Abstract
Dopamine (DA) replacement therapy with L-DOPA continues to be the primary treatment of Parkinson's disease; however, long-term therapy is accompanied by L-DOPA-induced dyskinesias (LID). Several experimental and clinical studies have established that Propranolol, a β-adrenergic receptor antagonist, reduces LID without affecting L-DOPA's efficacy. However, the exact mechanisms underlying these effects remain to be elucidated. The aim of the current study was to evaluate the anti-dyskinetic profile of Propranolol against a panel of DA replacement strategies, as well as elucidate the underlying neurochemical mechanisms. Results indicated that Propranolol, in a dose-dependent manner, reduced LID, without affecting motor performance. Propranolol failed to alter dyskinesia produced by the D1 receptor agonist, SKF81297 (0.08 mg/kg, sc), or the D2 receptor agonist, Quinpirole (0.05 mg/kg, sc). These findings suggested a presynaptic mechanism for Propranolol's anti-dyskinetic effects, possibly through modulating L-DOPA-mediated DA efflux. To evaluate this possibility, microdialysis studies were carried out in the DA-lesioned striatum of dyskinetic rats and results indicated that co-administration of Propranolol (20 mg/kg, ip) was able to attenuate L-DOPA- (6 mg/kg, sc) induced DA efflux. Therefore, Propranolol's anti-dyskinetic properties appear to be mediated via attenuation of L-DOPA-induced extraphysiological efflux of DA.
Keywords: Parkinson's disease, Propranolol, L-DOPA, Dyskinesia, Norepinephrine, Microdialysis
Introduction
Parkinson's disease (PD), a progressive neurodegenerative disorder, is characterized by the preferential loss of nigrostriatal dopamine (DA) neurons (Hornykiewicz, 2006). The classic symptoms of PD are the motor deficits typified by rigidity, postural imbalance, resting tremor, and akinesia. DA replacement therapy with L-DOPA continues to be the primary choice for symptomatic treatment in PD; however, chronic treatment usually leads to the manifestation of abnormal involuntary movements (AIMs), also known as L-DOPA-induced dyskinesia (LID; Ahlskog and Muenter, 2001).
The phenomenon of LID appears to be a result of supraphysiological striatal DA levels (Arai et al., 2008; de la Fuente-Fernandez et al., 2004; Pavese et al., 2006) and aberrant DA receptor signaling after L-DOPA administration (Cenci and Lindgren, 2007). Although accumulating evidence has validated the targeting of the serotonin (5-HT) system as a way to regulate release of L-DOPA-derived DA and LID (Carta et al., 2007; Eskow et al., 2009; Goetz et al., 2007; Kannari et al., 2002), emerging evidence also suggests that the noradrenaline (NA) system contributes to L-DOPA-derived DA release and uptake (Arai et al., 2008; Chotibut et al., 2014). Neuroanatomical lesioning of the adrenergic system produces either an attenuation (Barnum et al., 2012) or an exacerbation (Fulceri et al., 2007; Miguelez et al., 2011; Shin et al., 2014) of LID, indicating a role for the NA system in the induction of LID. Multiple studies have looked at modulation of adrenergic receptors in the treatment of LID, but the primary focus so far has been on the α-adrenergic receptors (Buck et al., 2010; Rommelfanger and Weinshenker, 2007; Savola et al., 2003), with α-adrenergic antagonists Idazoxan (Rascol et al., 2001) and Fipamezole (Lewitt et al., 2012) being moderately effective at the clinical level.
Importantly, a role for β-adrenergic receptors (βAR) against LID is supported by both pharmacological studies and neuroanatomical evidence. The β1 and β2 adrenergic (β1AR and β1AR, respectively) receptors are widely expressed in the striatum (Rainbow et al., 1984) and are relatively intact in PD patients (Waeber et al., 1991), making them a viable therapeutic target against LID. In vitro data from striatal slices shows that a population of presynaptic βAR facilitate DA release in a manner that is reversible with Propranolol (Goshima et al., 1991), suggesting a possible therapeutic mechanism. Behaviorally, both clinical and experimental studies have demonstrated the anti-dyskinetic potential of Propranolol, a non-selective β1AR antagonist (Carpentier et al., 1996; Barnum et al., 2012; Lindenbach et al., 2011). Interestingly, Barnum et al. (2012) showed that Propranolol's effects were not dependent upon an intact NA system, suggesting that its anti-LID efficacy is likely mediated via βAR on non-adrenergic neurons. Propranolol may additionally modify LID through post-synaptic modulation of striatal output neurons: recent evidence shows that the β1AR activate key striatal intracellular signaling pathways involved in LID (Hara et al., 2010; Meitzen et al., 2011). These potential effects remain to be established.
With that in mind, the present study utilized the hemi-parkinsonian rat model to test the anti-dyskinetic effects of Propranolol against dyskinesia produced by L-DOPA and the D1 and D2 receptor agonists, SKF81297 and Quinpirole respectively. Additionally, the effects of co-administration of Propranolol on L-DOPA's anti-parkinsionian effects were evaluated using the forepaw adjusting steps (FAS) test. Finally, striatal in vivo microdialysis was employed to evaluate if Propranolol modulated L-DOPA-induced DA efflux.
Materials and Methods
Animals
Adult male Sprague–Dawley rats were used (N=30, 225–250 g upon arrival; Taconic Farms, Hudson, NY, USA). Animals were housed in plastic cages (22 cm high, 45 cm deep and 23 cm wide) and had free access to food (Rodent Diet 5001; Lab Diet, Brentwood, MO, USA) and water. The colony room was maintained on a 12/12 h light/dark cycle (lights on at 0700 h) at a temperature of 22–23 °C. The guidelines of the Institutional Animal Care and Use Committee of Binghamton University and the “Guide for the Care and Use of Laboratory Animals” (8th Ed., National Academies Press, 2011) were followed and maintained throughout the study.
DA lesion and cannulation surgeries
One week after arrival, all rats received unilateral 6-hydroxydopamine hydrobromide (6-OHDA, Sigma, St. Loius, MO, USA) lesions of the left medial forebrain bundle to destroy DA neurons. Desipramine hydrochloride (25 mg/kg, ip; Sigma) was administered 30 min prior to the 6-OHDA injection to protect NA neurons. Rats were anesthetized with inhalant isoflurane (2-3%; Sigma) in oxygen (2.5 L/min) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). The following coordinates relative to bregma were used for the site of injection: AP, −1.8 mm; ML, +2.0 mm; DV, −8.6 mm, with the incisor bar positioned at 5 mm below the interaural line (Paxinos and Watson, 1998). A Hamilton syringe (Reno, NV, USA) attached to a 26-gauge needle was lowered into the target and 6-OHDA (12 μg) dissolved in 0.9% NaCl + 0.1% ascorbic acid, was infused at a rate of 2 μl/min for a total volume of 4 μl. The needle was withdrawn 5 min later. During the same surgery, rats from experiment 3 were implanted with plastic microdialysis guide cannulae (CMA 12 Elite; CMA Microdialysis, Stockholm, Sweden) targeting the dorsal striatum ipsilateral to the lesioned side (AP, +1.2 mm; ML, +2.5 mm; DV, −3.7 mm; relative to bregma; Paxinos and Watson, 1998). Five min pre-surgery and 1 d post-surgery, rats received an injection of buprenorphine hydrochloride (0.03 mg/kg, ip; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA, USA) as analgesic treatment. Rats were monitored for 3 weeks post-surgery in order to ensure full recovery before the beginning of experimental drug treatments.
