Abstract
Drug delivery to the brain for the treatment of pathologies with a CNS component is a significant clinical challenge. P-glycoprotein (PgP), a drug efflux pump in the endothelial cell membrane, is a major factor in preventing therapeutics from crossing the blood-brain barrier (BBB). Identifying PgP regulatory mechanisms is key to developing agents to modulate PgP activity. Previously, we found that PgP trafficking was altered concomitant with increased PgP activity and disassembly of high molecular weight PgP-containing complexes during acute peripheral inflammatory pain. These data suggest that PgP activity is post-translationally regulated at the BBB. The goal of the current study was to identify proteins that co-localize with PgP in rat brain microvessel endothelial cell membrane microdomains and use the data to suggest potential regulatory mechanisms. Using new density gradients of microvessel homogenates, we identified two unique pools (1,2) of PgP in membrane fractions. Caveolar constituents, caveolin1, cavin1 and cavin2, co-localized with PgP in these fractions indicating the two pools contained caveolae. A chaperone (Hsc71), protein disulfide isomerase and endosomal/lysosomal sorting proteins (Rab5, Rab11a) also co-fractionated with PgP in the gradients. These data suggest signaling pathways with a potential role in post-translational regulation of PgP activity at the BBB.
Keywords: caveolae, P-glycoprotein, blood brain barrier, protein disulfide isomerase, ATP synthase β subunit, Rab5
Introduction
One of the most difficult clinical challenges for treatment of pathologies with a CNS component is drug delivery across the blood-brain barrier (BBB). The BBB is comprised of an extensive network of non-fenestrated microcapillaries (Abbott et al. 2010). Each capillary lumen is surrounded by endothelial cells tethered to one another by tight junction protein complexes that limit paracellular diffusion (Abbott et al. 2010). BBB endothelial cells, with accompanying pericytes and astrocytes, provide a physical barrier between the blood and the CNS (Ballabh et al. 2004; Quaegebeur et al. 2011; Armulik et al. 2010). In addition to the physical barrier, cells of the BBB use biochemical mechanisms to limit transcellular movement of substances into the brain. The biochemical component of the barrier includes metabolizing enzymes and ATP-driven efflux pumps of the ATP-binding cassette transporter family (Borst and Elferink 2002; Miller and Cannon 2014). While the barrier function is required to prevent CNS infection and toxicity (Borst and Elferink 2002), it provides a challenge for effective drug delivery to the brain.
P-glycoprotein (MDR1/ABCB1, E.C. 3.6.3.44) is the major drug efflux pump expressed at the blood-brain barrier. P-glycoprotein (PgP) is a transmembrane protein that causes ATP-dependent efflux of substrates from the cytoplasm and lipid bilayer into the blood (Borst and Elferink 2002). The wide range of substrates that are extruded by PgP include drugs used to treat pain (morphine, other opioids), AIDS (antivirals), epilepsy, stroke, and glioblastoma among others (Seelbach et al. 2007; Sun et al. 2004; Potschka 2010; Miller et al. 2008; Spudich et al. 2006). An ability to alter CNS drug delivery for such a wide range of compounds has made PgP a major target in the design of treatment strategies for many diseases with a CNS component. Unfortunately, agents that directly inhibit PgP activity have not proven clinically viable (Thomas and Coley 2003; Liang and Aszalos 2006). The clinical failure of PgP inhibitors highlights the need to identify alternative strategies to modulate PgP activity to facilitate targeted drug delivery.
PgP is regulated by a combination of transcriptional and post-transcriptional mechanisms. Two recent reviews by the Miller group summarize the current knowledge of PgP regulation at the BBB (Miller and Cannon 2014; Miller 2010) and discuss the challenges the complex regulation presents for successfully manipulating PgP. These reviews present evidence indicating vascular endothelial growth factor (VEGF) signaling and TNFα/PKC/sphingolipid signaling can be manipulated in model systems to modulate PgP activity. Clearly, progress has been made in identifying signaling pathways that have the potential for clinical manipulation of PgP. However, our knowledge of the regulation of PgP activity, particularly under pathological conditions and at the post-transcriptional level, is incomplete. Pathology-specific molecular events and post-transcriptional regulation of PgP activity will complicate use of agents that target identified signaling pathways. A more complete understanding of the molecular events that alter PgP activity at the post-translational level and under specific pathological conditions is required for controlled drug delivery to the CNS.
Inflammation is both a primary pathology requiring medical treatment at the level of the CNS and a component of many CNS pathologies. Previous data measuring the effect of an inflammatory response on PgP at the BBB has produced contradictory results (Miller et al. 2008). The inflammatory response is complex; contradictions likely stem from the use of different models, inflammatory agents, timing of the measurements and other issues. Although contradictory, these data can be used to suggest molecular events that regulate PgP during the inflammatory response. Proteins involved in the suggested molecular events can be identified and the importance for the inflammatory response can be tested in these models. This approach will tease apart components of the complex effect of inflammation on PgP activity that can be targeted for controlled CNS drug delivery.
Using λ-carrageenan injection into the rat paw as a peripheral inflammatory pain (PIP) model, our laboratory found that PIP increases PgP activity in BBB endothelial cells (McCaffrey et al. 2012). Increased PgP activity is correlated with decreased morphine efficacy and accumulation in the brain (Seelbach et al. 2007). PIP also causes a redistribution of PgP in the BBB endothelial cell membrane fractions (McCaffrey et al. 2012). The altered PgP trafficking is accompanied by disassembly of high molecular weight disulfide-bonded protein complexes that contain PgP and caveolin1, a protein that contributes to caveolar scaffolding (McCaffrey et al. 2012; Parton and del Pozo 2013). These data imply that PgP activity is post-translationally regulated during inflammation by altering PgP-containing complexes and the membrane environment or location of PgP.
The goal of the current study was to: 1) use proteomics to identify proteins that co-fractionate with PgP in rat BBB endothelial cell membranes prior to a stimulus; 2) use the newly identified proteins to suggest signaling pathways with the potential to post-translationally regulate PgP activity; 3) test whether proteins which can modify disulfide bonds co-localize with PgP at the BBB; and 4) identify trafficking proteins that co-localize with PgP in vivo. Using biochemical gradients, we identified two unique pools of PgP in membrane fractions that migrated to different densities. We also found that a chaperone, a thiol oxidoreductase, caveolar constituents and endosomal/lysosomal sorting proteins co-fractionated with PgP in the gradients. These data indicate signaling pathways and molecular events with the potential to post-translationally modulate PgP activity.
