Abstract
Background
Clostridium difficile infection (CDI) severity has increased, especially among hospitalized elderly. We evaluated clinical factors to predict mortality following CDI.
Methods
We collected data from inpatients diagnosed with CDI at US academic medical center (HSR-IRB# 13630). We evaluated age, Charlson comorbidity index (CCI), admission from a long-term care facility (LTCF), intensive care unit (ICU) at time of diagnosis, white blood cell count (WBC), blood urea nitrogen (BUN), low body mass index (BMI), and delirium as possible predictors. A parsimonious predictive model was chosen using Akaike information criterion (AIC) and a best subsets model selection algorithm. Area under the ROC curve was used to assess the model’s comparative; with AIC as selection criterion for all subsets to measure fit and control for over-fitting.
Results
From 362 subjects, the selected model included CCI, WBC, BUN, ICU, and delirium. The logistic regression coefficients were converted to a points scale and calibrated so that each unit on the CCI contributed 2 points, ICU contributed 5, unit of WBC (natural log scale) contributed 3, unit of BUN contributed 5, and delirium contributed 11.
Discussion
Our model shows substantial ability to predict short term mortality in patients hospitalized with CDI.
Conclusion
Patients who were diagnosed in the ICU and developed delirium are at highest risk for dying within 30 days of CDI diagnosis.
Keywords: Clostridium difficile infection, predictive model, nosocomial infections, hospitalized patients, delirium
INTRODUCTION
Clostridium difficile (C. difficile) is an anaerobic bacterium that is one of the most common causes of antibiotic associated diarrhea and the main cause of healthcare onset infectious diarrhea (1–6). The incidence and severity of C. difficile infection (CDI) have increased dramatically in the past decade; with reported mortality rates over 15% in hospital epidemics (7–16). Elderly patients, especially those hospitalized with antibiotic exposure or in long-term care facilities (LTCF), have the greatest risk for infection and highest mortality rate (4–8;12–14;16).
C. difficile causes a toxin mediated intestinal inflammatory infection. The clinical manifestations of CDI can vary from asymptomatic carriage or mild self-resolving diarrhea to profuse diarrhea with pseudomembranous colitis, sepsis, and death. Current models to define severe CDI lack either sensitivity or specificity (17–19). There is no validated tool at CDI diagnosis to predict poor outcome (17;18). In this study, we evaluated clinical factors (demographics, co-morbidities, medications, laboratory values, and acute cognitive change) at CDI diagnosis. We present our findings to establish an evidence-based bedside tool that will allow clinicians to quickly identify hospitalized patients at highest risk of death following CDI.
METHODS
Hospitalized Cohort
IRB approval was obtained (HSR-IRB #13607). Sequential inpatients at our US academic hospital clinically diagnosed with CDI (May 2010–August 2011) were identified in the UVA Clinical Microbiology Laboratory once tested positive for via PCR for toxin B gene (BD GeneOhm™ Cdiff Assay, Becton, Dickinson and Company, Franklin Lakes, NJ or Xpert C. difficile, Cepheid, Sunnyvale, CA) based on manufactures instructions (20). Vital signs, laboratory values, and co-morbidities were obtained from the hospital electronic medical record. Most extreme value within 24 hours of CDI diagnosis was recorded. The Charlson comorbidity index was calculated for each subject from co-morbidities (21). Data was obtained by members of the research team and spot-checking and discrepancy resolution was done by the principle investigator. Delirium was identified retrospectively from medical record when acute cognitive change was cited by the clinical treatment team in medical record on the day of CDI diagnosis. Body-Mass index (BMI) used normative cut-offs for underweight (18.5 kg/m2) (22). Each subject was contacted via telephone for consent at 1month and, once consent was obtained, a semi-structured interview was performed with subject, surrogate, or facility staff to record outcomes. The medical record and state death registry was also reviewed for 30 day mortality.
