Abstract
In many species, females produce fewer offspring than they are capable of rearing, possibly because increases in current reproductive effort come at the expense of a female’s own survival and future reproduction. To test this, we induced female house wrens (Troglodytes aedon) to lay more eggs than they normally would and assessed the potential costs of increasing cumulative investment in the three main components of the avian breeding cycle – egg laying, incubation, and nestling provisioning. Females with increased clutch sizes reared more offspring in the first brood than controls, but fledged a lower proportion of nestlings. Moreover, nestlings of experimental females were lighter than those of control females as brood size and pre-fledging mass were negatively correlated. In second broods of the season, when females were not manipulated, experimental females laid the same number of eggs as controls, but experienced an intra-seasonal cost through reduced hatchling survival and a lower number of young fledged. Offspring of control and experimental females were equally likely to recruit to the breeding population, although control females produced more recruits per egg laid. The reproductive success of recruits from broods of experimental and control females did not differ. The manipulation also induced inter-seasonal costs to future reproduction, as experimental females had lower fecundity than controls when breeding at least two years after having their reproductive effort experimentally increased. Finally, females producing the modal clutch size of seven eggs in their first broods had the highest lifetime number of fledglings.
Keywords: body mass, brood size, clutch size, costs of reproduction, immunity, recruitment, reproductive success
Introduction
Understanding the relationship between reproductive effort and parental fitness is a central goal of life-history theory (Stearns, 1992; Roff, 2002). Lack (1947) first hypothesized that the clutch size of altricial birds (i.e., those species whose nestlings are fully dependent upon their parents for food and warmth at hatching) is shaped evolutionarily by the demands of nestling provisioning, and that altricial birds are selected to produce the maximum number of young for which they can provide adequate food. However, the results of many brood-manipulation studies of altricial species are inconsistent with Lack’s hypothesis (e.g., Finke et al., 1987; Robinson & Rotenberry, 1991; Sanz & Tinbergen, 1999) because parents can often successfully provision more nestlings than they normally attempt to rear (reviewed in Godfray et al., 1991; VanderWerf, 1992). If parents are capable of increasing their provisioning effort to rear additional nestlings, why do parents not routinely produce more offspring?
A widely accepted explanation, proposed initially by Williams (1966), is that clutch size is limited by reproductive costs that directly influence both parental survival (Dijkstra et al., 1990; Korpimäki & Rita, 1996) and future reproductive success (Gustafsson & Sutherland, 1988; Monaghan & Nager, 1997). Although tests for reproductive costs initially focused on costs associated with provisioning experimentally enlarged broods of nestlings, attention has subsequently shifted towards the possibility that reproductive costs associated with egg-production and incubation may also limit clutch size (see Monaghan & Nager, 1997; Nager, 2006).
Only a few studies have investigated whether fitness-related costs associated with egg production alone, independent of the other periods of the breeding cycle, might limit clutch size. The costs that have been documented come in the form of decreased local return rates in future years (Nager et al., 2001), decreased allocation to supernumerary eggs relative to earlier-laid eggs (Nager et al., 2000; Williams & Miller, 2003; Bowers et al., 2012; see also Williams, 2001), and decreased future reproduction (Bowers et al., 2012). More attention has also been paid to the possibility that metabolic and other demands placed on females during the incubation period exact reproductive costs and play a role in limiting clutch size (Thomson et al., 1998). The presence of supernumerary eggs within a nest decreases, on average, the surface area of each egg that is in contact with the brood patch of the female’s abdomen, which, in turn, can decrease incubation temperatures and hatching success (Reid et al., 2000; Nord & Nilsson, 2011). The production of additional eggs also may increase the length of the incubation period compared with that of normal-sized clutches (Dobbs et al., 2006; Nord & Nilsson, 2012).
These and other studies have demonstrated that significant reproductive costs can be incurred by increasing reproductive effort during the different stages of the nesting cycle in altricial and other species. It is not surprising, then, that in previous experiments on our study population of house wrens (Troglodytes aedon, Vieillot), manipulations of only a single stage of the reproductive cycle independent of the other stages (i.e., number of eggs laid [Bowers et al., 2012], number of eggs incubated [Baltz & Thompson, 1988; Dobbs et al., 2006], and number of nestlings reared [Finke et al., 1987; Harper et al., 1992; Bowers et al., 2014b]), have revealed disparate expressions of reproductive costs. However, to understand what actually limits clutch size in wild populations, reproductive effort must be increased in each period of the reproductive cycle to reveal how selection might act on clutch size (Heaney & Monaghan, 1995). The only study that has investigated the effects of manipulating all three periods of the breeding cycle in an altricial species revealed a cost of reproduction for females through decreased survival (Visser & Lessells, 2001). The lack of other similarly designed experiments has hindered our understanding of the selective forces shaping the evolution of clutch size and of the trade-offs involved between the number of offspring produced and residual reproductive value (i.e., effects on adult survival and expectation of future offspring).
In this study, we experimentally induced female house wrens to lay extra eggs to test the hypothesis that the number of offspring a female can produce is limited by the cumulative reproductive costs associated with producing more eggs, incubating more eggs, and provisioning more nestlings than she normally would. House wrens will produce supernumerary eggs if eggs are removed from the nest early in the egg-laying period (see Methods). As a result, females can be induced to produce more eggs (9 or more eggs) than they would normally (6-8 eggs). We predicted that if females increase reproductive effort in their current brood, they should suffer greater reproductive costs compared with unmanipulated females through one or more of the following ways: (i) the production of fewer surviving offspring or offspring of poorer quality; (ii) decreased probability of producing a second brood, or, if a second brood is attempted, having smaller clutch sizes and reduced reproductive success; (iii) lower likelihood of returning to breed and reduced reproductive success in future years; and (iv) lower recruitment and reproductive success of offspring surviving to breed in the local population.
Methods
Species and study site
House wrens are small (10–12 g), insectivorous songbirds with a widespread distribution in North America. Those in the study population return from their wintering grounds in April, breeding in central Illinois from late April to early September (Johnson, 2014). This study was carried out on the 130-ha Mackinaw Study Area (40°40’ N, 88°53’ W) ca. 30 km north of Illinois State University over four breeding seasons (2011-2014). The study site has 700 nestboxes in which 500-600 house wren nests are constructed each breeding season. Approximately 95% of the study population nests in these boxes (Drilling & Thompson, 1988), and, in any given year, we capture nearly every adult nesting on the site and ring all nestlings prior to fledging. Female house wrens lay one egg/day until their clutch is completed, and 50-70 % of those in our study population produce two broods of offspring each breeding season (Finke et al., 1987; Drilling & Thompson, 1988). Females on the study area produce, on average, clutches of 6.85 eggs (mode = 7 eggs) in May (early-season) declining to 5.5 eggs (mode = 6 eggs) in July-August (late-season) (Dobbs et al., 2006); this decline over the course of the breeding season is likely an adaptive response to a predictable seasonal decline in arthropod prey abundance (Kendeigh, 1979; Styrsky et al., 1999; Barnett et al., 2011).
