Abstract
Staphylococcus aureus , both methicillin susceptible and resistant, are now major community-based pathogens worldwide. The basis for this is multifactorial and includes the emergence of epidemic clones with enhanced virulence, antibiotic resistance, colonization potential, or transmissibility. Household reservoirs of these unique strains are crucial to their success as community-based pathogens. Staphylococci become resident in households, either as colonizers or environmental contaminants, increasing the risk for recurrent infections. Interactions of household members with others in different households or at community sites including schools and daycare facilities play a critical role in the ability of these strains to become endemic. Colonization density at these sites appears to play an important role in facilitating transmission. The integration of research tools including whole genome sequencing, mathematical modeling and social network analysis have provided additional insight into the transmission dynamics of these strains. Thus far, interventions designed to reduce recurrent infections among household members have had limited success, likely due to the multiplicity of potential sources for recolonization. The development of better strategies to reduce the number of household-based infections will depend on greater insight into the different factors that contribute to the success of these uniquely successful epidemic clones of S. aureus.
Keywords: Staphylococcus aureus, household transmission, community-associated
Staphylococcus aureus as a community pathogen
In 1960, Roodyn remarked that, “even in the comparative simplicity of a single household, the epidemiology of staphylococcal infections appears baffling” [1]. It has been 54 years since Roodyn’s publication on staphylococcal infections in the home, yet many might say that the dynamics of staphylococcal disease in the household, as well as in the community, continue to ‘baffle’ us. Understanding the basis for these community-based infections is critical because they have contributed to the ‘waves’ of staphylococcal infections, both methicillin-susceptible and resistant, that have occurred both locally and worldwide [2]. Since the 1980s, there has been a dramatic increase in the number of community-based infections due to methicillin-resistant S. aureus (MRSA), another example of the disturbing global trend of increasing antimicrobial resistance [3]. These infections have, for the most part, involved the skin and soft tissues, however 5–10% have been life threatening; these include septicemias and necrotizing pneumonias [4]. Until this community-based MRSA epidemic, the bulk of these infections occurred in the healthcare setting [4]. Now MRSA, in addition to methicillin susceptible S. aureus, is established as yet another antibiotic-resistant pathogen that frequently causes serious infections in the community [5].
It is noteworthy that the vast majority of community-associated (CA)-MRSA infections have been caused by a limited number of clones of S. aureus [3, 6–8]. In the United States, Canada, and South America the predominant clone has been pulsed field gel type USA300, or multilocus sequence type 8 (ST8) [3, 9, 10]. In other countries, different clones, and often not a single dominant clone, have been responsible for these infections [11, 12]. The success of these epidemic clones, even those that are methicillin susceptible, results from microbiologic determinants possessed by the clones themselves, environmental factors, and different types of exposures that increase the risk of infection [13, 14]. Despite the numerous reported outbreaks of CA-MRSA infections in vastly different settings, there have been several commonly identified factors associated with these different outbreaks [3]. These include crowding, limited access to suitable hygiene, damaged skin, and shared contaminated items or surfaces [15–18]. Interventions directed at these risks have reduced the spread of these bacteria and helped terminate outbreaks [19]. The success of CA-MRSA clones in epidemic settings has allowed them to become resident in communities throughout the world. In addition, the more successful clones have emerged as frequent nosocomial pathogens [20–22].
While the factors responsible for the transition of epidemic CA-MRSA clones to established endemic, community-based pathogens is incompletely understood, a recurring theme has been the role of the household as the epicenter for the introduction, persistence, and amplification of these successful clones. Investigators have noted the high frequency of recurrent infections among family members, the ready transmission of strains within the household, and the persistence of these strains on environmental surfaces in the home [23–26]. New strategies to prevent these infections require a better understanding of the risks associated with the spread of CA-MRSA among household members as well as their interactions with others in the community. In this review we discuss the crucial role of the home as a centerpiece for the establishment of S. aureus, both methicillin susceptible and resistant, as a remarkably successful community-based pathogen.
The intersection of community reservoirs with the household
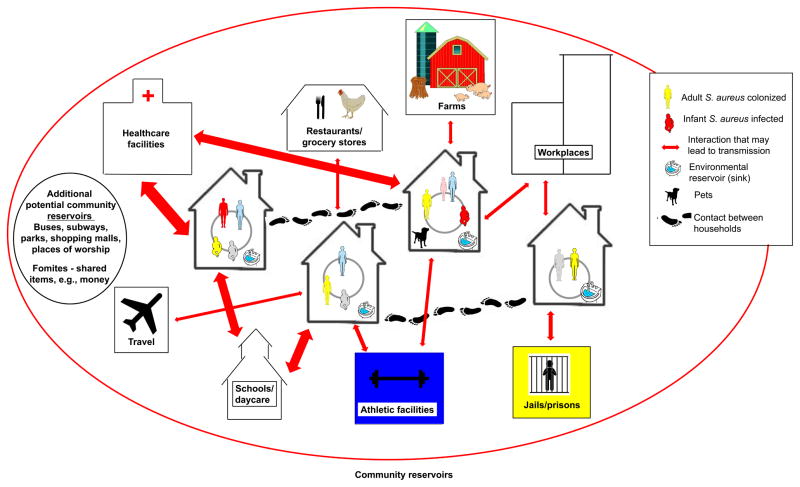
There is a dynamic interaction between community-based sources of CA-MRSA and the introduction of these clones into the household. This dynamic is perhaps best illustrated by the numerous reported outbreaks of CA-MRSA infections that have occurred in a variety of community-based reservoirs including sports clubs, day care facilities, jails, schools, and places of work [15–18]. People at these sites may then transport newly acquired strains of CA-MRSA into their homes [7, 17, 27, 28]. Research conducted in non-outbreak situations supports a similar hypothesis, with strains being transmitted bi-directionally between households and the community through settings (e.g., schools, daycare facilities, farms, and healthcare facilities) and activities (e.g., sports participation and travel), although more detail on this exchange of strains is needed [29–33]. Figure 1 illustrates the numerous potential pathways for the spread of S. aureus in a community. Interaction is shown among people within households, between households, and with community sites. The entry, diffusion and dissemination of S. aureus strains occurs through the flow of people, animals and shared objects, with infection also playing a role in transmission dynamics.
