Abstract
Cardiac dysfunction is a major consequence of sepsis/septic shock and contributes to the high mortality of sepsis. Innate and inflammatory responses mediated by Toll-like receptors (TLRs) play a critical role in sepsis-induced cardiac dysfunction. MicroRNA-146 was first identified as a negative regulator in innate immune and inflammatory responses induced by LPS. This study examined whether miR-146a will have a protective effect on sepsis-induced cardiac dysfunction. Lentivirus expressing miR-146a (LmiR-146a) or lentivirus expressing scrambled miR (LmiR-control) were delivered into the myocardium via the right carotid artery. Seven days after transfection, mice were subjected to CLP. Untransfected mice were also subjected to CLP-induced sepsis. Cardiac function was examined by echocardiography before and 6 h after CLP. In vitro studies showed that increased miR-146a levels suppresses LPS-induced IκBα phosphorylation and inflammatory cytokine production in both H9C2 cardiomyocytes and J774 macrophages. In vivo transfection of LmiR-146a attenuated sepsis-induced cardiac dysfunction. The values for EF% and FS% in LmiR-146a transfected CLP mice were significantly greater than in untransfected CLP control. LmiR-146a transfection prevented sepsis-induced NF-κB activity, suppressed IRAK and TRAF6 expression in the myocardium, and attenuated sepsis-induced inflammatory cytokine production in both plasma and peritoneal fluid. In addition, LmiR-146a transfection decreased sepsis-induced infiltration of neutrophils and macrophages into the myocardium. LmiR-146a can also transfect macrophages in the periphery. We conclude that miR-146a attenuates sepsis-induced cardiac dysfunction by preventing NF-κB activation, inflammatory cell infiltration, and inflammatory cytokine production via targeting of IRAK and TRAF6 in both cardiomyocytes and inflammatory monocytic cells.
Keywords: microRNA-146a, cardiac function, sepsis, NF-κB, TRAF6
Introduction
Severe sepsis and septic shock are major healthcare problems, affecting millions of people around the world each year. In the United States, over 1,66500 cases of sepsis occur each with a mortality rate up to 50% (1) (http://www.sepsisalliance.org). There is compelling evidence that cardiovascular dysfunction or septic cardiomyopathy is a major complication association with sepsis induced morbidity and mortality. Cardiac dysfunction in sepsis/septic shock is associated with mortality rates of up to 70% (2,3).
Innate immune and inflammatory responses, mediated by Toll-like receptors (TLRs), have been demonstrated to play a critical role in sepsis/septic shock induced cardiac dysfunction (4–8). TLR mediated signaling predominantly activates nuclear factor kappaB (NF-κB) which is an important transcription factor regulating the expression of immunoregulatory and pro-inflammatory mediators (9,10). We have shown that NF-κB activation plays an important role in mediating the development of sepsis/septic shock and cardiac dysfunction in sepsis/septic shock (4,5,11–13). Targeting TLR-mediated NF-κB activation signaling could be an efficient approach for the treatment and management of septic patients.
Recent studies have shown that specific microRNAs (miRs) play a critical role in the negative regulation of TLR/NF-κB mediated innate immune and inflammatory responses (14–19). MicroRNAs are 21 to 23 nucleotide non-protein-coding RNA molecules which have been identified as novel regulators of gene expression at the post-transcriptional level (14–16,20,21). Several miRs (miR-146a, miR-155, and miR-125, etc.) have been reported to regulate TLR-mediated NF-κB activation (17–19). MiR-181b has been reported to regulate NF-κB mediated vascular inflammation (22). Rescue of miR-181b levels reduced NF-κB signaling and leukocyte influx in the vascular endothelium and decreased lung injury and mortality in endotoxemic mice (22).
MiR-146 was first identified as a negative regulator in innate immune and inflammatory responses that are mediated by TLRs (23,24). Taganov et al have reported that stimulation of human monocytic THP-1 cells with lipopolysaccharides (LPS) rapidly induces the expression of both miR-146a and miR-146b (23,24). Interestingly, miR-146a directly targets IRAK1 and TRAF6 which are the key adapter molecules in the TLR/NF-κB pathway (9,10). Recent studies have shown that miR-146a is critical for the in vitro monocytic cell-based endotoxin tolerance (25,26) and inhibition of miR-146a can reverse endotoxin tolerance (26). We have previously reported that TLR-mediated NF-κB activation pathway plays a critical role in polymicrobial sepsis (4,27) and sepsis-induced cardiac dysfunction (5,12). However, the role of miR-46a in sepsis-induced cardiac dysfunction has not been investigated.
In the present study, we delivered lentivirus expressing miR-146a (LmiR-146a) into the myocardium through the right carotid artery and observed that increased expression of miR-146a protects against cardiac dysfunction induced by polymicrobial sepsis. We also demonstrated that increased expression of miR-146a markedly reduces the infiltration of macrophages and neutrophils into the myocardium and attenuates inflammatory response in both cardiomyocytes and macrophages via suppression of NF-κB binding activity by targeting IRAK1 and TRAF6. The data suggests that miR-146a could be a useful agent for protection against sepsis/septic shock induced cardiac dysfunction.
Materials and Methods
Animals
Male C57BL/6 mice were obtained from Jackson laboratory and were maintained in the Division of Laboratory Animal Resources, East Tennessee State University (ETSU). The experiments outlined in this manuscript conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication, 8th Edition, 2011). The animal care and experimental protocols were approved by the ETSU Committee on Animal Care.
CLP polymicrobial sepsis model
Cecal ligation and puncture (CLP) was performed to induce polymicrobial sepsis in mice as previously described (4,5,12,13,27,28). Briefly, the mice were anesthetized by 5.0% Isoflurane. A midline incision was made on the anterior abdomen and the cecum was exposed and ligated with a 4-0 suture. Two punctures were made through the cecum with an 18-gauge needle and feces were extruded from the holes. The abdomen was then closed in two layers. Sham surgically operated mice served as the surgery control group. Immediately following surgery, a single dose of resuscitative fluid (lactated Ringer’s solution, 50 ml/kg body weight) was administered by subcutaneous injection (5,13,28).
Echocardiography
M-mode tracings were used to measure left ventricular (LV) wall thickness, LV end-systolic diameter (LVESD), and LV end-diastolic diameter (LVEDD). Percent fractional shortening (%FS) and ejection fraction (EF%) were calculated as described previously (5,12,29).
