Abstract
Background
Little has been reported regarding patterns of oncologic care in American Indian/Alaska Natives (AI/ANs). Observed worse survival has been attributed to later-stage presentation. We aimed to evaluate racial differences in cancer-directed therapy and hospice care utilization in AI/ANs and non-Hispanic Whites (NHWs) with metastatic cancer.
Methods
The linked Surveillance, Epidemiology, and End Results (SEER)-Medicare claims database was accessed for AI/AN and NHW metastatic-cancer cases diagnosed 2001–2007. Utilization of cancer-directed therapy (surgery, radiation, and/or chemotherapy) and/or hospice services were compared between AI/ANs and NHWs. Minimally-adjusted (age, sex, diagnosis year) and fully-adjusted (also Klabunde comorbidity score, sociodemographic factors) regression models were used to estimate odds (ORs) and hazard ratios (HRs) for receipt of care.
Results
AI/ANs were younger, more likely to reside in the West, be unmarried, have lower income, and live in a non-urban setting than NHWs. Fewer AI/ANs received any cancer-directed therapy (57% vs. 61% NHWs) within 3 months of diagnosis; sociodemographic factors accounted for much of this difference (fully-adjusted HR 0.94, 95% confidence interval(CI) 0.83–1.08). We noted differences in hospice utilization between AI/ANs (52%) and NHWs (61%). A significant difference in hospice utilization remained after adjustment for sociodemographics (OR 0.78, 95%CI: 0.61–0.99).
Conclusion
Observed absolute differences in care for AI/ANs and NHWs with metastatic cancer were largely accounted for by adjusting for socioeconomics, comorbidities, and demographic factors. A significant association between race and hospice utilization was noted.
Impact
Efforts to improve metastatic-cancer care should focus on socioeconomic barriers and investigate the observed disparity in receipt of hospice services.
Keywords: health disparities, cancer outcomes, race, colorectal cancer, lung cancer, breast cancer
INTRODUCTION
Little has been documented regarding patterns of care for American Indian and Alaska Native (AI/AN) individuals after the diagnosis of metastatic cancer. AI/ANs are often not included, or are dramatically under-represented, in cancer treatment trials (1). AI/ANs have higher cancer-related mortality than non-Hispanic Whites (NHWs), with lung cancer being the most common cause of cancer-related death in AI/ANs, as in NHWs (2–4). This may be partly due to differences in prevention efforts and screening behaviors because AI/ANs tend to be diagnosed with cancers at a later stage compared to NHWs (5–7). It has yet to be evaluated how patterns of care in AI/ANs compare to NHWs after the diagnosis of metastatic disease.
Cancer control has improved over the last few decades, but this benefit appears to be greater for NHWs than AI/ANs and/or AI/ANs continue to have worse survival than NHWs (7–9). As noted with other diseases, AI/ANs may experience delays in time to presentation for care or receipt of care (10, 11). This raises the concern that AI/ANs with metastatic cancer may be less likely than NHWs to receive chemotherapy, which is potentially life-prolonging, as has been demonstrated for earlier stage cancers (9, 12, 13). AI/ANs may also undergo less surgery and/or radiation for palliation of symptoms.
Early use of palliative care services, which includes hospice services, can improve quality of life and, perhaps, survival (14). This is especially relevant as a high prevalence of pain symptoms has been reported for AI/ANs (15). However, nationally the median length of time on hospice is 20 days, with 36% of hospice users enrolling seven days or less before their death (16). Population statistics note that AI/ANs are underrepresented as hospice users, relative to their proportion of the population (17). While racial disparities in hospice services are well recognized, even a systematic review of hospice utilization in different minority populations did not have sufficient data to permit evaluation of hospice use in AI/ANs, limiting our understanding of this discrepancy (18). Therefore, we compared utilization of cancer-directed treatments and hospice services between AI/AN and NHW metastatic cancer patients age 65 years and older in the linked Surveillance, Epidemiology, and End Results cancer registry and Medicare claims database (SEER-Medicare).