Treatments and Procedures
Experiment 1. Effect of Propranolol administration on LID and motor performance
All rats in experiment 1 (n = 9) received L-DOPA methyl ester (12 mg/kg, sc; Sigma) + dl-Serine 2-(2,3,4-trihydroxybenzyl) hydrazide hydrochloride (benserazide; 15 mg/kg, sc; Sigma) once daily for 7 d. L-DOPA and benserazide were dissolved in Vehicle (VEH, 0.9% NaCl containing 0.1% ascorbic acid) and administered at a volume of 1.0 ml/kg. This priming regimen with L-DOPA has been demonstrated to produce a maximal and stable response after 1 week of treatment (Bishop et al., 2009; Dupre et al., 2011). Rats were observed for AIMs (see below) on days 1, 5, and 7 of L-DOPA priming. Only rats displaying a cumulative axial, limb and orolingual (ALO) AIMs score, of ≥ 45 on day 7 of priming, derived from measures taken for 1 min every 10 min over 3 h, were maintained for further study (n = 9 of 9, 100%). Importantly, this level of LID is associated with severe striatal DA depletion (>90%; Cenci and Lundblad, 2007; Dupre et al., 2011; Lindenbach et al., 2011; Winkler et al., 2002). Following L-DOPA priming, rats were tested for AIMs every 3-4 d in a within-subjects, counterbalanced design, receiving a pretreatment of VEH (dH2O) or the non-selective βAR antagonist, ±Propranolol (5 or 20 mg/kg, ip; Sigma) 5 min prior to injection of L-DOPA (4 mg/kg, sc) + benserazide (15 mg/kg, sc). Immediately following L-DOPA injections, rats were monitored for AIMs and rotations for 1 min every 10 min over 3 h.
In order to test the effects of Propranolol on L-DOPA's anti-parkinsonian efficacy, the FAS test (see below) was employed. All rats received the following three treatments in a within-subjects counterbalanced design: VEH + VEH, VEH + L-DOPA (4 mg/kg, sc) + benserazide (15 mg/kg, sc) or Propranolol (20 mg/kg, ip) + L-DOPA (4 mg/kg, sc) + benserazide (15 mg/kg, sc). To allow for peak L-DOPA plasma levels, testing began 1 h after L-DOPA injection. FAS testing occurred every 3-4 d following completion of the AIMs portion of experiment 1.
Experiment 2. Effect of Propranolol administration on D1 or D2 receptor-induced dyskinesia
Three weeks after 6-OHDA lesions, all rats in experiment 2 (n=9) received injections of the D1 receptor agonist (+)-SKF-81297 hydrobromide (SKF81297; 0.8 mg/kg, sc; Sigma) on three separate occasions 2-3 d apart to sensitize both D1 and D2 receptors (Dupre et al., 2007; Pollack and Yates, 1999). Contralateral rotations and AIMs were measured immediately after injections. Rats displaying ALO AIMs scores of ≥ 45 by the 3rd day of D1 receptor agonist priming were retained for further study (n = 9 of 9, 100%). Thereafter, rats were tested for AIMs every 3-4 d in a within-subjects design, receiving a pre-treatment of VEH (dH2O) or various doses of Propranolol (5 or 20 mg/kg, ip) 5 min prior to injection of vehicle (10% DMSO and 90% sterile saline), a low dose of the D1 receptor agonist SKF81297 (0.08 mg/kg, sc), or a low dose of the D2 receptor agonist (±)- Quinpirole dihydrochloride (Quinpirole; 0.05 mg/kg, sc; Sigma; Bhide et al., 2013; Dupre et al., 2007). Rats were monitored for AIMs and rotations for 1 min every 10 min over 3 h following the DA agonist injections. At least 48 h after final treatment, rats were rapidly decapitated and dorsal striata were dissected for subsequent analysis of monoamine content in order to quantify degree of DA depletion.
Experiment 3. Effect of Propranolol on extracellular striatal DA levels in LID
Three weeks after 6-OHDA lesions, all rats in experiment 3 (n=12) were primed with L-DOPA as described in experiment 1. Only rats displaying a cumulative ALO AIMs score of ≥ 45 on day 7 of priming were maintained for further study (n=12 of 12, 100%). Microdialysis testing commenced 2 d after the last day of L-DOPA priming. On test day, striatal probes (CMA 12 Elite; membrane length = 3 mm; 20000 Dalton; CMA Microdialysis) were inserted into rats' guide cannulae and locked into place so that the dialysis membrane extended −3.7 to −6.7 ventral to bregma (entirely within the striatum). Artificial cerebral spinal fluid (aCSF; containing, in mM: 128 NaCl, 2.5 KCl, 1.3 CaCl2, 2.1 MgCl2, 0.9 NaH2PO4, 2.0 Na2HPO4, and 1.0 glucose, pH of 7.4) was pushed through the probe at a constant flow rate of 2 μl/min. After 60 min of probe stabilization, striatal dialysate samples were collected every 20 min for 120 min to establish baseline levels. Following this, using a counterbalanced within-subjects design, rats received a pre-treatment of VEH (dH2O) or Propranolol (20 mg/kg, ip) 5 min prior to injection of L-DOPA (6 mg/kg, sc) + benserazide (15 mg/kg, sc). Sample fractions were collected every 20 min for 3 h and ALO AIMs were concurrently observed during this time. Each rat underwent this microdialysis procedure for 2 consecutive days.
Abnormal involuntary movements
Rats were monitored for AIMs using a procedure described in Lindenbach et al. (2011), Cenci et al. (1998) and Eskow et al. (2009). The AIMs model of dyskinesia utilizes distinct behavioral measures and demonstrates face validity with known anti-dyskinetic compounds (Lundblad et al., 2002; Taylor et al., 2005). On test days (between 0900 and 1400 h), rats were individually placed in plastic cylinders (22.2 cm × 24.5 cm) 5 min before pre-treatments. After L-DOPA injection, trained observers (with an inter-rater reliability of at least 95%), blinded to treatment condition, assessed each rat for exhibition of axial, limb, orolingual, and locomotor AIMs. In addition, both contralateral rotations (defined as complete 360° turns away from the lesioned side of the brain) and ipsilateral rotations (defined as complete 360° turns toward the lesioned side of the brain) were tallied and reported as the difference between contralateral and ipsilateral rotations. Dystonic posturing of the torso, involving positioning in a twisted manner directed toward the side of the body contralateral to the lesion, were referred to as “axial” AIMs. “Limb” AIMs were defined as rapid, purposeless movements of the forelimb located on the side of the body contralateral to the lesion. “Orolingual” AIMs were composed of repetitive openings and closings of the jaw and tongue protrusions. The movements were considered abnormal, as they occurred at times when the rats were not chewing or gnawing on food or other objects. Every 10 min for 3 h, rats were observed for an entire min. Rats were rated for AIMs and rotational behavior simultaneously during the entire min. During the AIMs observation periods, a dyskinesia duration-based score of 0–4 was assigned for each AIMs category: 0, not present; 1, present for <50% of the observation period (i.e., 1-29 s); 2, present for >50% or more of the observation period (i.e., 30-59 s); 3, present for the entire observation period (i.e., 60 s) and interrupted by a loud stimulus (a tap on the cylinder); or 4, present for the entire observation period but not interrupted by a loud stimulus.