Clutch-size manipulation
Nestboxes were checked twice weekly to determine the beginning of nest building. After the first egg was laid in the first clutch of a season, a treatment was randomly assigned to this nest and treatments were alternately applied to new nests thereafter in 2011, 2012, and 2013. Control (CON) females were unmanipulated. For the experimental (EXP) treatment, we manipulated the number of eggs produced by removing eggs 2-5 (leaving the first egg in the nest) as laid, thereby inducing females to produce extra eggs (Kennedy & Power, 1990; Bowers et al., 2012). Eggs that had been removed were stored in cotton-filled film canisters and held at room temperature (ca. 21°C) on a shelf in the laboratory. On the morning that egg 6 was laid, we returned eggs 2-5 to the nest. This procedure for storing eggs is unlikely to affect their hatchability. In a previous experiment on our study population, we increased clutch size using a similar approach, but removed a subset of eggs from the nest shortly after clutch completion to ensure that control and experimental females incubated clutches of similar size (Bowers et al., 2012). In that experiment, the hatchability of eggs produced by control and experimental females did not differ (E.K. Bowers, unpublished data). Females usually continued to lay 2-4 additional eggs before egg laying ceased and incubation began, resulting in significantly enlarged clutch sizes of 8-11 eggs for experimental females compared with control females (Fig. 1a). Nests occasionally failed prior to identifying the female (either because of nest depredation or abandonment); thus, our final sample of control and experimental females was 77 and 71, respectively, across the three breeding seasons. As eggs sometimes disappeared during the egg-laying period, we numbered the eggs with a non-toxic marker as they were laid to determine the total number of eggs produced (i.e., clutch size). To ensure that females were incubating enlarged clutches, we visited nests twice weekly and replaced any eggs that disappeared with replica dummy eggs, which are readily accepted and incubated, and all females assigned to the experimental group were retained for analyses (i.e., we did not omit experimental females from analyses if they lost eggs or nestlings). Once the eggs hatched, we monitored the success of each nest. Finally, if a female attempted a second brood later in the same season, we determined the reproductive success of these females in their unmanipulated, second-brood nests.
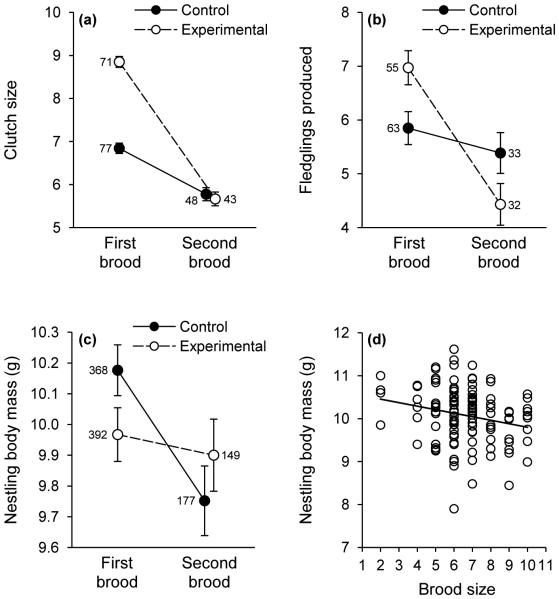
Fig. 1.
(a) Clutch sizes of first (manipulated) and second (unmanipulated) broods of control and experimental females within breeding seasons. (b) The number of offspring successfully fledged by control and experimental females in first and second broods. (c) Body mass of nestlings produced by control and experimental females prior to fledging from first and second broods within seasons. (d) Nestling body mass prior to fledging (brood means) in relation to brood size four days after hatching began in their nest.
Field procedures
Females were captured 5-6 d after incubation began (and, thus, after their treatment had been assigned) using either a permanently mounted sliding trapdoor attached to the nestbox or a mist net placed in front of the nestbox. Each female was ringed with a numbered, aluminium U. S. Geological Survey ring so that we could determine her future reproductive success during the current and any future breeding seasons. We measured mass to the nearest 0.1 g using a digital scale (Acculab Pocket Pro PP 201) and tarsus length to the nearest 0.1 mm using dial callipers. We monitored the future reproductive success of females in their second brood and in the following breeding season if they returned to breed.
Males were captured either in mist nets at the same time as the female, or during the nestling period using either the trapdoor or a mist net. Each male was ringed using a unique combination of three coloured, Darvic rings and one aluminium ring (two rings/leg). Unique colour-ring combinations made it possible to identify males without recapturing them. Mass and tarsus measurements were also recorded for captured males.
On brood-day 11 (brood-day 0 is the day hatching begins), we ringed each nestling with a numbered, aluminium ring, and measured their mass and tarsus length. We collected blood samples, performed the phytohaemagglutinin (PHA) skin test (see ‘Nestling immune response’ below), and measured haematocrit of all nestlings surviving to brood-day 11 from early-season nests. Blood was collected in heparinized microcapillary tubes and stored on ice in the field, and was processed later the same day.
Laboratory procedures
On the day of blood sampling, samples were centrifuged at 6,000 rpm for 60 s (Hematastat II, Separation Technology, Inc.) to separate red blood cells from plasma. Once the samples were centrifuged, we measured the volume of packed red blood cells three times and averaged the measurements to obtain a measure of haematocrit, which is expressed as the percentage of whole blood that is comprised of erythrocytes and is a widely used physiological indicator of condition (Ots et al., 1998; Bowers et al., 2014a; Sakaluk et al., 2014). Red blood cells were preserved in Queen’s Lysis buffer and stored at 4°C until they were used to determine nestling sex. Nestlings cannot be sexed using external morphology; therefore, we extracted DNA from the stored red blood cells and used polymerase chain reaction to determine nestling sex to identify any treatment effects on male and female nestlings (for details, see Kahn et al., 1998; Bowers et al., 2011).