MATERIALS AND METHODS
Study sample and follow-up
To identify cancer patients for this study, we used Medicare enrollment files and Indian Health Service Care System (IHSCS) records linked to SEER cancer registry data and Medicare enrollment and claims files (SEER-Medicare) between 2001 and 2007. IHSCS is a system of clinics that provide primary care services to AI/ANs, and is restricted to members of federally-recognized tribes for some states. NHWs were chosen as the comparison group, consistent with literature standards (8, 18). We included individuals who were: 1) diagnosed between 2001 and 2007 with SEER “distant” stage cancer (AJCC stage IV: Tx Nx M1) of the breast (female only), colorectum, lung, prostate, ovary, or stomach, 2) age 65 or older, 3) residing in a SEER catchment area, 4) enrolled in Medicare parts A and B ≥1 month prior to diagnosis, and 5) AI/AN or NHW. Individuals were excluded if: 1) they were enrolled in Medicare due to disability or end-stage renal disease, 2) had a prior malignancy, 3) diagnosed with cancer at death/autopsy, or 4) hospice enrollment data preceded the cancer diagnosis date. This resulted in 388 AI/AN and 75,871 NHW cases for analysis. Additional information on cohort development is detailed in supplemental Table S1 (online only). Follow-up for vital status through December 31, 2009 was obtained from SEER registry data.
Statistical Analysis
Descriptive characteristics were compared between AI/ANs and NHWs with two-sample t-test for continuous variables (age and Klabunde comorbidity index (19)) and chi-square test of homogeneity for the categorical variables. For patients with less than 12 months of enrollment prior to diagnosis, a Klabunde comorbidity score could not be calculated and was coded as null for this variable. For an alpha of 0.05, statistical significance was defined as a p-value of <0.05.
Cancer-Directed Therapy
The use of cancer-directed therapy (surgery, radiation, and/or chemotherapy) was compared between AI/AN and NHW case groups. “Cancer-directed therapy” was defined as surgical resection, radiation, or chemotherapy that was delivered after cancer diagnosis. Receipt of therapy was identified from Medicare part A and B claims. The specific CPT, ICD-9, HCPCS, DRG, and Revenue Codes claims are listed in supplementary Table S2 (online only). Chemotherapy was also evaluated separately from radiation and surgery because it is the major potentially life-prolonging intervention for metastatic cancer (12).
Utilization of cancer-directed therapy in 388 AI/ANs vs. 75,871 NHWs was described using cumulative incidence and compared with competing risks analysis, where death and hospice before the initiation of treatment were considered competing events. Both minimally adjusted hazard ratios (HRs; adjusted for age, sex, and diagnosis year) and fully adjusted HRs (additionally adjusted for marital status, SEER region, Klabunde score, zip-code-level median income, and type of residence [urban or rural]) were estimated. All metastatic cancer cases were included, followed by analyses for each of breast (female only), colorectal, lung, and prostate cancer cases. Secondary analysis for receipt of chemotherapy was additionally performed in all cases and by anatomic site. Using competing risk regression, treatment other than chemotherapy (including surgery, radiation, and hospice) and death were treated as competing events. Results were adjusted for diagnosis age, sex, SEER region (Midwest, Northeast, South, or West), diagnosis year, Klabunde co-morbidity score, marital status (single, married, divorced, separated, widowed, unknown), zip-code-level median income, and type of residence. These adjustment variables were selected a priori based on prior studies indicating that the factor was associated with race and with receipt of care or hospice utilization. We computed robust variance estimates for all models in order to relax assumptions. For the overall models combining all cancer types, we also clustered on cancer type to account for the correlation among patients. Statistical significance was defined as p<0.05 with two-tailed tests.
Hospice Utilization
Hospice care was separately evaluated, both in terms of ever enrollment and late utilization. Metastatic cancer meets the Medicare criteria of terminal illness, but one cannot determine a 6 month or less predicted prognosis from claims data (20). Thus, hospice use was retrospectively determined among those that died. Firstly, hospice enrollment was analyzed among individuals who died within two years of diagnosis (n=365 AI/AN, n=63,879 NHW). The two-year restriction served to remove bias based on enrollment year and length of available follow-up data as the last enrollment year (2007) had a maximum of two years of follow-up. The Medicare claims data were then examined for hospice enrollment prior to death and categorized as having any claims (“hospice utilizer”) or no claims (“non-utilizer”) prior to death. Secondly, late hospice use was analyzed among all hospice enrollees (n=190 AI/AN, n=44,010 NHW). Late hospice utilization was defined as enrollment ≤7 days prior to death.
Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (95% CIs), comparing AI/ANs to NHWs, for use vs. no utilization of hospice. Minimally adjusted and fully adjusted ORs and 95% CIs were calculated using the same adjustment variables as above. Analyses were performed in metastatic cases overall and for the top three cancer types for each gender (lung, colorectal, female breast, and male prostate cancer). A sensitivity analysis was additionally performed, adjusting for recent hospitalization (defined as hospitalization within 30 days of death) when evaluating the association between race and hospice enrollment given that hospital admission serves as an acute presentation of illness that might facilitate and/or lead to hospice enrollment. Again, we computed robust variance estimates for all models in order to relax assumptions. For the overall models combining all cancer types, we also clustered on cancer type to account for the correlation among patients. Statistical significance was defined as p<0.05 with two-tailed tests.
Research Ethics
Approval for this study was granted by the Institutional Review Boards of the Fred Hutchinson Cancer Research Center, State of Washington, Oregon Department of Public Health, Oregon Department of Health Services, California Rural Indian Health Board, Northwest Portland Area Indian Health Board, California Committee for the Protection of Human Subjects, and the California Department of Health Care Services.
RESULTS
Multiple differences between AI/AN and NHW individuals with metastatic cancer were noted (Table 1). On average, the AI/AN cancer patients were slightly younger than NHW patients at diagnosis (mean age 74.6 vs. 76.2 years, respectively; p<0.01). AI/ANs were more likely to reside in a West SEER region, be diagnosed in a later year of study enrollment, and live in a zip-code with lower median income (all p<0.05). Additionally, AIANs were less likely to live in an urban setting or be married. The distribution of cancers also varied by race group (p<0.01), though the most common cancer by far was lung cancer in both AI/ANs (59%) and NHWs (64%).
Table 1.
Comparison of demographics of American Indian/Alaska Natives (AI/ANs) and non-Hispanic Whites (NHW) at the time of metastatic cancer diagnosis, SEER-Medicare (2001–2007).
| Characteristic, n (%) | AI/AN N=388 |
NHW N=75,871 |
p-value |
|---|---|---|---|
|
| |||
| Diagnosis age, mean (SD) | 74.6 (6.6) | 76.2 (7.1) | <0.01a |
|
| |||
| Gender, male | 198 (51%) | 36,322 (48%) | 0.23b |
|
| |||
| Cancer type | <0.01 | ||
| Breast (female only) | 23 (6%) | 3,560 (5%) | |
| Lung | 229 (59%) | 48,302 (64%) | |
| Colorectal | 52 (13%) | 11,425 (15%) | |
| Prostate | 36 (9%) | 4,592 (6%) | |
| Ovarian | 27 (7%) | 5,586 (7%) | |
| Gastric | 21 (5%) | 2,406 (3%) | |
|
| |||
| SEER region | <0.01b | ||
| Midwest | 19 (5%) | 12,300 (16%) | |
| Northeast | 28 (7%) | 18,209 (24%) | |
| South | 11 (3%) | 15,648 (21%) | |
| West | 330 (85%) | 29,714 (39%) | |
|
| |||
| Diagnosis year | 0.03b | ||
| 2001 | 58 (15%) | 10,620 (14%) | |
| 2002 | 37 (10%) | 11,211 (15%) | |
| 2003 | 59 (15%) | 11,183 (15%) | |
| 2004 | 56 (14%) | 11,182 (15%) | |
| 2005 | 48 (12%) | 10,885 (14%) | |
| 2006 | 66 (17%) | 10,703 (14%) | |
| 2007 | 64 (17%) | 10,087 (13%) | |
|
| |||
| Klabunde, mean (SD) | 0.4 (0.5) | 0.4 (0.5) | 0.45a |
|
| |||
