 25.1 (49) | 41.7 (347) | 47.4 (551) | |
| NHL study | 54.8 (56) | 35.9 (222) | 45.3 (272) | |
| Ovarian cancer study | 20.1 (39) | 22.4 (150) | 7.3 (42) | |
| Red meat intake (g/1000 kcal/day)e | 22.9 (13.9–39.9) | 30.3 (20.2–41.9) | 35.4 (23.1–50.0) | <0.001 |
| White meat intake (g/1000 kcal/day)f | 12.7 (7.0–20.2) | 20.3 (13.7–33.0) | 20.4 (12.2–29.7) | 0.09 |
| Fruit intake (cups/1000 kcal/day) | 1.5 (0.9–2.0) | 1.2 (0.9–1.7) | 0.9 (0.6–1.4) | <0.001 |
| Vegetable intake (cups/1000 kcal/day) | 1.2 (1.0–1.7) | 1.4 (1.0–1.8) | 1.2 (0.9–1.5) | 0.002 |
Weighted column percent (unweighted n) for categorical variables and weighted median (IQR) for continuous variables
n=1,707 due to missing data on BMI
Includes current and recent (within previous 2 years) multivitamin use
Includes aspirin and ibuprofen use
Includes red and processed meat
Includes poultry and fish
P-value for Rao-Scott chi-square test for categorical variables and Wald’s F-test for continuous variables
Ten of the 77 markers studied were nominally associated (P-value for trend<0.05) with coffee drinking (Table 3) based on testing for trend in association across three levels of coffee consumption. Five markers were considered significant using a 20% FDR correction for multiple comparisons and one marker, IFNγ, was considered significant using a 5% FDR correction which was equivalent to the 5% Bonferroni corrected P-value (i.e. 0.05/77) (Figure 1). These five markers include various aspects of the immune and inflammatory response namely chemotaxis of monocytes/macrophages (IFNγ, CX3CL1/fractalkine, CCL4/MIP-1β), pro-inflammatory cytokines (sTNFRII) and regulators of cell growth (FGF-2), and were inversely associated with ORs ranging from 0.25 to 0.87 for heavy versus non-coffee drinkers (Table 3). Associations appeared monotonic for IFNγ, CX3CL1/fractalkine, CCL4/MIP-1β and sTNFRII but not for FGF-2. After excluding markers, including IFNγ and CX3CL1/fractalkine, with <25% detectability in any of the three nested case-control studies, 53 markers remained; of the eight markers that reached nominal significance, one, sTNFRII, met the 20% FDR correction (Supplementary Table S5).
Table 3.
Odds ratios (OR) for high versus low levelse for ten circulating inflammatory markers (P-trend<0.05) in three case-control studies nested within the PLCO Cancer Screening Trial (N=1,728)
| Non-drinkers | Moderate Coffee Drinkers (<2.5 cups/day) | Heavy Coffee Drinkers (≥2.5 cups/day) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammatory marker | OR | Low marker % (n)d |
High marker % (n)d |
OR | 95% CI | Low marker % (n)d |
High marker % (n)d |
OR | 95% CI | Low marker % (n)d |
High marker % (n)d |
P-trendf |
| IFNγa | 1.00 | 5.5 (83) | 4.6 (61) | 0.69 | (0.33,1.43) | 26.6 (418) | 19.6 (301) | 0.35 | (0.16,0.75) | 29.1 (465) | 14.5 (400) | 0.0003 |
| CX3CL1/fractalkinea | 1.00 | 8.0 (117) | 2.2 (27) | 0.46 | (0.19,1.10) | 38.3 (598) | (7.9) 121 | 0.25 | (0.10,0.64) | 39.1 (730) | 4.5 (135) | 0.0032 |
| CCL4/MIP-1β | 1.00 | 4.4 (69) | 5.8 (75) | 0.85 | (0.44,1.65) | 20.9 (348) | 25.4 (371) | 0.48 | (0.24,0.99) | 23.9 (452) | 19.7 (413) | 0.0050 |
| FGF-2a | 1.00 | 7.3 (95) | 2.9 (49) | 1.21 | (0.57,2.57) | 31.1 (501) | 15.1 (218) | 0.62 | (0.28,1.38) | 34.1 (599) | 9.5 (266) | 0.0080 |
| sTNFRII | 1.00 | 2.9 (46) | 7.2 (98) | 0.52 | (0.24,1.14) | 22.7 (345) | 23.6 (374) | 0.34 | (0.15,0.79) | 23.1 (395) | 20.4 (470) | 0.0112 |