Nestling immune response
We assessed nestling immunoresponsiveness using the PHA skin-swelling test. Injection of PHA into the wing web results in inflammation and swelling, and provides a measure of cutaneous immune activity (Smits et al., 1999; Martin et al., 2006). On brood-day 11, after blood sampling, we measured nestlings’ initial wing-web thickness to the nearest 0.01 mm using a digital thickness gauge (Mitutoyo no. 547–500) in three consecutive measurements to obtain a mean pre-injection thickness. Next, we injected the left wing-web with 50 µL of sterile phosphate buffered saline (PBS) in which 5 mg/mL of PHA (Sigma Aldrich cat. # L8754) was dissolved (Smits et al., 1999). After approximately 24 h, we again measured the wing-web thickness three times to obtain a mean post-injection thickness. We used the difference between the mean pre- and post-injection measures as a measure of cutaneous immune responsiveness.
Statistical analysis
All analyses were performed using SAS statistical software (SAS 9.3; SAS Institute, Cary, NC, USA) and all tests were two-tailed (α = 0.05). All means reported are least-squares means ± one standard error (SE) unless otherwise noted. We included year as a random effect and tested for two-way interactions between treatment and year in each analysis. We then obtained parsimonious models by removing non-significant (P > 0.15) terms, beginning with the interactions (reported only when significant). When two-way interactions were significant, we compared simple effects as a follow-up to test the effect of one factor (e.g., treatment) at each level of the other factor (e.g., first or second brood of the season). Conducting the experiment over three years resulted in some females (N = 5) being manipulated in multiple seasons; three control females from a previous breeding season were each randomly assigned to the control group in a subsequent year, one former control female was assigned to the experimental group in a subsequent year, and one former experimental female was also assigned to the experimental group in a subsequent year. In these instances, female identity was included as an additional random effect (in addition to year) to account for non-independence of observations (the experimental females were omitted from our analyses of inter-annual costs). For analyses involving nestlings, nest identity was included as a random effect (in addition to year and female identity) to control for non-independence of nestlings within broods. We used Satterthwaite’s degrees-of-freedom approximation in our analyses, which can result in non-integer denominator degrees of freedom.
We first analysed variation in clutch sizes, including all known females that completed a clutch. We determined the effect of the clutch manipulation on the clutch sizes of first and second broods of control and experimental females using a repeated-measures, general linear mixed model (LMM; PROC MIXED). We then analysed hatching success (number of eggs that hatched/number of eggs present at the end of incubation) and early hatchling survival (number of young present on brood-day 4/number of eggs that hatched) using LMMs with female identity as a random effect (in addition to year). We also analysed the number of offspring fledged from first and second broods within seasons using a similar approach. We then used a Cox proportional-hazards regression model (survival analysis; PROC PHREG) to analyse the length of a female’s interbrood interval (days between the fledging of her first brood and the initiation of her second clutch), and we included the fledging date of a female’s first brood as a covariate. We also used survival analysis (as above) to analyse the length of the apparent incubation period (days elapsed between clutch completion and the hatching of the first egg), the length of the nestling period (days from hatching of the first egg to fledging), and the length of the entire nesting cycle (days elapsed between laying of the first egg of the clutch and fledging). We then determined the effect of the manipulation on the likelihood that females fledging their first brood would attempt a second (unmanipulated) brood using a logistic-type generalized linear mixed model (GLMM; PROC GLIMMIX) with a binary response (double-brooded or not); we also included the initiation date of a female’s first brood as a covariate. We then tested for treatment effects on offspring phenotype, including their body mass, haematocrit, and immune responsiveness using LMMs. Both haematocrit and PHA responsiveness were measured in first broods only; each of these traits often covaries with body mass in our study species (Forsman et al., 2010; Bowers et al., in press), so we included body mass as a covariate in our analyses of haematocrit and PHA responsiveness.
Finally, we tested for lasting effects on the future reproductive success of females and the offspring they produced during the experiment. We first analysed female return rates to the study site in the years following the manipulation using a GLMM with binary responses (as above), and we included whether females were single-brooded or double-brooded during the previous breeding season as a main effect and female body mass as a covariate. We tested for effects on offspring recruitment in subsequent breeding seasons using a similar approach, but with nest and maternal identities as random effects (in addition to year). We also included brood number (first or second of the season) as a fixed factor to investigate differences between early- and late-season broods. We then used LMMs to analyse the future reproductive success (number of eggs produced) of returning females and that of recruited offspring in subsequent seasons (unmanipulated) to evaluate any inter-seasonal reproductive costs. We also analysed the first breeding date of females and offspring and their propensity to produce multiple broods on the study area in their subsequent breeding seasons using survival analysis and a GLMM with binary responses, respectively. We also tested for potential stabilizing selection on clutch size among unmanipulated females using a quadratic regression of a fitness measure (i.e., lifetime number of fledglings produced) on clutch size (scaled following Schielzeth 2010).
Results
Reproductive success within seasons
There was a significant interaction between treatment and brood number in their effect on clutch sizes (F1, 113 = 68.69, P < 0.0001). Experimental females produced more eggs in their first brood than control females (F1, 146 = 122.52, P < 0.0001; Fig. 1a), but clutch sizes in the second brood did not differ between control and experimental females (F1, 77.9 = 0.23, P = 0.6365; Fig. 1a). Clutch sizes declined significantly from the first to second broods for both control and experimental females (F1, 113 = 276.00, P < 0.0001; Fig. 1a).
Hatching success was significantly higher for control than for experimental females during both first and second broods (proportion of eggs that hatched: control = 0.899 ± 0.023, N = 106 broods; experimental = 0.843 ± 0.024, N = 94 broods; LMM: F1, 122 = 5.73, P = 0.0182), and tended to increase from the first to second brood (first brood = 0.849 ± 0.022, N = 127 broods; second brood = 0.893 ± 0.025, N = 73 broods; F1, 118 = 3.65, P = 0.0584), but there was no interaction between treatment and brood number (not shown). Analysing hatching success using events/trials syntax in a GLMM produced qualitatively similar results (not shown). The proportion of hatchlings that survived to brood-day 4 was significantly greater for control than for experimental females during both first and second broods (control = 0.949 ± 0.012, N = 101 broods; experimental = 0.905 ± 0.018, N = 88 broods; F1, 185 = 5.31, P = 0.0224), and this did not change from first to second broods, nor was there an interaction between treatment and brood number (data not shown).
