Abstract
The respiratory tract maintains immune homeostasis despite constant provocation by environmental antigens. Failure to induce tolerogenic responses to allergens incites allergic inflammation. Despite the understanding that antigen-presenting cells (APCs) have a crucial role in maintaining immune tolerance, the underlying mechanisms are poorly understood. Using mice with a conditional deletion of PPARγ in CD11c+ cells, we show that PPARγ performs two critical functions in CD11c+ cells to induce tolerance thereby preserving immune homeostasis. First, PPARγ was crucial for induction of retinaldehyde dehydrogenase (aldh1a2) selectively in CD103+ DCs, which we recently showed promotes Foxp3 expression in naïve CD4+ T cells. Second, in all CD11c+ cells, PPARγ was required to suppress expression of the Th17-skewing cytokines IL-6 and IL-23p19. Also, lack of PPARγ in CD11c+ cells induced p38 MAP kinase activity, which was recently linked to Th17 development. Thus, PPARγ favors immune tolerance by promoting Treg generation and blocking Th17 differentiation.
Introduction
Immune tolerance prevents unwarranted inflammatory immune responses to antigens that in susceptible individuals can lead to allergic diseases such as asthma (1). Antigen presenting cells (APCs) play a central role in the decision-making process between immune activation and tolerance (2). It is, therefore, important to understand the molecular mechanisms by which APCs mediate immune tolerance to be able to use the full potential of these cells for suppression of undesirable immune activation.
The molecule peroxisome proliferator-activated receptor gamma PPARγ is a member of the nuclear receptor superfamily. In addition to promoting adipocyte differentiation and glucose homeostasis, PPARγ also exerts anti-inflammatory effects (3). In the lung, PPARγ is expressed by multiple cell types including CD11c+ APCs (4), which have the unique dual ability to present antigens as well as express specific cytokines and co-stimulatory molecules that influence T cell differentiation. Using CD11c-Cre-PPARγfl/fl mice with conditional deletion of PPARγ in CD11c+ cells, we demonstrate a critical role for PPARγ in the maintenance of immune homeostasis in the airways.
Materials and Methods
Mice
PPARγfl/fl and CD11c-Cre mice (The Jackson Laboratory) were housed and bred under pathogen-free conditions in the Department of Laboratory Animal Resources (DLAR) at the University of Pittsburgh, to generate mice with cell-specific deletion of PPARγ in CD11c+ cells (CD11c-Cre-PPARγfl/fl) and littermate controls (PPARγfl/fl). OT-IIxThy1.1 transgenic mice, a gift from Dr. Lauren Cohn at Yale University (New Haven), were bred in the DLAR facility. All protocols involving animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh.
Induction of tolerance or inflammation in mice
Immunological tolerance in mice was established using a model of daily exposure to aerosolized ovalbumin (OVA; in PBS) (Sigma) as described previously (5). Airway inflammation was induced using a model previously described (6, 7). Briefly, mice were immunized with 100 µg OVA in the presence of the adjuvant cholera toxin (1 µg CT; List Biochemicals) for 3 consecutive days and subsequently challenged by OVA after a 5 d rest period.
Antibodies and flow cytometry
Cells were surface stained using FITC-, PE-, PE-Cy7-, APC-, PE-Texas Red- and PercP-Cy5.5–conjugated Abs to CD4, CD8α, B220, CD25, CD11b, CD11c, CD103, CD90.1, Ly6C, CD64 (BD Biosciences), CD45, CD80, CD86 (Biolegend), and MHCII (Southern Biotech). Intracellular staining for transcription factors (Foxp3, RORγt, GATA-3 and T-bet) and cytokines (IL-17A, IFN-γ, IL-5, IL-13, IL-6 and IL-23p19) was performed according to the manufacturer's instructions. Stained cells were examined on a FACSCalibur and FACSAria flow cytometers (BD Immunocytometry Systems) and the data were analyzed using FlowJo software (Tree Star).
Induction of Foxp3+ CD4+ T cells in vivo
Treg-depleted OT-IIxThy1.1 splenic CD4+ T cells were adoptively transferred i.v. (106 cells/recipient) into CD11c-Cre-PPARγfl/fl and PPARγfl/fl mice. 24 h following adoptive transfer, recipient mice were subjected to the tolerance model. Subsequently Foxp3 expression in CD4+CD90.1+ donor cells was assessed.