| TGF-α | 1.00 | 5.4 (76) | 4.8 (68) | 1.60 | (0.79,3.26) | 20.4 (328) | 25.8 (391) | 0.87 | (0.41,1.84) | 25.6 (397) | 8.0 (468) | 0.0189 |
| TNF-α | 1.00 | 3.2 (49) | 6.9(95) | 0.43 | (0.22,0.83) | 23.1 (328) | 23.2 (391) | 0.32 | (0.16,0.65) | 23.3 (408) | 20.3 (457) | 0.0261 |
| SAPb | 1.00 | 4.1 (21) | 2.3 (27) | 1.78 | (0.52,6.08) | 22.0 (151) | 27.6 (195) | 0.75 | (0.20,2.72) | 22.8 (212) | 21.2 (338) | 0.0324 |
| sTNFRI | 1.00 | 4.7 (59) | 5.5 (85) | 0.97 | (0.47,1.98) | 21.7 (343) | 24.5 (376) | 0.61 | (0.29,1.31) | 22.9 (405) | 20.7 (460) | 0.0330 |
| CCL19/MIP-3βc | 1.00 | 5.0 (50) | 5.6 (55) | 1.06 | (0.47,2.41) | 20.6 (265) | 25.4 (304) | 0.64 | (0.28,1.48) | 23.5 (424) | 20.0 (399) | 0.0340 |
Analyte detectable in less than 50% of samples
n=944, marker not measured in NHL and ovarian cancer studies
n=1497, marker not measured in ovarian cancer case-control study
Weighted row % (unweighted n)
ORs were estimated with weighted logistic regression. All models adjusted for NSAID use (yes or no); multivitamin use (yes or no); BMI category (0–25 kg/m2, 25–30 kg/m2, or ≥30 kg/m2); smoking status (no tobacco use, current smoker, quit 0-<10 y ago, quit 10 to <20 y ago, quit 20 to <30 y ago, quit 30 to <40 y ago, quit ≥40 y ago, or never smoked cigarettes but smoke pipe/cigars); age (≤59 y, 60–64 y, 65–69 y, or 70+ y); alcohol drinking (none, <1 drink/day, 1–3 drinks/day, or >3 drinks/day); education (≤11 y, 12 y or completed high school, some post high school education, or college graduate or post-graduate); sex (male or female); case-control study of origin (lung, NHL, or ovary); year of serum collection (calendar year); daily fruit intake (cups/1000 kcal/day); daily vegetable intake (cups/1000 kcal/day); daily red meat intake (g/1000 kcal/day); daily white meat (i.e. poultry and fish) intake (g/1000 kcal/day); estimated daily energy intake (kcal/day). Low marker was defined as below the median marker level or below the limit of detection (LOD) in the case of an analyte that is detectable in less than 50% of samples; high marker was defined as equal to or above the median marker level or detectable in the case of an analyte that is detectable in less than 50% of the samples.
Trend analysis treated coffee drinking as a single variable using the median value for each category of intake
Bold=Significant after 0.20 false discovery rate (FDR) correction
Figure 1.
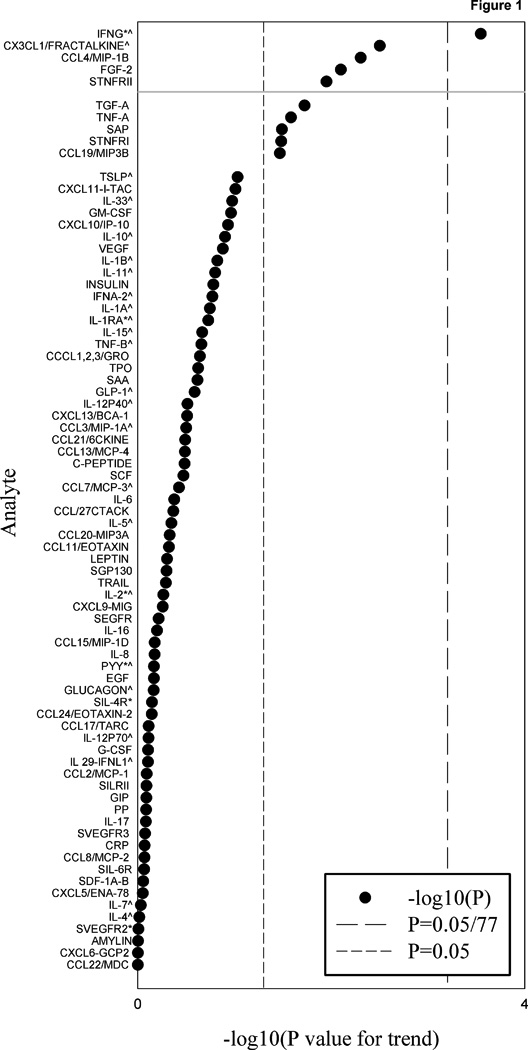
Summary of P-values for trend from tests of multivariable-adjusted association with coffee drinking for all analytes included in the analysis (N=77 markers). P-values for trend for analytes detectable in less than 50% of samples computed using Wald statistic of regression model parameters for dichotomized analyte level (undetectable vs. detectable). P-values for trend for all other analytes were computed using Wald statistic of regression model parameters for dichotomized analyte level (<median vs. ≥ median). All regression models were estimated with weighted logistic regression. Analytes with log-transformed intraclass correlations <70% in at least one of the three nested case-control studies are marked by an asterisk. Analytes with <25% detectability in at least one of the three nested case-control studies are marked by a caret. Analytes above the grey solid line met a 20% FDR criterion.