Construction of miR-146a into lentivirus expressing system
miR-146a was constructed into lentivirus expression vector using a lentivirus expressing system (Invitrogen corporation) as described previously (30). Briefly, the oligonucleotides for miR-146a were synthesized at Integrated DNA Technologies, annealed and ligated into pcDNATM6.2-GW/EmGFP-miR. The pcDNATM6.2-GW/EmGFP-miR cassette was subsequently transferred to pDONR221TM and finally pLenti6/V5-DEST by two sequential Gateway BP and LR recombinations. The lentiviral control vector contains a non-sense miR sequence that allows formation of a pre-miRNA hairpin predicted not to target any known vertebrate gene (Invitrogen Corporation). The viral particles were produced by third generation packaging in 293FT cells and Lentiviral stocks were concentrated using ultracentrifugation (30).
qPCR assay of microRNAs (miRs)
miRs were isolated from heart tissues or cultured cells using the mirVanaTM miR isolation kit (Ambion) as described previously (30). MiR-146a levels were quantified by qPCR using specific Taqman assays for miR (Applied Biosystems, USA) and specific primers for miR-146a (Applied Biosystems, primer identification numbers: 000468 for hsa-miR-146a and 001973 for snRU6). MiR-146a levels were quantified with the 2(−ΔΔct) relative quantification method that was normalized to the U6 small nucleolar RNA (snRU6).
In vitro experiments
Murine cardiac myocytes were isolated from murine hearts as described previously (31). Briefly, mouse hearts were isolated and the aorta was cannulated to a perfusion system. The hearts were arrested with a retrograde perfusion of calcium-free buffer and cardiac myocytes were dissociated using a collagenase-based enzymatic solution. Calcium was added into the isolated cardiac myocytes to produce isolated, quiescent, rod-shaped myocytes. The adult cardiomyocytes were cultured in 6 well plates coated with matrigel (BD Biosciences) in MEM supplemented with 1mg/ml BSA, 2mM glutamine, 100u/ml penicillin, 10uM Bleb and 0.1% ITS under 5% CO2 at 37°C. The murine macrophages (J774 cell line) were plated in 6 well plates at 1 × 105 cells/well according to our previous studies (5,13,30). To examine the effect of LPS on miR-146a expression, the cells were treated with and without LPS (0.1 μg/ml. Twelve hours after treatment, the cells were harvested for the examination of miR-146a by qPCR(30,31). To examine the effect of increased expression of miR-146a on LPS induced inflammatory responses, H9C2 cardiomyoblasts were transfected with miR-146a mimics (40 nM) or mimic control with Dy547 (Dharmacon) with Lipofectamine 2000 reagent (Invitrogen) as described previously (30). Six hours after transfection, the medium was replaced with fresh medium supplemented with 10% FBS and antibiotics. Two days after transfection, the cells were treated with and without LPS (1 μg/ml) for 24 hrs. The cells were harvested for analysis of the levels of IRAK1, TRAF6, as well as NF-κB binding activity. The medium were harvested for assessment of TNFα and IL-6. There were 4–6 replicates in each group.
In vivo transfection of lentivirus expressing miR-146a (LmiR-146a) into mouse hearts
Mice were transfected with LmiR-146a or LmiR-control through the right carotid artery as described previously (30,31). Briefly, mice were intubated and anesthetized with mechanical ventilation using 5% isoflurane. The anesthesia was maintained by inhalation of 1.5–2% isoflurane in 100% oxygen. Body temperature was maintained at 37°C by surface water heating. An incision was made in the middle of the neck and the right common carotid artery was carefully exposed. A micro-catheter was introduced into the isolated common carotid artery and positioned into the aortic root. One hundred microliters of LmiR-146a (1×108 PFU) or LmiR-Con was injected through the micro-catheter. The micro-catheter was gently removed and the common carotid artery was tightened before the skin was closed. Seven days after transfection, mice were subjected to CLP (4,5,12,13,27,28).
Tissue accumulation of neutrophils and macrophages
Neutrophil accumulation in heart tissues was examined by staining with naphtol AS-D Chloroacetate Esterase (Sigma-Aldrich, St. Louis, MO) as described previously (5,13,32). Macrophages in the myocardium were examined with the macrophage specific antibody F4/80 (1:50 dilution, Santa Cruz, CA) (5,13). Three slides from each block were evaluated, counterstained with hematoxylin, and examined with brightfield microscopy. Four different areas of each section were evaluated. The results are expressed as the numbers of macrophages/field (40x).
Immunohistochemistry staining
Immunohistochemistry was performed as described previously (5,13). Briefly, heart tissues were immersion-fixed in 4% buffered paraformaldehyde, embedded in paraffin, and cut at 5 um sections. The sections were stained with specific goat anti-ICAM-1 (1:50 dilution, Santa Cruz Biotechnology) and rabbit anti-VCAM-1 (1:50 dilution, Santa Cruz Biotechnology), respectively, and treated with the ABC staining system (Santa Cruz Biotechnology) according to the instructions of the manufacturer. Three slides from each block were evaluated, counterstained with hematoxylin, and examined with brightfield microscopy. Four different areas of each section were evaluated.
Western Blot
Western blot was performed as described previously (5,13,33). Briefly, the cellular proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred onto Hybond ECL membranes (Amersham Pharmacia, Piscataway, NJ). The ECL membranes were incubated with the appropriate primary antibody anti-IRAK1 (sc-7883, Santa Cruz Biotechnology), anti-TRAF6 (sc-7221, Santa Cruz Biotechnology), and anti-IκBα, respectively, followed by incubation with peroxidase-conjugated secondary antibodies (Cell Signaling Technology, Inc.) and analysis by the ECL system (Amersham Pharmacia, Piscataway). The signals were quantified using the G:Box gel imaging system by Syngene (Syngene, USA, Fredrick, MD).
Electrophoretic mobility shift assay (EMSA)
Nuclear proteins were isolated from heart samples as previously described (5,13,33). NF-κB binding activity was performed using a LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific, Waltham, MA) as described previously (4,5,27) in a 20 μl binding reaction mixture containing 1 x binding buffer, 50 ng poly dI:dC, 20 fmol of double stranded NF-κB consensus oligonucleotide which was end-labelled with Biotin, 15μg nuclear proteins. The binding reaction mixture was incubated at room temperature for 20 min and analyzed by electrophoresis, transferred to a nylon membrane. The biotin end-labeled DNA was detected using the Streptavidin-Horseradish peroxidase conjugate and the chemiluminescent substrate (4,5,27).
ELISA for cytokine assay
The levels of cytokines (TNFα, IL-1β and IL-6) were measured by ELISA using OptEIA cytokine kits (BD Biosciences) as described previously (5,13,30).
Statistical analysis
The data are expressed as mean ± SE. Comparisons of data between groups were made using one-way analysis of variance (ANOVA), and Tukey’s procedure for multiple-range tests was performed. The log-rank test was used to compare group survival trends. Probability levels of 0.05 or smaller were used to indicate statistical significance.