Real-time or semi-quantitative RT-PCR
Real-time PCR was performed for Ifng, Il5, Il13, Il17a, IL17f, Il6, Il23a, Il25, aldh1a2, pparg and hprt1 (Taqman Gene Expression Assays; Life Technologies). Expression for aldh1a2, pparg, Il23a and Il6 was calculated using the 2−ΔCt method (normalized to hprt1) and Ifng, Il5, Il13, Il17a, Il17f and Il25 were analyzed using the 2−ΔΔCt method (relative to expression in control; normalized to hprt1). Cre-mediated deletion of PPARγ was assessed using a semi-quantitative RT-PCR (Supplemental Fig. 1A).
ELISA for assaying OVA-specific serum IgE
ELISA to detect OVA-specific IgE in the serum was performed as previously described (6, 7).
p38 MAP kinase assay
p38 MAP kinase activity in cell extracts was performed using a non-radioactive p38 MAP kinase assay kit (Cell Signaling) following manufacturer’s instructions.
Statistical analyses
Student’s unpaired two-tailed t-test and one-way ANOVA with Tukey’s post hoc test was performed wherever applicable, using GraphPad Prism 5. Differences between groups were considered significant when p<0.05. *p< 0.05, **p<0.01, ***p< 0.001.
Results and Discussion
CD11c-specific PPARγ deficiency impairs induction of airway tolerance
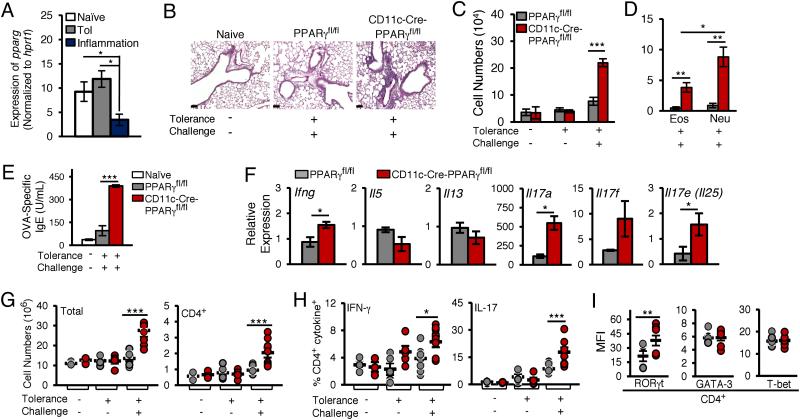
We recently showed an essential role of CD103+ dendritic cells (DCs) in induction of immune tolerance in the airways in response to a low dose of inhaled antigen (7). Compared to cells from naïve mice, CD103+ DCs from mice induced for tolerance upregulated expression of retinaldehyde dehydrogenase (aldh1a2), a key enzyme that catalyzes the generation of retinoic acid and together with TGF-β promotes de novo Foxp3 expression in naïve CD4+ T cells (7). The expression of this enzyme was however, downregulated when mice were immunized for inflammation (7). Since PPARγ has anti-inflammatory functions (3, 8)and previous in vitro studies showed increased aldh1a2 expression with PPARγ agonists (9, 10), we examined PPARγ expression in CD103+ DCs from naïve mice and mice immunized for tolerance or inflammation. As shown in Fig. 1A, the expression profile of PPARγ mirrored that of aldh1a2 (7). Given these results, we asked whether mice devoid of PPARγ expression selectively in APCs would be impaired in immune suppression/tolerance. We generated mice selectively deficient in PPARγ in CD11c+ APCs comprising both lung DCs and macrophages by breeding PPARγfl/fl mice (11) with transgenic mice expressing Cre recombinase driven by the CD11c promoter (CD11c-Cre mice). Efficient CD11c-specific Cre-mediated deletion of exons 1 and 2 of PPARγ was observed in the resulting CD11c-Cre-PPARγfl/fl mice (Supplemental Fig. 1A). Selective absence of PPARγ protein in CD11c+ cells was further confirmed (Supplemental Fig. 1B). No difference between total cell yields and numbers of various immune cells present in the spleens (Supplemental Fig. 1C and D) and lungs (Supplemental Fig. 1C and E) of naïve CD11c-Cre-PPARγfl/fl versus littermate controls (PPARγfl/fl) mice was noted except for an increase in CD103+ DC numbers. Given that our overall question was the role of PPARγ in pulmonary immune suppression, it was important to have PPARγ deleted in both DCs and macrophages since both of these cell types can process antigen and express pro-inflammatory cytokines that can influence T cell responses.