| Married status | 147 (38%) | 37,510 (49%) | <0.01b |
|
| |||
| Annual income, mean (SD) c | $37,424 (13,570) | $48,678 (19,152) | <0.01a |
|
| |||
| Residence, urban | 232 (60%) | 58,677 (77%) | <0.01b |
SD = standard deviation; AI/AN = American Indian/Alaska Native; NHW = non-Hispanic White
Two-sample t-test for difference in means
Chi-square test of homogeneity
Based on median income for the zip code of residence
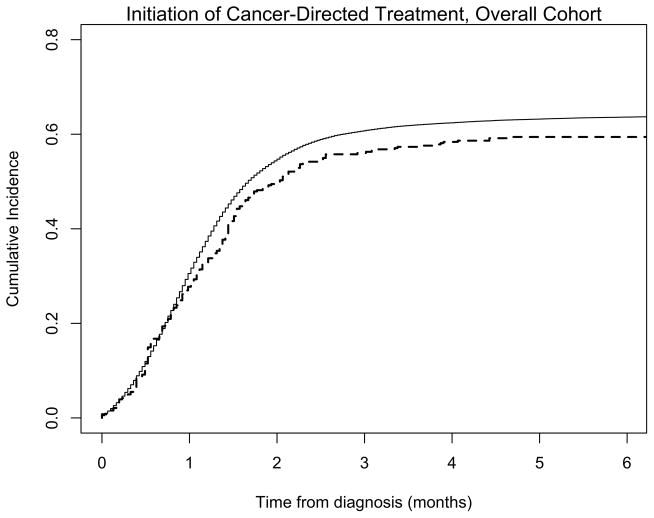
Overall, fewer AI/ANs received any cancer-directed therapy (218/388 [57%] AI/ANs vs. 46,509/75,871 [61%] NHWs) in the first three months following diagnosis (Figure 1). By competing-risks analysis, the HR for any treatment among AI/AN compared to NHW was 0.80 (95% CI: 0.74–0.86, p<0.01) for all cancers, adjusted for age, sex, and diagnosis year (Table 2). This difference was no longer significant (HR 0.94, 95% CI: 0.83–1.08, p=0.4) in the fully adjusted model. The observed differences in receipt of cancer-directed therapy appears to be largely driven by individuals with lung cancer, which was the most common metastatic cancer type for both AI/ANs and NHWs (114/229 [50%] of AI/ANs and 27,003/48,306 [56%] of NHWs had metastatic lung cancer). Female breast cancer comprised less than 10% of metastatic cases, but there was no evidence that AI/AN metastatic breast cancer patients were less likely to receive cancer-directed therapy than NHWs (fully adjusted HR 1.51, 95% CI: 0.91–2.50, p=0.11).
Figure 1. Cumulative incidence of use of cancer-directed therapy (surgery, radiation, or chemotherapy) in American Indian/Alaska Natives and non-Hispanic Whites with metastatic cancer.
In the overall cohort of individuals with metastatic cancer, a non-significant trend toward lower cancer-directed treatment use in AI/ANs (dashed line) compared to NHWs (solid line) was observed.
Table 2.
Utilization of cancer-directed treatments and chemotherapy within 3 months following diagnosis in American Indian/Alaska Native and non-Hispanic White metastatic cancer patients.
| Cancer type | Number of Patients | Cumulative incidence(3 months) | Minimally Adjusted HR a | p-value | Fully Adjusted HR ab | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| AI/AN | NHW | AI/AN | NHW | ||||||
| Cancer-directed treatment c | Overall | 388 | 75,871 | 57% | 61% | 0.80 (0.74, 0.86) | <0.01 | 0.94 (0.83, 1.08) | 0.40 |
| Breast | 23 | 3,560 | 65% | 63% | 1.19 (0.78, 1.83) | 0.43 | 1.51 (0.91, 2.50) | 0.11 | |
| Colorectal | 52 | 11,425 | 71% | 72% | 0.88 (0.64, 1.21) | 0.43 | 1.10 (0.79, 1.55) | 0.57 | |
| Lung | 229 | 48,302 | 50% | 56% | 0.78 (0.66, 0.92) | <0.01 | 0.89 (0.74, 1.06) | 0.20 | |
| Prostate | 36 | 4,592 | 61% | 73% | 0.72 (0.46, 1.13) | 0.15 | 0.87 (0.54, 1.41) | 0.57 | |
| Chemotherapy | Overall | 388 | 75,871 | 46% | 54% | 0.79 (0.68, 0.91) | <0.01 | 0.92 (0.78, 1.09) | 0.33 |
| Breast | 23 | 3,560 | d | 32% | 0.69 (0.41, 1.16) | 0.16 | 0.89 (0.50, 1.59) | 0.69 | |
| Colorectal | 52 | 11,425 | 36% | 47% | 0.69 (0.45, 1.06) | 0.09 | 0.85 (0.52, 1.37) | 0.50 | |
| Lung | 229 | 48,302 | 50% | 53% | 0.87 (0.71, 1.07) | 0.18 | 1.02 (0.82, 1.27) | 0.84 | |
| Prostate | 36 | 4,592 | 47% | 66% | 0.60 (0.40, 0.90) | 0.01 | 0.67 (0.44, 1.04) | 0.08 | |
AI/AN = American Indian/Alaska Native; NHW = non-Hispanic White; HR = hazard ratio
Adjusted for age, sex, and diagnosis year.