These five markers were modestly to weakly correlated; weighted Pearson correlation coefficients ranged from -0.054 for IFNγ and sTNFRI to 0.65 for sTNFRII and sTNFRI (Supplementary Table S6), and associations of coffee drinking with IFNγ, CCL4/MIP-1β and sTNFRII remained nominally significant following simultaneous adjustment for the other four markers (Supplementary Table S7). Additional analyses confirmed that our conclusions were unaltered when evaluating marker levels in quartiles (markers with >75% detection) or tertiles (markers with 50–75% detection) (data not shown). Finally, the magnitude and direction of the associations were similar when we modeled coffee intake as a continuous variable ln(coffee intake + c) (data not shown).
Sensitivity analyses revealed that associations were generally consistent across case-control study of origin (Supplementary Table S8). Tests for effect modification by sex (data not shown), smoking status, diabetes status, and BMI (Table 4) suggest that the associations are generally consistent across strata of sex, smoking, diabetes, and BMI (P-heterogeneity ≥ 0.10). However, associations of coffee drinking with levels of CCL4/MIP-1β (P-heterogeneity=0.006) and sTNFRII (P-heterogeneity =0.058) were stronger in women than in men, although in the same general direction for both sexes. In addition, associations with IFNγ (P-heterogeneity =0.043), CX3CL1/fractalkine (P-heterogeneity =0.015) and CCL4/MIP-1β (P-heterogeneity =0.064) appeared to be stronger in never-smokers than ever-smokers, although again of similar direction in both groups. Additionally, the association with sTNFRII was observed among those without self-reported diabetes only (P-heterogeneity =0.001).
Table 4.
Odds ratios (OR) for high versus low levelse for the top five circulating inflammatory markers in three case-control studies nested within the PLCO Cancer Screening Trial among never smokers, individuals without diabetes, normal BMI (18.5–25 kg/m2)
| Inflammatory marker |
Subgroup | Non- drinkers |
Moderate Coffee Drinkers (<2.5 cups/day) |
Heavy Coffee Drinkers (≥2.5 cups/day) |
P-trend | P-heterogeneityf | ||
|---|---|---|---|---|---|---|---|---|
| OR | OR | 95% CI | OR | 95% CI | ||||
| IFNγa | Overallb | 1.00 | 0.69 | (0.33,1.43) | 0.35 | (0.16,0.75) | 0.0003 | --- |
| Never smokersc | 1.00 | 0.63 | (0.35,1.61) | 0.19 | (0.06,0.58) | 0.0005 | 0.0434 | |
| Diabetes-freed | 1.00 | 0.76 | (0.36,1.60) | 0.38 | (0.17,0.84) | 0.0006 | 0.7554 | |
| Normal BMIe | 1.00 | 1.06 | (0.32,3.47) | 0.72 | (0.20,2.54) | 0.3019 | 0.4411 | |
| CX3CL1/ fractalkinea | Overallb | 1.00 | 0.46 | (0.19,1.10) | 0.25 | (0.10,0.64) | 0.0032 | --- |
| Never smokersc | 1.00 | 0.39 | (0.13,1.19) | 0.18 | (0.04,0.71) | 0.0443 | 0.0145 | |
| Diabetes-freed | 1.00 | 0.42 | (0.17,1.06) | 0.24 | (0.09,0.65) | 0.0073 | 0.9845 | |
| Normal BMIe | 1.00 | 2.08 | (0.43,10.16) | 1.13 | (0.20,6.32) | 0.3304 | 0.1140 | |
| CCL4/MIP-1β | Overallb | 1.00 | 0.85 | (0.44,1.65) | 0.48 | (0.24,0.99) | 0.0050 | --- |
| Never smokersc | 1.00 | 0.65 | (0.27,1.56) | 0.22 | (0.08,0.67) | 0.0033 | 0.0638 | |
| Diabetes-freed | 1.00 | 0.94 | (0.48,1.85) | 0.49 | (0.24,1.02) | 0.0034 | 0.9578 | |
| Normal BMIe | 1.00 | 1.05 | (0.35,3.11) | 0.48 | (0.14,1.61) | 0.0372 | 0.7972 | |
| FGF-2a | Overall b | 1.00 | 1.21 | (0.57,2.57) | 0.62 | (0.28,1.38) | 0.0080 | |
| Never smokersc | 1.00 | 0.83 | (0.34,2.03) | 0.32 | (0.10,1.03) | 0.0291 | 0.3399 | |
| Diabetes-freed | 1.00 | 1.14 | (0.53,2.48) | 0.54 | (0.23,1.24) | 0.0036 | 0.3721 | |
| Normal BMIe | 1.00 | 2.50 | (0.56,11.14) | 2.48 | (0.54,11.35) | 0.6746 | 0.3963 | |