Figure 1.
Graphical display of how S. aureus, including MRSA, spreads through the community. Possible pathways for the spread of S. aureus in a neighborhood. Household members share close physical contact with each other, their household environments (e.g., kitchen sinks) and their pets. These same people interact with members of other households, such as extended family members, friends, and neighbors. They also interact with community sites, such as healthcare facilities, athletic facilities, and schools or day care facilities. Travel may also introduce new strains into the community. As ongoing transmission events, either directly from person to person or mediated through fomites, new strains are periodically introduced into households. This entry, diffusion and dissemination of strains also occur at the community level through the flow of people, animals and objects. Some community members are persistently colonized while others are only temporarily colonized, sometimes long enough to transmit to another person and other times they clear colonization before transmission occurs. These dynamics are also affected by external factors, such as weather patterns. Infection also plays a role in S. aureus transmission dynamics. Based on a combination of exposure, host susceptibility and strain virulence factors, infections occur among a relatively small percentage of community members, which in turn increases the risk of transmission and infection among other household members, as well as their contacts in the community. In the figure the arrows are weighted based on the relative likelihood of S. aureus transmission.
Macal et al. [34] used agent-based modeling to assess CA-MRSA transmission dynamics. The authors simulated temporal and geographic trends in the incidence of CA-MRSA over a ten year period in Chicago, an area with a high incidence of infections [34]. The simulation indicated that the overwhelming majority of transmission events occurred within households. This was in large part due to the extensive degree of physical contact among household members, as well as the large amount of time people spend at home [35]. Of note, the model also suggested that colonization, rather than infection, was the primary source of the vast majority (95%) of transmission events. Schools and daycare centers played the greatest role in perpetuating the spread of MRSA among households. Outbreaks in jails and sports teams played a smaller role in MRSA transmission, although they often dominate popular perceptions. Athletic activities did account for a substantial proportion of colonization events. If confirmed in epidemiological studies, this analysis suggests that far greater attention needs to be placed on reducing MRSA colonization in the community, and in the household in particular, if the overall number of community-based infections is to be reduced.
The application of whole genome sequencing to address CA-MRSA transmission in communities
Recent studies have highlighted the limitations of traditional molecular typing tools in understanding the spread of pathogens in both the healthcare and community setting [36, 37]. The advent of whole genome sequencing (WGS) with its enhanced ability to discriminate among clones has provided a far greater understanding of how these spread within different settings. In particular, it has allowed for a more accurate determination of whether specific clones are unique or are involved in a transmission event [38, 39].
To date, only a limited number of studies have used WGS to investigate the evolution and spread of epidemic strains of CA-S. aureus in households or the community [40, 41]. A small longitudinal study of households following an index USA300 infection demonstrated limited genetic adaptation of isolates over a 15-months study period [40]. These included up to five non-synonymous single nucleotide point mutations (SNPs) as well as small genome rearrangements in tandem gene clusters. Based on a phylogenetic comparison, the study also highlighted that, despite the close epidemiological link between USA300 isolates collected from the same household, multiple different USA300 types were present. Among five USA300 isolates collected from the same household, one methicillin-susceptible S. aureus (600 SNPs) and one MRSA (75 SNPs) isolate were distinct from the initial infecting USA300 isolate, suggesting that new strains had been introduced into the household. Both pulsed field gel electrophoresis and spa-typing, the standard molecular typing tools, missed these differences and had classified all isolates as closely related. These results highlight the additional information and higher discriminatory power yielded by WGS that is critical to a more clear understanding of the transmission pattern among individuals in households.
A subsequent analysis of nearly 400 ST8 strains collected from 168 New York City households found that USA300 had likely been introduced into the community multiple times as several different clades were found to be endemic [41]. The granularity provided by this analytical technique was able to identify a far greater level of diversity than previously hypothesized. The analysis also identified clonal expansion of a fluoroquinolone resistant sub-clade, demonstrating the importance of antimicrobial resistance in the survival and spread of these strains in the community. Isolates collected from the members of a shared household were, on average, more closely related than those collected from other members of the same community, suggesting that individuals within a shared household frequently exchange colonizing S. aureus strains. These putative transmission events between household members confirm the importance of its role as a reservoir for S. aureus in the community setting. Despite the large sample collection, only a limited number of households shared closely related isolates. When applying the maximum pairwise-SNP distance of isolates (a measure of differences between pairs of strains) from households to community isolate pairs to determine a cut-off for possible USA300 transmission, several connections between households were identified (Figure 2) providing additional information on how these strains spread in the community. Based on the agent-based modeling simulations by Macal et al. [34] that highlighted the importance of colonization in transmission, this finding may in part be attributable to large, incompletely characterized colonizing community reservoirs.
Figure 2.