Materials and methods
Reagents
OptiPrep was obtained from Accurate Chemical (Westbury, NY). EDTA-free Complete proteinase inhibitor was purchased from Roche (Indianapolis, IN). Criterion TGX gels, tris(2-carboxyethyl)phosphine hydrochloride, Precision Plus prestained molecular weight standards, 4X sample loading buffer and Clarity chemiluminescence reagent were obtained from Bio-Rad (Hercules, CA). Coomassie Plus Protein Assay Reagent and BCA Protein Assay kit were purchased from ThermoFisher (Waltham, MA). Autoradiography film was purchased from ISC Bioexpress (Kaysville, UT). Antibodies were purchased from the following sources: P-glycoprotein (sc-8313), nucleoporin (sc25523) and cathepsin D (sc377299) (Santa Cruz Biotechnology, Dallas, TX); GLUT-1 (ab32551), ATP synthase beta subunit (ab14730), Annexin 3 (ab127924), Annexin V (ab14196), Rab5 (ab13253), protein disulfide isomerase (ab2792), von Willebrand factor (ab6994), Hsc71 (ab19136), thioredoxin (ab86255) and PTRF (ab48824) from AbCam (Cambridge, MA); QSOX1 (12713-1-AP – Proteintech, Chicago, IL); caveolin-1 (610060 - BD Biosciences, San Jose, CA,); COX IV (4850) and Rab11a (2413) (Cell Signaling, Danvers, MA); and HRP-linked goat anti-rabbit and HRP-linked goat anti-mouse (GEHealthcare, Piscataway, NJ). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Animals and treatments
All animal protocols were written in accordance with the guidelines of the National Institutes of Health and approved by the University of Arizona Institutional Animal Care and Use Committee. Female Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN) were maintained under standard 12h light/12h dark conditions with food and water provided ad libitum. Three hours prior to sacrifice, 100 μl 0.9% NaCl or λ carrageenan (3% in 0.9% NaCl) was injected subcutaneously in the left hind paw. Naïve animals received no injection.
Cell culture
bEnd.3 mouse brain endothelial cell culture cells were obtained from ATCC (Manassas, VA). Cells were maintained in Dulbeco’s Modified Eagle Medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 I.U. penicillin and 100 μg/ml streptomycin (Mediatech, Inc. Manassas, VA) at 37°C in a humidified 5% CO2 environment. Cells were subcultured every 3–4 days by incubating with 0.25% trypsin: 0.53 mM EDTA solution (Gemini Bioproducts, Woodland, CA) and then diluting the resulting cell suspension into new medium. Cells harvested for immunoprecipitation were ~80% confluent and between passages 5 and 17.
Microvessel isolation and fractionation
Microvessels were isolated as previously described (McCaffrey et al. 2012). Briefly, rats were anesthetized with sodium pentobarbital (64 mg/kg), decapitated and the brains placed in ice-cold buffer A (136.9 mM NaCl; 2.7 mM KCl; 1 mM CaCl2; 1.5 mM KH2PO4, 8.1 mM Na2HPO4; 0.5 mM MgCl2; 5 mM glucose; 1 mM sodium pyruvate, pH7.4) supplemented with Roche EDTA-free Complete Protease Inhibitor cocktail, Sigma protease inhibitor cocktail and 2 mM phenylmethylsulfonyl fluoride. All subsequent steps were performed on ice or at 4°C. After removal of the choroid plexis and meninges, three or four rat brains were pooled and homogenized in 20 ml buffer A using a Potter-Elvehjem homogenizer (20 strokes at moderate speed) followed by 8 strokes in a glass dounce homogenizer by hand. Homogenate was mixed with 30% Ficoll in buffer A (10 ml homogenate/15 ml 30% Ficoll) and centrifuged for 20 min at 5800 × g in a Sorvall SS-34 rotor. The supernatant was discarded and the pellet resuspended in 10 ml buffer A supplemented with 1% bovine serum albumin using 2 strokes with the Potter-Elvehjem homogenizer. The suspension was gravity filtered through a 300 μm mesh filter and microvessels collected on a 40 μm mesh filter. Microvessels retained on the 40 μm mesh filter were resuspended in buffer A with 1% bovine serum albumin and pelleted by centrifugation (10 min at 1500 × g). The pellet was washed twice in buffer B (20 mM Tris-HCl; 250 mM sucrose; 1 mM CaCl2; 1 mM MgCl2, pH 7.8) supplemented with Roche EDTA-free Complete Protease Inhibitor cocktail, Sigma protease inhibitor cocktail and 2 mM phenylmethylsulfonyl fluoride. The microvessel pellet was overlaid with buffer B and stored at −20°C until use. This protocol results in a 9-fold enrichment of the endothelial form of GLUT1 over that found in brain homogenates (McCaffrey et al. 2007).
Rat cerebral microvessels were homogenized and fractionated as previously described (McCaffrey et al. 2012) with minor modifications. Microvessel pellets were rapidly thawed and passed through a 21-gauge needle 20 times. Protein concentration in the lysate was measured using the Coomassie Plus Protein Assay Reagent according to the manufacturer’s instructions. Aliquots of microvessel homogenate containing 300 μg protein were diluted to 1.87 ml in buffer B and subsequently mixed with 60% OptiPrep to make a final concentration of 35% OptiPrep. The resulting OptiPrep/microvessel homogenate was overlaid with a discontinuous stepwise gradient consisting of 10/15/20/25/30% OptiPrep diluted in buffer B without ions or protease inhibitors.
Gradients were centrifuged and fractionated as previously described (McCaffrey et al. 2012). Briefly, gradients were centrifuged in a Beckman SW28.1 swinging bucket rotor for 90 min at 52,000 × g. Twenty-four fractions were collected from each gradient using a Biocomp Gradient Station (Frederickton, NB, Canada) starting from the top. Each fraction was aliquotted for measurement of refractive index, protein concentration and specific proteins by gel electrophoresis. Aliquots were stored at −20°C until analysis. Fraction density was calculated by measuring absorbance at 340 nm using a plate reader (TECAN GENios, TECAN U.S., Inc., Research Triangle Park, NC) and converting the absorbance readings to density using the conversion table that accompanies the OptiPrep solution. Protein concentration was measured using the BCA Protein Assay kit according to the manufacturer’s protocol and by reading the absorbance on a plate reader (TECAN GENios).
Proteomics
The fraction that contained the major portion of the PgP (Fraction 12) from each of 29 independent gradients (representing material from a total of 87 rats) was pooled. SDS was added to the sample (final concentration = 1%) and the sample concentrated by centrifugation using Amicon 10,000 MWCO concentrators (Millipore, Billerica MA) according to the manufacturer’s protocol. The samples were then mailed to Kendrick Labs (Madison, WI) for further concentration by dialysis, 2-D gel electrophoresis using ampholines in the pH range of 3 –10 and protein identification. The most abundant proteins in the molecular size range of 14–80 kDa were identified by LC-MS/MS using the Kendrick Labs protocol (Darie et al. 2011; Sokolowska et al. 2012a; Sokolowska et al. 2012b). Briefly, proteins were identified by digesting the proteins with trypsin and analyzing the peptide mixture by reversed phase liquid chromatography and MS using a NanoAcuity UPLC (Micromass/Waters, Milford, MA) coupled to a Q-TOF Micro MS (Micromass/Waters, Milford, MA). Peptides were loaded onto a 100 μm × 10 nm NanoAquity BEH130 C18 1.7 μm UPLC column (Waters, Milford, MA). Peptides were eluted using a 150 minute gradient of 2–80% organic solvent (acetonitrile containing 0.1% formic acid) with a flow rate of 400 nl/min. The aqueous solvent was 0.1% formic acid in HPLC water.