There was a significant interaction between treatment and brood number in their effect on the number of offspring fledged (F1, 84 = 17.49, P < 0.0001; Fig. 1b). Despite experimental nests having lower hatching success and hatchling survival, these nests produced more fledglings than controls from the first brood of the season (F1, 158 = 173.78, P = 0.0003; Fig. 1b), but experimental females fledged fewer young than controls in the second brood (F1, 163 = 4.78, P = 0.0302; Fig. 1b). The number of offspring fledged by experimental females declined significantly from the first to the second brood (F1, 81.7 = 50.07, P < 0.0001; Fig. 1b), whereas the number of offspring fledged by control females did not differ significantly between first and second broods (F1, 85.4 = 1.81, P = 0.1816; Fig. 1b). The total number of fledglings produced in the breeding season did not differ between control and experimental females (F1, 105 = 0.09, P = 0.7668; Fig. 1b). Similarly, the proportion of eggs laid that produced fledglings was significantly higher for control than for experimental females (mean ± SE proportion fledged; control: 0.867 ± 0.033; experimental: 0.795 ± 0.033; LMM: F1, 108 = 6.23, P = 0.014), and this was the case throughout breeding seasons, as there was no interaction between treatment and brood number (data not shown), nor was there a difference between first and second broods within seasons in the proportion of offspring laid as an egg that survived to fledge (F1, 93.5 = 2.14, P = 0.147).
Effects on the duration of breeding attempts
There was a significant interaction between treatment and brood number (first or second brood of the season) on the incubation period (survival analysis: parameter estimate ± SE = 1.230 ± 0.314, Wald = 15.38, P < 0.0001). Means ± SE reported in this sub-section are arithmetic means (excluding failed nests). The incubation period for experimental females was significantly longer than for controls within the first brood (control = 11.95 ± 0.13 d, N = 73 clutches; experimental = 12.33 ± 0.14 d, N = 61 clutches), but not within the second brood (control = 11.2 ± 0.12 days, N = 40 clutches; experimental = 11.0 ± 0.10 days, N = 38 clutches). Incubation periods declined significantly from first to second broods (first brood = 12.12 ± 0.10 d, N = 134 clutches; second brood mean = 11.10 ± 0.08 d, N = 78 clutches).
The nestling period did not differ between control and experimental females (Wald = 2.14, P = 0.1435) in either the first brood (control = 15.1 ± 0.18 d, N = 63 broods; experimental = 14.9 ± 0.20 d, N = 55 broods) or the second brood (control = 14.7 ± 0.27 d, N = 33 broods; experimental = 14.5 ± 0.2 d, N = 32 broods), and there was no interaction between treatment and brood number (not shown). There was, however, an interaction between treatment and brood number in their effect on the length of the nesting cycle (Wald = 11.70, P = 0.0006), with experimental females taking approximately two more days, on average, to complete their first nesting cycle than control females (control = 33.9 ± 0.22 d, N = 63 broods; experimental = 36.1 ± 0.28 d, N = 55 broods). In second broods within seasons, the length of the nesting cycle did not differ between treatments (control = 31.7 ± 0.3 d, N = 33 broods; experimental = 31.2 ± 0.27 d, N = 32 broods).
Effects on females within seasons
The clutch-size manipulation did not affect female body mass (F1, 153 = 0.00, P = 0.9477), nor was there an interaction between treatment and brood number. Female body mass declined from first to second broods equally for control and experimental females (first brood = 12.17 ± 0.08 g, N = 148 females; second brood = 11.84 ± 0.09 g, N = 91 females; F1, 99.6 = 19.30, P < 0.0001).
Experimental females were equally as likely as controls to attempt a second brood (control: 40/77 females; experimental: 35/71 females; F1, 135.1 = 0.00, P = 0.9988). Females producing their first brood earlier in the year were more likely to initiate a second brood than those fledging their first broods later in the season (GLMM: parameter estimate ± SE = −0.093 ± 0.029, F1, 145 = 10.23, P = 0.0017).
The interbrood interval did not differ between control (median = 12 d, N = 40 females) and experimental females (median = 9 d, N = 35 females) (survival analysis: parameter estimate ± SE = 0.068 ± 0.233, Wald = 0.09, P = 0.7706). The date that females fledged their first brood marginally affected the length of their interbrood interval, with females fledging their first broods earlier in the breeding season taking slightly less time to initiate a second brood (parameter estimate ± SE = −0.027 ± 0.016, Wald = 3.09, P = 0.0787).
Effects on offspring within seasons
There was an interaction between treatment and brood number in their effect on offspring body mass prior to fledging (LMM: F1, 103 = 4.51, P = 0.0360; Fig. 1c). Within first broods, experimental females produced lighter offspring, on average, than control females (F1, 80.6 = 4.34, P = 0.0403), but not during their second brood (F1, 62.2 = 1.61, P = 0.2088). Indeed, increases in brood size had a negative effect on nestling body mass across all broods (LMM: parameter estimate ± SE = −0.083 ± 0.030, F1, 180 = 7.59, P = 0.0065; Fig. 1d). Male nestlings were heavier than female nestlings (males = 10.05 ± 0.06 g, N = 383; females = 9.93 ± 0.06 g, N = 443; F1, 720 = 8.33, P = 0.0040). There was no difference in the body mass of those control and experimental nestlings that would eventually survive and recruit to the local population as adults (F1, 25.6 = 0.41, P = 0.5273).
Haematocrit did not differ significantly between the offspring of control and experimental females (control = 40.0 ± 0.5 %, N = 398 nestlings; experimental = 41.2 ± 0.6 %, N = 393 nestlings; F1, 109 = 2.47, P = 0.1186). Haematocrit also did not differ significantly between male and female nestlings (F1, 717 = 1.20, P = 0.2733), but this trait was positively correlated with nestling body mass (parameter estimate ± SE = 0.983 ± 0.248, F1, 803 = 15.77, P < 0.0001).
There was a marginally non-significant interaction between treatment and sex in their effect on nestlings’ PHA responsiveness (F1, 707 = 3.49, P = 0.0623). Within control broods, female nestlings had higher PHA responsiveness than males (LS means ± SE: female = 0.755 ± 0.062 mm, N = 171; male = 0.691 ± 0.063 mm, N = 213; F1, 350 = 6.04, P = 0.0145), but this difference disappeared within experimental broods (female = 0.713 ± 0.065 mm, N = 189; male = 0.711 ± 0.065 mm, N = 208; F1, 358 = 0.01, P = 0.9069).