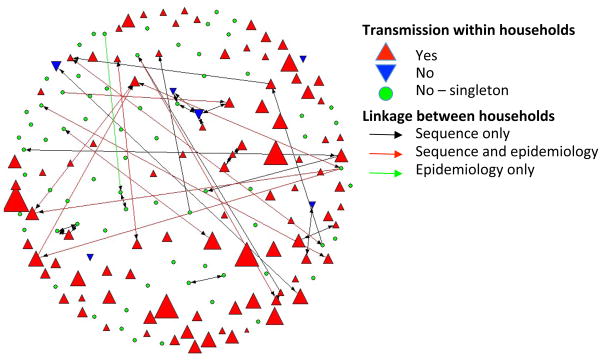
Transmission of the epidemic S. aureus strain ST8 (USA300) within and between households in Northern Manhattan based on sequence and epidemiological analysis. Upright red triangles indicate transmission within households. The size of the triangle corresponds with the number of isolates per household. Downward blue triangles indicate multiple unrelated ST8 isolates, and green highlights single ST8 isolate households. Linkages between households are shown as black lines (identical sequences), red lines (sequence and epidemiological connection), or green lines (epidemiological but no sequence link). Reprinted with permission from PNAS [41].
Alam et al. [42] more recently reported a WGS analysis of 146 isolates collected longitudinally over 6 months from 21 households in Chicago and Los Angeles. In this study there was also evidence for very limited sequence diversity and close clustering of isolates in monophyletic lineages within a household. In about half of the households, the index infection isolate was the connection to the household-specific branch. These data suggest that the other cases were caused by isolates already resident in the household prior to the index infection, emphasizing the importance of decolonization for household members of an infected individual. As in the earlier longitudinal study by Uhlemann et al. [40]. Alam et al. [42] detected evidence for horizontal gene transfer and homologous recombination; they speculated that these were derived from co-resident S. aureus species. The authors noted that the fluoroquinolone-resistant USA300 clone was more widespread in Los Angeles and New York but less so in Chicago. More studies are needed to identify if household transmission differs between varying urban environments.
Staphylococcus aureus transmission and disease within the household
Several studies have highlighted the household’s role as the primary reservoir for S. aureus in the community [24, 25, 41, 43–58]. The events that follow a CA-MRSA infection in a household include an increase in: (i) the risk of infections among other household members [26, 44, 45, 48–51]; (ii) MRSA colonization among other household members [46, 47, 52–57, 59]; and (iii) contamination of environmental surfaces [24, 25, 58]. These reports have described epidemic clones that ‘ping pong’ among family members [26, 51], resulting in high rates of recurrent infection. Eradicating S. aureus carriage from household members and the environment in an effort to reduce the frequency of these infections has achieved mixed results [60, 61].
Research on the spread of S. aureus within the household has identified a diverse set of risk factors for intra-member transmission. A study of CA-MRSA spread among household contacts of infected children found that bathing the child and sharing lotions with the child increased the risk of transmission [62]. Knox et al. [24] observed that the presence of a young child increased the odds of intra-household S. aureus transmission. Cook et al. [26] reported the potential for heterosexual transmission of CA-MRSA among sexual partners in a household. A prospective study in the Netherlands found that prolonged household exposure to MRSA, the age of the individual infected, the diagnosis of eczema in the infected individual, and the number of household members increased the risk of transmission to contacts within the home; the observed risk factors in this study may however not be applicable to countries with a high incidence of CA-MRSA infections [63]. Multiple studies have suggested that domesticated animals play a role in household transmission of S. aureus and that transmission between humans and their pets is likely bi-directional [43, 64–70]. A recent study that used WGS showed that 46 MRSA isolates cultured from cats and dogs in the United Kingdom were interspersed throughout the epidemic MRSA-15 pandemic clade and clustered with a population of human isolates from the same lineage [71].
A comprehensive study looking at the spread of clinical CA-MRSA strains to household members of infected adults and children from two major U.S. cities found that USA300 was more transmissible than other strain types [72]. This same study also found that the index having had a previous skin infection in the past year also increased the risk of transmission to other household members, implicating the interplay between colonization and infection in S. aureus transmission dynamics. This study was strengthened by the large sample size, geographic diversity, surveillance of multiple body sites, and characterization of the S. aureus isolates at the molecular level. Recent skin infection and recent cephalexin use were also found to be independently associated with household transmission.
In contrast, other studies have shown that certain MRSA strains, for example the livestock-associated strain CC398 that has been associated with infections among pig farmers in the Netherlands, are less transmissible than other strains [73, 74].
A recent prospective study found that ‘colonization pressure,’ a measure used in healthcare settings to reflect the magnitude of a microorganism reservoir within a particular unit, was associated with persistent MRSA colonization among infected index outpatients but not recurrent skin and soft tissue infections [75]. Rodriquez et al. [76] reported a high level of strain relatedness among household contacts of children with both methicillin susceptible and resistant CA-S. aureus infections, while additionally observing that a substantial proportion of index cases were colonized with strains discordant from the one causing infection or from strains colonizing their household contacts, again suggesting the introduction of multiple strain types from outside sources.
The risk factors for transmission of community-associated S. aureus clones may differ from those associated with healthcare-associated strains [6, 77–80]. For household spread of healthcare-associated MRSA clones, risk factors have included the presence of a skin condition, close physical contact among household members, and participating in the care of an individual with an infection [63, 81, 82]. A recent analysis pooled common features of CA-MRSA household transmission from three studies performed in geographically diverse settings Melbourne, Australia, New York, USA and Breda, Netherlands [83]. Similar levels of CA-MRSA household transmission were observed across these settings and several common risk factors for household transmission were identified. These included nasal colonization of the index patient with the clinical isolate and the number of children in the household. The number of common risk factors that could be measured across the different studies limited the study.