Forepaw adjusting steps
The effect of DA lesion and subsequent drug treatment on motor ability was assessed using the FAS test, which assesses akinesia, a cardinal symptom of PD; rats with >80% striatal DA depletion show marked motor deficits on this test (Chang et al., 1999; Olsson et al., 1995). Rats were held such that they had only one free forelimb; for each trial, rats were moved laterally across a table at a steady rate of 90 cm/10 s. Each stepping test consisted of six trials for each forepaw, alternating between forehand and backhand movement (for more detail see Eskow et al., 2007). “Percent intact” stepping was derived by summing steps with the lesioned forelimb and dividing them by the sum of steps with the intact forelimb and multiplying by 100. Lower scores indicate a greater motoric impairment.
High performance liquid chromatography
Reverse-phase high-performance liquid chromatography (HPLC) coupled to electrochemical detection was performed based on a protocol by Kilpatrick et al. (1986). For experiment 2, striatal tissue was analyzed for abundance of DA, the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), 5-HT, the 5-HT metabolite 5-hydroxyindolacetic acid (5-HIAA) and NA. Tissue was homogenized in ice-cold perchloric acid (0.1 M) with 1% ethanol and 0.02% EDTA. The homogenates were spun for 45 min at 14,000 g (maintained at 4 °C) and aliquots of supernatant were taken for subsequent analysis. For experiment 3, in order to determine extracellular concentrations of DA and DOPAC, striatal dialysis samples (20 μl each) were collected every 20 min and loaded directly into the column.
The system used equipment manufactured by ESA (currently owned by Thermo Scientific; Chelmsford, MA, USA) and consisted of an autoinjector (Model 542), a solvent delivery system (Model 582), a C-18 column (100×4.6mm, 5 μm packing), a Coulochem III electrochemical detector connected to an analytical cell (model 5011A) located immediately after the column, a Model 5020 guard cell positioned prior to the autoinjector, and an external pulse dampener. Samples were chromatographically separated using a mobile phase composed of 90 mM sodium dihydrogen phosphate (monobasic, anhydrous), 0.05 mM EDTA, 1.7 mM octane sulfonic acid, 10% (v:v) acetonitrile, and adjusted to pH 3.0 with o-phosphoric acid. The guard cell potential was set to +500 mV (for tissue) or +700 mV (for dialysate); two analytical electrodes were used with the first electrode set at -150 mV and the second electrode set at +250 mV. As DA and DOPAC eluted from the column and passed through the analytical cell, they were oxidized at the second electrode and a spike in current was generated. This signal was recorded as a trace of electrode current versus time by EZChrom Elite software (ESA) via a Scientific Software, Inc. (SS420χ) module. Each compound was detected as a peak in this trace, and the EZChrom Elite software was used to analyze chromatographs. Peak areas were converted to picograms using a standard curve (1e-6 M to 1e-9 M). For tissue analysis, values were adjusted to wet tissue weights and expressed as picograms (of monoamine) per milligram (of tissue). For dialysate, values were normalized to injected dialysate volume and expressed as percentage of baseline levels.
Statistical analyses
Main effects of treatment and individual time points for ALO AIMs were analyzed using repeated measures non-parametric Friedman tests and Wilcoxon signed-rank post hocs when appropriate. Analysis of rotations, striatal dialysate and DA turnover ratio were analyzed with 2-way (treatment × time) repeated-measures ANOVAs and Fisher's least significant difference post hoc comparisons when appropriate. One-way repeated-measures ANOVAs followed by Fisher's post hoc tests were employed for analysis of FAS data. The Fisher's post hoc test was used since it allowed for analysis of pair-wise a priori predictions. Striatal tissue monoamine content was analyzed using paired-samples t-tests. Though rare, if microdialysis data points were outside of 2 standard deviations of group means, they were considered outliers, and thus not included in the data analyses. All analyses were performed with Statistica software '98 (Statsoft Inc., Tulsa, OK, USA). Alpha was set at 0.05.
Results
Effect of 6-OHDA on striatal monoamine tissue content
Striatal tissue from animals in experiment 2 (n = 9; off treatment) were analyzed via HPLC to verify 6-OHDA-induced DA depletion and examine potential changes in striatal 5-HT and/or NA levels between the lesioned vs. intact striata. 6-OHDA caused a 99% decrease in striatal DA (intact = 4710 ± 212 pg/mg; lesion = 67 ± 26 pg/mg; t8 = 20.85, p < .001) and 93% decrease in striatal DOPAC (intact = 2574 ± 90 pg/mg; lesion = 179 ± 82 pg/mg; t8 = 21.98, p < .001). Despite desipramine pre-treatment, there was a 50% loss of NA on the lesion side (intact = 34 ± 4 pg/mg; lesion = 17 ± 5 pg/mg; t8 = 5.44, p = .001). There was also a 47% decrease in striatal 5-HT content (intact = 173 ± 17 pg/mg; lesion = 109 ± 6 pg/kg; t8 = 3.60, p = .007), corresponding with a 23% increase in 5-HIAA (intact = 353 ± 13 pg/kg; lesion = 434 ± 29 pg/mg; t8 = 3.01, p = .017).
Effect of Propranolol on L-DOPA-induced ALO AIMs and rotations
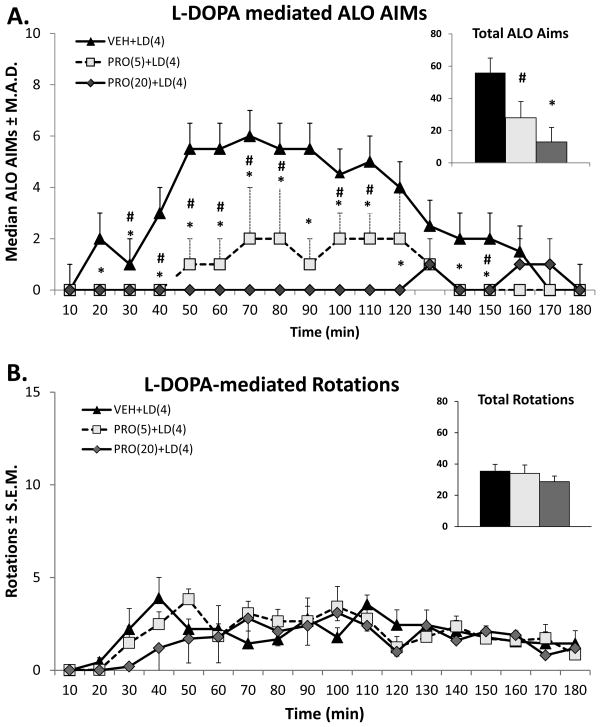
The anti-dyskinetic potential of Propranolol against LID was evaluated at two different doses (5 and 20 mg/kg). As can be observed in Figure 1, Propranolol reduced ALO AIMs, but not rotations, observed after L-DOPA (4 mg/kg) in a dose-dependent manner.
Figure 1.