Results
Endotoxin increases miR-146a expression in cardiac myocytes and macrophages
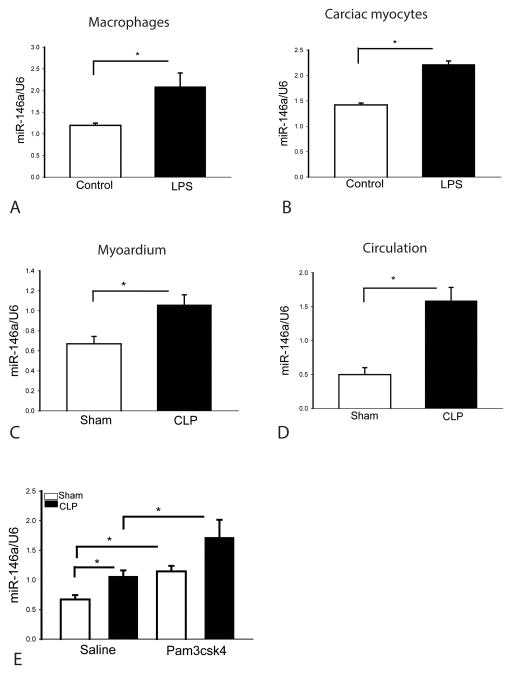
LPS stimulation of monocytes increased the expression of miR146a which has been demonstrated to be a negative regulator of the TLR-mediated NF-κB activation pathway (23,30). We observed that LPS stimulation increased the levels of miR-146a in macrophages compared with the untreated group (Figure 1A). Interestingly, stimulation of adult cardiac myocytes with LPS also increased the expression of miR-146a, when compared with untreated control (B). The data suggests that LPS stimulation up-regulates the expression of miR-146a in both cardiac myocytes and macrophages.
Figure 1. Endotoxin and sepsis increase miR-146a expression in macrophages, cardiac myocytes, heart tissue and serum.
(A) Macrophages (J774) and (B) cardiac myocytes isolated from adult mouse hearts were treated with LPS. Six 6 hours after treatment, the cells were harvested for miR-146a quantitation by qPCR. There were 4–6 replicates in each group. Polymicrobial sepsis increased miR-146a levels in the myocardium (C) and serum (D). Mice were subjected to CLP and sham surgical operation served as sham control. Six hours after CLP, hearts and serum were harvested for assay of miR-146a by qPCR. There were 6 mice/group. (E) Pam3CSK4 treatment increases miR-146a expression in the myocardium. Mice were treated with and without Pam3CSK4 one hour prior to CLP. Sham surgical operation served as sham control. Six hours after CLP, hearts were harvested for miR-146a quantitation by qPCR. There were 4 mice/group. * p<0.05 compared with indicated groups.
Polymicrobial sepsis increases the levels of miR-146a in the myocardium and in circulation
It is unclear whether polymicrobial sepsis will induce the expression of miR-146a. We induced sepsis by CLP and harvested hearts and serum 6 hours after CLP for examination of miR-146a by qPCR. As shown in Figure 1C and D, polymicrobial sepsis increases the expression of miR-146a in the myocardium (C) and circulation (D). The data indicates that miR-146a expression can be induced by polymicrobial sepsis and may serve as an early protective response during sepsis.
Pam3CSK4 treatment increased expression of miR-146a in the myocardium
We have previously reported that treatment of mice with the TLR2 agonist, Pam3CSK4, attenuated cardiac dysfunction in polymicrobial sepsis (12). We examined whether Pam3CSK4 treatment will regulate the expression of miR-146a in the myocardium. Mice were treated with and without Pam3CSK4 one hour prior to CLP. Six hours after CLP, hearts were harvested for analysis of miR-146a levels by qPCR. Polymicrobial sepsis increased the expression of miR-146a in the myocardium. However, Pam3CSK4 treatment significantly increased the levels of miR-146a by 70% in sham control compared with the untreated sham mice (Figure 1E). The levels of miR-146a in Pam3CSK4 treated CLP mice were markedly greater than in untreated CLP mice (Figure 1E). The data indicates that miR-146a expression can be induced by polymicrobial sepsis and may serve as an early protective response during sepsis. Pam3CSK4 administration, in the presence or absence of sepsis, increases myocardial levels of miR-146a, indicating that miR-146a may play a cardioprotective role in cardiac dysfunction in sepsis.
Increased miR-146a expression suppresses LPS-induced inflammatory cytokine production in cardiomyocytes
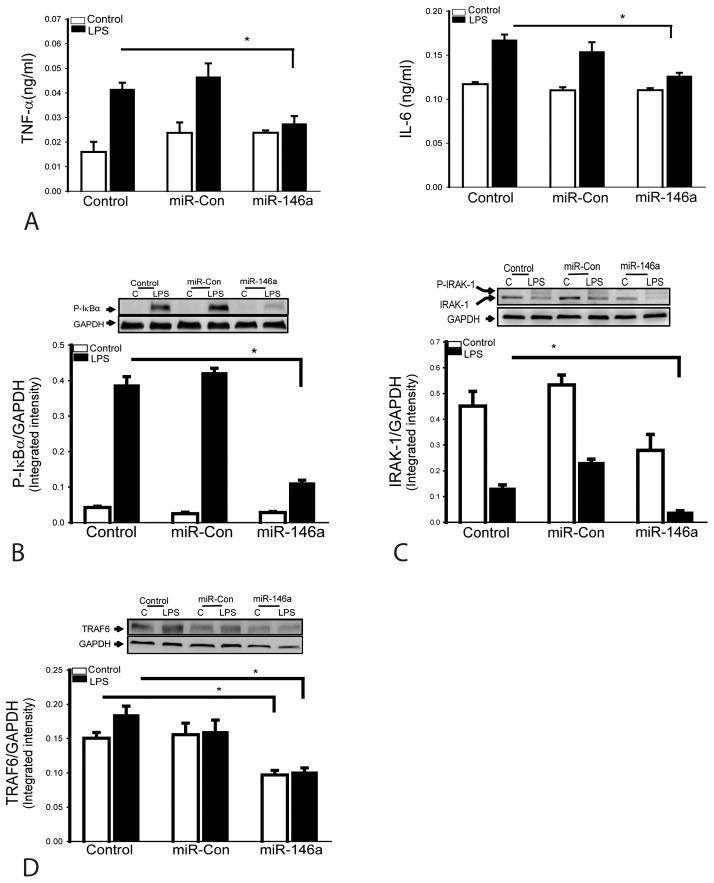
To determine whether increased miR-146a levels will attenuate LPS-induced inflammatory cytokine production in cardiomyocytes, we transfected H9C2 cardiomyocytes with miR-146a mimics before the cells were stimulated with LPS. Scrambled miR mimics served as miR-control. Figure 2 shows that transfection of cardiomyocytes with miR-146a mimics suppressed LPS-induced inflammatory cytokine production (A). Transfection of miR-146a mimics also suppressed LPS-induced IκBα phosphorylation (B), indicating that increased miR-146a levels attenuates LPS-induced NF-κB activity. In addition, transfection of miR-146a mimics suppressed the expression of TRAF6 (C) and IRAK1 (D) which are important components in TLR-mediated NF-κB activation (9,10). These data indicate that miR-146a serves as a negative regulator of endotoxin-induced inflammatory cytokine production in cardiomyocytes.