FIGURE 1.
CD11c-specific PPARγ deficiency impairs airway tolerance induction. (A) Expression of pparg mRNA in CD103+ lung DCs from naïve, tolerized and OVA/CT mice, relative to hprt1. (B-I) PPAR γ fl/fl and from CD11c-Cre-PPAR γ fl/fl mice were tested for induction of tolerance as described. 24 hours after the last challenge, various parameters of inflammation were analyzed. (B) Histological assessment of lung sections after PAS staining. Scale bar, 100 µm and magnification 20X. (C) Total cells, (D) eosinophil (Eos) and neutrophil (Neu) numbers in the BALF (n=6). (E) OVA-specific serum IgE levels. (F) qRT-PCR analysis of gene expression in the lungs of mice subjected to the sequential tolerance and inflammation model, relative to expression in naïve mice. (G) Total and CD4+ T cell numbers (n=4) and (H) frequency of IL-17A- and IFN-γ-expressing lung CD4+ T cells in mice (n=6) subjected to indicated conditions. (I) Expression of transcription factors RORγt, GATA-3 and T-bet in lung CD4+ T cells from PPARγfl/fl and CD11c-Cre-PPARγfl/fl mice (n=6) subjected sequentially to tolerance and inflammation models. Numbers shown represent mean ± SEM mean fluorescence intensities (MFIs). Data in (A-B) and (E-F) are representative of two independent experiments and data in (C) and (G-I) are representative of three independent experiments; symbols in the graphs represent data from individual mice, horizontal lines show the mean and error bars denote SEM.
To examine the role of PPARγ in induction of tolerance, CD11c-Cre-PPARγfl/fl and PPARγfl/fl mice were first tolerized using a low dose of inhaled antigen (Ovalbumin-OVA) mimicking ambient exposure to environmental antigens (5, 12). To test for tolerance establishment, the mice were then challenged using a model of lung inflammation involving sensitization with OVA and the mucosal adjuvant cholera toxin (CT) followed by repeated exposure to aerosolized OVA(6, 13). 24 hours after the last OVA challenge, histological examination of lung sections revealed increased cellular infiltration around bronchovascular bundles in CD11c-Cre-PPARγfl/fl mice as compared to that in the controls (Fig. 1B and Supplemental Fig. 2A). Assessment of total cell counts in the BAL fluid also yielded similar results (Fig. 1C). Differential cell count analysis of the BAL fluid samples revealed a more significant increase in the number of neutrophils and eosinophils, but not macrophages, in CD11c-Cre-PPARγfl/fl mice as compared to that in PPARγfl/fl mice (Fig. 1D and Supplemental Fig. 2B). Similar data were obtained for OVA-specific serum IgE levels (Fig. 1E). When analyzed for expression of cytokines expressed by T cells, the expression of multiple IL-17 family genes was higher in the lungs of CD11c-Cre-PPARγfl/fl mice. A less pronounced increase in IFN-γ levels was also observed (Fig. 1F). However, the levels of the type 2 cytokines, IL-5 and IL-13, were not enhanced in the lungs of the CD11c-Cre-PPARγfl/fl mice (Fig. 1F).
We further investigated the effect of CD11c-specific PPARγ loss on CD4+ T cells frequency and function. An increase in the numbers of both total cells as well as CD4+ T cells was observed in the lungs of CD11c-Cre-PPARγfl/fl mice (Fig. 1G). As CD4 is expressed by T cells as well as by other cell types such as DCs, a gating strategy was employed to distinguish between CD4+ T cells and CD4+ DCs. It was observed that CD4+SSClo gated cells were essentially CD4+ T cells with no contaminating CD4+ CD11c+ DCs (Supplemental Fig. 2C). Consistent with the gene expression data, loss of PPARγ expression in CD11c+ cells caused a significant increase in the frequency of IL-17A+ CD4+ and IFN-γ+ CD4+ T cells (Fig. 1H). No significant change was noted in IL-13 or IL-5 expression by the CD4 T cells in the two groups (Supplemental Fig. 2D). When analyzed for the expression of key transcription factors associated with T helper lineages, while no significant difference was noted in the expression of T-bet and GATA-3 in CD4+ T cells between the two groups of mice, the expression of RORγt was higher in CD4+ T cells from CD11c-Cre-PPARγfl/fl mice (Fig. 1I & Supplemental Fig. 2E), an observation that was in line with increased expression of IL-17A in the CD4+ T cells (Fig. 1H).