Effects of Propranolol (PRO) on L-DOPA (LD)-induced A) ALO AIMs and B) rotations. Five min after pretreatments with Vehicle or PRO (5 or 20 mg/kg, ip), rats (n=9) received treatments with LD (4 mg/kg + benserazide 15 mg/kg, sc). Symbols demonstrate median ALO AIMs ± median average deviation (M.A.D.) and mean rotations ± standard error of the mean (S.E.M.) every 10 min over the 3 h sampling period immediately after LD. *p < 0.025 for PRO(20)+LD(4) vs. VEH+LD(4), #p < 0.025 for PRO(5)+LD(4) vs. VEH+LD(4). For the inset graphs in panels A and B, bars denote the median ALO AIMs + M.A.D. and mean rotations + S.E.M. for unilateral 6-OHDA-lesioned rats for the entire 3 h period after LD injection.
An overall main effect of treatment was observed upon analysis of ALO AIMs (χ2 = 31.2, p < 0.001; Figure 1A, inset) and post hoc analysis revealed dose-dependent differences (p < 0.05). Time point analyses with the Wilcoxon signed-rank test revealed that the anti-LID efficacy of the highest dose of Propranolol (20 mg/kg) began at the 20 min time point and was maintained for up to 150 min, with the exception of 130 min time point, after L-DOPA (all p's < 0.025). The lower dose of Propranolol (5 mg/kg) reduced AIMs from 30 to 80 and at 100, 110 and 150 min after L-DOPA injection (p's < 0.025).
Administration of L-DOPA resulted in the protracted expression of contralateral rotations in DA-lesioned animals (Figure 1B). A repeated measures 2-way ANOVA revealed that there was main effect of time (F(17,408) = 6.03, p < 0.001), but no treatment effect (F(2,24) = 0.86, p = 0.44) nor significant interaction between treatment and time (F(34,408) = 1.4, p = 0.06).
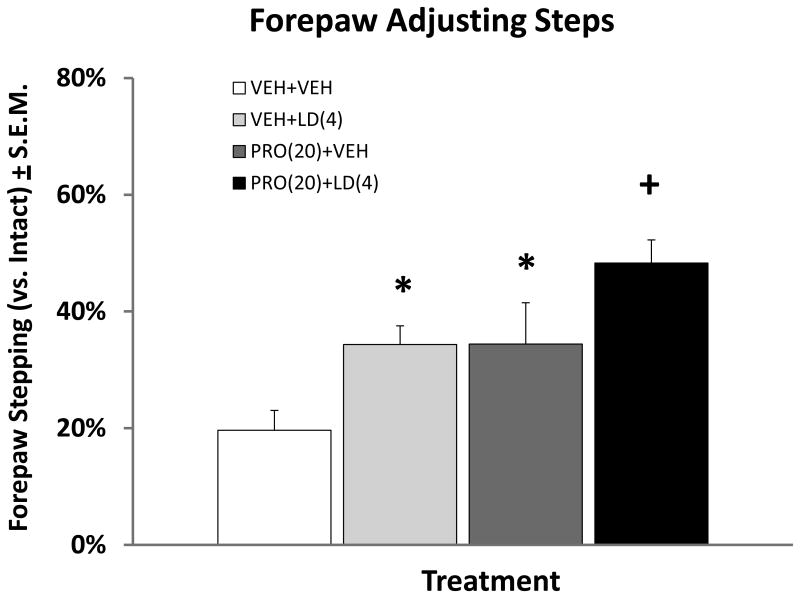
Effect of Propranolol on L-DOPA improvements on forepaw stepping
The FAS test was performed to confirm the anti-parkinsonian effects of L-DOPA on stepping performance and to assess the impact of Propranolol co-administration on L-DOPA efficacy. As seen in Figure 2, main effects of treatment were found (F(3,44) = 8.39; p < 0.001) and post-hoc comparisons between the groups indicated that treatment with Propranolol (20 mg/kg) alone or L-DOPA (4 mg/kg) alone provided equivalent improvements in stepping when compared to VEH-treated controls (p < 0.025). Importantly, co-administration of Propranolol with L-DOPA appeared to provide an additional improvement of motor performance relative to L-DOPA monotherapy (p < 0.05).
Figure 2.
Effects of Propranolol (PRO) and L-DOPA (LD) on forepaw adjusting steps. Five min after pretreatments with Vehicle (VEH) or PRO (20 mg/kg, ip), rats (n=10) received treatments of VEH or LD (4 mg/kg + benserazide 15 mg/kg, sc), leading to four treatment conditions [VEH+VEH, PRO(20)+VEH, VEH+LD(4) and PRO(20)+LD(4)]. Sixty min after VEH or LD, forepaw adjusting steps were counted. Bars denote the average percent stepping in the lesioned forepaw compared to the intact forepaw ± standard error of the mean (S.E.M.) *p < 0.025 when compared with VEH+VEH, + p < 0.05 when compared with all.
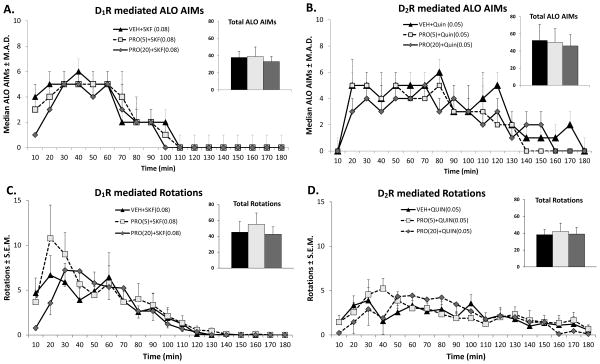
Effect of Propranolol on ALO AIMs and rotations produced by D1 and D2 agonists
In order to evaluate whether Propranolol acts upon DA receptor signaling, we evaluated the anti-dyskinetic efficacy of Propranolol (5 and 20 mg/kg) against direct DA agonists. Previous studies have confirmed that the D1 receptor agonist SKF81297 (0.08 mg/kg) and the D2 receptor agonist Quinpirole (0.05 mg/kg) produce dyskinetic behavior in primed, 6-OHDA lesioned animals (Bhide et al., 2013; Dupre et al., 2007).
As shown in Figures 3A & B, both SKF81297 and Quinpirole produced a robust AIMs expression that lasted for about 100 and 180 min after the administration of each respective direct DA agonist. Propranolol, at both the tested doses, was unable to reverse dyskinesia produced by SKF81297 (χ2 = 1.77, p = 0.41; Figure 3A, inset) or Quinpirole (χ2 = 0.67, p = 0.71; Figure 3B, inset).
Figure 3.
Effects of Propranolol (PRO) on direct DA agonist-induced ALO AIMs (A,C) and rotations (B,D). Five min after pretreatments with Vehicle (VEH) or PRO (5 or 20 mg/kg, ip), rats (n=9) received treatments with either the D1 receptor (D1R) agonist SKF81297 (SKF; 0.08 mg/kg, sc) or the D2 receptor (D2R) agonist Quinpirole (Quin; 0.05mg/kg, sc). Symbols demonstrate median ALO AIMs ± median absolute deviations (M.A.D.) and mean rotations ± standard error of the mean (S.E.M.) every 10 min over the 3 h sampling period immediately after SKF or Quin. For the inset graphs in panels, bars denote the median ALO AIMs + M.A.D. and mean rotations + S.E.M. for the entire 3 h period after SKF or Quin.