Figure 2. MiR-146a suppresses LPS-induced inflammatory cytokine production in cardiomyocytes by suppressing NF-κB activation pathway.
H9C2 cardiomyocytes were transfected with miR-146a mimics or miR-scrambled control. Forty-eight hours after transfection, the cells were treated with LPS. Transfection of miR-146a prevents LPS-induced inflammatory cytokine production (A), decreases the levels of IκBα phosphorylation (B), IRAK1 (C) and TRAF6 (D). There were 4 replicates in each group. * p<0.05 compared with indicated groups.
Increased in vivo expression of miR-146a attenuates cardiac dysfunction and improves survival following CLP sepsis
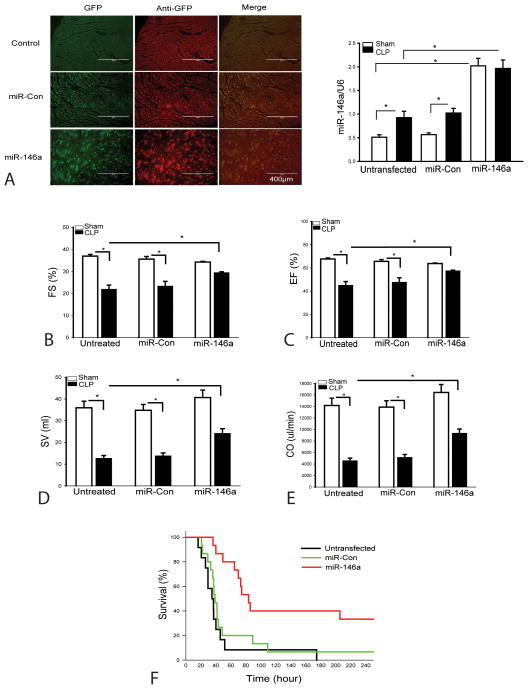
To determine the role of miR-146a in cardiac function in sepsis/septic shock, we constructed a lentiviral vector expressing miR-146a (LmiR-146a) and transfected LmiR-146a into the myocardium via the right carotid artery (30). Lentiviral expressing scrambled miR served as control (LmiR-control). Seven days after transfection, mice were subjected to CLP. Cardiac function was measured 6 hours after CLP. Figure 3A shows that myocardium can be efficiently transfected with LmiR-146a via the right carotid artery as the evidenced by immunohistochemistry staining and qPCR assay. The levels of miR-146a were significantly increased by 2.9 fold in sham control and by 1.4 fold in CLP group, when compared with untransfected respective controls. As shown in Figure 3(B–E), LmiR-146a transfection markedly attenuates sepsis induced cardiac dysfunction. CLP induced significant cardiac dysfunction as evidenced by decreased (B) ejection fraction (EF%, 40.6%), (C) fractional shortening (%FS, 33.4%), (D) stroke volume (SV, 64.7%), and (E) cardiac output (CO, 67.8%) compared with baseline values. In contrast, transfection of LmiR-146a into the myocardium significantly attenuated CLP-induced cardiac dysfunction. Transfection of LmiR-146a increased EF% by 26%, %FS by 20.6%, SV by 74.8% and CO by 81.4% when compared with the untransfected CLP group. LmiR-146a transfection also markedly improves survival rate of CLP sepsis (Figure 3F). In the untransfected CLP group, the mice began to die at 16 h after CLP. Fifty percent of the control septic mice died by 34 h and 100% mortality occurred at 175 h after CLP. However, the septic mice transfected with LmiR-146a did not show the onset of mortality until 40 h after CLP. The median survival time (time to 50% mortality) was 80 h and 33% of the LmiR-146a transfected mice survived for the duration of the study, i.e. 10 days after CLP. Transfection of LmiR-control did not alter CLP-induced cardiac dysfunction and mortality outcome.
Figure 3. Transfection with LmiR-146a attenuates cardiac dysfunction and improves survival in polymicrobial sepsis.
LmiR-146a or LmiR-Con was transfected into the myocardium. Seven days after transfection, mice were subjected to polymicrobial sepsis. Cardiac function was measured by echocardiography 6 h after CLP. (A) Transfection of LmiR-146a into the myocardium and increased the myocardial miR-146a levels. (B–E) LmiR-146a transfection improves cardiac function in CLP septic mice. EF: Ejection fraction; FS: Fractional shortening; SV: stroke volume; CO: cardiac output. There were 6 mice/group. (F) Transfection of LmiR-146a improves survival outcome. There were 12–15/group. * p<0.05 compared with indicated groups.
Increased cardiac expression of miR-146a attenuates sepsis-induced infiltration of macrophages and neutrophils into the myocardium
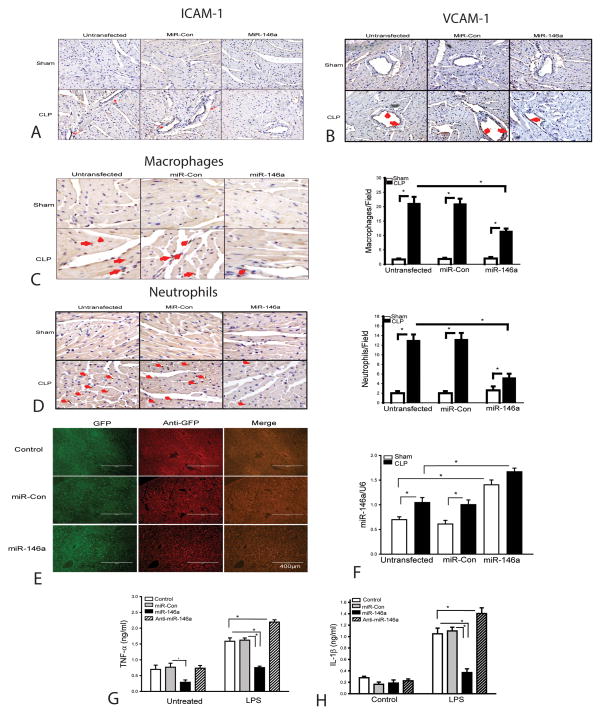
It is well known that the infiltration of macrophages and neutrophils into the myocardium contributes to cardiac dysfunction during the development of sepsis/septic shock (34,35). We have previously reported that macrophage and neutrophil infiltration into the myocardium were markedly increased following CLP(5,13). Increased expression of adhesion molecules, such as VCAM-1 and ICAM-1 plays a critical role in mediating inflammatory cell infiltration into the tissues (34–36). We examined the effect of miR-146a on sepsis-induced expression of adhesion molecules and infiltration of neutrophils and macrophages into the myocardium. As shown in Figure 4A and B, there is more positive staining of ICAM and VCAM1 in the septic myocardium, when compared with sham control. Figure 4C shows that sepsis markedly increased macrophage accumulation in the myocardium by 11.5 fold when compared with sham control. Sepsis also induced neutrophil infiltration into the myocardium. As shown in Figure 4D, positive staining of neutrophils in the myocardium of septic mice was significantly increased by 5.5 fold compared with sham control. However, transfection of LmiR-146a attenuated sepsis-stimulated expression of myocardial ICAM-1 and VCAM1 (Figure 4A and B), significantly decreased the infiltration of macrophages by 45.7% (C) and neutrophils by 60.0% (D) into the septic myocardium, when compared with untransfected CLP mice. Transfection of LmiR-control did not affect sepsis-induced expression of ICAM1 or VCAM1, or infiltration of macrophages and neutrophils into the myocardium.