Carry-over effects on females
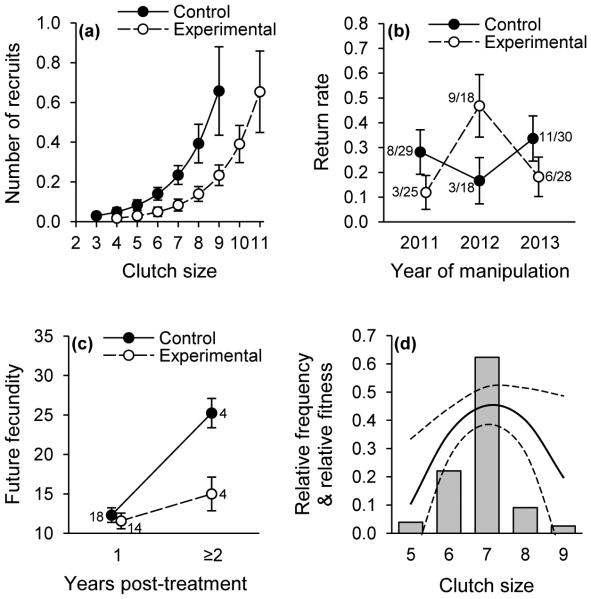
There was a marginally non-significant interaction between treatment and the year of the manipulation in determining whether females returned to breed at the study site in subsequent years (F1, 137.1 = 3.02, P = 0.0519; Fig. 2b). The interaction arose primarily because return rates differed among years for experimental females (F2, 48 = 4.45, P = 0.0169) but not for controls (F2, 57 = 0.59, P = 0.5568), as there was no significant difference between treatments within either year (2011: F1, 40 = 1.38, P = 0.2471; 2012: F1, 23 = 3.10, P = 0.0916; 2013: F1, 40 = 3.31, P = 0.0764; Fig. 2b). Whether a female produced one or two broods within a breeding season did not affect the likelihood that she would return to breed in subsequent years (F1, 136.5 = 1.54, P = 0.2171), nor did a female’s body mass predict whether she would return to breed in subsequent years (F1, 137.7 = 0.04, P = 0.8511).
Fig. 2.
(a) Number of recruits (± SE) in relation to clutch size and treatment. (b) Rates at which females returned to breed in the population in the year following their inclusion in the experiment. (c) Cumulative future fecundity (number of eggs produced) by females that were included in the experiment and not manipulated in subsequent breeding seasons. (d) Distribution of clutch sizes among unmanipulated females (relative frequency; filled bars); curves represent the relative fitness (i.e., the lifetime number of fledglings relative to the population maximum) of these females (quadratic regression ± 95% confidence limits).
There was no difference between control and experimental females’ fecundity in the year immediately following the manipulation, but effects on their future fecundity arose when breeding at least two years after the manipulation (LMM: F1, 33 = 9.17, P = 0.0047; Fig. 2c). There was no difference between control and experimental females’ fecundity when breeding one year after the manipulation (F1, 30 = 0.30, P = 0.5890), but experimental females had significantly lower fecundity when breeding in future years (F1, 6 = 13.10, P = 0.0111; Fig. 2c). This long-term effect on females’ fecundity was not attributable to increased longevity of control relative to experimental birds (Wald = 0.11, P = 0.7356), or to differences in breeding dates in subsequent years (day of the year: control females = 139.6 ± 3.2, N = 22; experimental females = 135.6 ± 1.9, N = 18; Wald = 1.60, P = 0.2057); the likelihood of being double-brooded in subsequent breeding seasons also did not differ between treatments (control: 11/22; experimental: 10/18; F1, 37 = 0.01, P = 0.9164).
Our selection analysis suggested significant stabilizing selection on clutch size (Fig. 2d), as there was a hump-shaped relationship between a female’s lifetime number of fledglings produced in the study population and her initial clutch size (quadratic term: estimate ± SE = −0.166 ± 0.062, F1, 68.5 = 7.06, P = 0.0098; linear term: estimate ± SE = −0.142 ± 0.097, F1, 69.7 = 2.15, P = 0.1470). The lifetime number of fledglings that females produced was maximized when producing the modal clutch size of 7 eggs in their first brood (Fig. 2d).
Offspring recruitment and reproductive success
Among individual offspring, their probability of recruiting as a breeder to the study population did not differ significantly between maternal treatments (proportion of offspring ± SE: control = 0.046 ± 0.009, N = 546 fledglings; experimental = 0.040 ± 0.008, N = 529 fledglings; F1, 144.4 = 0.17, P = 0.677). For any given clutch size, however, control females produced more recruits to the breeding population than did experimental females (control = 0.221 ± 0.070; experimental = 0.075 ± 0.034; F1, 236 = 5.33, P = 0.0218; effect of clutch size: estimate ± SE = 0.525 ± 0.138, F1, 236 = 14.48, P = 0.0002; Fig. 2a). The proportion of first-brood offspring recruited into the breeding population exceeded that of second-brood offspring (first brood = 0.059 ± 0.009, N = 747 fledglings; second brood = 0.006 ± 0.004, N = 323 fledglings; F1, 1067 = 10.15, P = 0.0015), but there was no interaction between treatment and brood number, indicating that the differences in recruitment between groups persisted between first and second broods.
The date that recruited offspring initiated their first brood during their first breeding season as an adult did not differ significantly between maternal treatment groups (day of the year: control = 150.8 ± 4.2, N = 25 recruits; experimental = 152.3 ± 5.0, N = 21 recruits; Wald = 0.26, P = 0.6121). Also, the total number of eggs produced by unmanipulated recruits in subsequent breeding seasons did not differ significantly between maternal treatments (mean ± SE number of eggs: control = 8.9 ± 0.9, N = 22 recruits; experimental = 11.1 ± 1.0, N = 17 recruits; F1, 36 = 2.51, P = 0.1221). Finally, the likelihood that recruits would be double-brooded in subsequent breeding seasons also did not differ between maternal treatments (control = 7/25 recruits; experimental = 4/21; F1, 43 = 0.31, P = 0.5810).
Discussion
Our results are consistent with the hypothesis (Williams, 1966) that optimal clutch size is shaped by reproductive costs incurred at different stages of the reproductive cycle. The experimental increase in reproductive effort during the three stages of the nesting cycle strongly affected female fitness. Although female house wrens were capable of producing and incubating more eggs and successfully rearing more nestlings during the first brood than they normally attempt, and the number of recruits produced to the breeding population did not differ between control and experimental females, control females produced more recruits to the breeding population per egg laid than experimental females, a finding that is consistent with the hypothesis that natural selection favours the level of investment that maximizes the net fitness returns for parents per unit of investment (Trivers 1972, 1974). Reduced fitness of experimental females was brought about through a combination of both intra-annual costs (i.e., reduced hatchling survival and size) and inter-annual costs (i.e., lower fecundity in future breeding seasons), with the net result that females producing the modal clutch size of seven eggs had the highest expected lifetime reproductive success (Fig. 2d).