Adjusted for zip code-level median income, marital status, SEER region, type of residence, and Klabunde comorbidity score
Cancer-directed treatment is defined as receipt of cancer-directed surgery, radiation, or chemotherapy.
To protect privacy, SEER-Medicare restricts reporting of actual values for cell sizes <11.
Chemotherapy utilization followed a similar trend as cancer-directed therapies collectively (Table 2). Lower utilization at three months was seen in the AI/ANs (179/388 [46%] vs. 40,894/75,871 [54%] NHWs). Similarly, the HR for overall utilization was no longer significant after full adjustment (minimally adjusted HR 0.79, 95% CI: 0.68–0.91, p<0.01; fully adjusted HR 0.92, 95% CI: 0.78–1.09, p=0.33). All four anatomic site sub-analyses followed the same trend.
Among individuals who were observed to die within two years of diagnosis, there was less use of hospice services by AI/ANs, with 190 (52%) enrolling in hospice prior to death versus 38,733 (61%) of NHWs (Table 3). The OR for the association between AI/AN race and lower hospice utilization was significant in both the minimally adjusted model (OR 0.68, 95% CI: 0.55–0.83, p<0.01) and the fully adjusted model (OR 0.78, 95% CI: 0.61–0.99, p=0.04). Additionally, within each cancer type, AI/ANs showed less use of hospice services compared to NHWs. Comparing between groups, AI/AN lung cancer patients were higher users, while prostate cancer patients were the least likely to use hospice services. However, only the association seen in prostate cancer remained significant after full adjustment (OR 0.39, 95% CI: 0.17–0.93, p=0.03). In a sensitivity analysis, hospitalization within the 30 days prior to death was not associated with higher rates of hospice enrollment (data not shown). Late hospice utilization (Table 3) was not significantly lower in AI/ANs compared with NHWs (fully adjusted OR 0.82, 95% CI: 0.56–1.19, p=0.3).
Table 3.
Utilization of hospice care in American Indian/Alaska Natives and non-Hispanic Whites with metastatic cancer.
| Cancer type | Number of Utilizers/Eligible At risk c | Proportion | Minimally Adjusted OR a | p-value | Fully Adjusted OR ab | p-value | |||
|---|---|---|---|---|---|---|---|---|---|
| AI/AN | NHW | AI/AN | NHW | ||||||
| Hospice | Overall | 190/365 | 38,733/63,879 | 52% | 61% | 0.68 (0.55, 0.83) | <0.01 | 0.78 (0.61, 0.99) | 0.04 |
| Breast | d | 1,323/2,225 | d | 60% | 0.59 (0.24, 1.43) | 0.25 | 0.51 (0.19, 1.37) | 0.18 | |
| Colorectal | 29/51 | 5,699/9,080 | 52% | 63% | 0.71 (0.41, 1.23) | 0.23 | 0.95 (0.48, 1.91) | 0.90 | |
| Lung | 120/222 | 26,756/44,383 | 54% | 60% | 0.76 (0.59, 1.00) | 0.05 | 0.91 (0.66, 1.25) | 0.56 | |
| Prostate | d | 1,400/2,523 | d | 56% | 0.34 (0.15, 0.75) | 0.01 | 0.39 (0.17, 0.93) | 0.03 | |
| Late utilization | Overall | 49/190 | 13,664/44,010 | 26% | 31% | 0.76 (0.55, 1.06) | 0.11 | 0.82 (0.56, 1.19) | 0.30 |
| Breast | d | 564/1,817 | d | 31% | 0.95 (0.24, 3.71) | 0.94 | 1.59 (0.37, 7.00) | 0.53 | |
| Colorectal | d | 1,967/6,849 | d | 29% | 0.79 (0.33, 1.85) | 0.58 | 1.04 (0.43, 2.52) | 0.94 | |
| Lung | 31/120 | 9,316/28,580 | d | 33% | 0.72 (0.47, 1.08) | 0.11 | 0.74 (0.47, 1.15) | 0.18 | |
| Prostate | d | 607/2,107 | d | 29% | 1.20 (0.30, 4.83) | 0.80 | 0.79 (0.16, 4.03) | 0.78 | |
AI/AN = American Indian/Alaska Native; NHW = non-Hispanic White; OR = odds ratio
Adjusted for age, sex, and diagnosis year.