Figure 4. Transfection of LmiR-146a attenuates sepsis-induced macrophage and neutrophil infiltration into the myocardium and prevents macrophage inflammatory response.
LmiR-146a or LmiR-Con was transfected into the myocardium. Seven days after transfection, mice were subjected to polymicrobial sepsis. Hearts were harvested and sectioned for immunohistochemical staining of ICAM-1 (A) and VCAM-1 (B), macrophages (C), neutrophils (D). The bar graphs show the numbers of infiltrating macrophages (C) and neutrophils (D) in five heart fields. N=5/group. (E, F) LmiR-146a transfection increases the levels of miR-146a in the liver tissue n=4/group. (G, H) transfection of miR-146a mimics prevents LPS-induced inflammatory cytokine production in J774 macrophages. There were 6 replicates/group. * p<0.05 compared with indicated groups.
Hepatic localization of miR-146a following LmiR-146a administration
Figure 3A clearly shows that delivery of LmiR-146a via the right carotid artery can efficiently transfect the myocardium. However, we could not exclude the possibility that the lentiviral vector could travel via the circulation and transfect cells in the peripheral organs. To address this issue, we analyzed the miR-146a levels in liver tissue before and after LmiR-146a transfection. As shown in Figure 4E, immunohistochemistry staining indicates that the liver was transfected with LmiR-146a. LmiR-146a transfection increased the levels of miR-146a by 100% in sham control and by 67% in CLP mice, when compared with untransfected respective controls (F). In addition, the GFP positive cells appear to be projecting into the hepatic sinusoidal lumen and they appear to be consistent with Kupffer cell localization (Figure 4E). Thus, we conclude that administration of LmiR-146a can result in transfection of macrophages in the liver and perhaps other organs.
Transfection of macrophages with miR-146a attenuates pro-inflammatory responses
The data shown in Figure 4E indicate that transfection with LmiR-146a can result in Kupffer cell uptake and expression of miR-146a. This suggests that in the periphery LmiR-146a may be uptaken by macrophages which play a key role in inflammatory responses and the response to septic disease (34,35). This raised the question of whether macrophage transfection with miR-146a altered macrophage inflammatory responses. To address this question, we examined the effect of transfection of miR-146a mimics on LPS-induced inflammatory response in macrophages (J774). Transfection of miR-146a mimics prevents LPS-induced inflammatory cytokine production (Figure 4G and H). The data suggests that suppression of macrophage inflammatory response may be on important mechanism by which transfection of LmiR-146a attenuates sepsis-induce cardiac dysfunction.
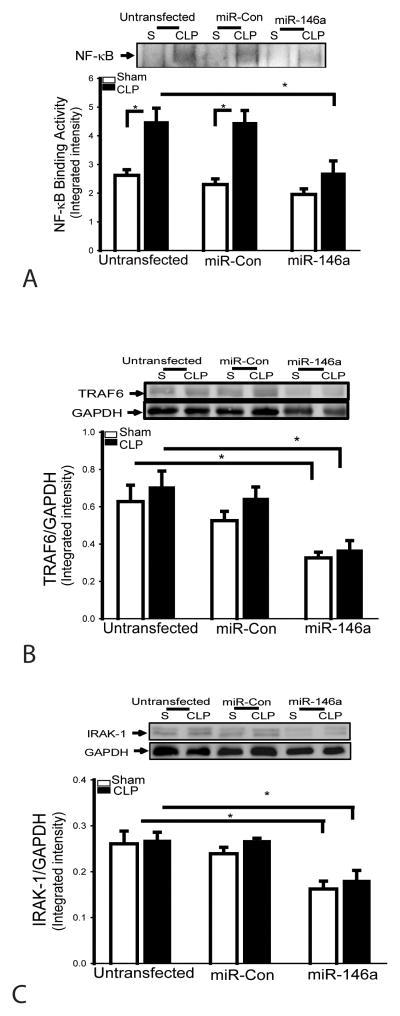
Transfection of LmiR-146a prevents sepsis-induced myocardial NF-κB binding activity and suppresses TRAF6 and IRAK1 expression
NF-κB is an important transcriptional factor that regulates the expression of inflammatory cytokines and adhesion molecules during sepsis/septic shock (9,10). We examined the effect of miR-146a on NF-κB binding activity in the myocardium in CLP septic mice. Figure 5A shows that myocardial NF-κB binding activity in CLP septic mice was significantly increased by 71.2% compared with sham control. However, LmiR-146a transfection prevented sepsis-induced NF-κB binding activity in the myocardium. TRAF6 and IRAK1 are important components in TLR/IL-R mediated NF-κB activation pathways (9,10). We then examined the effect of miR-146a on the expression of TRAF6 and IRAK1 in the presence and absence of CLP-induced sepsis. Figures 5B and C show that the levels of TRAF6 and IRAK1 in LmiR-146a transfected hearts were significantly decreased by 48.1% and 37.8%, respectively, compared with untransfected mice. Transfection of LmiR-control did not alter sepsis-induced myocardial NF-κB binding activity and the levels of TRAF6 and IRAK1 in the myocardium.
Figure 5. Increased expression of LmiR-146a prevents sepsis-induced NF-κB binding activity and suppresses the expression of TRAF6 and IRAK in the myocardium.