Immunoblots
Gradient fractions were prepared, proteins separated by SDS-PAGE and proteins transferred to membranes as previously described (McCaffrey et al. 2012). Briefly, gradient fractions were mixed with 4X sample loading buffer supplemented with reducing agent, tris(2-carboxyethyl)phosphine hydrochloride, or water. Samples were incubated for 10 min at 70°C and centrifuged for 5 min at 16,000 × g at 21°C prior to gel electrophoresis. Proteins were separated by SDS-PAGE on Criterion TGX precast gels using Tris-glycine-SDS running buffer and transferred to polyvinyldifluoride membranes (Imobilon, Millipore, Waltham, MA) using Genie Electroblotters (Idea Scientific, Minneapolis, MN). Blots were probed and proteins detected as previously described (McCaffrey et al. 2012). Bands were visualized by incubating with chemiluminescence reagents and exposing the blots to film. To visualize multiple bands on the same blot, blots were stripped with Restore Western Blot Stripping Buffer (Pierce, Thermo Fisher, Waltham, MA) or 0.2 M NaOH before reprobing with another antibody.
Immunoprecipitation
Co-immunoprecipitations were done using the Co-immunoprecipitation kit from Pierce (ThermoFisher, Waltham, MA) using the manufacturer’s protocol. Briefly, 10 μg PgP antibody was linked to 25 μl resin. Samples were lysed in the kit lysis buffer and protein concentration measured using the BCA Protein Assay kit. The sample was pre-cleared using the control agarose resin and the sample incubated with the antibody linked or control resin overnight at 4°C. Samples were eluted and prepared for gel electrophoresis according to the manufacturer’s instructions. Proteins were separated by SDS-PAGE and proteins identified by immunoblotting.
Image processing
Films were scanned and cropped, and then auto contrast was used on the entire cropped portion prior to compiling the figure. For some proteins, only a portion of the gradient was run on the gel; scans of these blots were resized to fit the images of the complete gradients.
Results
Isolation of the PgP-containing fraction from proteins that do not enter the gradient
Our long term goal is to identify proteins and signaling pathways involved in the post-translational regulation of PgP. We expect some regulatory proteins to bind PgP directly. However, we expect other proteins, particularly those that regulate PgP by altering the PgP microenvironment or participate in signaling and trafficking events, to be located in membranes or vesicles adjacent to PgP. These proteins are unlikely to bind PgP. To maximize our ability to identify proteins both bound to PgP and in the same membrane microenvironment, our approach was to do a proteomics analysis to identify proteins that co-fractionated with PgP in density gradients of rat microvessel lysates. We previously showed that PgP is located in fractions 15 and 16 in a stepwise 5–30% detergent-free OptiPrep gradient in control animals (Figure 1A). In λ carrageenan-treated animals, the major pool of PgP is located in a denser fraction, fraction 19. These fractions contain PgP and a number of other proteins. In particular, the higher density fractions are not well separated from the bulk of the proteins that do not enter the gradient. An increase in the signal to noise ratio was necessary to identify proteins that are in the same membrane microdomain as PgP and avoid an analysis that resulted in identification of the most abundant proteins in the cell. To prepare a sample enriched for proteins bound or close to PgP in the membrane, the first step was to alter and redefine the gradients to achieve greater separation between the PgP containing fractions, and the proteins that do not enter the gradient.
Figure 1.
Localization of P-glycoprotein in detergent-free OptiPrep gradients loaded with rat microvessel isolates. Panel A. Localization of P-glycoprotein in stepwise 5–30% OptiPrep gradient fractions in saline and λ carrageenan-treated animals. These samples were analyzed in the presence of reducing equivalents. Protein concentration (□) and density (■) of each fraction is plotted above the immunoblot. Values are the mean ± S.E.M. (n = 6). This figure is redrawn from our previously published figure (McCaffrey et al. 2012). Panel B. Localization of PgP in detergent-free 10–35% stepwise OptiPrep gradient fractions in saline-treated animals. This immunoblot is representative of at least three independent gradients; each gradient contains microvessels isolated from a pool of 3–4 rats. These samples were analyzed in the absence of reducing equivalents. Protein concentration (○) and density (■) of the fractions is plotted above the immunoblot. Values are the mean ± S.D. (n = 6). Abbreviations: PgP – P-glycoprotein; SAL – saline-treated; CARR – λ carrageenan- treated.
We used a stepwise 10–35% OptiPrep gradient to increase the separation between PgP-containing fractions and the bulk of the proteins loaded on the gradient. Under these conditions, the main PgP containing fraction was fraction 12 (Figure 1B). The density in fraction 12 of the 10–35% gradients was 1.117 g/ml (Figure 1B) which is similar to the density of fraction 15 in the 5–30% gradients used previously in our laboratory. Migration of PgP to a similar density fraction in the two gradients suggests that fraction 12 in the 10–35% gradient now contains the same PgP co-localizing proteins that were previously located in fraction 15 in the 5–30% gradient. Furthermore, the major PgP-containing pool in the 10–35% OptiPrep gradients was separated to a greater degree from the bulk of the proteins that did not enter the gradient (fraction 24) (compare Figure 1 Panel A with Panel B).
The altered gradient resulted in the identification of a second, unique, PgP-containing pool that migrated to a density of 1.130 g/ml (fraction 15). The higher density of the fraction containing the second PgP pool suggests some of the PgP is contained in alternate lipoprotein complexes and potentially a different subcellular location.
PgP co-fractionates with plasma membrane markers
Stepwise OptiPrep gradients separate lipoprotein complexes based on density. Microscopy studies indicate that a major portion of the PgP in endothelial cells is located in the plasma membrane (Bendayan et al. 2006). We previously showed that: 1) the PgP pool at a density of 1.12 g/ml co-fractionated with plasma membrane proteins (McCaffrey et al. 2012); and 2) our microvessel isolation procedure enriched the lysate for endothelial cells (McCaffrey et al. 2007). To comfirm that PgP was localized in fractions that contain other plasma membrane proteins in our redefined gradients, we tested for the presence of plasma membrane proteins and markers of other subcellular compartments in the gradients. As shown in Figure 2, PgP was located in the same fraction as the glucose transporter, GLUT-1, which localizes to the plasma membrane (Cornford and Hyman 2005). Fraction 12 also contained von Willebrand factor (vWF). Von Willebrand factor is an endothelial cell protein that is secreted from the luminal surface of microvessel endothelial cells (Zanetta et al. 2000). Identification of von Willebrand factor also confirms that we are measuring proteins from endothelial cells. Fraction 12, and to a lesser extent fraction 15, contained cathepsin D, which is a lysosomal marker (Luzio et al. 2007), suggesting lysosomal membranes are also contained in these fractions. PgP did not co-localize in a substantial way with nucleoporin, a marker for the nuclear membrane (Guan et al. 1995). We did not detect mitochondrial membrane proteins in our gradients as indicated by the lack of cytochrome c oxidase subunit IV (COX IV) immunoreactivity in the gradient fractions.