Adjusted for zip code-level median income, marital status, SEER region, type of residence, and Klabunde comorbidity score
For overall use, eligibility was determined based on those that died within 2 years of diagnosis. For late hospice use, all hospice users were considered eligible.
To protect privacy, SEER-Medicare restricts reporting of actual values for cell sizes <11.
DISCUSSION
Neither receipt of cancer-directed chemotherapy, radiation, and/or surgery nor of chemotherapy specifically was significantly different between AI/ANs and NHWs. However, a significantly lower use of hospice services was observed in AI/ANs compared to NHWs. This difference was not seen when comparing late utilization of hospice services.
After adjusting for factors that are known to influence access to care, AI/AN race was not associated with less use of cancer-directed therapy. For utilization of cancer-directed therapy, the minimally-adjusted HR estimates suggest that there are disparities in care between AI/ANs and NHWs. However, our fully-adjusted HR estimates indicate that these disparities may be largely explained by differences in social and demographic factors between AI/ANs and NHWs. Though prior studies note differences in stage at presentation may account for some difference in cancer outcomes, this would not explain the differences observed in this analysis restricted to metastatic-stage cancer patients (13, 21). In our analysis, marital status and zip-code level median income were significantly different between AI/ANs and NHWs. This suggests that differences in social support and resources, rather than race per se, are the primary factors influencing access and utilization of care (22). Apparent disparities in the use of healthcare among AI/AN Veterans unselected for a specific diagnosis were similarly accounted for by adjusting for sociodemographics and insurance coverage (23). This data recommends that enhancing available options for resource-poor individuals may have the greatest positive impact on observed disparities in care received for metastatic cancer.
Even when accounting for sociodemographic factors that could influence use of hospice services, AI/ANs had significantly lower utilization of hospice care compared to NHWs. As in previous reports, we observed racial differences in utilization of hospice services(18, 24). Expanding upon what has been previously shown (24), we further examined hospice enrollment seven or less days prior to death to valuate if there was a greater amount of late enrollment in hospice care among AI/ANs than NHWs that might be consistent with observed delays at other time points of cancer care (10, 11). In both groups, late use was less than the population average of 36% enrollment in the last week of life (16). However, there was no significant difference between AI/ANs and NHWs observed in the fully adjusted model. In this assessment, younger age, married status, urban residence, and higher income were associated with greater hospice utilization. Our analysis is the first with sufficient numbers of AI/AN to test associations between common factors, including race, that may influence use of initial and end-of-life treatments (18). Additional investigation is needed to understand the root-cause for the observed differences, such as differences in cultural opinions about the utility of hospice or prior positive experiences with friends/relatives in hospice.
Our study strengths include a selection of study participants from the SEER-Medicare database linked to IHSCS in the recent past. Though prior studies have evaluated cancer care in AI/ANs, these have been limited by inclusion of no or few stage IV cases, analysis restricted to a certain region of the country, and/or earlier years of Medicare enrollment, resulting in potentially less relevant results to an evaluation of the experience of AI/ANs with metastatic cancer nationwide (13, 21, 25, 26). Given the relatively small numbers of AI/AN cancer patients in the SEER-Medicare database, this analysis cannot make inferences about specific sub-groups of AI/ANs, such as individual cancer types. Additionally, due to the inherent limitations of the SEER-Medicare resource, non-hospice palliative care and supportive care measures, including over-the-counter medications and oral prescription drugs, were not examined. Non-surgical palliative interventions, such as stenting for colonic obstruction, were also not evaluated in this analysis.