LmiR-146a or LmiR-Con was transfected into the myocardium. Seven days after transfection, mice were subjected to polymicrobial sepsis. LmiR-146a transfection prevents sepsis-induced NF-κB binding activity (A), suppresses TRAF6 (B) and IRAK1 expression (C). n=5–6/group. * p<0.05 compared with indicated groups.
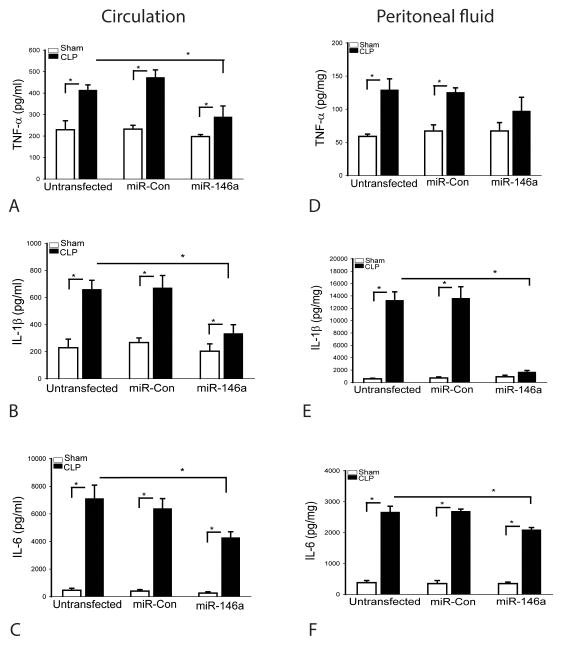
In vivo transfection of LmiR-146a prevents sepsis-induced production of inflammatory cytokines
Inflammatory cytokines, such as IL-1β and TNFα, as well as IL-6 play an important role in the inhibition of cardiac contractility (34). We examined the effect of increased expression of miR-146a on inflammatory cytokine production following CLP. Figures 6(A–C) show that sepsis induced by CLP markedly increased the circulating levels of TNFα (A) by 0.8 fold, IL-1β (B) by 1.9 fold and IL-6 (C) by 14.4 fold, when comparison with sham control. The levels of TNFα (D), IL-1β (E) and IL-6 (F) in peritoneal fluid were also increased by 1.2 fold, by 22.2 fold, and 6.0 fold, respectively, compared with sham control. In contrast, transfection of LmiR-146a significantly attenuates TNFα, IL-1β and IL-6 production in serum and IL-1β and IL-6 in the peritoneal fluid. Transfection of LmiR-control did not alter sepsis-induced increases in the production of inflammatory cytokines in the peritoneal fluid and plasma.
Figure 6. Transfection of LmiR-146a attenuates sepsis-induced inflammatory cytokine production in the circulation and peritoneal cavity.
Seven days after transfection of LmiR-146a or LmiR-con, mice were subjected to polymicrobial sepsis. Serum and peritoneal cavity fluids were harvested for examination of inflammatory cytokine production. Increased expression of miR-146a attenuates sepsis induced TNFα (A), IL-1β (B), and IL-6 (C) production in serum and in peritoneal fluid (E–F). n=5–6/group. * p<0.05 compared with indicated groups.
Discussion
The present study demonstrated that increased expression of miR-146a significantly attenuates cardiac dysfunction, decreases pro-inflammatory responses and improves survival outcome in polymicrobial sepsis. To the best of our knowledge, this is the first report that in vivo transfection of LmiR-146a significantly attenuates cardiac dysfunction, suppresses the pro-inflammatory phenotype and improves survival in CLP-induced sepsis/septic shock. We demonstrated that the mechanisms by which miR-146a protects the myocardium from sepsis-induced cardiac dysfunction involve inhibition of inflammatory responses in both cardiomyocytes and macrophages via inhibition of NF-κB binding activity by targeting IRAK1 and TRAF6. Transfection of LmiR-146a also reduces the infiltration of inflammatory cells into the myocardium following CLP-induced sepsis/septic shock. When taken together, these data indicate that in vivo transfection with miR-146a can have both local (organ) and systemic effects that contribute to the attenuation of sepsis induced organ injury and septic sequelae.
Innate immune and inflammatory responses mediated by TLRs play an important role in cardiac dysfunction in sepsis/septic shock (4–8). TLR-mediated signaling predominately activates NF-κB(10) which is an important transcription factor directly regulating inflammatory cytokine production and adhesion molecule expression that are involved in cardiac dysfunction in sepsis/septic shock (4,5,11–13). Therefore, suppression of NF-κB activation may be an important approach for the attenuation of cardiac dysfunction in sepsis/septic shock.
MicroRNA-146a has been demonstrated to be a negative regulator of the NF-κB activation pathway (23,30). Taganov et al reported that stimulation of THP-1 cells with LPS and cytokines significantly increased the expression of mature miR-146a (23). These authors found that there are several NF-κB binding sites in the promoter of miR-146a, suggesting that LPS and/or cytokine induced miR-146a expression is NF-κB dependent (23,37). We have observed that LPS stimulation significantly increased the expression of miR-146a in both cardiomyocytes and macrophages. Similarly, the levels of miR-146a were also markedly increased in the myocardium and serum following CLP-induced sepsis/septic shock. The data is consistent with the report by Taganov et al that activation of NF-κB regulates miR-146a expression (23). However, we have demonstrated that transfection of miR-146a mimics into H9C2 cardiomyocytes suppresses IRAK1 and TRAF6, resulting in decreasing IκBα phosphorylation and NF-κB binding activity as well as inflammatory cytokine production following LPS stimulation. Collectively, the data suggests that miR-146a is a negative regulator in TLR/NFκB mediated inflammatory responses in both cardiomyocytes and macrophages (23,30,37).
We have previously reported that TLR-mediated NF-κB activation pathway contributes to polymicrobial sepsis (4,27) and sepsis-induced cardiac dysfunction (5,12). To investigate whether increased expression of miR-146a will attenuate cardiac dysfunction in sepsis, we delivered lentivirus expressing miR-146a (LmiR-146a) into the myocardium through the right carotid artery seven days before induction of CLP-sepsis. We observed that increased myocardial expression of miR-146a significantly attenuates CLP sepsis-induced cardiac dysfunction. LmiR-146a transfection also improves survival outcome following CLP-induced sepsis/septic shock. We observed that the myocardium was efficiently transfected with LmiR-146a when the LmiR-146a was delivered through the right carotid artery. This suggests that miR-146 may target the TLR-mediated NF-κB pathway, leading to reduced inflammatory cytokine production in cardiac myocytes. Indeed, our in vitro data shows increased expression of miR-146a in cardiomyocytes suppresses the expression of IRAK1 and TRAF6 and prevents LPS-induced activation of NF-κB and inflammatory cytokine production. In vivo data also demonstrated that transfection of LmiR-146a into the myocardium prevents sepsis induced myocardial NF-κB binding activity and attenuates sepsis induced increases in the levels of TNFα, IL-1β and IL-6 in the serum and peritoneal cavity. More importantly, increased expression of miR-146a suppresses IRAK and TRAF6 in the myocardium. Thomas et al reported that LPS administration induces IRAK1 activation in hearts (38). Hearts isolated from IRAK1 deficient mice showed resistance to LPS-induced contractile dysfunction and attenuation of LPS-induced activation of NF-κB pathway (38). Collectively, the data suggest that IRAK1 plays a role in cardiac dysfunction in sepsis and that it may be a potential target for cardioprotection in sepsis/septic shock.