In recent years, environmental contamination has been recognized as a potential mediator of S. aureus transmission and possible reinfection within the household [24, 25, 43, 58, 80, 84]. The role of the environment in S. aureus infections has been previously explored in the healthcare setting [85, 86] and in certain community settings, such as among injection drug users [87]. In an analysis that included many of the previously identified risk factors for CA-MRSA household transmission, Knox et al. [24] found that environmental contamination with the clinical isolate was by far the most important predictor of the spread of the clinical isolate among non-index household members. Uhlemann et al. [25] found that recurrent infections were more common within households where the isolate that resulted in clinic infection was detected on environmental surfaces; the infections were predominantly caused by USA300. These epidemic strains also are capable of prolonged survival in households. USA300 infection was found in 63% of households at three months where there was an antecedent infection [58]. In a longitudinal study, Miller et al. confirmed these earlier studies showing that patients with a S. aureus skin infection were more likely to have a recurrent infection within 6 months in households with environmental MRSA contamination. Index MRSA colonization and other household member MRSA colonization were not identified as independent predictors of recurrent infections among indexes or infections among household contacts [88]. The importance of environmental contamination in S. aureus infection is further supported by the limited success of body-site decolonization interventions designed to prevent recurrent infections within the household [61, 89]. Alternatively, environmental contamination may be a surrogate marker of colonization of multiple body sites, which itself may serve as an unrecognized staphylococcal reservoir in the community setting [90].
Recent studies have begun to look at MRSA transmission among individuals within a household by relationship and contact type, a level of detail that is likely needed given the complexity of relations within households [62, 63, 81, 82]. These studies found that being a closer contact and participating in the care of a person with an infection were both risk factors for transmission. WGS will be able to more clearly define the interaction among household members in order to untangle the complexity of S. aureus transmission.
Limitations of research on the transmission of CA-MRSA within the household
To date, the majority of research identifying risk factors for infection has been primarily restricted to retrospective case-control studies. As a result, studies of CA-MRSA household transmission are often limited to analyses of cross-sectional data collected after a household index infection has occurred. Therefore, neither the directionality nor the source of transmission may be ascertained and shared strains, the standard proxy measure for transmission, potentially indicate a shared exposure. Analyses of transmission are also usually limited to the spread of the clinical isolate. Furthermore, these household studies have mostly relied on nasal colonization to assess household transmission. This likely underestimates the true burden of S. aureus carriage among individuals in the household and the community. Several studies have now documented the enhanced capacity of CA-MRSA strains, such as USA300, to colonize multiple body sites including the oropharynx, axilla, groin, inguinal canal, rectum and perineum [59, 72, 80, 88, 91–97]. The extent of colonization of these sites has varied among studies, although many of them note that a substantial proportion of carriage is missed when only the anterior nares is sampled [72, 95, 97]. Future studies will need to further delineate these factors by culturing multiple body sites and the environment.
Interventions to reduce the incidence of S. aureus infections in the home
As noted above, households with a previously infected individual are at increased risk of recurrent infection [26, 44, 45, 48–51]. In addition to the household risks, patients recently discharged from healthcare facilities with a history of staphylococcal infections or with evidence of S. aureus colonization are also at increased risk of recurrent infection [98, 99]. Several studies have investigated the efficacy of intervention strategies to reduce the incidence of recurrent infections in the household. While these interventions have been partially successful in reducing colonization, recurrent infections have continued to occur despite these efforts.
Among the first efforts to eradicate epidemic strains of S. aureus from the household was a study by Bocher et al. [100]. This investigation applied the ‘search and destroy’ strategy used in Scandinavian healthcare facilities to the home environment. The investigators worked with patients and healthcare workers colonized with ST22, a MRSA strain present in Vejle County, Denmark. Using a combination of topical mupirocin for those nasally colonized, systemic antibiotics for throat-colonized subjects combined with extensive environmental cleaning, they were able to successfully eradicate colonization in most households. Of note, persistent colonization was most commonly found in subjects with throat or multiple site colonization and among those with chronic diseases.
In more recent studies, several different intervention strategies have been used. In a randomized trial, Fritz et al. [61] found that a combination of diluted bleach baths and topical intranasal mupirocin was the most effective strategy to eradicate colonization (nares, inguinal fold, and axilla sampled) in children. However, depending on the intervention they received (hygiene education for all groups, mupirocin, or mupirocin and bleach baths), 43–54% of the cohort still experienced recurrent infections after six months. In a subsequent household based study, the same group investigated the efficacy of an individual versus a household based decolonization strategy [101]. They found that the household based decolonization approach, despite failing to reduce colonization more than the individual-based approach, was more successful in reducing the number of recurrent infections over a 12-month period, although the number of recurrent infections remained high among both groups. In a small prospective study using nasal mupirocin and hexachlorophene body wash with oral trimethoprim-sulfamethoxazole, Miller et al. [60] noted a significantly reduced number of infections in the six months following the intervention. In another randomized clinical trial, Kaplan et al. [89] found that diluted bleach baths in addition to routine hygiene measures were not more effective in reducing recurrent infections among children than routine hygiene measures alone; within 12 months, recurrent infection requiring medical attention occurred among 17% of children receiving bleach baths compared to 21% among the control group.
As outlined above, certain epidemiological and biological aspects of CA-S. aureus likely account for the limited success of the intervention strategies aimed at reducing recurrent infections within households. First, the spread of S. aureus within the household is complex, involving multiple mechanisms of transmission as well as the frequent introduction of new and old strains from the community into the household [41, 72, 76]. The factors contributing to CA-S. aureus spread require careful consideration when designing measures to interrupt transmission pathways within the home. Second, the burden and persistence of CA-S. aureus carriage may correlate with the pattern of colonization as well as the total number of body sites colonized. To date, most studies have not examined extra-nasal S. aureus colonization and in particular the contribution of pharyngeal colonization to the risk of persistent colonization and reinfection. In order to quantify the true effect of decolonization strategies, longitudinal assessments of staphylococcal carriage at multiple body sites will be needed. Third, the virulence, transmissibility and persistence of CA-S. aureus varies by clonal type, with epidemic strains such as USA300 accounting for a large burden of invasive and recurrent staphylococcal infections [72, 102, 103]. It has been hypothesized that clonal lineage is an important factor to consider when designing, implementing, and assessing intervention strategies. As a growing number of household-based studies have supplemented epidemiological data with molecular typing, further clarification of the role of clonal lineages will be established. Finally, environmental contamination appears to play an important role in the persistence of particular strains in the home and may also increase the risk of recurrent infections [24, 25, 58]. At present, few intervention studies have assessed the prevalence and significance of this important issue.