There is growing evidence that early specialty palliative care involvement improves quality of life and care (14). At a minimum, hospice involvement near the end of life can assist with supportive care, patient/family comfort, and financial burden (27). Acute changes in health status, such as would be reflected with a performance status score, are not available with this type of claims-based analysis. Thus, it is difficult to objectively assess when a person is “ready” or eligible for hospice services. Accordingly, we used death in a metastatic cancer patient as a surrogate for meeting the hospice eligibility criteria of a terminal diagnosis and a 6 month or less prognosis (20). Other groups whose focus was only on the last 6 months of life may miss longer term hospice users (24). We then retrospectively examined the records of those who died for utilization of hospice services. Sensitivity analyses to evaluate recent hospitalization prior to death did not significantly affect our results, arguing against differences in acute medical illness affecting likelihood of hospice enrollment as the cause of the observed difference in hospice utilization.
In conclusion, while AI/ANs included in the SEER-Medicare data who were diagnosed with metastatic cancer have lower receipt of cancer-directed therapy overall and of chemotherapy than NHWs, these differences are accounted for by socioeconomic, comorbid illness, and other demographic factors. A significant association with AI/AN race is observed when evaluating utilization of hospice services. Because little has been published on this topic, it is important to note that differences in care between AI/AN and NHW metastatic cancer patients is partially attributable to factors for which interventions may be developed. Future efforts to improve receipt of cancer-directed care and utilization of hospice among AI/ANs should focus on barriers of care related to social support, transportation, financial resources, and other socioeconomic issues and on investigation into the observed disparity in receipt of hospice services.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT: Research reported in this manuscript was supported by the Hutchinson Institute for Cancer Outcomes Research (HICOR) and National Institutes of Health (NIH) R01 CA125231 to S. Ramsey. Additional sources of support include NIH T32-CA009515 (SS) and KL2 TR000421 (ABH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Indian Health Service.
Footnotes
DISCLOSURES: The authors declare no conflicts of interest.
References
- 1.Hawk ET, Habermann EB, Ford JG, Wenzel JA, Brahmer JR, Chen MS, Jr, et al. Five National Cancer Institute-designated cancer centers’ data collection on racial/ethnic minority participation in therapeutic trials: a current view and opportunities for improvement. Cancer. 2014;120 (Suppl 7):1113–21. doi: 10.1002/cncr.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holck P, Day GE, Provost E. Mortality trends among Alaska Native people: successes and challenges. International journal of circumpolar health. 2013:72. doi: 10.3402/ijch.v72i0.21185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plescia M, Henley SJ, Pate A, Underwood JM, Rhodes K. Lung cancer deaths among American Indians and Alaska Natives, 1990–2009. American journal of public health. 2014;104 (Suppl 3):S388–95. doi: 10.2105/AJPH.2013.301609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiggins CL, Espey DK, Wingo PA, Kaur JS, Wilson RT, Swan J, et al. Cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113:1142–52. doi: 10.1002/cncr.23734. [DOI] [PubMed] [Google Scholar]
- 5.Steele CB, Cardinez CJ, Richardson LC, Tom-Orme L, Shaw KM. Surveillance for health behaviors of American Indians and Alaska Natives-findings from the behavioral risk factor surveillance system, 2000–2006. Cancer. 2008;113:1131–41. doi: 10.1002/cncr.23727. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher MC, Slattery ML, Lanier AP, Ma KN, Edwards S, Ferucci ED, et al. Prevalence and predictors of cancer screening among American Indian and Alaska native people: the EARTH study. Cancer causes & control : CCC. 2008;19:725–37. doi: 10.1007/s10552-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson-Jennings MD, Tarraf W, Xavier Hill K, Gonzalez HM. United States colorectal cancer screening practices among American Indians/Alaska Natives, blacks, and non-Hispanic whites in the new millennium (2001 to 2010) Cancer. 2014 doi: 10.1002/cncr.28855. [DOI] [PubMed] [Google Scholar]