Figures 3C & D illustrate that both SKF81297 and Quinpirole produced robust expression of rotations that temporally mirrored the expression of ALO AIMs. Analysis with 2-way repeated-measures ANOVA revealed that there was a time effect for both SKF81297 (F(17,408) = 17.9, p < 0.001) and Quinpirole (F(17,408) = 5.9, p < 0.001), but no effect of Propranolol treatment (F(2,24) = 0.26, p = 0.77 & F(2,24) = 0.65, p = 0.94, respectively). Importantly, there was no significant interaction between treatment and time for both SKF81297 (F(34,408) = 1.15, p = 0.26) and Quinpirole (F(34,408) = 1.23, p = 0.18).
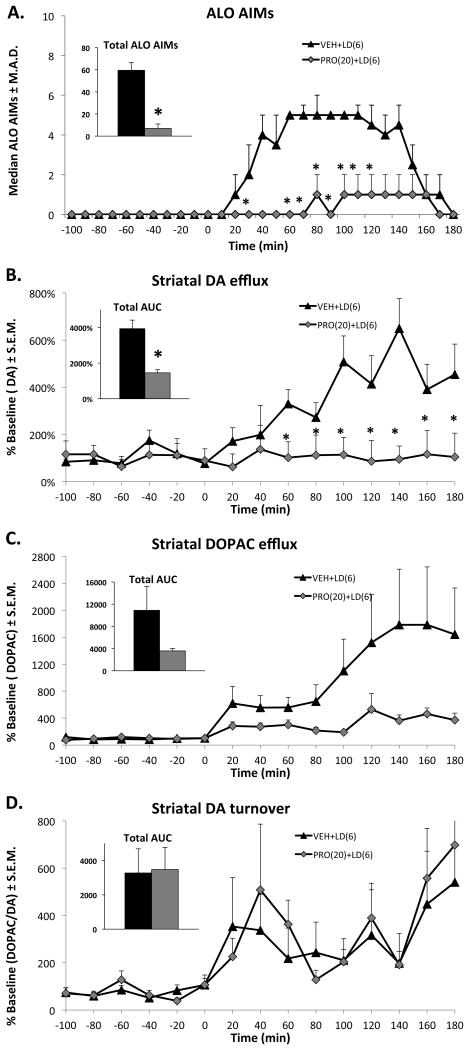
Effect of Propranolol on L-DOPA-induced DA and DOPA efflux in the striatum
Aberrant DA release produced by L-DOPA is implicated in the manifestation of LID (de la Fuente-Fernandez et al., 2004). Propranolol's anti-dyskinetic efficacy against L-DOPA, but not against direct DA agonists, suggested a pre-synaptic mechanism affecting L-DOPA-induced changes in striatal neurochemistry. In order to evaluate the effect of Propranolol (20 mg/kg) on L-DOPA- (6 mg/kg) induced DA efflux, intrastriatal microdialysis coupled with HPLC was performed in 6-OHDA-lesioned animals. In support of experiment 1, L-DOPA was able to produce a robust expression of ALO AIMs in these animals that was attenuated by Propranolol (χ2 = 5.44, p = 0.019; Figure 4A, inset). Time analyses using the Wilcoxon-signed rank test revealed that Propranolol attenuated ALO AIMs at 30 and 60-120 min after L-DOPA administration.
Figure 4.
Effects of Propranolol (PRO) on L-DOPA- (LD) induced A) ALO AIMs, B) Dopamine (DA), C) 3,4-Dihydroxyphenylacetic acid (DOPAC) and D) DA turnover efflux in the DA-lesioned striatum. Five min after pretreatments with VEH or PRO (20 mg/kg, ip), unilateral DA-lesioned rats (n=9) received treatments with the LD (6 mg/kg, sc). Symbols demonstrate median ALO AIMs and mean striatal DA, DOPAC efflux or DA turnover ratios (DOPAC/DA) ± SE.M. every 10 or 20 min over the sampling period. For the inset graphs in panels, bars denote the median + M.A.D or means + S.E.M for the 3 h period after the LD. *p < 0.05 when compared vs. VEH+LD.
Importantly, Propranolol was able to reduce L-DOPA-induced DA efflux in the striatum. Analysis with a 2-way ANOVA revealed a time effect (F(14,154) = 5.9, p < 0.001) and a treatment effect (F(1,14) = 7.6, p < 0.025), as well as a time X treatment interaction (F(14,154) = 5.6, p < 0.001). Post-hoc analysis using the LSD test revealed that Propranolol attenuated L-DOPA-induced DA efflux 60-180 min after L-DOPA administration. For DOPAC, a 2-way repeated measures ANOVA revealed a time effect (F(14,196) = 1.9, p < 0.05), but no significant treatment effect (F(1,14) = 0.86, p = 0.37) or time X treatment interaction (F(14,196) = 0.84, p = 0.62). Propranolol did not have any effect on striatal DA turnover after L-DOPA administration, as statistical analyses revealed no effects of time (F(14,154) = 1.4, p = 0.21), treatment (F(1,14) = 0.6, p = 0.52) or time X treatment (F(14,154) = 0.53, p = 0.91).
Discussion
In the present study, Propranolol dose-dependently attenuated AIMs, but not contralateral rotations induced by acute L-DOPA administration and importantly did not alter L-DOPA's ability to improve stepping in lesioned animals, suggesting that the anti-LID effect is not related to motor suppressant action. Additionally, Propranolol's anti-dyskinetic effects did not extend to dyskinesia produced by direct D1 and D2 receptor agonists, thereby suggesting a pre-synaptic mechanism of action, specifically modulation of L-DOPA-derived DA efflux. This was further confirmed by in vivo microdialysis demonstrating that Propranolol strongly suppressed striatal DA efflux produced by L-DOPA administration.
These findings are a key mechanistic extension of previous studies (Lindenbach et al., 2011) that evaluated the anti-dyskinetic potential of Propranolol against the development and expression of LID. The current study evaluated the effects of Propranolol, across different doses, on the acute expression of ALO AIMs and rotations after L-DOPA administration. The study employed two doses of L-DOPA (4 and 6 mg/kg); the lower dose was chosen with the aim of producing equivalent dyskinesia with the other DA agonist treatments, while the higher dose of L-DOPA was utilized to produce robust changes in striatal DA neurochemistry (Lindgren et al., 2010). Treatment with Propranolol resulted in attenuation of LID at both L-DOPA doses in agreement with previous experimental (Barnum et al., 2012; Buck and Ferger, 2010; Dekundy et al., 2007) and clinical findings (Carpentier et al., 1996). In fact, compared to Lindenbach et al., (2011) and Barnum et al., (2012), Propranolol seemed to be more effective in attenuating LID. Although not directly comparable, methodological differences such as L-DOPA dose and data representation (Mean vs. Median) may have contributed. Importantly, Propranolol did not appear to affect L-DOPA's therapeutic efficacy, as judged from the FAS test, often employed to quantify motor deficits and their reversal in L-DOPA-treated DA-lesioned animals (Chang et al., 1999; Winkler et al., 2002). Admittedly, in the study, we could not entirely rule out motor suppressant effects of higher doses of Propranolol against LID in the first 60 min of treatment or how such effects might have altered L-DOPA's anti-parkinsonian efficacy. Our lab has previously reported that Propranolol at higher doses (20-40 mg/kg) reduced spontaneous activity in motion chambers (Lindenbach et al., 2011). However, it should be noted that in the current study when Propranolol (20 mg/kg) was given with DA agonists, no anti-dyskinetic effects were observed, suggesting that motor suppression was not occurring under those treatment conditions. Thus, Propranolol's anti-dyskinetic properties coupled with its purported anti-parkinsonian benefits, with or without L-DOPA, further support its potential as an adjunct to L-DOPA therapy in the treatment of PD. Interestingly, Propranolol at both the tested doses was not able to reduce contralateral rotations produced by administration of L-DOPA. This observation is of interest considering the debate surrounding the meaning/interpretation of drug induced contralateral rotations. Some studies have suggested that rotations are a proxy of dyskinesia (Cenci and Lundblad, 2007), while others suggest rotations are difficult to interpret since rotational activity can be affected by a change in parkinsonian or dyskinetic status, or could simply be due to a change in DA signaling in the intact (non-lesioned) striatum (Lane et al., 2006). Findings from this study further support the dissociation of contralateral rotations from LID.