CD11c-specific PPARγ deficiency impairs de novo Foxp3 expression in CD4+ T cells and specific loss of aldh1a2 expression in CD103+ DCs
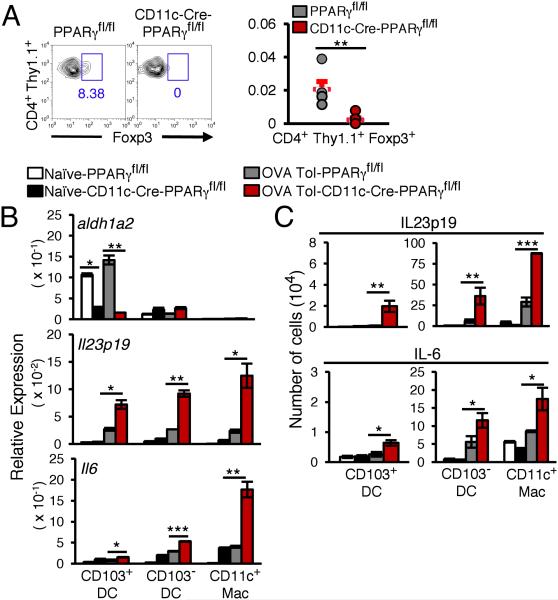
With the inability of the CD11c-Cre-PPARγfl/fl mice to mount airway tolerance, we next asked whether PPARγ deficiency in the APCs prevented de novo Foxp3 induction in naïve T cells required to generate iTregs whose involvement in establishment of airway tolerance has been established by us and others (5, 7, 14). CD4+CD25− splenic T cells from donor OT-II mice bred with Thy1.1 mice were adoptively transferred into CD11c-Cre-PPARγfl/fl and PPARγfl/fl mice. The recipient mice were subjected to the tolerance-inducing regimen using inhaled OVA at the end of which the lungs were harvested and Foxp3 expression in the donor cells (CD4+ Thy1.1+) was analyzed. Absence of PPARγ expression in CD11c+ cells prevented Foxp3 induction in the transferred cells, which was however, readily apparent in cells transferred into PPARγfl/fl mice (Fig. 2A). We observed that the expression of aldh1a2, the key enzyme responsible for retinoic acid (RA) production and induction of airway tolerance (7) was restricted to CD103+ DCs isolated from control tolerized mice but the expression was significantly reduced in PPARγ-deficient CD103+ DCs (Fig. 2B).
FIGURE 2.
PPARγ deficiency in CD11c+ cells impairs de novo Foxp3 induction in CD4+ T cells and promotes expression of pro-inflammatory cytokines. (A) Representative flow cytometry data and number of de novo induced CD4+ Thy1.1+ Foxp3+ T cells in PPARγfl/fl and CD11c-Cre-PPARγfl/fl mice are shown. Symbols in the graphs represent data from individual mice; horizontal lines show the mean and error bars denote SEM (n=6 mice). (B) Expression of aldh1a2, Il6 and Il23a (p19) relative to that of hprt1. (C) Various CD11c+ cells subsets expressing IL-6 and IL-23p19 from naïve and tolerized CD11c-Cre-PPARγfl/fl and PPARγfl/fl mice (n=6 mice). Data shown are mean ± SEM of two independent experiments.