- 8.White MC, Espey DK, Swan J, Wiggins CL, Eheman C, Kaur JS. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. American journal of public health. 2014;104 (Suppl 3):S377–87. doi: 10.2105/AJPH.2013.301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fesinmeyer MD, Goulart B, Blough DK, Buchwald D, Ramsey SD. Lung cancer histology, stage, treatment, and survival in American Indians and Alaska Natives and whites. Cancer. 2010;116:4810–6. doi: 10.1002/cncr.25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim G, Ford KL, Chiriboga DA, Sorkin DH. Racial and ethnic disparities in healthcare use, delayed care, and management of diabetes mellitus in older adults in California. Journal of the American Geriatrics Society. 2012;60:2319–25. doi: 10.1111/jgs.12003. [DOI] [PubMed] [Google Scholar]
- 11.Livingston SE, Townshend-Bulson LJ, Bruden DL, McMahon BJ, Homan CE, Gove JE, et al. Treatment eligibility in Alaska Native and American Indian persons with hepatitis C virus infection. International journal of circumpolar health. 2012;71:1–7. doi: 10.3402/ijch.v71i0.18445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Referenced with permission from The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines(r)) for Senior Adult Oncology V.2.2014. (c) National Comprehensive Cancer Network, Inc 2014. All rights reserved. Accessed October 9, 2014. To view the most recent and complete version of the guideline, go online to www.nccn.org. NATIONAL COMPREHENSIVE CANCER NETWORK(r), NCCN(r), NCCN GUIDELINES(r), and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
- 13.Smith CB, Bonomi M, Packer S, Wisnivesky JP. Disparities in lung cancer stage, treatment and survival among American Indians and Alaskan Natives. Lung cancer. 2011;72:160–4. doi: 10.1016/j.lungcan.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Parikh RB, Temel JS. Early specialty palliative care. The New England journal of medicine. 2014;370:1075–6. doi: 10.1056/NEJMc1400243. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez N, Garroutte E, Kundu A, Morales L, Buchwald D. A review of the experience, epidemiology, and management of pain among American Indian, Alaska Native, and Aboriginal Canadian peoples. The journal of pain : official journal of the American Pain Society. 2011;12:511–22. doi: 10.1016/j.jpain.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Hospice and Palliative Care Organization. [Accessed December 11, 2014];Facts and Figures: 2012 edition. 2011 http://www.nhpco.org/sites/default/files/public/Statistics_Research/2012_Facts_Figures.pdf.
- 17.United States Census Bureau. [Accessed October 9, 2014];American Indian and Alaska Native Heritage Month: November 2012. http://www.census.gov/newsroom/releases/archives/facts_for_features_special_editions/cb12-ff22.html.
- 18.Cohen LL. Racial/ethnic disparities in hospice care: a systematic review. Journal of palliative medicine. 2008;11:763–8. doi: 10.1089/jpm.2007.0216. [DOI] [PubMed] [Google Scholar]
- 19.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Medicare and Medicaid Services. [Accessed October 9, 2014];Medicare Hospice Benefits, Revised August 2013. http://www.medicare.gov/publications/pubs/pdf/02154.pdf.
- 21.Cueto CV, Szeja S, Wertheim BC, Ong ES, Tsikitis VL. Disparities in treatment and survival of white and Native American patients with colorectal cancer: a SEER analysis. Journal of the American College of Surgeons. 2011;213:469–74. doi: 10.1016/j.jamcollsurg.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Barnes PM, Adams PF, Powell-Griner E. Health characteristics of the American Indian or Alaska Native adult population: United States, 2004–2008. National health statistics reports. 2010:1–22. [PubMed] [Google Scholar]
- 23.Johnson PJ, Carlson KF, Hearst MO. Healthcare disparities for American Indian veterans in the United States: a population-based study. Medical care. 2010;48:563–9. doi: 10.1097/MLR.0b013e3181d5f9e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guadagnolo BA, Huo J, Buchholz TA, Petereit DG. Disparities in hospice utilization among American Indian Medicare beneficiaries dying of cancer. Ethnicity & disease. 2014;24:393–8. [PubMed] [Google Scholar]
- 25.Javid SH, Varghese TK, Morris AM, Porter MP, He H, Buchwald D, et al. Guideline-concordant cancer care and survival among American Indian/Alaskan Native patients. Cancer. 2014;120:2183–90. doi: 10.1002/cncr.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson RT, Adams-Cameron M, Burhansstipanov L, Roubidoux MA, Cobb N, Lynch CF, et al. Disparities in breast cancer treatment among American Indian, Hispanic and non-Hispanic White Women Enrolled in Medicare. Journal of health care for the poor and underserved. 2007;18:648–64. doi: 10.1353/hpu.2007.0071. [DOI] [PubMed] [Google Scholar]
- 27.Gazelle G. Understanding hospice--an underutilized option for life’s final chapter. The New England journal of medicine. 2007;357:321–4. doi: 10.1056/NEJMp078067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.