TRAF6 plays a crucial role in the induction of inflammatory responses via activation of IKK, leading to NF-κB activation (9,10). TRAF6 is utilized by TLR/IL-1R to activate NF-κB and MAPK signaling pathways (9,10). TRAF6 also contains a RING domain that confers E3 ligase activity (39) which induces TRAF6 autoubiquitination via catalyzation of lysine-63 (K63) polyubiquitination. K63 polyubiquitination activates IKK and MAPKs, as well as in RIG-I signal transduction at multiple steps (39). Therefore, suppression of TRAF6 will significantly down-regulate inflammatory responses mediated by NF-κB and MAPK signaling pathways. We have observed that the levels of TRAF6 in the myocardium were significantly reduced following transfection of LmiR-146a, which may be an important mechanism by which LmiR-146 transfection attenuates cardiac dysfunction in sepsis/septic shock.
Infiltration of macrophages and neutrophils into the myocardium contributes to cardiac dysfunction in sepsis/septic shock (34,35). Activated macrophages also release chemokines such as MCP-1 and KC, which attract neutrophils into the myocardium (36). Infiltrated macrophages and neutrophils release inflammatory cytokines, including TNF-α and IL-1β which are the important suppressors of cardiac function (34). In addition, macrophages release macrophage inhibitory factor (MIF) which contributes to cardiac dysfunction and correlates with sepsis severity (40). In the present study, we observed that polymicrobial sepsis resulted in significantly increased numbers of macrophages and neutrophils in the myocardium. In contrast, macrophage and neutrophil infiltration into the myocardium was markedly attenuated in the mice transfected with LmiR-146a. To examine whether macrophages, peripheral to the heart, can be transfected by administration of LmiR-146a, we examined liver tissue and observed that LmiR-146a is delivered into the liver. More specifically, we observed miR-146a in what appear to be hepatic sinusoid macrophages, i.e. Kuppfer cells. In vitro data showed that transfection of macrophages with miR-146a mimics prevents LPS-induced inflammatory cytokine production. Thus, we conclude that administration of LmiR-146a results in myocardial transfection as well as macrophages in major organs, such as the liver. These data also suggest that the mechanisms of miR-146a attenuation in sepsis may be both local and systemic. This is an important finding because it suggests that systemic administration of LmiR-146a may be a viable approach for the management of septic disease.
It is well known that increased expression of adhesion molecules, such as ICAM-1 and VCAM-1 plays a critical role in recruitment of macrophages and neutrophils into the myocardium in sepsis (34–36) and is associated with myocardial dysfunction induced by LPS(41). We observed that transfection of LmiR-146a into the myocardium attenuates sepsis induced increases in the expression of adhesion molecules in the myocardium, suggesting that increased expression of miR-146a in the myocardium suppresses sepsis-induced adhesion molecule expression, resulting in attenuation of the infiltration of macrophages and neutrophils into the myocardium.
In summary, the present study demonstrated that miR-146a plays a protective role in sepsis induced cardiac dysfunction and survival outcome. The mechanisms involve suppression of sepsis induced activation of the NF-κB pathway in both cardiomyocytes and macrophages and decreases in inflammatory cell infiltration into the myocardium. Increased miR-146a expression may be an effective approach for prevention and/or treatment of sepsis induced cardiac dysfunction.
Acknowledgments
Funding:
This work was supported, in part, by NIH HL071837, GM083016, GM53522, GM093878 and C06RR0306551.
Footnotes
Disclosure: None
Reference List
- 1.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gille-Johnson P, Smedman C, Gudmundsdotter L, Somell A, Nihlmark K, Paulie S, Andersson J, Gardlund B. Circulating Monocytes are Not the Major Source of Plasma Cytokines in Patients with Sepsis. Shock. 2012;38:577–583. doi: 10.1097/SHK.0b013e3182746e52. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes CJ, Jr, de Assuncao MSC. Myocardial Dysfunction in Sepsis: A Large, Unsolved Puzzle. Critical Care Research and Practice. 2012 doi: 10.1155/2012/896430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams DL, Ha T, Li C, Kalbfleisch JH, Schweitzer J, Vogt W, Browder IW. Modulation of tissue toll-like receptor 2 and 4 during the early phases of polymicrobial sepsis correlates with mortality. Crit Care Med. 2003;31:1808–1818. doi: 10.1097/01.CCM.0000069343.27691.F3. [DOI] [PubMed] [Google Scholar]
- 5.Gao M, Ha T, Zhang X, Liu L, Wang X, Kelley J, Singh K, Kao R, Gao X, Williams D, Li C. Toll-like receptor 3 plays a central role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2012;40:2390–2399. doi: 10.1097/CCM.0b013e3182535aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avlas O, Fallach R, Shainberg A, Porat E, Hochhauser E. Toll-like receptor 4 stimulation initiates an inflammatory response that decreases cardiomyocyte contractility. Antioxid Redox Signal. 2011;15:1895–1909. doi: 10.1089/ars.2010.3728. [DOI] [PubMed] [Google Scholar]
- 7.Feng Y, Zou L, Zhang M, Li Y, Chen C, Chao W. MyD88 and Trif signaling play distinct roles in cardiac dysfunction and mortality during endotoxin shock and polymicrobial sepsis. Anesthesiology. 2011;115:555–567. doi: 10.1097/ALN.0b013e31822a22f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou L, Feng Y, Chen YJ, Si R, Shen S, Zhou Q, Ichinose F, Scherrer-Crosbie M, Chao W. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2010;38:1335–1342. doi: 10.1097/CCM.0b013e3181d99e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R, Preston-Hurlburt PC, Jr, Janeway A. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 10.Zhang G, Ghosh S. Toll-like receptor-mediated NF-κB activation: a phylogenetically conserved paradigm in innate immunity. The Journal of Clinical Investigation. 2001;107:13–19. doi: 10.1172/JCI11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams DL, Ha T, Li C, Kalbfleisch JH, Laffan JJ, Ferguson DA. Inhibiting early activation of tissue nuclear factor-κB and nuclear factor interleukin 6 with (1-->3)-β-D-glucan increases long-term survival in polymicrobial sepsis. Surgery. 1999;126:54–65. doi: 10.1067/msy.1999.99058. [DOI] [PubMed] [Google Scholar]