Figure 2.
The primary P-glycoprotein pool co-fractionates with plasma membrane markers. Representative immunoblots showing the relative location of PgP and other membrane markers in the gradient fractions. These blots are representative of at least three independent gradients; each gradient contains microvessels isolated from a pool of 3–4 rats. These samples were analyzed in the presence of reducing equivalents. Abbreviations: PgP – P-glycoprotein; GLUT1 – glucose transporter1; vWF – von Willebrand factor.
Proteomics identifies proteins that co-fractionate with PgP
To identify proteins bound to PgP or in the same membrane microenvironment we pooled the 12th fraction from 29 gradients and identified the most prominent proteins in the 14–80 kDa range on the resulting 2-D gel by LC-MS/MS. Table 1 lists the proteins we identified. Our list included a chaperone, Hsc71, and proteins that have a potential role in signaling cascades, e.g. the annexins and ATP synthase β subunit (ATPB). There was also a substantial amount of actin in this fraction.
Table 1.
Proteins identified by limited proteomics analysis of pooled Fraction 12 samples.
| Protein | Accession # |
|---|---|
| AT-rich interactive domain-containing protein | 29129900 |
| Annexin V | 28373862 |
| Guanine nucleotide binding protein beta subunit | 13937391 |
| Annexin A3 | 149046865 |
| ATP synthase beta subunit | 1374715 |
| Heat shock cognate 71 | 13242237 |
| Protein disulfide isomerase | 8393322 |
Confirmation of protein co-fractionation with PgP
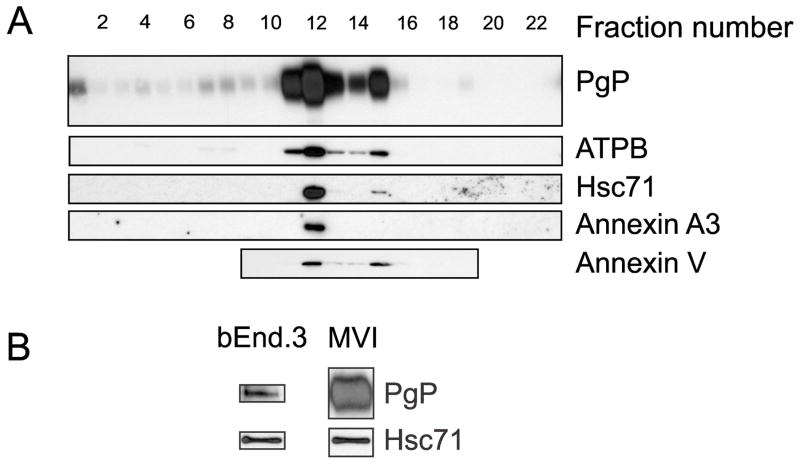
To confirm our proteomics results, we tested for the presence of several of the proteins identified by proteomics in the gradient fractions. As shown in Figure 3A, there was a substantial amount of ATPB, Hsc71, annexin 3 and annexin V in the fractions that contained the bulk of the PgP. Annexin 3 and annexin V both bind to lipids and can be found at the plasma membrane (Monastyrskaya et al. 2009). Hsc71 is known to bind PgP when both proteins are purified and incubated together in vitro (Loo and Clarke 1995). We performed co-immunoprecipitation experiments to test whether PgP and Hsc71 are binding partners in bEnd.3 mouse brain endothelial cell culture cells and in rat microvessels. As shown in Figure 3B, Hsc71 co-immunoprecipitated with PgP from both sources indicating these proteins are binding partners in vivo. ATPB is often used as a marker of mitochondria, because it is a subunit of the F1/F0 ATPase involved in mitochondrial ATP generation (Leyva et al. 2003). Co-localization of ATPB with plasma membrane markers and a lack of mitochondrial markers in the gradient fractions containing PgP, suggest that a portion of ATPB is located at the plasma membrane of rat brain microvessel endothelial cells.
Figure 3.
Proteins identified via proteomics co-fractionate with PgP in the density gradient fractions. Panel A. Representative immunoblots showing the relative location of PgP and a subset of the proteins identified by proteomics in the gradient fractions. These blots are representative of at least three independent gradients; each gradient contains microvessels isolated from a pool of 3–4 rats. Abbreviations: PgP – P-glycoprotein; ATPB – ATP synthase subunit β; Hsc71 – heat shock cognate 71. Panel B. Representative immunoblots showing co-immunoprecipitation of Hsc71 with PgP using a PgP antibody in bEnd.3 cell lysates and isolated microvessel lysates (MVI). These blots are representative of three independent lysates where each MVI lysate contains microvessels from a pool of 3 rats. Lines indicate the images are from different blots. These samples were analyzed in the presence of reducing equivalents.
Protein disulfide isomerase co-fractionates with and is bound to PgP
Previously, we found that a percentage of the PgP is contained in high molecular weight complexes (McCaffrey et al. 2012). After a PIP stimulus, the high molecular weight complexes disassemble concomitant with an increase in PgP activity and localization of PgP to a gradient fraction with increased density (McCaffrey et al. 2012). Figure 4A shows the loss of the high molecular weight complexes in the presence of the PIP stimulus. Addition of reducing equivalents to the control sample caused disassembly of the high molecular weight complexes similar to the effect of a PIP stimulus (Figure 4A).
Figure 4.
Protein disulfide isomerase fractionates with and is bound to PgP. Panel A: Representative immunoblots of the main PgP pool in microvessel lysates from saline- and λ carrageenan-treated rats. Equivalent amounts of microvessel lysate protein were loaded on the gradients. Samples were incubated in the absence (NR) or presence (R) of reducing equivalents prior to loading on the gels. Vertical lines indicate that the samples are from different blots. Panel B. Representative immunoblots showing the relative location of PgP and two thiol oxidoreductase proteins in the gradient fractions. These blots are representative of at least three independent gradients; each gradient contains microvessels isolated from a pool of 3–4 rats. The samples were analyzed in the presence of reducing equivalents. Abbreviations: PgP – P-glycoprotein; HMW – high molecular weight; PDI – protein disulfide isomerase; QSOX1 – quiescin sulfhydral oxidase1. Panel C. Representative immunoblots showing co-immunoprecipitation of PDI with PgP using a PgP antibody in bEnd.3 cell lysates and isolated microvessel lysates (MVI). These blots are representative of three independent lysates where each MVI lysate contains microvessels from a pool of 3 rats. Lines indicate the images are from different blots. These samples were analyzed in the presence of reducing equivalents.