| sTNFRII | Overallb | 1.00 | 0.52 | (0.24,1.14) | 0.34 | (0.15,0.79) | 0.0112 | --- |
| Never smokersc | 1.00 | 0.38 | (0.15,0.97) | 0.25 | (0.09,0.73) | 0.0418 | 0.9181 | |
| Diabetes-freed | 1.00 | 0.52 | (0.24.1.14) | 0.38 | (0.17,0.89) | 0.0497 | 0.0008 | |
| Normal BMIe | 1.00 | 0.76 | (0.22,2.60) | 0.58 | (0.16,2.29) | 0.4104 | 0.3062 | |
Analytes detectable in less than 50% of samples
Estimated with weighted logistic regression. All models adjusted for NSAID use (yes or no);multivitamin use (yes or no); BMI category (0–25 kg/m2, 25–30 kg/m2, ≥30 kg/m2); smoking status (no tobacco use, current smoker, quit 0-<10 y ago, quit 10 to <20 y ago, quit 20 to <30 y ago, quit 30 to <40 y ago, quit ≥40 y ago, or never smoked cigarettes but smoke pipe/cigars); age (≤59 y, 60–64 y, 65–69 y, or 70+ y); alcohol drinking (none, <1 drink/day, 1–3 drinks/day, or >3 drinks/day); sex (male or female) (lung and NHL studies only); year of serum collection (calendar year); education (≤11 y, 12 y or completed high school, some post high school education, or college graduate or post-graduate); daily fruit intake (cups/1000 kcal/day); daily vegetable intake (cups/1000 kcal/day); daily red meat intake (g/1000 kcal/day); daily white meat (i.e. poultry and fish) intake (g/1000 kcal/day); estimated daily energy intake (kcal/day)
Estimated with weighted logistic regression. All models adjusted for covariates in overall modelb except smoking status
Estimated with weighted logistic regression. All models adjusted for covariates in overall modelb; 4 individuals with missing diabetes status excluded
Estimated with weighted logistic regression. All models adjusted for covariates in overall modelb except BMI category
P-value for Wald X2 test of global significance for the cross-product term(s) for each stratifying variable and coffee drinking; 21 individuals excluded from analysis of coffee*BMI category interaction due to missing BMI
Inflammatory markers, including CRP and IL-6, that have been previously studied in relation to coffee drinking were not statistically significant overall or among nonsmokers (data not shown); however, heavy coffee drinking was significantly associated with lower circulating levels of insulin among non-smokers only (OR=0.30; 95% CI 0.10, 0.96; P-value=0.03)(data not in table). Of the markers that were considered herein and statistically significantly associated with coffee intake in other population studies, CRP, IL-6, SAA, and insulin, but not leptin, were statistically significantly (P-value<0.05) positively correlated with at least one of the five primary markers of interest. Weighted Pearson correlation coefficients for these previously identified markers and the current ones were generally weak and ranged from 0.08 for IFNγ and SAA to 0.27 for sTNFRII and CRP (data not in table). Adjustment for CRP, IL-6, or SAA considerably attenuated the inverse association between coffee intake and sTNFRII, although associations for other identified markers were unaffected (data not shown).
DISCUSSION
In the most comprehensive study of coffee drinking and systemic markers of inflammation and immunity to date, we found that heavier coffee consumption was associated with lower levels of a number of different inflammatory markers, including chemokines (CX3CL1/fractalkine, CCL4/MIP-1β), cytokines (IFNγ, sTNFRII) and basic fibroblast growth factor (FGF-2). Our findings were generally consistent among never and ever smokers, among those within the normal BMI range, and among those not reporting diabetes suggesting that confounding by these risk factors, which have been associated with chronic inflammation, is not a likely explanation for our findings.