Concluding remarks and future directions
A combination of epidemiological, genomic, and modeling studies have provided considerable insight into the factors that contribute to the spread of CA-MRSA within the community and the household, however numerous questions remain. For example, while there has been a major focus on defining the epidemiology of CA-MRSA, methicillin sensitive S. aureus has been relatively understudied and the likely interaction among MRSA and methicillin sensitive S. aureus strain types remains largely undefined. Also, the implicit distinction between HA- and CA-S. aureus is made due to the very different nature of these settings; however, there is clearly interaction between them that merits further exploration. Of interest, in this regard, is the observed variance in the incidence of CA-MRSA infections among countries with a high incidence of HA-MRSA, an observation that remains incompletely understood. The ultimate goal of our research efforts is to develop strategies that will successfully reduce CA-MRSA transmission and infections. The evolution of analytic techniques will allow for enhanced depth in the analysis of our research questions (Box 1). Uhlemann et al. [41, 42] illustrated the potential that more precise molecular techniques have for defining how CA-MRSA spreads beyond households and establishes itself in communities [25]. Macal et al. [34] demonstrated the capacity of mathematical modeling to help understand the dynamics of S. aureus transmission and infection in the community setting [34]. The collection and analysis of detailed social network data could help to further strengthen studies, and work is needed to explore overcoming the challenges of conducting this type of research in community settings.
Box 1. Outstanding questions.
Is colonization density, as reflected by the number of colonized household members or environmental sites contaminated, the critical parameter for CA-MRSA persistence?
Which bacterial virulence determinants are necessary for survival in environmental settings?
What are the most effective intervention strategies to prevent recurrent CA-MRSA infections in homes and is oral antibiotic therapy a necessary component of these interventions?
What is the role of the environment in recurrent household infections?
What is the explanation for the regional variation in epidemic clones of CA-MRSA?
The application of these methods will help us to sort out the complexity of staphylococcal spread among households that Roodyn described so aptly over 50 years ago. Without improved information our efforts to further reduce the spread of this dangerous pathogen and prevent infections will be limited.
Highlights.
The household is a major community-based reservoir for methicillin-resistant and methicillin-susceptible S. aureus.
Whole genome sequencing allows enhanced discrimination of strains and, as a result, provides a more accurate understanding of their transmission.
Environmental contamination may contribute to recurrent S. aureus infections.
Interventions directed at reducing household S. aureus infections have had mixed success.
Acknowledgments
This work was supported by the National Institutes of Health (NIH/NIAID) to FL (R01 AI077690, R01 AI077690-S1 and R21 AI103562) and to ACU (K08 AI090013) and the Paul A. Marks Scholarship. We appreciate the assistance of Skye Peebles in the preparation of Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roodyn L. Staphylococcal infections in general practice. Br Med J. 1954;2:1322–1325. doi: 10.1136/bmj.2.4900.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridkin SK, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. [DOI] [PubMed] [Google Scholar]
- 5.Dukic VM, et al. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. PLoS One. 2013;8:e52722. doi: 10.1371/journal.pone.0052722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold BC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 7.Grundmann H, et al. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–885. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 8.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–182. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimmo GR. USA300 abroad: global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2012;18:725–734. doi: 10.1111/j.1469-0691.2012.03822.x. [DOI] [PubMed] [Google Scholar]
- 10.Reyes J, et al. Dissemination of methicillin-resistant Staphylococcus aureus USA300 sequence type 8 lineage in Latin America. Clin Infect Dis. 2009;49:1861–1867. doi: 10.1086/648426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deleo FR, et al. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang YY, Huang YC. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Asia. Lancet Infect Dis. 2013;13:698–708. doi: 10.1016/S1473-3099(13)70136-1. [DOI] [PubMed] [Google Scholar]
- 13.Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol. 2010;64:143–162. doi: 10.1146/annurev.micro.112408.134309. [DOI] [PubMed] [Google Scholar]
- 14.Lowy FD. Secrets of a superbug. Nat Med. 2007;13:1418–1420. doi: 10.1038/nm1207-1418. [DOI] [PubMed] [Google Scholar]
- 15.Bancroft EA. Antimicrobial resistance: it’s not just for hospitals. JAMA. 2007;298:1803–1804. doi: 10.1001/jama.298.15.1803. [DOI] [PubMed] [Google Scholar]
- 16.Baggett HC, et al. Community-onset methicillin-resistant Staphylococcus aureus associated with antibiotic use and the cytotoxin Panton-Valentine leukocidin during a furunculosis outbreak in rural Alaska. J Infect Dis. 2004;189:1565–1573. doi: 10.1086/383247. [DOI] [PubMed] [Google Scholar]
- 17.Kazakova SV, et al. A clone of methicillin-resistant Staphylococcus aureus among professional football players. N Engl J Med. 2005;352:468–475. doi: 10.1056/NEJMoa042859. [DOI] [PubMed] [Google Scholar]
- 18.Campbell KM, et al. Risk factors for community-associated methicillin-resistant Staphylococcus aureus infections in an outbreak of disease among military trainees in San Diego, California, in 2002. J Clin Microbiol. 2004;42:4050–4053. doi: 10.1128/JCM.42.9.4050-4053.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Outbreaks of community-associatied methicillin-resistant Staphylococcus aureus skin infection - Los Angeles County, California, 2002–2003. Morb Mortal Wkly Rep. 2003;52:88–89. [PubMed] [Google Scholar]