Despite the accumulating evidence for Propranolol's beneficial effects in PD, the mechanism(s) by which it conveys its effects remains unexplored. Recent research has suggested the possibility that antagonism at the β1AR could play a role in reducing LID via post-synaptic mechanisms. Specifically, β1AR antagonism reduces phosphorylation of dopamine- and cyclic-AMP-regulated phosphoprotein of 32 kDa (DARPP-32; Hara et al., 2010); these findings are clinically important because high levels of phosphorylated DARPP-32 in medium spiny neurons of the striatum are implicated in the expression of LID (Picconi et al., 2003; Santini et al., 2007). In order to test the hypothesis that Propranolol's anti-dyskinetic effects were produced by modulating post-synaptic DA receptor activity, we employed direct D1 & D2 receptor agonists SKF81297 and Quinpirole, respectively, at doses known to produce AIMs of a magnitude similar to the dose of L-DOPA used in this study (Dupre et al., 2007). Both D1 & D2 receptors play a critical role in the expression of LID and their direct stimulation produces dyskinesia in both experimental and clinical settings (Bhide et al., 2013; Delfino et al., 2004; Dupre et al., 2007; Monville et al., 2005; Rascol et al., 2001).
In the current study, both SKF81297 and Quinpirole produced robust ALO AIMs and contralateral rotations, but these behaviors were not affected by Propranolol administration. This lack of effect against direct DA agonist-induced dyskinesia suggests that Propranolol's behavioral and neurochemical effects against dyskinesia are largely upstream of post-synaptic medium spiny neuron modulation. At the same time, L-DOPA-induced changes in medium spiny neuron opioid transcription that are associated with LID (Cenci et al., 1998) were attenuated by propranolol (Lindenbach et al., 2011). Thus, propranolol modifies post-synaptic signal transduction caused by L-DOPA, but, in light of the present results, it is likely that this post-synaptic outcome is driven by pre-synaptic modulation of striatal DA levels.
Therefore, to elucidate the possible presynaptic mechanism underlying Propranolol's effects we evaluated Propranolol's effects on L-DOPA-induced increases in striatal DA efflux. This was supported by neuroanatomical evidence that β1AR and β1AR, the target for Propranolol, are expressed highly in the striatum (Rainbow et al., 1984) and that microinjection of Propranolol into the striatum reduced LID (Lindenbach et al., 2011). In addition, βAR antagonists are known to modulate striatal DA release in vitro, as Propranolol was able to attenuate L-DOPA-induced DA release from striatal slices of MPTP-treated mice (Goshima et al., 1991) and attenuated DA release produced by the β1/2AR agonist (-) isoproterenol in rat striatal slices (Reisine et al., 1982). Importantly, Propranolol (20 mg/kg) was able to completely attenuate both L-DOPA-induced ALO AIMs and the supraphysiological striatal DA efflux. The attenuation of ALO AIMs followed reductions in striatal DA levels in a temporal manner for the most part; it can be observed during the initial phase that Propranolol administration tends to reduce L-DOPA derived dopamine. It is possible that DA levels have to be above a certain “threshold” to manifest dyskinesia and Propranolol is able to blunt that. Interestingly, the concentrations of DA in animals treated with L-DOPA alone continued to be elevated even after ALO AIMs activity had subsided. Although the temporal effects of Propranolol on striatal DOPAC levels mirror the pattern seen with striatal DA (cf. Figure 4B, C), the omnibus ANOVA revealed no significant effects of Propranolol on DOPAC (and no treatment X time interaction), so time point comparisons were not deemed appropriate. However, the DOPAC/DA ratio did not differ significantly between treatment groups (Figure 4D), suggesting that Propranolol's attenuation of elevated DA concentrations is not due to pharmacokinetic factors, such as altered monoamine oxidase activity. Thus, it seems that Propranolol provides anti-LID effects by tempering L-DOPA-induced DA efflux in the striatum.
Interestingly, recent evidence suggests that augmented NA neurotransmission in the striatum, after acute L-DOPA positively correlates with induction of LID and that modulation of aberrant NA is accompanied by a reduction in L-DOPA-associated behaviors (Buck and Ferger, 2010; Ostock et al., 2014; Wang et al., 2014). Thus, Propanolol may have been acting at NA receptors to directly influence NA cell activity that normally contributes to LID. However, previous work has shown that even when NA neurons are compromised, βAR blockade reduced LID (Barnum et al., 2012), implicating mechanisms orthogonal to NA release.
Thus, although the exact mechanism by which Propranolol reduces LID remains opaque, this study provides further evidence that neurochemical changes in striatum underlie its anti-dyskinetic effects. It is conceivable that Propranolol administration could be reducing central L-DOPA levels, an effect similarly observed with Idazoxan by Buck et al., (2010), leading to lower DA levels and thus reduced LID. A thorough evaluation of central L-DOPA levels following Propranolol administration would shed further light. Another possible mechanism may involve reduced firing of the raphe-striatal serotonergic neurons, whose involvement in LID is well established. (-) Propranolol, at low doses, has been shown to suppress 5-HT neuron firing in the dorsal raphe nucleus (Fornal et al., 1994). Therefore, Propranolol by suppressing 5-HT neuronal activity could reduce efflux of DA derived from 5-HT terminals.
By extension, the present study provides support for the use of βAR agents and perhaps other NA compounds, as adjuncts along with L-DOPA, in the treatment of PD. It is important to note that Propranolol is successfully employed in the clinic to treat essential and PD-related tremor (Fox, 2013). While autonomic problems in PD patients, notably hypotension, could contraindicate the use of Propranolol in some PD patients, Carpentier et al (1996) reported a reasonable therapeutic window that treated LID while concomitantly avoiding side effects of Propranolol. In conclusion, the findings from this study establish that Propranolol provides relief against LID by normalizing the supraphysiological DA efflux caused by L-DOPA. Together with Propranolol's lack of efficacy against dyskinesia produced by DA agonists, this study provides evidence that pre-synaptic striatal βAR are powerful modulators of LID.
Acknowledgments
This work was supported by National Institutes of Health grant R01-NS059600 (to CB) and the Center for Development and Behavioral Neuroscience at Binghamton University.
The authors wish to thank Dr. Corinne Y. Ostock, Dr. Karen L. Eskow Jaunarajs, Dr. Kristin B. Dupre and Thomas Button for assistance with behavioral testing and chromatography.