Despite increasing their reproductive effort and successfully fledging more offspring than control females during their first brood, experimental females were equally likely to produce a second brood, did not take longer to initiate a second brood, and did not produce smaller second-brood clutches. However, experimental females suffered reduced hatching success and hatchling survival in both their first and second broods, and produced fewer fledglings than control females from their second, unmanipulated broods. One possible reason for the reduction in hatchling survival, particularly during the second brood, is that the effort expended by experimental females in raising their first brood made them less competent parents than control females later in the season. In house wrens, only the female incubates eggs, and, early in the nestling period, females are solely responsible for brooding their altricial young; females also provision their nestlings with food at a higher rate than do their social mates (Barnett et al., 2011; Bowers et al., 2014b). Experimental females, therefore, may have had to increase foraging for themselves to offset the costs of their increased reproductive effort earlier in the season, and consequently been less effective than control females at brooding and provisioning their second-brood hatchlings. Additionally, previous work on the study population has shown that increased egg production generates a trade-off between egg number and quality (Bowers et al., 2012). Eggs produced beyond the normal number receive reduced allocation of maternal resources, including protein and lipids (Nager et al., 2000; Williams, 2001; Williams & Miller, 2003), and such a decrease in egg quality may have contributed to the reduction in hatchling survival within experimental nests in both first and second broods within seasons. In addition to generating costs through effects on a female’s subsequent reproduction within and across breeding seasons, the production of supernumerary eggs in the first brood significantly increased the length of the incubation period and the length of the breeding cycle in which offspring are confined to a nest, which can often affect the risk of nest depredation (e.g., Julliard et al., 1997; Lima, 2009; see also Zanette et al., 2006).
We did not detect the expected negative relationship between increased reproductive effort and the likelihood that females would return to breed in future years (for similar results, see Young, 1996; Bowers et al., 2014b). Similarly, previous studies in which extra nestlings only were placed in the nest during the nestling period did not find evidence of negative effects on second broods in house wrens in both our population and another (Finke et al., 1987; Robinson & Rotenberry, 1991, respectively). These earlier results called into question the existence of reproductive costs as originally predicted by Lack (1947); however, failure to detect costs of increased brood size may be caused, at least in part, by an increase in site fidelity or a female’s reproductive effort if the artificial increase in reproductive success positively influences a female’s perception of the quality of breeding sites (Bowers et al., 2014b). For example, on our study site, females re-nesting after nest failure are more likely to move farther from their original nest site than successful females, and those fledging more offspring in a breeding season are more likely to return the following year than less-successful females, suggesting that females associate nest sites with differences in reproductive success (Drilling & Thompson, 1988; see also Nolan, 1978; Weatherhead & Boak, 1986; Danchin et al., 1998; Doligez et al., 1999; Schjorring et al., 2000; Hoover, 2003). The experimental females in the current study experienced the same direct benefits of producing and raising an enlarged brood (as well as producing and incubating more eggs), and this may have directly influenced their decision to be double-brooded. Such a process could also explain why females that produce enlarged clutches but that do not benefit from fledging more young (i.e., those not reaping the full benefits associated with producing a larger clutch), have a decreased likelihood of producing a second brood, an increased interbrood interval, and reduced success of their second brood (e.g., de Heij, 2006; Bowers et al., 2012). Caution, therefore, should be used when extrapolating the generality of brood-manipulation studies, as the responses of individual animals are often context-dependent (Reznick, 1985; McNamara & Houston, 1996).
Contrary to our predictions, experimental and control females did not differ in mass, suggesting that experimental females did not experience an immediate trade-off between self-maintenance and reproductive effort in producing an enlarged first brood. However, increased clutch and brood sizes did result in experimental females producing lighter offspring (Fig. 1c). Previous experiments on the study population in which only brood size was enlarged did not reveal brood-size effects on nestling mass (Finke et al., 1987; Harper et al., 1992; but see Bowers et al., 2014b); thus, as in the case of reduced hatchling survival, the treatment effect on offspring mass in the current study may be attributable, at least in part, to maternal effects manifested through the egg, in addition to reduced per-capita resource availability of nestlings, because experimental increases in clutch size affect pre-natal allocation to eggs (Nager et al., 2000; Williams, 2001; Williams & Miller, 2003; Bowers et al., 2012). Many studies, including the current study, have shown a negative relationship between nestling mass and brood size (Nur, 1984; Dijkstra et al., 1990; Arnold, 1993, Sanz & Tinbergen, 1999; Pettifor et al., 2001; Mitchell et al., 2011; Clairardin et al., 2011; Bowers et al., 2015). It is well-documented that post-fledging survival in a wide range of species is positively correlated with fledgling body mass (e.g., Krementz et al., 1989; Tinbergen & Boerlijst, 1990; Hochachka & Smith, 1991; Magrath, 1991; Both et al., 1999; Monrós et al., 2002; Schwagmeyer & Mock, 2008; Mitchell et al., 2011). This is also true of tropical house wrens (Young, 1996) and house wrens in our central-Illinois population (Bowers et al., 2014a).
In conclusion, the results of this study demonstrate the role that increased cumulative reproductive effort in the egg-production, egg-incubation, and nestling-provisioning periods of the reproductive cycle plays in shaping the optimal clutch size of an altricial bird. Increasing clutch size in the first brood resulted in the production of more, but lighter, fledglings, and over the course of the breeding season control females produced more recruits to the breeding population per egg laid than experimental females. Furthermore, experimental females suffered intra-seasonal costs in the form of reduced hatchling survival in their second brood and inter-annual costs in the form of lower fecundity in future breeding seasons.
Acknowledgments
This work was supported by grants from the National Science Foundation (IBN-0316580, IOS-0718140, and IOS-1118160) and the National Institutes of Health (R15HD076308-01) to SKS and CFT, student awards from the Beta Lambda Chapter of the Phi Sigma Biological Sciences Honor Society and the American Museum of Natural History’s Frank M. Chapman Memorial Fund to CJH, and Research Internships in Science and Engineering (RISE) from the Deutscher Akademischer Austauschdienst. We thank the ParkLands Foundation (Merwin Preserve) and the Sears and Butler families for allowing us to use their properties, and R. Bowden and V. Borowicz for their support and guidance throughout this study. We thank two anonymous reviewers for their helpful comments on the manuscript, and M. Strange, M. Sakaluk, R. Machett, J. Chamberlain, C. Loebach, C. McGrath, R. Goeker, A. Voigt, J. Pomahac, T. Martini, P. Kohlmeier, C. Lothery, M. Lawler, K. Ringer, D. Nietz, and M. Schuld for assistance in the field and laboratory.