- 20.Diep BA, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 21.King MD, et al. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 22.Seybold U, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 23.Fritz SA, et al. Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J Infect. 2009;59:394–401. doi: 10.1016/j.jinf.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knox J, et al. Environmental contamination as a risk factor for intra-household Staphylococcus aureus transmission. PLoS One. 2012;7:e49900. doi: 10.1371/journal.pone.0049900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhlemann AC, et al. The environment as an unrecognized reservoir for community-associated methicillin resistant Staphylococcus aureus USA300: a case-control study. PLoS One. 2011;6:e22407. doi: 10.1371/journal.pone.0022407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook HA, et al. Heterosexual transmission of community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2007;44:410–413. doi: 10.1086/510681. [DOI] [PubMed] [Google Scholar]
- 27.Begier EM, et al. A high-morbidity outbreak of methicillin-resistant Staphylococcus aureus among players on a college football team, facilitated by cosmetic body shaving and turf burns. Clin Infect Dis. 2004;39:1446–1453. doi: 10.1086/425313. [DOI] [PubMed] [Google Scholar]
- 28.Saravolatz LD, et al. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982;96:11–16. doi: 10.7326/0003-4819-96-1-11. [DOI] [PubMed] [Google Scholar]
- 29.Hota B, et al. Community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections at a public hospital: do public housing and incarceration amplify transmission? Arch Intern Med. 2007;167:1026–1033. doi: 10.1001/archinte.167.10.1026. [DOI] [PubMed] [Google Scholar]
- 30.Jaureguiberry S, et al. Transmission of Panton-Valentine leukocidin-producing Staphylococcus aureus from returning travelers to household contacts. Int J Dermatol. 2011;50:705–708. doi: 10.1111/j.1365-4632.2010.04858.x. [DOI] [PubMed] [Google Scholar]
- 31.Stenhem M, et al. Imported methicillin-resistant Staphylococcus aureus, Sweden. Emerg Infect Dis. 2010;16:189–196. doi: 10.3201/eid1602.081655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradley SF, et al. Methicillin-resistant Staphylococcus aureus: colonization and infection in a long-term care facility. Ann Intern Med. 1991;115:417–422. doi: 10.7326/0003-4819-115-6-417. [DOI] [PubMed] [Google Scholar]
- 33.Adcock PM, et al. Methicillin-resistant Staphylococcus aureus in two child care centers. J Infect Dis. 1998;178:577–580. doi: 10.1086/517478. [DOI] [PubMed] [Google Scholar]
- 34.Macal CM, et al. Modeling the transmission of community-associated methicillin-resistant Staphylococcus aureus: a dynamic agent-based simulation. J Transl Med. 2014;12:124. doi: 10.1186/1479-5876-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leech JA, et al. It’s about time: a comparison of Canadian and American time-activity patterns. J Expo Anal Environ Epidemiol. 2002;12:427–432. doi: 10.1038/sj.jea.7500244. [DOI] [PubMed] [Google Scholar]
- 36.Harris SR, et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science. 2010;327:469–474. doi: 10.1126/science.1182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koser CU, et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366:2267–2275. doi: 10.1056/NEJMoa1109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris SR, et al. Whole-genome sequencing for analysis of an outbreak of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect Dis. 2013;13:130–136. doi: 10.1016/S1473-3099(12)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardy JL, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364:730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 40.Uhlemann AC, et al. Toward an understanding of the evolution of Staphylococcus aureus strain USA300 during colonization in community households. Genome Biol Evol. 2012;4:1275–1285. doi: 10.1093/gbe/evs094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhlemann AC, et al. Molecular tracing of the emergence, diversification, and transmission of S. aureus sequence type 8 in a New York community. Proc Natl Acad Sci U S A. 2014;111:6738–6743. doi: 10.1073/pnas.1401006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alam MT, et al. Transmission and microevolution of USA300 MRSA in U.S. households: evidence from whole-genome sequencing. mBio. 2015;6:e00054–00015. doi: 10.1128/mBio.00054-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis MF, et al. Household transmission of meticillin-resistant Staphylococcus aureus and other staphylococci. Lancet Infect Dis. 2012;12:703–716. doi: 10.1016/S1473-3099(12)70156-1. [DOI] [PubMed] [Google Scholar]
- 44.Huijsdens XW, et al. Multiple cases of familial transmission of community-acquired methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2006;44:2994–2996. doi: 10.1128/JCM.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.L’Heriteau F, et al. Community-acquired methicillin-resistant Staphylococcus aureus and familial transmission. JAMA. 1999;282:1038–1039. doi: 10.1001/jama.282.11.1038. [DOI] [PubMed] [Google Scholar]
- 46.Busato CR, et al. Staphylococcus aureus nasopharyngeal carriage rates and antimicrobial susceptibility patterns among health care workers and their household contacts. Braz J Infect Dis. 1998;2:78–84. [PubMed] [Google Scholar]
- 47.Wagenvoort J, et al. Transmission of methicillin-resistant Staphylococcus aureus within a household. Eur J Clin Microbiol Infect Dis. 1997;16:399–400. doi: 10.1007/BF01726373. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto T, et al. Super-sticky familial infections caused by Panton-Valentine leukocidin-positive ST22 community-acquired methicillin-resistant Staphylococcus aureus in Japan. J Infect Chemother. 2012;18:187–198. doi: 10.1007/s10156-011-0316-0. [DOI] [PubMed] [Google Scholar]