Prior studies of coffee drinking and systemic inflammation have focused on a smaller group of inflammatory markers, and the results have been inconsistent. Ten of the previously examined inflammatory markers overlap with the 77 markers that were evaluated in our study (16–21, 23). We observed a similar inverse association between coffee consumption and sTNFRII as reported in the Nurses’ Health Study (18). We also found null associations for coffee drinking and four markers (monocyte chemoattractant protein-1 (CCL2/MCP-1), IL-1β, IL-1ra, and insulin) consistent with the results from prior studies (17, 20, 21). Our null results for CRP, leptin, IL-6, serum amyloid-A (SAA) and tumor necrosis factor-α (TNF-α) are, however, consistent with some (17, 20, 21) but not all (16, 19, 20, 23) prior studies. Several observational studies have found inverse associations between coffee drinking and CRP in women (16, 18–20) and men (20). In contrast, a Greek study observed associations between moderate to heavy coffee consumption and higher levels of CRP as well as higher levels of IL-6, SAA and TNF-α (23). In addition to chance, one possible explanation for the discrepant results between this study and our own is differences in coffee preparation method. In the Greek study, 88% of men and 92% of women reported drinking Greek-style unfiltered coffee.
Evidence from feeding studies suggests that the lipid-enriched fraction containing cafestol, which is present at markedly higher concentrations in unfiltered coffee, has a hypercholesterolemic effect (37) and may contribute to endothelial dysfunction (38). However, two small feeding trials of paper filtered coffee and systemic inflammation did not find associations with CRP (17, 21), IL-6 (17, 21), leptin (17), SAA (17), or TNF-α (21), as in our study. Nevertheless, additional observational studies with detailed information on coffee preparation and feeding trials with a range of coffee preparations are needed to examine this hypothesis.
Historically, coffee was regarded as a cardiovascular risk factor owing to findings from case-control studies (39, 40). Prospective cohort studies, which are less prone to selection bias and recall bias, have largely failed to reproduce these findings (41). In contrast, the more recent epidemiologic evidence suggests inverse associations with cardiovascular disease (41), total and cardiovascular disease mortality (2–4) as well as type 2 diabetes (10), Parkinson’s disease (42), chronic liver disease (11), and certain cancers (5, 6, 8). One hypothesis, which may explain the numerous associations of coffee drinking with health outcomes, is that constituents of coffee, such as polyphenols, have immunomodulatory and anti-inflammatory properties (22). For example, decaffeinated coffee consumption has been shown to reduce hepatic concentrations of INFγ and TNF-α in an animal model of nonalcoholic steatohepatitis (NASH), which is marked by a chronic inflammatory state (43). Chlorogenic acid, a constituent of both caffeinated and decaffeinated coffee, has been shown to improve insulin resistance through reduced gluconeogenesis and inflammation in the liver and reduced glucose absorption in the gut (44), and caffeic acid, a metabolite of chlorogenic acid, has been shown to inhibit the TNF-α induced inflammatory response in human endothelial cells (45). Intake of caffeine, a naturally occurring compound in coffee, has been shown to improve endothelial function and reduce inflammation in patients with and without coronary artery disease (46), and in vitro and in vivo studies suggest that methylxanthines, such as caffeine and theophylline, alter inflammatory and immune cell function at concentrations that are reflective of human exposure (47). More specifically, caffeine has been shown to decrease TNF-α expression in blood cells (48) and theophylline, which is used to treat asthma and chronic obstructive pulmonary disease (COPD), is believed to exert some of its therapeutic effects by down regulating inflammation (49). Finally, kahweol, a coffee diterpene, has been shown to inhibit nuclear factor (NF)-κB (50), a protein complex that controls DNA transcription and directly regulates expression of cytokines/chemokines, growth factors and immunoreceptors including CX3CL1/fractalkine (51, 52), CCL4/MIP-1β (53), and IFNγ (54).
Stratified analyses and tests for heterogeneity suggest that associations between coffee drinking and inflammatory markers do not vary considerably by sex, smoking status or diabetes status. In several instances, however, there was evidence of a quantitative interaction in which the association differed in magnitude but not direction across subgroups. For example, associations appeared to be stronger among women than men for CCL4/MIP-1β, among never smokers than ever smokers for IFNγ and CX3CL1/fractalkine, and among those without diabetes than those with diabetes for sTNFRII. Given the sizable number of statistical tests conducted in secondary analyses, these results may be due to chance and should be interpreted with caution.
Strengths of our study include its broad characterization of coffee drinking and variations in systemic immune and inflammatory markers measured using validated technology, a relatively large sample size, the use of innovative design methods to weight the analyses to the population-based screening arm of the PLCO cohort, and our consideration of multiple comparisons in the primary analysis (36). In addition, detailed adjustment for smoking and subgroup analyses in never smokers suggests that the observed associations are not explained by residual confounding by smoking; moreover, none of the statistically significant markers reported herein overlap with inflammatory and immune markers that were statistically significantly associated with smoking in this population (29). Limitations of our study include the cross-sectional design, which precludes evaluation of changes in marker level over time, and the lack of detailed information on brewing method as well as whether coffee was caffeinated or decaffeinated. In addition, 24 of the 77 markers considered in this analysis had low detectability (<25%) in at least one of the three nested case-control studies. Future studies of these markers should consider more sensitive assays than the multiplex assays used herein. Our findings may not be generalizable to non-White or younger populations. Finally, in spite of a relatively large sample size, we had only modest statistical power to test interactions.