Statistical Analysis
Predictive model
All statistical analysis was done with R version 3.1.1 (23). We identified 9 clinically obtained potential predictors of interest (age, admission from a long-term care facility (LTCF), Charlson comorbidity index score, peripheral white blood cell count (WBC), low BMI (≤18.5 kg/m2), serum albumin, blood urea nitrogen (BUN), diagnosed in intensive care unit (ICU), antibiotic use at time of diagnosis, and delirium). BUN was selected to reflect the acute changes seen with dehydration instead of assessing for underlying chronic kidney disease, which is accounted for in the Charlson co-morbidity index. Wilcoxon rank sum test was used to determine significance. A parsimonious predictive model was chosen using an all subsets selection algorithm (24) with AIC (25) as the model selection criterion. The model was directly calculated from the retained variables. The area under the Receiver Operating Characteristic (ROC) curve (AUC) was used to assess the predictive ability of the model. We used bootstrap model validation to assess the optimism in the AUC (26).
Points Scale
The logistic regression coefficients were converted to a points scale calibrated by scaling the regression coefficients to make 1 point on the Charlson scale equivalent to 2 points and rounding the remaining coefficients to the nearest integer.
RESULTS
Enrolled hospitalized cohort
There were 1,022 patients screened from fecal samples that tested positive for C. difficile in the clinical microbiology laboratory. Of these, 591 were not inpatients, 29 had chronic diarrhea, and 40 were < 18 years old. We enrolled the 362 hospitalized adult subjects who did not have chronic diarrhea and followed them for 30 days after CDI diagnosis or until death. Subjects in our cohort had a median age of 63.5 years (interquartile range (IQR) 51.6, 72.9) and Charlson comorbidity score of 5 points (IQR 3, 7). In our cohort, 17% of subjects (n=61) died within 30 days of CDI diagnosis, see Table I.
Table I.
Cohort Demographics
| Cohort | Alive (n=301) | Died (n=61) | P Value | |
|---|---|---|---|---|
| Age in years (median, IQR) | 63.5 (51.6, 72.9) | 61.8 (50,72.5) | 69.4 (60.9,75.4) | 0.001 |
| WBC | 11 (6.8, 16.6) | 10.2 (6.6,15.7) | 13.3 (8.2, 21.3) | 0.015 |
| Charlson comorbidity score | 5 (3, 7) | 4 (3, 6) | 7 (5, 9) | <.001 |
| Serum albumin (n=164) | 2.9 (2.5, 3.4) | 3.0 (2.5, 3.5) | 2.6 (2.2, 2.9) | <.001 |