These data suggest that PIP causes oxidation, reduction or rearrangement of disulfide bonds in the high molecular weight complex. We tested for the presence of proteins that could catalyze these reactions in fraction 12 which contains the high molecular weight complexes. Protein disulfide isomerase (PDI), a thiol oxidoreductase which catalyzes the formation and rearrangement of disulfide bonds (Wilkinson and Gilbert 2004), co-fractionated with PgP. A portion of the PDI was also in higher density fractions that did not contain PgP. We tested whether PDI was a PgP binding partner in bEnd.3 mouse brain endothelial cell culture cells and rat microvessels. Using co-immunoprecipitation, we found that PDI was bound to PgP in both the cell culture cells and rat microvessels (Figure 4). Quiescin sulphydral oxidase1 (QSOX1), which catalyzes the formation of disulfide bonds (Heckler et al. 2008), did not co-localize with PgP, but appeared solely in the denser gradient fractions. Thioredoxin1, a protein responsible for reducing disulfides (Arner and Holmgren 2000), was undetectable in the gradient fractions.
Caveolar and trafficking proteins co-fractionate with PgP
The movement of PgP to higher density gradient fractions after a PIP stimulus, suggests that PgP protein trafficking/location is altered during acute inflammatory pain concomitant with increased PgP activity (McCaffrey et al. 2012). We tested for two groups of caveolar/trafficking proteins that have the potential to contribute to PgP movement after a stimulus. We first tested for the presence of caveolin1, cavin1 (polymerase I and transcript release factor/PTRF) and cavin2 (serum deprivation response protein/SDPR), each of which are important for the formation of caveolae (Parton and del Pozo 2013). Caveolin1, cavin1 and cavin2 were found in the same gradient fractions as the two pools of PgP (Figure 5). Cavin2 appeared as a doublet in the microvessel lysates; the appearance of a doublet is tissue type specific (Hansen et al. 2013). The second group of proteins we investigated was the Rab proteins. Rab5 and Rab11a are involved in the endosomal/lysosomal trafficking pathway and have been implicated in PgP trafficking events (Kelly et al. 2012; Hutagalung and Novick 2011; Fu and Arias 2012). Rab11a and Rab5 were both found in the fractions containing PgP.
Figure 5.

Caveolar and trafficking proteins co-fractionate with PgP. Representative immunoblots showing the location of caveolin1, cavin1, cavin2, Rab11a and Rab5 relative to PgP in the gradient fractions. These blots are representative of at least three independent gradients; each gradient contains microvessels isolated from a pool of 3–4 rats. The samples were analyzed in the presence of reducing equivalents. Abbreviations: PgP – P-glycoprotein; CAV1 – caveolin1.
Discussion
Our biochemical characterization of proteins that co-fractionate with PgP in vivo suggests molecular events and signaling pathways that have the potential to activate PgP. A chaperone, a thiol oxidoreductase, caveolar constituents and endosomal/lysosomal sorting proteins co-fractionate with PgP in density gradients of microvessel membranes. Although our analysis in the current proteomics study is based on samples from saline-injected rats, our data indicate that this is reflective of non-stressed animals. Gradients of microvessels isolated from naïve animals show the same pattern of PgP fractionation as the saline-treated animals (Supplementary data). GLUT1 and ATPB also co-localize with PgP in the naïve animals (Supplementary data). Several molecular events and signaling pathways suggested by the proteins identified in the current study have a post-transcriptional component that could regulate PgP activity. Our data mining using proteomics directs us toward pathways to test for their ability to modulate PgP activity and alter CNS drug delivery at the BBB.
Our data are consistent with a portion of the PgP being located in caveolae in BBB endothelial cells. A caveolar location for a portion of PgP was suggested by biochemical studies in cell culture cells (Jodoin et al. 2003; Demeule et al. 2000) and shown by electron microscopy in naïve BBB endothelial cells (Bendayan et al. 2006). Caveolin1, cavin1 and cavin2 are found in both the fractions that contain the main PgP pools. These three proteins are responsible for the formation of caveolae (Parton and del Pozo 2013). Caveolin1 forms the caveolar scaffold (Sowa 2012). Cavin1 is required for the formation of caveolae, in part by recruiting caveolin1 (Hill et al. 2008) and cavin2 controls caveolar size and stability (Hansen et al. 2013). Immunoprecipitation studies indicate that a small percentage of PgP is bound to caveolin1 in endothelial cells in culture, rat capillaries and astrocytes (Jodoin et al. 2003; Barakat et al. 2007) (Demeule et al. 2000; Ronaldson et al. 2004). Although caveolin1 forms the caveolar scaffold, it is also located in non-caveolar membranes and performs trafficking functions (Zheng et al. 2011; Parton and del Pozo 2013). The finding that cavin1 and cavin2 are prominent proteins in both of our gradient fractions indicates that caveolae are a component of the rat brain microvessel membranes isolated by our biochemical technique.
Fractions that contain the higher and lower density PgP pools both contain caveolar proteins. This suggests that there are at least two caveolar populations in vivo with different compositions or subcellular locations. There are several possible explanations for our finding of two caveolar populations that co-fractionate with PgP. One possibility, illustrated in Figure 6, is that there are caveolae with different lipoprotein content that affect caveolar density. Caveolae are dynamic structures that can fuse or undergo endocytosis (Parton and del Pozo 2013) which may account for the observed alterations in density. Another possibility is that the caveolae in the two populations are attached to different intracellular structural proteins, such as actin filaments and microtubules. Alternate attachments could indicate caveolae from different subcellular locations such as luminal and abluminal endothelial cell membranes. Both membranes contain caveolae and PgP (Bendayan et al. 2006). Our ability to biochemically separate the two caveolar populations will allow us to identify signaling events unique to each population and determine the significance of these signals for PgP activity/trafficking.
Figure 6.
Model of caveolae in the two fractions that contain caveolar proteins. P-glycoprotein is located in two pools of different densities. These pools contain caveolar proteins suggesting that there are two types of P-glycoprotein-containing caveolae with different lipoprotein compositions or subcellular locations in rat brain microvessels.
A caveolar location for PgP may be critical for PgP activity (Wang et al. 2014). Membrane cholesterol content is linked to PgP activity; a basal concentration is required for PgP activity (Troost et al. 2004; Jodoin et al. 2003). Cholesterol is concentrated in caveolae (Sowa 2012). Both cavin1 and cavin2 are involved in regulating caveolar cholesterol content (Parton and del Pozo 2013). Caveolae in plasma membranes contain a multitude of proteins, many of which are involved in signaling cascades. Within the caveolae, autocrine signaling via release of small molecules that bind to neighboring caveolar proteins initiates signaling cascades (Head et al. 2014). Release of reactive oxygen/nitrogen species within the caveolae can also directly modulate enzymatic activity (Rath et al. 2009; Oshikawa et al. 2010).