In conclusion, our study finds inverse associations between coffee drinking and systemic levels of a number of different inflammatory markers including four markers (CX3CL1/fractalkine, CCL4/MIP-1β, IFNγ and FGF-2) that have not been previously considered in human studies of coffee drinking. Higher coffee drinking has been associated with lower risk of mortality (2–4) and numerous chronic diseases (5, 6, 8, 10, 42). If these associations were a result of coffee’s effects on the immune system, then our data identifies promising markers for future studies aiming to understand whether immune and inflammatory markers mediate the association between coffee and disease. Randomized feeding trials exploring the impact of coffee or coffee constituents, such as caffeine, on a broad array of inflammatory and immune markers are needed to validate the associations observed in our study. In addition, prospective-based studies (e.g. nested case-control studies in large cohorts) of coffee drinking and health that can measure variations in immune and inflammatory markers, including the markers examined herein, may provide insight into possible mediators of observed coffee-disease associations and ultimately elucidate the potentially etiologic role of coffee drinking in the development of disease.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Dr. Yan Li of the University of Maryland’s Joint Program for Survey Methodology as well as Mr. Craig Williams, Mr. Michael Curry and Michael Furr of Information Management Services, Inc., who were compensated for statistical programming.
Financial Support: This work was supported in part by the Yale–NCI pre-doctoral training grant T32 CA105666 to S.T. Mayne, and by the Intramural Research Program of the National Institutes of Health, National Cancer Institute (E. Loftfield, M.S. Shiels, B.I. Graubard, H.A. Katki, A.K. Chaturvedi, B. Trabert, L.A. Pinto, T.J. Kemp, N. Wentzensen, A. Hildesheim, R. Sinha, N.D. Freedman).
Footnotes
The opinions and conclusions expressed in this article are solely the views of the author(s) and do not necessarily reflect those of the Food and Drug Administration.
Conflict of Interest: None to disclose.
REFERENCES
- 1.National Coffee Association. National Coffee Drinking Trends. New York, NY: National Coffee Association; 2014. (Market research infographic). [Google Scholar]
- 2.Je Y, Giovannucci E. Coffee consumption and total mortality: a meta-analysis of twenty prospective cohort studies. Br J Nutr. 2014;111:1162–1173. doi: 10.1017/S0007114513003814. [DOI] [PubMed] [Google Scholar]
- 3.Crippa A, Discacciati A, Larsson SC, Wolk A, Orsini N. Coffee Consumption and Mortality From All Causes, Cardiovascular Disease, and Cancer: A Dose-Response Meta-Analysis. Am J Epidemiol. 2014;180:763–775. doi: 10.1093/aje/kwu194. [DOI] [PubMed] [Google Scholar]
- 4.Freedman ND, Park Y, Abnet CC, Hollenbeck AR, Sinha R. Association of coffee drinking with total and cause-specific mortality. N Engl J Med. 2012;366:1891–1904. doi: 10.1056/NEJMoa1112010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Je Y, Giovannucci E. Coffee consumption and risk of endometrial cancer: findings from a large up-to-date meta-analysis. Int J Cancer. 2012;131:1700–1710. doi: 10.1002/ijc.27408. [DOI] [PubMed] [Google Scholar]
- 6.Galeone C, Turati F, La Vecchia C, Tavani A. Coffee consumption and risk of colorectal cancer: a meta-analysis of case-control studies. Cancer Causes Control. 2010;21:1949–1959. doi: 10.1007/s10552-010-9623-5. [DOI] [PubMed] [Google Scholar]
- 7.Sinha R, Cross AJ, Daniel CR, Graubard BI, Wu JW, Hollenbeck AR, et al. Caffeinated and decaffeinated coffee and tea intakes and risk of colorectal cancer in a large prospective study. Am J Clin Nutr. 2012;96:374–381. doi: 10.3945/ajcn.111.031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sang LX, Chang B, Li XH, Jiang M. Consumption of coffee associated with reduced risk of liver cancer: a meta-analysis. BMC Gastroenterol. 2013;13:34. doi: 10.1186/1471-230X-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Discacciati A, Orsini N, Wolk A. Coffee consumption and risk of nonaggressive, aggressive and fatal prostate cancer--a dose-response meta-analysis. Ann Oncol. 2014;25:584–591. doi: 10.1093/annonc/mdt420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 11.Torres DM, Harrison SA. Is it time to write a prescription for coffee? Coffee and liver disease. Gastroenterology. 2013;144:670–672. doi: 10.1053/j.gastro.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Akash MS, Rehman K, Chen S. Effects of coffee on type 2 diabetes mellitus. Nutrition. 2014;30:755–763. doi: 10.1016/j.nut.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Arnlov J, Vessby B, Riserus U. Coffee consumption and insulin sensitivity. JAMA. 2004;291:1199–1201. doi: 10.1001/jama.291.10.1199-b. [DOI] [PubMed] [Google Scholar]