| BUN | 21 (11, 35) | 18 (10, 33) | 31 (21, 53) | <.001 |
| BMI | 25.5 (22.3, 33) | 25.9 (22.3, 33.1) | 25.3 (22.2, 32.7) | 0.545 |
| Admitted from LTCF (%) | 94 (26.0) | 68 (22.6) | 26 (42.6) | 0.002 |
In addition to the predictors considered above, we also considered serum albumin. Since this lab test was only performed for 87% of subject in our cohort, it was excluded from further consideration. Additionally, 100% of subject who died were on antibiotics at the time of CDI diagnosis, making it impossible to estimate an associated odds; it was therefore excluded from the model. Therefore, the predictive model was directly calculated from the 5 retained variables: Charlson score, ICU at diagnosis, WBC, BUN, and delirium (Table II).
Table II. Predictive Model.
The mortality risk associated with each variable was calibrated from the regression coefficients into a points scale listed below
| Coefficients | Standard Error |
z value | Pr(>|z|) | Points | |
|---|---|---|---|---|---|
| Intercept* | −5.765 | 0.881 | −6.545 | 5.94e–11 | NA |
| Charlson score* | 0.205 | 0.057 | 3.632 | 0.000 | 2 |
| ln(WBC) | 0.316 | 0.203 | 1.562 | 0.118 | 3 |
| ln(BUN) † | 0.490 | 0.232 | 2.109 | 0.035 | 5 |
| Diagnosed in ICU | 0.495 | 0.232 | 1.470 | 0.141 | 5 |
| Delirium* | 1.079 | 0.320 | 3.369 | 0.001 | 11 |
p<0.001;
p<0.010
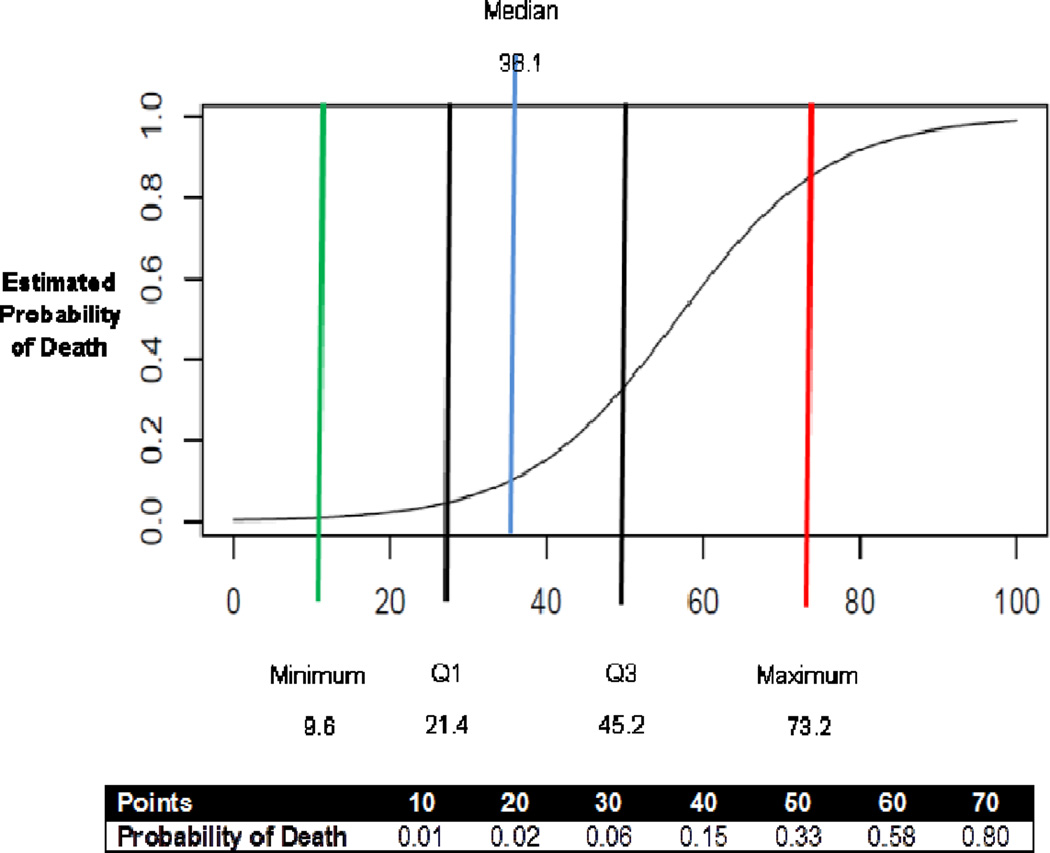
The area under the ROC curve is 0.804 (Figure 1). The regression coefficients and their standard errors are shown in Table II. The bootstrap estimate of optimism was −0.034; suggesting that this model applied to a novel cohort is expected to have a AUC of 0.770.
Figure 1.
Point scale and probability of death following hospitalization with CDI
Points Scale
The calibration of the regression coefficients into a points scale is shown in Table II. With this model, a 1 point corresponds to approximately an 11% increase in the odds of death within 30 days (Figure 1).
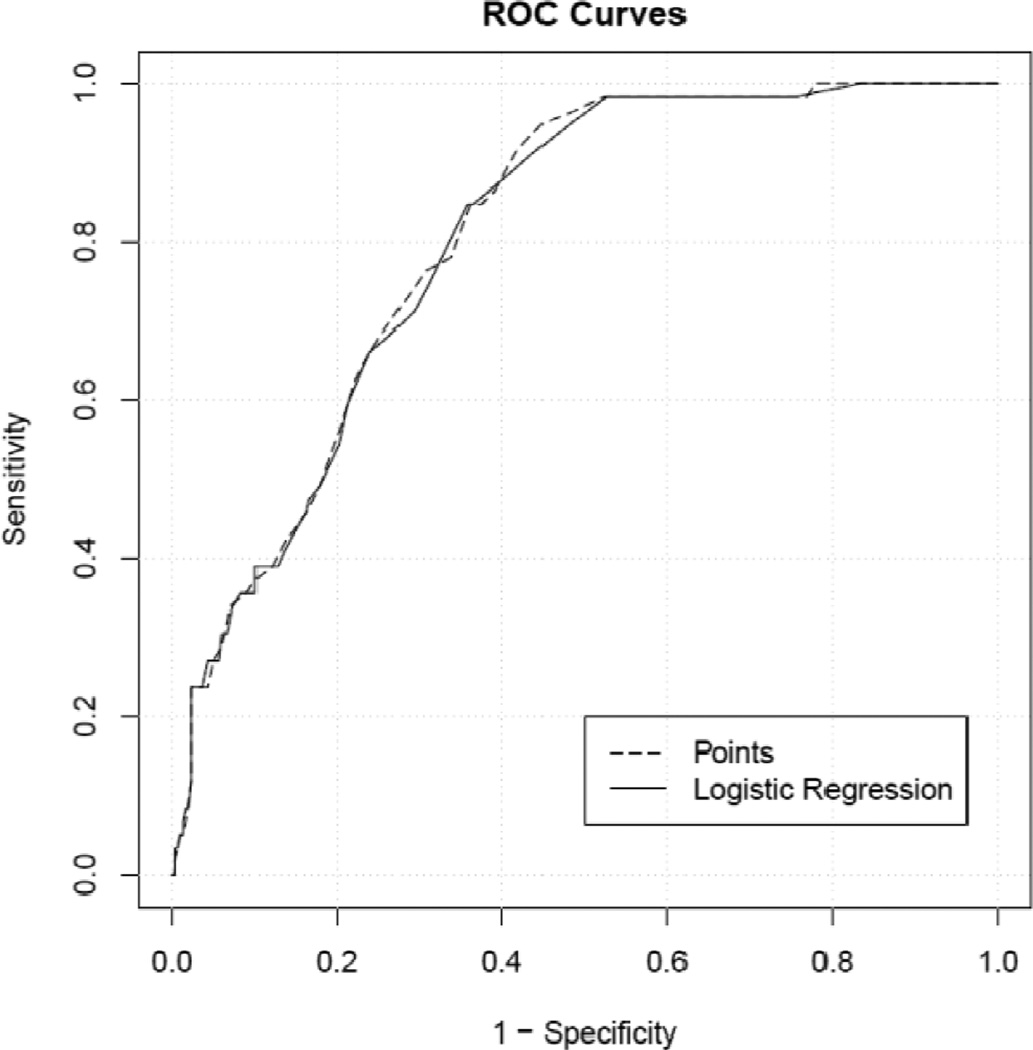
The points score is almost as effective in determining survival, as compared to the raw logistic regression model, as shown by the nearly identical ROC curves (Figure 2). In our 30 day predictive model, delirium was the factor most strongly predictive of death following CDI.
Figure 2.
ROC Curves for Logistic Regression and Points Scale
DISCUSSION