References
- Arnold TW. Fledging success in experimentally manipulated broods of house wrens. Wilson Bull. 1993;105:448–454. [Google Scholar]
- Baltz ME, Thompson CF. Successful incubation of experimentally enlarged clutches by house wrens. Wilson Bull. 1988;100:70–79. [Google Scholar]
- Barnett CA, Clairardin SG, Thompson CF, Sakaluk SK. Turning a deaf ear: A test of the manipulating androgens hypothesis in house wrens. Anim. Behav. 2011;81:113–120. [Google Scholar]
- Both C, Visser ME, Verboven N. Density-dependent recruitment rates in great tits: the importance of being heavier. Proc. R. Soc. Lond. B. 1999;266:465–469. [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Adaptive sex allocation in relation to hatching synchrony and offspring quality in house wrens. Am. Nat. 2011;177:617–629. doi: 10.1086/659630. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Experimentally increased egg production constrains future reproduction of female house wrens. Anim. Behav. 2012;83:495–500. [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014a;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Nietz D, Thompson CF, Sakaluk SK. Parental provisioning in house wrens: effects of varying brood size and consequences for offspring. Behav. Ecol. 2014b;25:1485–1493. [Google Scholar]
- Bowers EK, Thompson CF, Sakaluk SK. Persistent sex-by-environment effects on offspring fitness and sex-ratio adjustment in a wild bird population. J. Anim. Ecol. 2015;84:473–486. doi: 10.1111/1365-2656.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. Am. Nat. doi: 10.1086/681017. In press. doi:10.1086/681017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clairardin SG, Barnett CA, Sakaluk SK, Thompson CF. Experimentally increased in ovo testosterone leads to increased plasma bactericidal activity and decreased cutaneous immune response in nestling house wrens. J. Exp. Biol. 2011;214:2778–2782. doi: 10.1242/jeb.054833. [DOI] [PubMed] [Google Scholar]
- Danchin E, Boulinier T, Massot M. Conspecific reproductive success and breeding habitat selection: implications for the study of coloniality. Ecology. 1998;79:2415–2428. [Google Scholar]
- de Heij ME, Van den Hout PJ, Tinbergen JM. Fitness cost of incubation in great tits (Parus major) is related to clutch size. Proc. R. Soc. B. 2006;273:2353–2361. doi: 10.1098/rspb.2006.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C, Bult A, Bijlsma S, Daan S, Meijer T, Zijlstra M. Brood size manipulation in the kestrel (Falco tinnunculus): effects on offspring and parent survival. J. Anim. Ecol. 1990;59:269–285. [Google Scholar]
- Dobbs RC, Styrsky JD, Thompson CF. Clutch size and the costs of incubation in the house wren. Behav. Ecol. 2006;17:849–856. [Google Scholar]
- Doligez B, Danchin E, Colbert J, Gustafsson L. The use of conspecific reproductive success for breeding habitat selection in a non-colonial, hole-nesting species, the Collared Flycatcher. J. Anim. Ecol. 1999;68:1193–1206. [Google Scholar]
- Drilling NE, Thompson CF. Natal and breeding dispersal in house wrens (Troglodytes aedon) Auk. 1988;105:480–491. [Google Scholar]
- Finke MA, Milinkovich DJ, Thompson CF. Evolution of clutch size: an experimental test in the house wren (Troglodytes aedon) J. Anim. Ecol. 1987;56:99–114. [Google Scholar]
- Forsman AM, Sakaluk SK, Thompson CF, Vogel LA. Cutaneous immune activity, but not innate immune responsiveness, covaries with mass and environment in nestling house wrens (Troglodytes aedon) Physiol. Biochem. Zool. 2010;83:512–518. doi: 10.1086/649894. [DOI] [PubMed] [Google Scholar]
- Godfray HCJ, Partridge L, Harvey PH. Clutch size. Ann. Rev. Ecol. Syst. 1991;22:409–429. [Google Scholar]
- Gustafsson L, Sutherland WJ. The costs of reproduction in the collard flycatcher Ficedula albicollis. Nature. 1988;335:813–815. [Google Scholar]
- Harper GR, Juliano SA, Thompson CF. Hatching asynchrony in the house wren, Troglodytes aedon: a test of the brood-reduction hypothesis. Behav. Ecol. 1992;3:76–83. [Google Scholar]
- Heaney V, Monaghan P. A within-clutch trade-off between egg production and rearing in birds. Proc. R. Soc. Lond. B. 1995;261:361–365. [Google Scholar]
- Hochachka W, Smith JNM. Determinants and consequences of nestling condition in song sparrows. J. Anim. Ecol. 1991;60:995–1008. [Google Scholar]
- Hoover JP. Decision rules for site fidelity in a migratory bird, the prothonotary warbler. Ecology. 2003;84:416–430. [Google Scholar]
- Johnson LS. House wren (Troglodytes aedon) In: Poole A, editor. The Birds of North America. Cornell Lab of Ornithology and American Ornithologists’ Union. 380. 2nd Ithaca, NY: 2014. [Google Scholar]
- Julliard R, McCleery RH, Clobert J, Perrins CM. Phenotypic adjustment of clutch size due to nest predation in the great tit. Ecology. 1997;78:394–404. [Google Scholar]
- Kahn NW, St. John J, Quinn TW. Chromosome-specific intron size differences in the avian CHD gene provide an efficient method for sex identification in birds. Auk. 1998;115:1074–1078. [Google Scholar]
- Kendeigh SC. Invertebrate populations of the deciduous forest: fluctuations and relation to weather. Illinois Biol. Monogr. 1979;50:1–107. [Google Scholar]
- Kennedy ED, Power HW. Experiments on indeterminate laying in house wrens and European starlings. Condor. 1990;92:861–865. [Google Scholar]
- Korpimäki E, Rita H. Effects of brood size manipulations on offspring and parental survival in the European kestrel under fluctuating food conditions. Écoscience. 1996;3:264–273. [Google Scholar]
- Krementz DG, Nichols JD, Hines JE. Postfledging survival of European starlings. Ecology. 1989;70:646–655. [Google Scholar]
- Lack D. The significance of clutch-size. Ibis. 1947;89:302–352. [Google Scholar]
- Lima SL. Predators and the breeding bird: behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 2009;84:485–513. doi: 10.1111/j.1469-185X.2009.00085.x. [DOI] [PubMed] [Google Scholar]
- Magrath RD. Nestling weight and juvenile survival in the blackbird, Turdus merula. J. Anim. Ecol. 1991;60:335–351. [Google Scholar]
- Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. Phytohemagglutinin-induced skin swelling birds: histological support for a classic immunoecological technique. Funct. Ecol. 2006;20:290–299. [Google Scholar]