Abbreviations
- 5-HIAA
5-hydroxyindolacetic acid
- 5-HT
Serotonin
- 6-OHDA
6-hydroxydopamine
- AIMs
Abnormal involuntary movements
- βAR
β-adrenergic receptor
- DA
Dopamine
- DOPAC
3,4-dihydroxyphenylacetic acid
- HPLC
High-performance liquid chromatography
- LID
L-DOPA-induced dyskinesia
- FAS
Forepaw adjusting steps
- NA
Noradrenaline
- PD
Parkinson's disease
- S.E.M
Standard error of the mean
Footnotes
The authors declare that they have no conflicts of interest.
References
- Ahlskog JE, Muenter MD. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord. 2001;16:448–458. doi: 10.1002/mds.1090. [DOI] [PubMed] [Google Scholar]
- Arai A, Tomiyama M, Kannari K, Kimura T, Suzuki C, Watanabe M, Kawarabayashi T, Shen H, Shoji M. Reuptake of L-DOPA-derived extracellular DA in the striatum of a rodent model of Parkinson's disease via norepinephrine transporter. Synapse. 2008;62:632–635. doi: 10.1002/syn.20535. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Bhide N, Lindenbach D, Surrena MA, Goldenberg AA, Tignor S, Klioueva A, Walters H, Bishop C. Effects of noradrenergic denervation on L-DOPA-induced dyskinesia and its treatment by α- and β-adrenergic receptor antagonists in hemiparkinsonian rats. Pharmacol Biochem Behav. 2012;100:607–615. doi: 10.1016/j.pbb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide N, Lindenbach D, Surrena MA, Goldenberg AA, Bishop C, Berger SP, Paquette MA. The effects of BMY-14802 against L-DOPA- and dopamine agonist-induced dyskinesia in the hemiparkinsonian rat. Psychopharmacology. 2013;227:533–544. doi: 10.1007/s00213-013-3001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, Krolewski DM, Eskow KL, Barnum CJ, Dupre KB, Deak T, Walker PD. Contribution of the striatum to the effects of 5-HT1A receptor stimulation in L-DOPA-treated hemiparkinsonian rats. J Neurosci Res. 2009;87:1645–1658. doi: 10.1002/jnr.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, Taylor JL, Kuhn DM, Eskow KL, Park JY, Walker PD. MDMA and fenfluramine reduce L-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. Eur J Neurosci. 2006;23:2669–2676. doi: 10.1111/j.1460-9568.2006.04790.x. [DOI] [PubMed] [Google Scholar]
- Buck K, Ferger B. The selective alpha1 adrenoceptor antagonist HEAT reduces L-DOPA-induced dyskinesia in a rat model of Parkinson's disease. Synapse. 2010;64:117–126. doi: 10.1002/syn.20709. [DOI] [PubMed] [Google Scholar]
- Buck K, Voehringer P, Ferger B. The alpha(2) adrenoceptor antagonist idazoxan alleviates L-DOPA-induced dyskinesia by reduction of striatal dopamine levels: an in vivo microdialysis study in 6-hydroxydopamine-lesioned rats. J Neurochem. 2010;112:444–452. doi: 10.1111/j.1471-4159.2009.06482.x. [DOI] [PubMed] [Google Scholar]
- Carpentier AF, Bonnet AM, Vidailhet M, Agid Y. Improvement of levodopa-induced dyskinesia by propranolol in Parkinson's disease. Neurology. 1996;46:1548–1551. doi: 10.1212/wnl.46.6.1548. [DOI] [PubMed] [Google Scholar]
- Carta AR, Frau L, Lucia F, Pinna A, Annalisa P, Pontis S, Silvia P, Simola N, Nicola S, Schintu N, Nicoletta S, Morelli M, Micaela M. Behavioral and biochemical correlates of the dyskinetic potential of dopaminergic agonists in the 6-OHDA lesioned rat. Synapse. 2008;62:524–533. doi: 10.1002/syn.20527. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lee CS, Björklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998 Aug;10(8):2694–706. 1998. [PubMed] [Google Scholar]
- Cenci MA, Lindgren HS. Advances in understanding L-DOPA-induced dyskinesia. Curr Opin Neurobiol. 2007;17:665–671. doi: 10.1016/j.conb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci. 2007 Oct;Chapter 9(Unit 9):25. doi: 10.1002/0471142301.ns0925s41. 2007. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007a;30:236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cenci MA. L-DOPA-induced dyskinesia: cellular mechanisms and approaches to treatment. Parkinsonism Relat Disord. 2007b;13:S263–267. doi: 10.1016/S1353-8020(08)70014-2. [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Chotibut T, Fields V, Salvatore MF. Norepinephrine transporter inhibition with desipramine exacerbates L-DOPA-induced dyskinesia: role for synaptic dopamine regulation in denervated nigrostriatal terminals. Mol Pharmacol. 2014;86:675–685. doi: 10.1124/mol.114.093302. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, Ruth TJ, Stoessl AJ. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson's disease: implications for dyskinesias. Brain. 2004;127:2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Delfino MA, Stefano AV, Ferrario JE, Taravini IRE, Murer MG, Gershanik OS. Behavioral sensitization to different dopamine agonists in a parkinsonian rodent model of drug-induced dyskinesias. Behav Brain Res. 2004;152:297–306. doi: 10.1016/j.bbr.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Eskow KL, Negron G, Bishop C. The differential effects of 5-HT(1A) receptor stimulation on dopamine receptor-mediated abnormal involuntary movements and rotations in the primed hemiparkinsonian rat. Brain Res. 2007;1158:135–143. doi: 10.1016/j.brainres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Dupre KB, Ostock CY, Eskow Jaunarajs KL, Button T, Savage LM, Wolf W, Bishop C. Local modulation of striatal glutamate efflux by serotonin 1A receptor stimulation in dyskinetic, hemiparkinsonian rats. Exp Neurol. 2011;229:288–299. doi: 10.1016/j.expneurol.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C. The role of the dorsal raphe nucleus in the development, expression, and treatment of L-dopa-induced dyskinesia in hemiparkinsonian rats. Synapse. 2009;63:610–620. doi: 10.1002/syn.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskow KL, Gupta V, Alam S, Park JY, Bishop C. The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav. 2007;87:306–314. doi: 10.1016/j.pbb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Marrosu F, Metzler CW, Tada K, Jacobs BL. Effects of the putative 5-hydroxytryptamine1A antagonists BMY 7378, NAN 190 and (-)-propranolol on serotonergic dorsal raphe unit activity in behaving cats. J Pharmacol Exp Ther. 1994 Sep;270(3):1359–66. 1994. [PubMed] [Google Scholar]
- Fox SH. Non-dopaminergic treatments for motor control in Parkinson's disease. Drugs. 2013;73:1405–1415. doi: 10.1007/s40265-013-0105-4. [DOI] [PubMed] [Google Scholar]
- Fulceri F, Biagioni F, Ferrucci M, Lazzeri G, Bartalucci A, Galli V, Ruggieri S, Paparelli A, Fornai F. Abnormal involuntary movements (AIMs) following pulsatile dopaminergic stimulation: severe deterioration and morphological correlates following the loss of locus coeruleus neurons. Brain Res. 2007 Mar 2;1135(1):219–29. doi: 10.1016/j.brainres.2006.12.030. 2007. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Damier P, Hicking C, Laska E, Müller T, Olanow CW, Rascol O, Russ H. Sarizotan as a treatment for dyskinesias in Parkinson's disease: a double-blind placebo-controlled trial. Mov Disord. 2007;22:179–186. doi: 10.1002/mds.21226. [DOI] [PubMed] [Google Scholar]