We propose a model of clinical factors that can predict 30 day mortality following CDI in hospitalized patients. With 5 simple, low or no cost clinical factors known at CDI diagnosis (WBC, BUN, Charlson score, location at diagnosis, and delirium), clinicians can use this tool to enhance the early recognition of high risk patients with CDI, implement a more intensive treatment regimen, and aid in the decision for earlier surgical consultation. Likewise, this model can provide an evidence-based, objective definition for severe CDI that could be used in future research studies or clinical guidelines to define severe CDI.
There have been multiple recent studies that have identified univariate risk factors associated with poor outcome following CDI (17–19). Zar, et al. published a scoring system to define severe CDI (≥ 2 points) and determined that patients with severe CDI had better outcomes on oral vancomycin, as compared to metronidazole, and has since served as the basis for treatment guidelines (5). Additionally, in 2011, Fujitani, et al. evaluated eight clinical scales, including the scoring system published by Zar, et al., to determine the predictive value of each. They found the Hines VA Index most predictive of severe CDI (κ-score, 0.69 [95% confidence interval, 0.59–0.83]) (17–19;27). This index utilized clinical factors (fever, abnormal radiology, blood pressure and peripheral white blood cell count) to correlate with higher disease severity. However, neither severity index provided evidence to support the weighting of each factor.
A unique variable identified as a highly significant predictive variable for 30-day mortality is delirium. Delirium is under-recognized, under-diagnosed, and under-reported in hospitalized patients (28–30). Although we did not perform systematic or objective assessments to verify all cases of delirium in this study, regression analysis estimated the highest point for this variable. This observation is clinically relevant because previous studies have shown that hospitalized patients with delirium have worse outcomes (28–30). However, the role of delirium in predicting severe outcomes from CDI has not been reported. Moreover, the causes of delirium are multi-factorial and often reversible (28–30). Its detection early on may indicate the need to identify reversible etiologies, which when addressed promptly, may potentially improve outcome.