- 14.Wu T, Willett WC, Hankinson SE, Giovannucci E. Caffeinated coffee, decaffeinated coffee, and caffeine in relation to plasma C-peptide levels, a marker of insulin secretion, in U.S. women. Diabetes Care. 2005;28:1390–1396. doi: 10.2337/diacare.28.6.1390. [DOI] [PubMed] [Google Scholar]
- 15.Lecoultre V, Carrel G, Egli L, Binnert C, Boss A, MacMillan EL, et al. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am J Clin Nutr. 2014;99:268–275. doi: 10.3945/ajcn.113.069526. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- 17.Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91:950–957. doi: 10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 18.Williams CJ, Fargnoli JL, Hwang JJ, van Dam RM, Blackburn GL, Hu FB, et al. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: a prospective cohort study. Diabetes Care. 2008;31:504–507. doi: 10.2337/dc07-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs S, Kroger J, Floegel A, Boeing H, Drogan D, Pischon T, et al. Evaluation of various biomarkers as potential mediators of the association between coffee consumption and incident type 2 diabetes in the EPIC-Potsdam Study. Am J Clin Nutr. 2014;100:891–900. doi: 10.3945/ajcn.113.080317. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita K, Yatsuya H, Muramatsu T, Toyoshima H, Murohara T, Tamakoshi K. Association of coffee consumption with serum adiponectin, leptin, inflammation and metabolic markers in Japanese workers: a cross-sectional study. Nutr Diabetes. 2012;2:e33. doi: 10.1038/nutd.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa TA, Rogero MM, Mioto BM, Tarasoutchi D, Tuda VL, Cesar LA, et al. Paper-filtered coffee increases cholesterol and inflammation biomarkers independent of roasting degree: a clinical trial. Nutrition. 2013;29:977–981. doi: 10.1016/j.nut.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Tangney CC, Rasmussen HE. Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep. 2013;15:324. doi: 10.1007/s11883-013-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zampelas A, Panagiotakos DB, Pitsavos C, Chrysohoou C, Stefanadis C. Associations between coffee consumption and inflammatory markers in healthy persons: the ATTICA study. Am J Clin Nutr. 2004;80:862–867. doi: 10.1093/ajcn/80.4.862. [DOI] [PubMed] [Google Scholar]
- 24.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 25.Hayes RB, Sigurdson A, Moore L, Peters U, Huang WY, Pinsky P, et al. Methods for etiologic and early marker investigations in the PLCO trial. Mutat Res. 2005;592:147–154. doi: 10.1016/j.mrfmmm.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, et al. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013;105:1871–1880. doi: 10.1093/jnci/djt309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trabert B, Pinto L, Hartge P, Kemp T, Black A, Sherman ME, et al. Pre-diagnostic serum levels of inflammation markers and risk of ovarian cancer in the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial. Gynecol Oncol. 2014;135:297–304. doi: 10.1016/j.ygyno.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purdue MP, Hofmann JN, Kemp TJ, Chaturvedi AK, Lan Q, Park JH, et al. A prospective study of 67 serum immune and inflammation markers and risk of non-Hodgkin lymphoma. Blood. 2013;122:951–957. doi: 10.1182/blood-2013-01-481077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiels MS, Katki HA, Freedman ND, Purdue MP, Wentzensen N, Trabert B, et al. Cigarette smoking and variations in systemic immune and inflammation markers. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaturvedi AK, Kemp TJ, Pfeiffer RM, Biancotto A, Williams M, Munuo S, et al. Evaluation of multiplexed cytokine and inflammation marker measurements: a methodologic study. Cancer Epidemiol Biomarkers Prev. 2011;20:1902–1911. doi: 10.1158/1055-9965.EPI-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 32.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 33.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 34.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Department of Agriculture. Design and Operation: The Continuing Survey of Food Intakes by Individuals and the Diet and Health Knowledge Survey, 1994–96. Washington, DC: Agricultural Research Service, U.S. Department of Agriculture; 1997. (Nationwide Food Surveys Report no. 96-1). [Google Scholar]