A portion of the PDI, a protein with thiol oxidoreductase activity (Wilkinson and Gilbert 2004), co-fractionates with and binds to PgP. PDI is located in the endoplasmic reticulum where it is involved in protein folding; however, it is also found on the cell surface of some cell types and can be secreted (Mezghrani et al. 2000; Zai et al. 1999). QSOX1 is located in the endoplasmic reticulum and/or Golgi as well as secreted (Tury et al. 2004; Ilani et al. 2013). A portion of the PDI is located in fractions containing QSOX1, suggesting that these fractions (19–22) contain endoplasmic reticulum and Golgi proteins. However, we found that a portion of the PDI co-fractionates with plasma membrane markers and PgP suggesting that some of the PDI is bound to PgP at the plasma membrane of the BBB endothelial cells. Rearrangement of disulfide bonds in PgP increases ATPase activity (Urbatsch et al. 2001); maximal PgP activity requires a specific disulfide bond configuration (Loo et al. 2013). Previously, we also showed that a PIP stimulus elicited disassembly of a high molecular weight complex that contains PgP and caveolin1 concomitant with increased PgP activity (McCaffrey et al. 2012). It is tempting to speculate that PDI is responsible for disassembly of the high molecular weight complexes and rearrangement of disulfide bonds within PgP to increase PgP activity.
Identification of ATPB, annexin 3 and annexin V as prominent proteins in fractions that contain PgP suggests that signaling pathways with the potential to impact PgP activation could be located near PgP in BBB endothelial cell membranes in vivo. One of the exciting findings of this study was the presence of ATPB. This protein is a component of the F1/F0 ATPase in the mitochondria (Leyva et al. 2003); however, it can also be found in the plasma membrane of some cell types including endothelial cells (Wang et al. 2006; Shin and Kim 2010; Bae et al. 2004). Mitochondrial membrane proteins were not detected in the gradient fractions, which indicates that ATPB is likely located in BBB endothelial cell plasma membranes. In the plasma membrane, ATPB synthesizes ATP and releases it into the extracellular domain in response to cellular stresses including inflammation (Wang et al. 2006; Idzko et al. 2014). Once in the extracellular domain, ATP can bind to purinergic receptors (Idzko et al. 2014). Both the ATPB and purinergic receptors are located in caveolae (Wang et al. 2006; D’ Ambrosi and Volonte 2013). Autocrine signaling within the caveolae is certainly possible; ATP can be released into the caveolae by ATPB where it can bind caveolar purinergic receptors to initiate a signaling cascade.
Annexin 3 and annexin V are both Ca+2-dependent phospholipid-binding proteins that can bind the plasma membrane (Monastyrskaya et al. 2009). At the plasma membrane, they can block access of phospholipase A2 (PLA2) to its phospholipid substrate (Buckland and Wilton 1998) and may influence Ca+2 signaling (Monastyrskaya et al. 2009). Both PLA2 activation and Ca+2 signaling occur during inflammation. The literature suggests links between ATPB, annexin 3 and annexin V and the inflammatory response. In the current study we found that these proteins co-fractionate with PgP-containing membranes. Future studies will determine the effect of the indicated signaling pathways on basal PgP activity and activation of PgP during inflammation.
Our analysis of PgP co-fractionating proteins identified several proteins involved in trafficking pathways. Co-fractionation of Rab5 and Rab11a with PgP indicates that endosomal/lysosomal pathways are potentially involved in PgP trafficking in BBB endothelial cells in vivo. The endosomal/lysosomal sorting pathway receives vesicles containing newly synthesized proteins and proteins in endocytic vesicles from the cell surface (Hutagalung and Novick 2011). These vesicles are sorted by the Rab proteins resulting in some vesicles moving to the plasma membrane while others are targeted to lysosomes (Hutagalung and Novick 2011). Rab 5 mediates endocytosis and is a marker of the early endosome (Hutagalung and Novick 2011; Spang 2009). Rab11a is involved with endocytic sorting and recycling of the endosomes to the plasma membrane (Hutagalung and Novick 2011; Kelly et al. 2012). Both proteins influence PgP trafficking in cell culture cells or hepatocytes (Fu and Arias 2012); however, their role in rat brain microvessel endothelial cells in vivo is unknown. A portion of the PgP in naïve rat brain endothelial cells is contained in intracellular vesicles (Bendayan et al. 2006). There is some evidence that PgP endocytosis occurs in rat microvessel endothelial cells. Hawkins et al. found that, in response to a VEGF signaI, a portion of PgP was rapidly internalized (Hawkins et al. 2010). Our previous data show that PgP trafficking events occur during PIP (McCaffrey et al. 2012); however, whether these include endocytosis and/or recycling/degradation is unknown. PgP also co-fractionated with cathepsin D, a lysosomal protein, indicating lysosomal membranes are in the PgP-containing fractions. This suggests that some of the PgP could be targeted for degradation through the endosomal/lysosomal degradation pathway. Taken together, these data are consistent with Rab-mediated endocytic cycling playing a role in the modulation of PgP activity and trafficking at the BBB in vivo. We also found that Hsc71 co-immunoprecipitated with PgP in rat microvessel lysates and bEnd.3 cells indicating this protein is a PgP binding partner. Hsc71 has chaperone functions; however, it is also implicated in trafficking events at the plasma membrane, particularly in endocytic cycling (Lu et al. 2007; Chanoux et al. 2013). Identification of Hsc71 as a PgP binding partner suggests it could participate in PgP trafficking events.
Identification of proteins involved in post-translational regulation of PgP activity is a key component in our understanding of PgP regulation that will impact our ability to modulate PgP activity in the clinic. The proteins we identified in this study, which co-fractionate with PgP in rat brain microvessels, suggest several signaling pathways and molecular events that have the potential to modulate PgP activity at the post-translational level. The finding that PgP co-fractionates with PDI suggests that PDI could catalyze disassembly of high molecular weight PgP-containing complexes and rearrangement of internal disulfide bonds to optimize PgP activity. Location of two of the Rab proteins, Rab5 and Rab11a, in the same fractions as PgP, as well as Hsc71 as a PgP binding partner, indicate that these proteins are potentially involved in PgP trafficking events. Trafficking events would affect the location of PgP and ability of PgP to efflux drug into the capillary lumen. The indication that there are two different caveolar populations that co-fractionate with PgP and that ATPB is likely at the endothelial cell surface near PgP in vivo, present new, exciting possibilities for investigating how PgP activity is regulated within membrane microdomains at the BBB. Measuring the ability of the newly identified proteins and suggested pathways to modulate PgP activity will indicate novel targets for drug development to treat CNS disorders.