Another unique variable we evaluated was admission from LTCF. As we have previously published, residents from LTCF may be at increased risk for C. difficile acquisition, as well as specifically ribotype 027, due to physical frailty, increased healthcare exposure, and live in closer contact as compared to patients in the community (31). Additionally, LTCF residents may have worse CDI due to less diversity and therefore, decreased effectiveness of their microbiome due to a narrower diet and higher rates of antibiotic use (32).
Our study has several limitations. First, we excluded the use of antibiotics for this model since all of the patients who died were on antibiotics, antibiotics are strongly associated with death, but we are statistically unable to quantify the size of the effect. Additionally, a limitation of this study is the retrospective identification of subjects with delirium, based on notation in the medical record. In this study, we did not perform systematic or objective assessments to verify all cases of delirium. However, this study presents a novel association and hypothesis for further investigation. The use of delirium as a poor prognostic indicator needs to be evaluated in prospective studies with objective, validated assessments, such as the Confusion Assessment Method (33). Further investigation is needed to move beyond an association and begin to further understand the connection between inflammation and cognitive and functional decline (7;9;12;34;35). Indeed, we have previously shown that undetected intestinal inflammation is common among nursing home residents independent of diarrhea (36). Whether intestinal inflammation has a role in cognitive and functional decline during CDI is unknown. Further limitation of our proposed predictive models include it’s being based on clinical factors; there may be additional benefit to include potential fecal biomarkers of bacterial characteristics or inflammatory response. Therefore, we are working to determine the predictive benefit of analyzing fecal biomarkers for mortality and relapse. Since this study did not have a control group of C. difficile negative subjects; we are unable to determine if our findings are specific to patients with CDI. Additionally, a potential limitation of the applicability of this model is the need for calculating the natural log for WBC and BUN in this model. However, this is can be overcome at the bedside using the scientific calculators included standard with most smartphones and handheld computing devices that might enable targeted attention to alterable risk factors.
Conclusion
In our cohort study of hospitalized patients with CDI, patients who were admitted from a long-term care facility, diagnosed in the intensive care unit, and developed delirium were at highest risk for dying within 30 days of CDI diagnosis.
Highlights.
Goal: predict 30 day mortality for inpatients with C. difficile infection (CDI)
Predictive factors: Charlson Co-morbidity Index (CCI), WBC, BUN, ICU, and delirium
Weighted risk: delirium=11; ICU=5; unit of CCI=2, unit of WBC=3, unit of BUN= 5
Delirium and ICU carry the highest risk mortality risk within 30 days of CDI
Acknowledgements
We thank the UVA Clinical Microbiology laboratory staff for their assistance with stool collection and our research team who assisted with specimen collection, subject follow-up, and data extraction, as well as our collaborators at TechLab, Inc. for preforming stool analysis.
Financial support: This study received grant support from NIH/NIAID (1K23AI074681), UVA General Medicine Research Taskforce, and TechLab, Inc. (Blacksburg, VA)
Dr. Archbald-Pannone reports grants from NIH/NIAID, grants from TechLab INC, grants from UVA General Medicine Research Taskforce during the conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of Interest: All authors report no conflicts of interest relevant to this article.
Author Contributions Drs. Archbald-Pannone, Guerrant, and Warren were involved with the development and design of the study, acquisition of subjects and data, interpretation of data, and preparation of manuscript. All authors were involved in analysis, data interpretation, and manuscript preparation.