- 36.Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67:850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Zock PL, Katan MB, Merkus MP, van Dusseldorp M, Harryvan JL. Effect of a lipid-rich fraction from boiled coffee on serum cholesterol. Lancet. 1990;335:1235–1237. doi: 10.1016/0140-6736(90)91302-q. [DOI] [PubMed] [Google Scholar]
- 38.Bonita JS, Mandarano M, Shuta D, Vinson J. Coffee and cardiovascular disease: in vitro, cellular, animal, and human studies. Pharmacol Res. 2007;55:187–198. doi: 10.1016/j.phrs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Jick H, Miettinen OS, Neff RK, Shapiro S, Heinonen OP, Slone D. Coffee and myocardial infarction. N Engl J Med. 1973;289:63–67. doi: 10.1056/NEJM197307122890203. [DOI] [PubMed] [Google Scholar]
- 40.Hennekens CH, Drolette ME, Jesse MJ, Davies JE, Hutchison GB. Coffee-Drinking and Death Due to Coronary Heart-Disease. New Engl J Med. 1976;294:633–636. doi: 10.1056/NEJM197603182941203. [DOI] [PubMed] [Google Scholar]
- 41.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129:643–659. doi: 10.1161/CIRCULATIONAHA.113.005925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu R, Guo X, Park Y, Huang X, Sinha R, Freedman ND, et al. Caffeine intake, smoking, and risk of Parkinson disease in men and women. Am J Epidemiol. 2012;175:1200–1207. doi: 10.1093/aje/kwr451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitaglione P, Morisco F, Mazzone G, Amoruso DC, Ribecco MT, Romano A, et al. Coffee reduces liver damage in a rat model of steatohepatitis: the underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52:1652–1661. doi: 10.1002/hep.23902. [DOI] [PubMed] [Google Scholar]
- 44.Tunnicliffe JM, Shearer J. Coffee, glucose homeostasis, and insulin resistance: physiological mechanisms and mediators. Appl Physiol Nutr Metab. 2008;33:1290–1300. doi: 10.1139/H08-123. [DOI] [PubMed] [Google Scholar]
- 45.Moon MK, Lee YJ, Kim JS, Kang DG, Lee HS. Effect of caffeic acid on tumor necrosis factor-alpha-induced vascular inflammation in human umbilical vein endothelial cells. Biol Pharm Bull. 2009;32:1371–1377. doi: 10.1248/bpb.32.1371. [DOI] [PubMed] [Google Scholar]
- 46.Shechter M, Shalmon G, Scheinowitz M, Koren-Morag N, Feinberg MS, Harats D, et al. Impact of Acute Caffeine Ingestion on Endothelial Function in Subjects With and Without Coronary Artery Disease. Am J Cardiol. 2011;107:1255–1261. doi: 10.1016/j.amjcard.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 47.Hasko G, Cronstein B. Methylxanthines and inflammatory cells. Handb Exp Pharmacol. 2011:457–468. doi: 10.1007/978-3-642-13443-2_18. [DOI] [PubMed] [Google Scholar]
- 48.Chavez-Valdez R, Wills-Karp M, Ahlawat R, Cristofalo EA, Nathan A, Gauda EB. Caffeine modulates TNF-alpha production by cord blood monocytes: the role of adenosine receptors. Pediatr Res. 2009;65:203–208. doi: 10.1203/PDR.0b013e31818d66b1. [DOI] [PubMed] [Google Scholar]
- 49.Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, Zeng D. Effect of a specific and selective A(2B) adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther. 2007;320:1246–1251. doi: 10.1124/jpet.106.112250. [DOI] [PubMed] [Google Scholar]
- 50.Shen T, Park YC, Kim SH, Lee J, Cho JY. Nuclear Factor-kappa B/Signal Transducers and Activators of Transcription-1-Mediated Inflammatory Responses in Lipopolysaccharide-Activated Macrophages Are a Major Inhibitory Target of Kahweol, a Coffee Diterpene. Biol Pharm Bull. 2010;33:1159–1164. doi: 10.1248/bpb.33.1159. [DOI] [PubMed] [Google Scholar]
- 51.Ahn SY, Cho CH, Park KG, Lee HJ, Lee S, Park SK, et al. Tumor necrosis factor-alpha induces fractalkine expression preferentially in arterial endothelial cells and mithramycin A suppresses TNF-alpha-induced fractalkine expression. Am J Pathol. 2004;164:1663–1672. doi: 10.1016/s0002-9440(10)63725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhavsar PK, Sukkar MB, Khorasani N, Lee KY, Chung KF. Glucocorticoid suppression of CX3CL1 (fractalkine) by reduced gene promoter recruitment of NF-kappaB. FASEB J. 2008;22:1807–1816. doi: 10.1096/fj.07-094235. [DOI] [PubMed] [Google Scholar]
- 53.Widmer U, Manogue KR, Cerami A, Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J Immunol. 1993;150:4996–5012. [PubMed] [Google Scholar]
- 54.Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, et al. Interaction of NF-kappaB and NFAT with the interferon-gamma promoter. J Biol Chem. 1997;272:30412–30420. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