CD11c-specific PPARγ deficiency augments expression of pro-inflammatory cytokines in CD11c+ cells
Given the increase in expression of Th17 family cytokines in the lungs of CD11c-Cre-PPARγfl/fl mice after antigen exposure, we next examined the expression of the Th17-skewing cytokines IL-6 and IL-23 in the various CD11c+ subsets. All PPARγ-deficient CD11c+ subsets examined were found to express significantly higher levels of Il6 and Il23a (IL-23p19) mRNA as compared to CD11c+ cells from control mice (Fig. 2B). We also examined intracellular expression of IL-6 and IL-23p19 in various CD11c+ subsets from both naïve and tolerized CD11c-Cre-PPARγfl/fl and PPARγfl/fl mice. In line with the T cell cytokine data, there was a significant increase in the number of IL-6- and IL-23p19-expressing cells among all the subsets examined in the tolerized CD11c-Cre-PPARγfl/fl mice as compared to that in PPARγfl/fl mice (Fig. 2C).
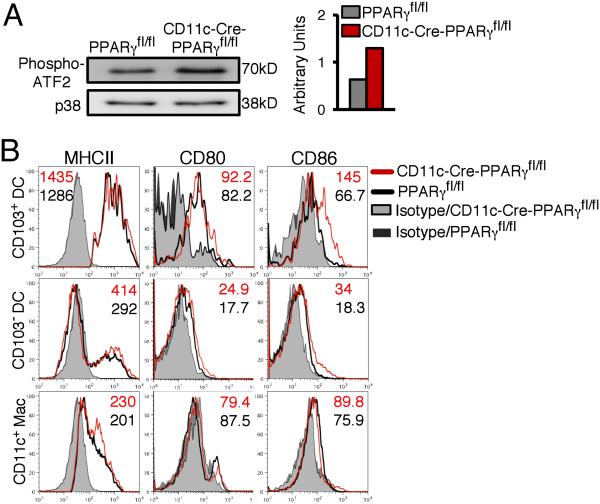
Increased p38 MAP kinase activity and expression of CD86 in PPARγ-deficient DCs
Recently, deletion of p38 MAP kinase (MAPK) in CD11c+ cells was shown to cause reduced IL-6 production and CD86 expression with impairment of Th17 differentiation (15). We wondered whether absence of PPARγ in CD11c+ cells converted the DCs to a Th17-promoting phenotype with increased p38 MAPK activity (as measured by immune complex kinase assay) and CD86 expression, which was found to be the case (Figs. 3A, 3B and Supplemental Fig. 2F).
FIGURE 3.
PPARγ deficiency in CD11c+ cells promotes p38 MAPK activity and CD86 expression. (A) Immunoprecipitated phospho-p38 from lung CD11c+ cells incubated with the substrate ATF-2 and phospho-ATF-2 was detected by immunoblotting as a measure of p38 MAPK activity. Total p38 MAP kinase was assessed to ensure equivalent protein loading. Bar graph represents densitometric quantification. (B) MHC II, CD80 and CD86 expression on various CD11c+ cell subsets. Numbers shown represent MFIs. Data shown are representative of two independent experiments.
While previous studies have associated PPARγ function in T cells with Treg promotion (16) and Th17 suppression (17) in the context of synthetic PPARγ agonists, our study has identified an important role of CD11c+ cell-specific PPARγ in response to low dose inhaled antigen only, which mimics natural exposure to environmental antigens. Since no synthetic agonist of PPARγ was used and yet PPARγ function was required for immune suppression in our study, it is likely that antigen inhalation causes PPARγ activation by endogenous ligands, many of which have been previously described (3). Pollen-associated lipid mediators such as E1-phytoprostanes (PPE1) were previously shown to activate PPARγ and inhibit LPS-induced IL-12 production in human DCs by transrepression of NF-κB (18).
We show that PPARγ is critical for the expression of the enzyme aldh1a2 in lung DCs, which catalyzes retinoic acid production and together with TGF-β promotes Foxp3 expression in naïve CD4+ T cells (7, 19-24). Among the 3 broad CD11c+ cell subsets, CD103+, CD103− and alveolar macrophages, it is the CD103+ subset that was found to possess the selective ability to express aldh1a2 in a PPARγ-dependent fashion during tolerance However, just the generation of induced/peripheral Tregs does not always guarantee establishment of antigen-induced tolerance. As our study (13) and those of others (25-27) have shown, Tregs are phenotypically unstable and dysfunctional under inflammatory conditions and thus for the stabilization of newly minted Tregs (28), induction of pro-inflammatory cytokines in all other CD11c+ cells that can process antigens needs to be suppressed, which we show is also ensured by PPARγ. We also detected increased p38 MAPK activity in PPARγ deficient CD11c+ cells, concomitant with the development of a Th17 immune response in the CD11c-Cre-PPARγfl/fl mice. This was consistent with the dependence of Th17 immune response on p38 MAPK activity in DCs, as recently reported (15). Thus, we demonstrate a CD11c+ cell-intrinsic function of PPARγ whose collective goal in response to inhaled antigen is to not only promote de novo Foxp3 expression in T cells via aldh1a2 expression in CD103+ DCs but to also actively dampen expression of pro-inflammatory cytokines in all CD11c+ cells to preserve Treg function.