REFERENCES
- 1.Bartlett JG. Clinical practice: Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145:758–764. doi: 10.7326/0003-4819-145-10-200611210-00008. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S12–S18. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett JG. Antibiotic-associated colitis. Dis Mon. 1984:1–54. [PubMed] [Google Scholar]
- 5.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 6.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 7.Zilberberg MD. Clostridium difficile-related hospitalizations among US adults, 2006. Emerg Infect Dis. 2009;15:122–124. doi: 10.3201/eid1501.080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redelings MD, Sorvillo F, Mascola L. Increase in Clostridium difficile-related mortality rates, United States, 1999–2004. Emerg Infect Dis. 2007;13:1417–1419. doi: 10.3201/eid1309.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 10.Pépin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol. 2007;102:2781–2788. doi: 10.1111/j.1572-0241.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 11.Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173:1037–1042. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JR, Barrett LJ, Kotloff K, Guerrant RL. A rapid test for infectious and inflammatory enteritis. Arch Intern Med. 1994;154:2660–2664. doi: 10.1001/archinte.1994.00420230043006. [DOI] [PubMed] [Google Scholar]
- 13.Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 14.CDC. Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1201–1205. [PubMed] [Google Scholar]
- 15.Zollner-Schwetz I, Hogenauer C, Joainig M, et al. Role of Klebsiella oxytoca in antibioticassociated diarrhea. Clin Infect Dis. 2008;47:e74–e78. doi: 10.1086/592074. [DOI] [PubMed] [Google Scholar]
- 16.Baker SS1, Faden H, Sayej W, Patel R, Baker RD. Increasing incidence of community-associated atypical Clostridium difficile disease in children. Clin Pediatr. 2010;49:644–647. doi: 10.1177/0009922809360927. [DOI] [PubMed] [Google Scholar]
- 17.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 18.Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:220–228. doi: 10.1086/658336. [DOI] [PubMed] [Google Scholar]
- 19.Belmares J, Gerding DN, Parada JP, Miskevics S, Weaver F, Johnson S. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55:495–501. doi: 10.1016/j.jinf.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Boone JH, Archbald-Pannone LR, Wickham KN, et al. Ribotype 027 Clostridium difficile infections with measurable stool toxin have increased lactoferrin and are associated with a higher mortality. Eur J Clin Microbiol Infect Dis. 2014;33:1045–1051. doi: 10.1007/s10096-013-2043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 22.National Institutes of Health. National Heart Lung and Blood Institute and North American Association for the Study of Obesity. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. 2000;10 [Google Scholar]
- 23.R Development Core Team. R Foundation for Statistical Computing. Vienna, Austria: 2014. R: A language and environment for statistical computing. [Google Scholar]
- 24.McLeod A, Xu C. R package versoin 0.33 using AIC as the selection criterion. University of Western Ontario; 2011. Bestglm: Best subset GLM. [Google Scholar]
- 25.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1976;19:716–723. [Google Scholar]
- 26.Efron B, Tibshirani RJ. An intruductino to the bootstrap. CRC Press. 1998;248 [Google Scholar]
- 27.Belmares J, Gerding DN, Tillotson G, Johnson S. Measuring the severity of Clostridium difficile infection: implications for management and drug development. Expert Rev Anti Infect Ther. 2008;6:897–908. doi: 10.1586/14787210.6.6.897. [DOI] [PubMed] [Google Scholar]
- 28.Pompei P, Foreman M, Rudberg MA, Inouye SK, Braund V, Cassel CK. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42:809–815. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 29.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 30.Thomas C, Kreisel SH, Oster P, Driessen M, Arolt V, Inouye SK. Diagnosing delirium in older hospitalized adults with dementia: adapting the confusion assessment method to international classification of diseases, tenth revision, diagnostic criteria. J Am Geriatr Soc. 2012;60:1471–1477. doi: 10.1111/j.1532-5415.2012.04066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Archbald-Pannone LR, Boone JH, Carman RJ, Lyerly DM, Guerrant RL. Clostridium difficile ribotype 027 is most prevalent among inpatients admitted from long-term care facilities. J Hosp Infect. 2014;88:218–221. doi: 10.1016/j.jhin.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 33.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56:823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leslie DL, Zhang Y, Holford TR, Bogardus ST, Leo-Summers LS, Inouye SK. Premature death associated with delirium at 1-year follow-up. Arch Intern Med. 2005;165:1657–1662. doi: 10.1001/archinte.165.14.1657. [DOI] [PubMed] [Google Scholar]
- 35.Inouye SK, Zhang Y, Jones RN, Kiely DK, Yang F, Marcantonio ER. Risk factors for delirium at discharge: development and validation of a predictive model. Arch Intern Med. 2007;167:1406–1413. doi: 10.1001/archinte.167.13.1406. [DOI] [PubMed] [Google Scholar]
- 36.Archbald-Pannone L, Sevilleja J, Guerrant R. Diarrhea, Clostridium difficile, and Intestinal Inflammation in Residents of a Long-Term Care Facility. J Am Med Dir Assoc. 2010;11:263–267. doi: 10.1016/j.jamda.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]