Supplementary Material
Acknowledgments
Funding for this study comes from NIH grants DA 011271 and NS 042652 to T.P.D. A portion of this work was submitted to the Honors College, University of Arizona by Yifeng Zhang as partial fulfillment of the requirements for graduation with honors. A portion of this work was presented in poster format at the Barriers of the CNS Gordon Research Conference 2014.
Abbreviations
- AIDS
acquired immune deficiency syndrome
- ATPB
ATP synthase β subunit
- BBB
blood brain barrier
- CARR
λ carrageenan-injected animals
- cavin1
Polymerase 1 and transcription release factor/PTRF
- cavin2
serum deprivation response protein/SDPR
- COX IV
cytochrome c oxidase subunit IV
- GLUT1
glucose transporter1
- HRP
horseradish peroxidase
- Hsc71
heat shock cognate71
- LC
liquid chromatography
- MS
mass spectrometry
- MWCO
molecular weight cut-off
- PDI
protein disulfide isomerase
- PgP
P-glycoprotein (MDR1/ABCB1)
- PIP
peripheral inflammatory pain
- PKC
protein kinase C
- PLA2
phospholipase A2
- QSOX1
quiescin sulfhydral oxidase 1
- SAL
saline injected animals
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Footnotes
The authors have no conflicts of interest to declare.
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Bae TJ, Kim MS, Kim JW, Kim BW, Choo HJ, Lee JW, Kim KB, Lee CS, Kim JH, Chang SY, Kang CY, Lee SW, Ko YG. Lipid raft proteome reveals ATP synthase complex in the cell surface. Proteomics. 2004;4:3536–3548. doi: 10.1002/pmic.200400952. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Barakat S, Demeule M, Pilorget A, Regina A, Gingras D, Baggetto LG, Beliveau R. Modulation of p-glycoprotein function by caveolin-1 phosphorylation. J Neurochem. 2007;101:1–8. doi: 10.1111/j.1471-4159.2006.04410.x. [DOI] [PubMed] [Google Scholar]
- Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P- glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54:1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- Buckland AG, Wilton DC. Inhibition of secreted phospholipases A2 by annexin V. Competition for anionic phospholipid interfaces allows an assessment of the relative interfacial affinities of secreted phospholipases A2. Biochim Biophys Acta. 1998;1391:367–376. doi: 10.1016/s0005-2760(98)00026-5. [DOI] [PubMed] [Google Scholar]
- Chanoux RA, Shubin CB, Robay A, Suaud L, Rubenstein RC. Hsc70 negatively regulates epithelial sodium channel trafficking at multiple sites in epithelial cells. Am J Physiol Cell Physiol. 2013;305:C776–C787. doi: 10.1152/ajpcell.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford EM, Hyman S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx. 2005;2:27–43. doi: 10.1602/neurorx.2.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’ Ambrosi N, Volonte C. Metabotropic purinergic receptors in lipid membrane microdomains. Curr Med Chem. 2013;20:56–63. [PubMed] [Google Scholar]
- Darie CC, Deinhardt K, Zhang G, Cardasis HS, Chao MV, Neubert TA. Identifying transient protein-protein interactions in EphB2 signaling by blue native PAGE and mass spectrometry. Proteomics. 2011;11:4514–4528. doi: 10.1002/pmic.201000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeule M, Jodoin J, Gingras D, Beliveau R. P-glycoprotein is localized in caveolae in resistant cells and in brain capillaries. FEBS Lett. 2000;466:219–224. doi: 10.1016/s0014-5793(00)01087-5. [DOI] [PubMed] [Google Scholar]
- Fu D, Arias IM. Intracellular trafficking of P-glycoprotein. Int J Biochem Cell Biol. 2012;44:461–464. doi: 10.1016/j.biocel.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T, Muller S, Klier G, Pante N, Blevitt JM, Haner M, Paschal B, Aebi U, Gerace L. Structural analysis of the p62 complex, an assembly of O-linked glycoproteins that localizes near the central gated channel of the nuclear pore complex. Mol Biol Cell. 1995;6:1591–1603. doi: 10.1091/mbc.6.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CG, Shvets E, Howard G, Riento K, Nichols BJ. Deletion of cavin genes reveals tissue-specific mechanisms for morphogenesis of endothelial caveolae. Nat Commun. 2013;4:1831. doi: 10.1038/ncomms2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Rigor RR, Miller DS. Rapid loss of blood-brain barrier P-glycoprotein activity through transporter internalization demonstrated using a novel in situ proteolysis protection assay. J Cereb Blood Flow Metab. 2010;30:1593–1597. doi: 10.1038/jcbfm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head BP, Patel HH, Insel PA. Interaction of membrane/lipid rafts with the cytoskeleton: impact on signaling and function: membrane/lipid rafts, mediators of cytoskeletal arrangement and cell signaling. Biochim Biophys Acta. 2014;1838:532–545. doi: 10.1016/j.bbamem.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckler EJ, Alon A, Fass D, Thorpe C. Human quiescin-sulfhydryl oxidase, QSOX1: probing internal redox steps by mutagenesis. Biochemistry. 2008;47:4955–4963. doi: 10.1021/bi702522q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani T, Alon A, Grossman I, Horowitz B, Kartvelishvily E, Cohen SR, Fass D. A secreted disulfide catalyst controls extracellular matrix composition and function. Science. 2013;341:74–76. doi: 10.1126/science.1238279. [DOI] [PubMed] [Google Scholar]
- Jodoin J, Demeule M, Fenart L, Cecchelli R, Farmer S, Linton KJ, Higgins CF, Beliveau R. P-glycoprotein in blood-brain barrier endothelial cells: interaction and oligomerization with caveolins. J Neurochem. 2003;87:1010–1023. doi: 10.1046/j.1471-4159.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- Kelly EE, Horgan CP, McCaffrey MW. Rab11 proteins in health and disease. Biochem Soc Trans. 2012;40:1360–1367. doi: 10.1042/BST20120157. [DOI] [PubMed] [Google Scholar]
- Leyva JA, Bianchet MA, Amzel LM. Understanding ATP synthesis: structure and mechanism of the F1-ATPase (Review) Mol Membr Biol. 2003;20:27–33. doi: 10.1080/0968768031000066532. [DOI] [PubMed] [Google Scholar]
- Liang XJ, Aszalos A. Multidrug transporters as drug targets. Curr Drug Targets. 2006;7:911–921. doi: 10.2174/138945006778019264. [DOI] [PubMed] [Google Scholar]
- Loo TW, Bartlett MC, Clarke DM. Human P-glycoprotein contains a greasy ball-and-socket joint at the second transmission interface. J Biol Chem. 2013;288:20326–20333. doi: 10.1074/jbc.M113.484550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. P-glycoprotein. Associations between domains and between domains and molecular chaperones. J Biol Chem. 1995;270:21839–21844. doi: 10.1074/jbc.270.37.21839. [DOI] [PubMed] [Google Scholar]