Supplementary Material
Acknowledgments
This work was supported by US National Institutes of Health grants AI 048927 (to A.R.), AI 100012 (to P.R.), HL 113956 (to A.R. and P.R.) and HL HL114453 (to P.R.).
Footnotes
Disclosures
The authors declare no competing financial interests.
References
- 1.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu. Rev. Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 3.Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur J Pharmacol. 2006;533:101–109. doi: 10.1016/j.ejphar.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 5.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J. Clin. Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oriss TB, Ostroukhova M, Seguin-Devaux C, Dixon-McCarthy B, Stolz DB, Watkins SC, Pillemer B, Ray P, Ray A. Dynamics of Dendritic Cell Phenotype and Interactions with CD4+ T Cells in Airway Inflammation and Tolerance. J. Immunol. 2005;174:854–863. doi: 10.4049/jimmunol.174.2.854. [DOI] [PubMed] [Google Scholar]
- 7.Khare A, Krishnamoorthy N, Oriss TB, Fei M, Ray P, Ray A. Cutting edge: inhaled antigen upregulates retinaldehyde dehydrogenase in lung CD103+ but not plasmacytoid dendritic cells to induce Foxp3 de novo in CD4+ T cells and promote airway tolerance. J. Immunol. 2013;191:25–29. doi: 10.4049/jimmunol.1300193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammad H, de Heer HJ, Soullie T, Angeli V, Trottein F, Hoogsteden HC, Lambrecht BN. Activation of peroxisome proliferator-activated receptor-gamma in dendritic cells inhibits the development of eosinophilic airway inflammation in a mouse model of asthma. Am J Pathol. 2004;164:263–271. doi: 10.1016/s0002-9440(10)63116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Housley WJ, O'Conor CA, Nichols F, Puddington L, Lingenheld EG, Zhu L, Clark RB. PPARgamma regulates retinoic acid-mediated DC induction of Tregs. J Leukoc Biol. 2009;86:293–301. doi: 10.1189/jlb.1208733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szatmari I, Pap A, Ruhl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–2362. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen- specific gamma delta T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamoorthy N, Khare A, Oriss TB, Raundhal M, Morse C, Yarlagadda M, Wenzel SE, Moore ML, Peebles RS, Ray A, Ray P. Early infection with respiratory syncytial virus impairs regulatory T cell function and increases susceptibility to allergic asthma. Nat. Med. 2012;18:1525–1530. doi: 10.1038/nm.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Huang G, Wang Y, Vogel P, Kanneganti TD, Otsu K, Chi H. Signaling via the kinase p38alpha programs dendritic cells to drive TH17 differentiation and autoimmune inflammation. Nat Immunol. 2012;13:152–161. doi: 10.1038/ni.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanisms. J Immunol. 2007;178:4129–4135. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- 17.Klotz L, Burgdorf S, Dani I, Saijo K, Flossdorf J, Hucke S, Alferink J, Nowak N, Beyer M, Mayer G, Langhans B, Klockgether T, Waisman A, Eberl G, Schultze J, Famulok M, Kolanus W, Glass C, Kurts C, Knolle PA. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilles S, Mariani V, Bryce M, Mueller MJ, Ring J, Jakob T, Pastore S, Behrendt H, Traidl-Hoffmann C. Pollen-derived E1-phytoprostanes signal via PPAR-gamma and NF-kappaB-dependent mechanisms. J Immunol. 2009;182:6653–6658. doi: 10.4049/jimmunol.0802613. [DOI] [PubMed] [Google Scholar]
- 19.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 22.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 23.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 24.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O'Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, Belkaid Y. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.