- 49.Yabe S, et al. Spread of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone among family members in Japan. J Infect Chemother. 2010;16:372–374. doi: 10.1007/s10156-010-0087-z. [DOI] [PubMed] [Google Scholar]
- 50.Amir NH, et al. Spread of community-acquired meticillin-resistant Staphylococcus aureus skin and soft-tissue infection within a family: implications for antibiotic therapy and prevention. J Med Microbiol. 2010;59:489–492. doi: 10.1099/jmm.0.015925-0. [DOI] [PubMed] [Google Scholar]
- 51.Jones TF, et al. Family outbreaks of invasive community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;42:e76–78. doi: 10.1086/503265. [DOI] [PubMed] [Google Scholar]
- 52.Zafar U, et al. Prevalence of nasal colonization among patients with community-associated methicillin-resistant Staphylococcus aureus infection and their household contacts. Infect Control Hosp Epidemiol. 2007;28:966–969. doi: 10.1086/518965. [DOI] [PubMed] [Google Scholar]
- 53.Hugo Johansson P, et al. High prevalence of MRSA in household contacts. Scand J Infect Dis. 2007;39:764–768. doi: 10.1080/00365540701302501. [DOI] [PubMed] [Google Scholar]
- 54.Lautenbach E, et al. The impact of household transmission on duration of outpatient colonization with methicillin-resistant Staphylococcus aureus. Epidemiol Infect. 2010;138:683–685. doi: 10.1017/S0950268810000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ho PL, et al. Molecular epidemiology and household transmission of community-associated methicillin-resistant Staphylococcus aureus in Hong Kong. Diagn Microbiol Infect Dis. 2007;57:145–151. doi: 10.1016/j.diagmicrobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Stone A, et al. Staphylococcus aureus nasal colonization among pediatric cystic fibrosis patients and their household contacts. Pediatr Infect Dis J. 2009;28:895–899. doi: 10.1097/inf.0b013e3181a3ad0a. [DOI] [PubMed] [Google Scholar]
- 57.Huang YC, et al. Nasal carriage of methicillin-resistant Staphylococcus aureus in household contacts of children with community-acquired diseases in Taiwan. Pediatr Infect Dis J. 2007;26:1066–1068. doi: 10.1097/INF.0b013e31813429e8. [DOI] [PubMed] [Google Scholar]
- 58.Eells SJ, et al. Persistent environmental contamination with USA300 methicillin-resistant Staphylococcus aureus and other pathogenic strain types in households with S. aureus skin infections. Infect Control Hosp Epidemiol. 2014;35:1373–1382. doi: 10.1086/678414. [DOI] [PubMed] [Google Scholar]
- 59.Cluzet VC, et al. Duration of colonization and determinants of earlier clearance of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2015 doi: 10.1093/cid/civ075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller LG, et al. Prospective investigation of nasal mupirocin, hexachlorophene body wash, and systemic antibiotics for prevention of recurrent community-associated methicillin-resistant Staphylococcus aureus infections. Antimicrob Agents Chemother. 2012;56:1084–1086. doi: 10.1128/AAC.01608-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fritz SA, et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis. 2012;54:743–751. doi: 10.1093/cid/cir919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nerby JM, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2011;30:927–932. doi: 10.1097/INF.0b013e31822256c3. [DOI] [PubMed] [Google Scholar]
- 63.Mollema FP, et al. Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol. 2010;48:202–207. doi: 10.1128/JCM.01499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bramble M, et al. Potential role of pet animals in household transmission of methicillin-resistant Staphylococcus aureus: a narrative review. Vector Borne Zoonotic Dis. 2010;11:617–620. doi: 10.1089/vbz.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simoons-Smit AM, et al. Transmission of Staphylococcus aureus between humans and domestic animals in a household. Eur J Clin Microbiol Infect Dis. 2000;19:150–152. doi: 10.1007/s100960050450. [DOI] [PubMed] [Google Scholar]
- 66.Manian FA. Asymptomatic nasal carriage of mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MRSA) in a pet dog associated with MRSA infection in household contacts. Clin Infect Dis. 2003;36:e26–28. doi: 10.1086/344772. [DOI] [PubMed] [Google Scholar]
- 67.Weese JS, et al. Suspected transmission of methicillin-resistant Staphylococcus aureus between domestic pets and humans in veterinary clinics and in the household. Vet Microbiol. 2006;115:148–155. doi: 10.1016/j.vetmic.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Gomez-Sanz E, et al. Clonal dynamics of nasal Staphylococcus aureus and Staphylococcus pseudintermedius in dog-owning household members. Detection of MSSA ST(398) PLoS One. 2013;8:e69337. doi: 10.1371/journal.pone.0069337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faires MC, et al. An investigation of methicillin-resistant Staphylococcus aureus colonization in people and pets in the same household with an infected person or infected pet. J Am Vet Med Assoc. 2009;235:540–543. doi: 10.2460/javma.235.5.540. [DOI] [PubMed] [Google Scholar]
- 70.Hanselman BA, et al. Coagulase positive staphylococcal colonization of humans and their household pets. Can Vet J. 2009;50:954–958. [PMC free article] [PubMed] [Google Scholar]
- 71.Harrison EM, et al. A shared population of epidemic methicillin-resistant Staphylococcus aureus 15 circulates in humans and companion animals. mBio. 2014;5:e00985–00913. doi: 10.1128/mBio.00985-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller LG, et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis. 2012;54:1523–1535. doi: 10.1093/cid/cis213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verkade E, et al. Transmission of methicillin-resistant Staphylococcus aureus CC398 from livestock veterinarians to their household members. PLoS One. 2014;9:e100823. doi: 10.1371/journal.pone.0100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcia-Graells C, et al. Dynamic of livestock-associated methicillin-resistant Staphylococcus aureus CC398 in pig farm households: a pilot study. PLoS One. 2013;8:e65512. doi: 10.1371/journal.pone.0065512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez M, et al. Measurement and impact of colonization pressure in households. J Pediatric Infect Dis Soc. 2013;2:147–154. doi: 10.1093/jpids/pit002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodriguez M, et al. Molecular epidemiology of Staphylococcus aureus in households of children with community-associated S aureus skin and soft tissue infections. J Pediatr. 2014;164:105–111. doi: 10.1016/j.jpeds.2013.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorak EJ, et al. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin Infect Dis. 1999;29:797–800. doi: 10.1086/520437. [DOI] [PubMed] [Google Scholar]