- McNamara JN, Houston AI. State-dependent life histories. Nature. 1996;380:215–221. doi: 10.1038/380215a0. [DOI] [PubMed] [Google Scholar]
- Mitchell GW, Guglielmo CG, Wheelwright NT, Freeman-Gallant CR, Norris DR. Early life events carry over to influence pre-migratory condition in a free-living songbird. PLoS One. 2011;6:e28838. doi: 10.1371/journal.pone.0028838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P, Nager RG. Why don’t birds lay more eggs? Trends Ecol. Evol. 1997;12:270–274. doi: 10.1016/s0169-5347(97)01094-x. [DOI] [PubMed] [Google Scholar]
- Monrós JS, Belda EJ, Barba E. Post-fledging survival of individual great tits: the effect of hatching date and fledging mass. Oikos. 2002;99:481–488. [Google Scholar]
- Nager RG, Monaghan P, Houston DC. Within-clutch trade-offs between the number and quality of eggs: experimental manipulations in gulls. Ecology. 2000;81:1339–1350. [Google Scholar]
- Nager RG, Monaghan P, Houston DC. The cost of egg production: increased egg production reduces future fitness in gulls. J. Avian Biol. 2001;32:159–166. [Google Scholar]
- Nager RG. The challenges of making eggs. Ardea. 2006;94:323–346. [Google Scholar]
- Nolan V. The ecology and behavior of the prairie warbler Dendroica discolor. Ornithol. Monogr. 1978;26:1–595. [Google Scholar]
- Nord A, Nilsson J. Incubation temperature affects growth and energy metabolism in blue tit nestlings. Am. Nat. 2011;178:639–651. doi: 10.1086/662172. [DOI] [PubMed] [Google Scholar]
- Nord A, Nilsson J. Context-dependent costs of incubation in the pied flycatcher. Anim. Behav. 2012;84:427–436. [Google Scholar]
- Nur N. The consequences of brood size for breeding blue tits. II. Nestling weight, offspring survival and optimal brood size. J. Anim. Ecol. 1984;53:497–517. [Google Scholar]
- Ots I, Murumägi A, Hõrak P. Haematological health state indices of reproducing great tits: methodology and sources of natural variation. Funct. Ecol. 1998;12:700–707. [Google Scholar]
- Pettifor RA, Perrins CM, McCleery RH. The individual optimization of fitness: variation in reproductive output, including clutch size, mean nestling mass and offspring recruitment, in manipulated broods of great tits Parus major. J. Anim. Ecol. 2001;70:62–79. [Google Scholar]
- Reid JM, Monaghan P, Ruxton GD. Resource allocation between reproductive phases: the importance of thermal conditions in determining the cost of incubation. Proc. R. Soc. Lond. B. 2000;267:37–41. doi: 10.1098/rspb.2000.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reznick D. Costs of reproduction: an evaluation of the empirical evidence. Oikos. 1985;44:257–267. [Google Scholar]
- Robinson KD, Rotenberry JT. Clutch size and reproductive success of house wrens rearing natural and manipulated broods. Auk. 1991;108:277–284. [Google Scholar]
- Roff DA. Life History Evolution. Sinauer Associates; Sunderland, Massachusetts, USA: 2002. [Google Scholar]
- Sakaluk SK, Wilson AJ, Bowers EK, Johnson LS, Masters BS, Johnson BGP, Vogel LA, Forsman AM, Thompson CF. Genetic and environmental variation in condition, cutaneous immunity, and haematocrit in house wrens. BMC Evol. Biol. 2014;14:242. doi: 10.1186/s12862-014-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz JJ, Tinbergen JM. Energy expenditure, nestling age, and brood size: an experimental study of parental behavior in the great tit Parus major. Behav. Ecol. 1999;10:598–606. [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Meth. Ecol. Evol. 2010;1:103–113. [Google Scholar]
- Schjorring S, Gregersen J, Bregnballe T. Sex difference in criteria determining fidelity towards breeding sites in the great cormorant. J. Anim. Ecol. 2000;69:214–223. [Google Scholar]
- Schwagmeyer PL, Mock DW. Parental provisioning and offspring fitness: size matters. Animal Behaviour. 2008;75:291–298. [Google Scholar]
- Smits JE, Bortolotti GR, Tella JL. Simplifying the phytohemagglutinin skin test technique in studies of avian immunocompetence. Funct. Ecol. 1999;13:567–572. [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford University Press; New York, New York, USA: 1992. [Google Scholar]
- Styrsky JD, Eckerle KP, Thompson CF. Fitness-related consequences of egg mass in nestling house wrens. Proc. R. Soc. Lond. B. 1999;266:1253–1258. [Google Scholar]
- Tinbergen JM, Boerlijst MC. Nestling weight and survival in individual great tits (Parus major) J. Anim. Ecol. 1990;59:1113–1127. [Google Scholar]
- Thomson DL, Monaghan P, Furness RW. The demands of incubation and avian clutch size. Biol. Rev. 1998;73:293–304. [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual Selection and the Descent of Man,1871–1971. Aldine; Chicago: 1972. pp. 136–178. [Google Scholar]
- Trivers RL. Parent-offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- VanderWerf E. Lack’s clutch size hypothesis: an examination of the evidence using meta-analysis. Ecology. 1992;73:1699–1705. [Google Scholar]
- Visser ME, Lessells CM. The costs of egg production and incubation in great tits (Parus major) Proc. R. Soc. Lond. B. 2001;268:1271–1277. doi: 10.1098/rspb.2001.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherhead PJ, Boak KA. Site infidelity in song sparrows. Anim. Behav. 1986;34:1299–1310. [Google Scholar]
- Williams GC. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am. Nat. 1966;100:687–690. [Google Scholar]
- Williams TD. Experimental manipulation of female reproduction reveals an intraspecific egg size-clutch size trade-off. Proc. R. Soc. Lond. B. 2001;268:423–428. doi: 10.1098/rspb.2000.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TD, Miller M. Individual and resource-dependent variation in ability to lay supranormal clutches in response to egg removal. Auk. 2003;120:481–489. [Google Scholar]
- Young BE. An experimental analysis of small clutch size in tropical house wrens. Ecology. 1996;77:472–488. [Google Scholar]
- Zanette L, Clinchym M, Smith JNM. Food and predators affect egg production in song sparrows. Ecology. 2006;87:2459–2467. doi: 10.1890/0012-9658(2006)87[2459:fapaep]2.0.co;2. [DOI] [PubMed] [Google Scholar]