- 12.Ha T, Lu C, Liu L, Hua F, Hu Y, Kelley J, Singh K, Kao RL, Kalbfleisch J, Williams DL, Gao X, Li C. TLR2 ligands attenuate cardiac dysfunction in polymicrobial sepsis via a phosphoinositide-3-kinase dependent mechanism. Am J Physiol Heart Circ Physiol. 2010;298:H984–H991. doi: 10.1152/ajpheart.01109.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao M, Ha T, Zhang X, Wang X, Liu L, Kalbfleisch J, Singh K, Williams D, Li C. The Toll-like Receptor 9 Ligand, CpG-Oligodeoxynucleotide, Attenuates Cardiac Dysfunction in Polymicrobial Sepsis, Involving Activation of Both Phosphoinositide 3 Kinase/AKT and and Extracellular-Signal-Related Signaling. J Infect Dis. 2013;207:1471–1479. doi: 10.1093/infdis/jit036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheedy FJ, O’Neill LAJ. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis. 2008;67:iii50–iii55. doi: 10.1136/ard.2008.100289. [DOI] [PubMed] [Google Scholar]
- 15.Taganov KD, Boldin MP, Baltimore D. MicroRNAs and Immunity: Tiny Players in a Big Field. Immunity. 2007;26:133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: Novel players in the regulation of normal immune function and inflammation. Seminars in Cancer Biology. 2008;18:131–140. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Quinn SR, O’Neill LA. A trio of microRNAs that control Toll-like receptor signalling. Int Immunol. 2011;23:421–425. doi: 10.1093/intimm/dxr034. [DOI] [PubMed] [Google Scholar]
- 18.O’Neill LA, Sheedy FJ, McCoy CE. MicroRNAs: the fine-tuners of Toll-like receptor signalling. Nature Reviews Immunology. 2011;11:163–175. doi: 10.1038/nri2957. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nature Reviews Immunology. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 20.van Rooij E, Marshall WS, Olson EN. Toward MicroRNA-Based Therapeutics for Heart Disease: The Sense in Antisense. Circ Res. 2008;103:919–928. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroen B, Heymans S. MicroRNAs and Beyond: The Heart Reveals Its Treasures. Hypertension. 2009;54:1189–1194. doi: 10.1161/HYPERTENSIONAHA.109.133942. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Registry MICU, Blackwell TS, Baron RM, Feinberg MW. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. The Journal of Clinical Investigation. 2012;122:1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. PNAS. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Chen X-P, Li Y-J. MicroRNA-146a and Human Disease. Scand J Immunol. 2010;71:227–231. doi: 10.1111/j.1365-3083.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 25.Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011;8:388–403. doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Gazzar M, Church A, Liu T, McCall CE. MicroRNA-146a regulates both transcription silencing and translation disruption of TNF-α during TLR4-induced gene reprogramming. J Leukoc Biol. 2011;90:509–519. doi: 10.1189/jlb.0211074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-Kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 28.Ha T, Hua F, Grant D, Xia Y, Ma J, Gao X, Kelley J, Williams DL, Kablfleisch J, Browder IW, Li C. Glucan phosphate attenuates cardiac dysfunction and inhibits cardiac MIF expression and apoptosis in septic mice. Am J Physiol Heart Circ Physiol. 2006;291:H1910–H1918. doi: 10.1152/ajpheart.01264.2005. [DOI] [PubMed] [Google Scholar]
- 29.Collino F, Derigibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles Derived from Adult Human Bone Marrow and Tissue Specific Mesenchymal Stem Cells Shuttle Selected Pattern of miRNAs. PLoS ONE. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Ha T, Liu L, Zou J, Zhang X, Kalbfleisch J, Gao X, Williams D, Li C. Increased expression of microRNA-164a decreases myocardial ischemia/reperfusion injury. Cardiovascular Research. 2013;97:432–442. doi: 10.1093/cvr/cvs356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Ha T, Zou J, Ren D, Liu L, Zhang X, Kalbfleisch J, Gao X, Williams D, Li C. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102:385–395. doi: 10.1093/cvr/cvu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Z, Hu Y, Wu W, Ha T, Kelley J, Deng C, Chen Q, Li C, Li J, Li Y. The TIR/BB-loop mimetic AS-1 protects the myocardium from ischaemia/reperfusion injury. Cardiovascular Research. 2009;84:442–451. doi: 10.1093/cvr/cvp234. [DOI] [PubMed] [Google Scholar]
- 33.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against Myocardial Ischemia/Reperfusion Injury in TLR4 Deficient Mice is Mediated through a Phosphoinositide 3-Kinase Dependent Mechanism. J Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 34.Alves-Filho JC, de Freitas A, Spiller F, Souto FO, Cunha CQ. The role of neutrophils in severe sepsis. Shock. 2008;30:3–9. doi: 10.1097/SHK.0b013e3181818466. [DOI] [PubMed] [Google Scholar]
- 35.Cavaillon J-M, Adib-Conquy M. Monocytes/macrophages and sepsis. Crit Care Med. 2005;33:S506–S509. doi: 10.1097/01.ccm.0000185502.21012.37. [DOI] [PubMed] [Google Scholar]
- 36.Raeburn CD, Calkins CM, Zimmerman MA, Song Y, Ao L, Banerjee A, Harken AH, Meng X. ICAM-1 and VCAM-1 mediate endotoxemic myocardial dysfunction independent of neutrophil accumulation. Am J Physiol Regulatory Integrative Comp Physiol. 2002;283:R477–R486. doi: 10.1152/ajpregu.00034.2002. [DOI] [PubMed] [Google Scholar]
- 37.Zhao JL, Rao D, Boldin MP, Taganov KD, O’Connell RM, Baltimore D. NF-κB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. PNAS. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas JA, Haudek SB, Koroglu T, Tsen MF, Bryant DD, White DJ, Kusewitt DF, Horton JW, Giroir BP. IRAK1 deletion disrupts cardiac Toll/IL-1 signaling and protect against contractile dysfunction. Am J Physiol Heart Circ Physiol. 2003;285:H597–H606. doi: 10.1152/ajpheart.0655.2001. [DOI] [PubMed] [Google Scholar]
- 39.Chen ZJ. Ubiquitination in signaling to and activation if IKK. Immunological Reviews. 2012;246:95–106. doi: 10.1111/j.1600-065X.2012.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nature Reviews Immunology. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]