- 78.Moreno F, et al. Methicillin-resistant Staphylococcus aureus as a community organism. Clin Infect Dis. 1995;21:1308–1312. doi: 10.1093/clinids/21.5.1308. [DOI] [PubMed] [Google Scholar]
- 79.Naimi TS, et al. Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996–1998. Clin Infect Dis. 2001;33:990–996. doi: 10.1086/322693. [DOI] [PubMed] [Google Scholar]
- 80.Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46:752–760. doi: 10.1086/526773. [DOI] [PubMed] [Google Scholar]
- 81.Lucet JC, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med. 2009;169:1372–1378. doi: 10.1001/archinternmed.2009.217. [DOI] [PubMed] [Google Scholar]
- 82.Calfee DP, et al. Spread of methicillin-resistant Staphylococcus aureus (MRSA) among household contacts of individuals with nosocomially acquired MRSA. Infect Control Hosp Epidemiol. 2003;24:422–426. doi: 10.1086/502225. [DOI] [PubMed] [Google Scholar]
- 83.Knox J, et al. Community-associated methicillin-resistant Staphylococcus aureus transmission in households of infected cases: a pooled analysis of primary data from three studies across international settings. Epidemiol Infect. 2014;24:1–12. doi: 10.1017/S0950268814000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fritz SA, et al. Contamination of environmental surfaces with Staphylococcus aureus in households with children infected with methicillin-resistant S. aureus. JAMA Pediatr. 2014;168:1030–1038. doi: 10.1001/jamapediatrics.2014.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect. 2009;73:378–385. doi: 10.1016/j.jhin.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 86.Dancer SJ. Importance of the environment in meticillin-resistant Staphylococcus aureus acquisition: the case for hospital cleaning. Lancet Infect Dis. 2008;8:101–113. doi: 10.1016/S1473-3099(07)70241-4. [DOI] [PubMed] [Google Scholar]
- 87.Gwizdala RA, et al. Staphylococcus aureus colonization and infection among drug users: identification of hidden networks. Am J Public Health. 2011;101:1268–1276. doi: 10.2105/AJPH.2010.300028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller LG, et al. Staphylococcus aureus skin infection recurrences among household members: an examination of host, behavioral, and pathogen-level predictors. Clin Infect Dis. 2015;60:753–763. doi: 10.1093/cid/ciu943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaplan SL, et al. Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis. 2014;58:679–682. doi: 10.1093/cid/cit764. [DOI] [PubMed] [Google Scholar]
- 90.Harbarth S, et al. Risk factors for persistent carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2000;31:1380–1385. doi: 10.1086/317484. [DOI] [PubMed] [Google Scholar]
- 91.Miller M, et al. Staphylococcus aureus in the community: colonization versus infection. PLoS One. 2009;4:e6708. doi: 10.1371/journal.pone.0006708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee CJ, et al. Staphylococcus aureus oropharyngeal carriage in a prison population. Clin Infect Dis. 2011;52:775–778. doi: 10.1093/cid/cir026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miko BA, et al. High prevalence of colonization with Staphylococcus aureus clone USA300 at multiple body sites among sexually transmitted disease clinic patients: an unrecognized reservoir. Microbes Infect. 2012;14:1040–1043. doi: 10.1016/j.micinf.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peters PJ, et al. Methicillin-resistant Staphylococcus aureus colonization in HIV-infected outpatients is common and detection is enhanced by groin culture. Epidemiol Infect. 2011;139:998–1008. doi: 10.1017/S0950268810002013. [DOI] [PubMed] [Google Scholar]
- 95.Eveillard M, et al. Evaluation of a strategy of screening multiple anatomical sites for methicillin-resistant Staphylococcus aureus at admission to a teaching hospital. Infect Control Hosp Epidemiol. 2006;27:181–184. doi: 10.1086/500627. [DOI] [PubMed] [Google Scholar]
- 96.Mermel LA, et al. Methicillin-resistant Staphylococcus aureus colonization at different body sites: a prospective, quantitative analysis. J Clin Microbiol. 2011;49:1119–1121. doi: 10.1128/JCM.02601-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lautenbach E, et al. Surveillance cultures for detection of methicillin-resistant Staphylococcus aureus: diagnostic yield of anatomic sites and comparison of provider- and patient-collected samples. Infect Control Hosp Epidemiol. 2009;30:380–382. doi: 10.1086/596045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dantes R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 100.Bocher S, et al. The search and destroy strategy prevents spread and long-term carriage of methicillin-resistant Staphylococcus aureus: results from the follow-up screening of a large ST22 (E-MRSA 15) outbreak in Denmark. Clin Microbiol Infect. 2010;16:1427–1434. doi: 10.1111/j.1469-0691.2009.03137.x. [DOI] [PubMed] [Google Scholar]
- 101.Fritz SA, et al. Mupirocin and chlorhexidine resistance in Staphylococcus aureus in patients with community-onset skin and soft tissue infections. Antimicrob Agents Chemother. 2013;57:559–568. doi: 10.1128/AAC.01633-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moran GJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 103.Limbago B, et al. Characterization of methicillin-resistant Staphylococcus aureus isolates collected in 2005 and 2006 from patients with invasive disease: a population-based analysis. J Clin Microbiol. 2009;47:1344–1351. doi: 10.1128/JCM.02264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]