- Lu HA, Sun TX, Matsuzaki T, Yi XH, Eswara J, Bouley R, McKee M, Brown D. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem. 2007;282:28721–28732. doi: 10.1074/jbc.M611101200. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Quigley CA, Nametz N, Seelbach MJ, Campos CR, Brooks TA, Egleton RD, Davis TP. Tight junctions contain oligomeric protein assembly critical for maintaining blood-brain barrier integrity in vivo. J Neurochem. 2007;103:2540–2555. doi: 10.1111/j.1471-4159.2007.04943.x. [DOI] [PubMed] [Google Scholar]
- McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, Demarco K, Laracuente ML, Ronaldson PT, Davis TP. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem. 2012;122:962–975. doi: 10.1111/j.1471-4159.2012.07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezghrani A, Courageot J, Mani JC, Pugniere M, Bastiani P, Miquelis R. Protein-disulfide isomerase (PDI) in FRTL5 cells. pH-dependent thyroglobulin/PDI interactions determine a novel PDI function in the post-endoplasmic reticulum of thyrocytes. J Biol Chem. 2000;275:1920–1929. doi: 10.1074/jbc.275.3.1920. [DOI] [PubMed] [Google Scholar]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood- brain barrier. Trends Pharmacol Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Bauer B, Hartz AM. Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy. Pharmacol Rev. 2008;60:196–209. doi: 10.1124/pr.107.07109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DS, Cannon RE. Signaling pathways that regulate basal ABC transporter activity at the blood- brain barrier. Curr Pharm Des. 2014;20:1463–1471. doi: 10.2174/13816128113199990457. [DOI] [PubMed] [Google Scholar]
- Monastyrskaya K, Babiychuk EB, Draeger A. The annexins: spatial and temporal coordination of signaling events during cellular stress. Cell Mol Life Sci. 2009;66:2623–2642. doi: 10.1007/s00018-009-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, Poole LB, Fukai T, Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PLoS One. 2010;5:e10189. doi: 10.1371/journal.pone.0010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- Potschka H. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia. 2010;51:1333–1347. doi: 10.1111/j.1528-1167.2010.02585.x. [DOI] [PubMed] [Google Scholar]
- Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Rath G, Dessy C, Feron O. Caveolae, caveolin and control of vascular tone: nitric oxide (NO) and endothelium derived hyperpolarizing factor (EDHF) regulation. J Physiol Pharmacol. 2009;60(Suppl 4):105–109. [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan M, Gingras D, Piquette-Miller M, Bendayan R. Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. J Neurochem. 2004;89:788–800. doi: 10.1111/j.1471-4159.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- Seelbach MJ, Brooks TA, Egleton RD, Davis TP. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102:1677–1690. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- Shin S, Kim KS. Human brain endothelial ATP synthase beta-subunit is mannose-insensitive binding target of FimH. FEMS Microbiol Lett. 2010;303:156–162. doi: 10.1111/j.1574-6968.2009.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska I, Dorobantu C, Woods AG, Macovei A, Branza-Nichita N, Darie CC. Proteomic analysis of plasma membranes isolated from undifferentiated and differentiated HepaRG cells. Proteome Sci. 2012a;10:47. doi: 10.1186/1477-5956-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowska I, Gawinowicz MA, Ngounou Wetie AG, Darie CC. Disulfide proteomics for identification of extracellular or secreted proteins. Electrophoresis. 2012b;33:2527–2536. doi: 10.1002/elps.201200182. [DOI] [PubMed] [Google Scholar]
- Sowa G. Caveolae, caveolins, cavins, and endothelial cell function: new insights. Front Physiol. 2012;2:120. doi: 10.3389/fphys.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A. On the fate of early endosomes. Biol Chem. 2009;390:753–759. doi: 10.1515/BC.2009.056. [DOI] [PubMed] [Google Scholar]
- Spudich A, Kilic E, Xing H, Kilic U, Rentsch KM, Wunderli-Allenspach H, Bassetti CL, Hermann DM. Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat Neurosci. 2006;9:487–488. doi: 10.1038/nn1676. [DOI] [PubMed] [Google Scholar]
- Sun J, He ZG, Cheng G, Wang SJ, Hao XH, Zou MJ. Multidrug resistance P-glycoprotein: crucial significance in drug disposition and interaction. Med Sci Monit. 2004;10:RA5–14. [PubMed] [Google Scholar]
- Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10:159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- Troost J, Lindenmaier H, Haefeli WE, Weiss J. Modulation of cellular cholesterol alters P-glycoprotein activity in multidrug-resistant cells. Mol Pharmacol. 2004;66:1332–1339. doi: 10.1124/mol.104.002329. [DOI] [PubMed] [Google Scholar]
- Tury A, Mairet-Coello G, Poncet F, Jacquemard C, Risold PY, Fellmann D, Griffond B. QSOX sulfhydryl oxidase in rat adenohypophysis: localization and regulation by estrogens. J Endocrinol. 2004;183:353–363. doi: 10.1677/joe.1.05842. [DOI] [PubMed] [Google Scholar]
- Urbatsch IL, Gimi K, Wilke-Mounts S, Lerner-Marmarosh N, Rousseau ME, Gros P, Senior AE. Cysteines 431 and 1074 are responsible for inhibitory disulfide cross-linking between the two nucleotide-binding sites in human P-glycoprotein. J Biol Chem. 2001;276:26980–26987. doi: 10.1074/jbc.M010829200. [DOI] [PubMed] [Google Scholar]
- Wang T, Chen Z, Wang X, Shyy JY, Zhu Y. Cholesterol loading increases the translocation of ATP synthase beta chain into membrane caveolae in vascular endothelial cells. Biochim Biophys Acta. 2006;1761:1182–1190. doi: 10.1016/j.bbalip.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu T, Bai Y, Liao H, Qiu S, Chang Z, Liu Y, Yan X, Guo H. Polymerase I and transcript release factor acts as an essential modulator of glioblastoma chemoresistance. PLoS One. 2014;9:e93439. doi: 10.1371/journal.pone.0093439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B, Gilbert HF. Protein disulfide isomerase. Biochim Biophys Acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Zai A, Rudd MA, Scribner AW, Loscalzo J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J Clin Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetta L, Marcus SG, Vasile J, Dobryansky M, Cohen H, Eng K, Shamamian P, Mignatti P. Expression of Von Willebrand factor, an endothelial cell marker, is up-regulated by angiogenesis factors: a potential method for objective assessment of tumor angiogenesis. Int J Cancer. 2000;85:281–288. doi: 10.1002/(sici)1097-0215(20000115)85:2<281::aid-ijc21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Zheng YZ, Boscher C, Inder KL, Fairbank M, Loo D, Hill MM, Nabi IR, Foster LJ. Differential impact of caveolae and caveolin-1 scaffolds on the membrane raft proteome. Mol Cell Proteomics. 2011;10:M110. doi: 10.1074/mcp.M110.007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.