- Goshima Y, Misu Y, Arai N, Misugi K. Nanomolar L-dopa facilitates release of dopamine via presynaptic beta-adrenoceptors: comparative studies on the actions in striatal slices from control and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated C57 black mice, an animal model for Parkinson's disease. Jpn J Pharmacol. 1991;55:93–100. doi: 10.1254/jjp.55.93. [DOI] [PubMed] [Google Scholar]
- Hara M, Fukui R, Hieda E, Kuroiwa M, Bateup HS, Kano T, Greengard P, Nishi A. Role of adrenoceptors in the regulation of dopamine/DARPP-32 signaling in neostriatal neurons. J Neurochem. 2010;113:1046–1059. doi: 10.1111/j.1471-4159.2010.06668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm. 2006;70:9–15. doi: 10.1007/978-3-211-45295-0_3. [DOI] [PubMed] [Google Scholar]
- Kannari K, Kurahashi K, Tomiyama M, Maeda T, Arai A, Baba M, Suda T, Matsunaga M. [Tandospirone citrate, a selective 5-HT1A agonist, alleviates L-DOPA-induced dyskinesia in patients with Parkinson's disease] No To Shinkei. 2002;54:133–137. [PubMed] [Google Scholar]
- Kilpatrick IC, Jones MW, Phillipson OT. A semiautomated analysis method for catecholamines, indoleamines, and some prominent metabolites in microdissected regions of the nervous system: an isocratic HPLC technique employing coulometric detection and minimal sample preparation. J Neurochem. 1986;46:1865–1876. doi: 10.1111/j.1471-4159.1986.tb08506.x. [DOI] [PubMed] [Google Scholar]
- Lane EL, Cheetham SC, Jenner P. Does contraversive circling in the 6-OHDA-lesioned rat indicate an ability to induce motor complications as well as therapeutic effects in Parkinson's disease? Exp Neurol. 2006;197:284–290. doi: 10.1016/j.expneurol.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Lewitt PA, Hauser RA, Lu M, Nicholas AP, Weiner W, Coppard N, Leinonen M, Savola JM. Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study) Neurology. 2012;79:163–169. doi: 10.1212/WNL.0b013e31825f0451. [DOI] [PubMed] [Google Scholar]
- Lindenbach D, Ostock CY, Eskow Jaunarajs KL, Dupre KB, Barnum CJ, Bhide N, Bishop C. Behavioral and cellular modulation of L-DOPA-induced dyskinesia by beta-adrenoceptor blockade in the 6-hydroxydopamine-lesioned rat. J Pharmacol Exp Ther. 2011;337:755–765. doi: 10.1124/jpet.111.179416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson's disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem. 2010;112:1465–1476. doi: 10.1111/j.1471-4159.2009.06556.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Miguelez C, Aristieta A, Cenci MA, Ugedo L. The locus coeruleus is directly implicated in L-DOPA-induced dyskinesia in parkinsonian rats: an electrophysiological and behavioural study. PLoS One. 2011;6(9):e24679. doi: 10.1371/journal.pone.0024679. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monville C, Torres EM, Dunnett SB. Validation of the l-dopa-induced dyskinesia in the 6-OHDA model and evaluation of the effects of selective dopamine receptor agonists and antagonists. Brain Res Bull. 2005;68:16–23. doi: 10.1016/j.brainresbull.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostock CY, Lindenbach DL, Goldenberg AA, Kampton E, Bishop C. Effects of noradrenergic denervation by anti-DBH-saporin on behavioral responsivity to L-DOPA in the hemi-parkinsonian rat. Behav Brain Res. 2014;270:75–85. doi: 10.1016/j.bbr.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, Piccini P. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology. 2006;67:1612–7. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003 May;6(5):501–6. doi: 10.1038/nn1040. 2003. [DOI] [PubMed] [Google Scholar]
- Pollack AE, Yates TM. Prior D1 dopamine receptor stimulation is required to prime D2-mediated striatal Fos expression in 6-hydroxydopamine-lesioned rats. Neuroscience. 1999;94:505–514. doi: 10.1016/s0306-4522(99)00338-3. [DOI] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Arnulf I, Peyro-Saint Paul H, Brefel-Courbon C, Vidailhet M, Thalamas C, Bonnet AM, Descombes S, Bejjani B, Fabre N, Montastruc JL, Agid Y. Idazoxan, an alpha-2 antagonist, and L-DOPA-induced dyskinesias in patients with Parkinson's disease. Mov Disord. 2001;16:708–713. doi: 10.1002/mds.1143. [DOI] [PubMed] [Google Scholar]
- Reisine TD, Chesselet MF, Lubetzki C, Chéramy A, Glowinski J. A role for striatal beta-adrenergic receptors in the regulation of dopamine release. Brain Res. 1982;241:123–130. doi: 10.1016/0006-8993(82)91235-5. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, Hervé D, Greengard P, Fisone G. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savola JM, Hill M, Engstrom M, Merivuori H, Wurster S, McGuire SG, Fox SH, Crossman AR, Brotchie JM. Fipamezole (JP-1730) is a potent alpha2 adrenergic receptor antagonist that reduces levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinson's disease. Mov Disord. 2003;18:872–883. doi: 10.1002/mds.10464. [DOI] [PubMed] [Google Scholar]
- Shin E, Rogers JT, Devoto P, Bjorklund A, Carta M. Noradrenaline neuron degeneration contributes to motor impairments and development of L-DOPA-induced dyskinesia in a rat model of Parkinson's disease. Exp Neurol. 2014;257:25–38. doi: 10.1016/j.expneurol.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Ullrich T, Rice KC, Walker PD. Serotonin 2A receptor antagonist treatment reduces dopamine D1 receptor-mediated rotational behavior but not L-DOPA-induced abnormal involuntary movements in the unilateral dopamine-depleted rat. Neuropharmacology. 2006;50:761–768. doi: 10.1016/j.neuropharm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Bishop C, Walker PD. Dopamine D1 and D2 receptor contributions to L-DOPA-induced dyskinesia in the dopamine-depleted rat. Pharmacol Biochem Behav. 2005;81:887–893. doi: 10.1016/j.pbb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Waeber C, Rigo M, Chinaglia G, Probst A, Palacios JM. Beta-adrenergic receptor subtypes in the basal ganglia of patients with Huntington's chorea and Parkinson's disease. Synapse. 1991;8:270–280. doi: 10.1002/syn.890080405. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang HS, Wang T, Huang C, Liu J. L-DOPA-induced dyskinesia in a rat model of Parkinson's disease is associated with the fluctuational release of norepinephrine in the sensorimotor striatum. J Neurosci Res. 2014;92:1733–1745. doi: 10.1002/jnr.23439. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Björklund A, Cenci MA. L-DOPA-induced dyskinesia in the intrastriatal 6-hydroxydopamine model of parkinson's disease: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2002;10:165–186. doi: 10.1006/nbdi.2002.0499. [DOI] [PubMed] [Google Scholar]