Abstract
Background
US esophageal adenocarcinoma (EAC) incidence increased over five-fold between 1975 and 2009. Symptomatic gastroesophageal reflux disease (sGERD) elevates the risk for EAC. However, a simple calculation suggests that changes in sGERD prevalence can explain at most ~16% of this trend. Importantly, a mechanistic understanding of the influence of sGERD and other factors (OF) on EAC is lacking.
Methods
A multiscale model was developed to estimate temporal trends for sGERD and OF, and their mechanistic role during carcinogenesis. Model calibration was to Surveillance, Epidemiology, and End Results (SEER) incidence and age-dependent sGERD data using maximum likelihood and Markov chain Monte Carlo (MCMC) methods.
Results
Among men, 77.8% [95% Credibility Interval (CI) = 64.9 – 85.6%] of the incidence trend is attributable to OF, 13.4% [95% CI = 11.4 – 17.3%] to sGERD, and 8.8% [95% CI = 4.2 – 13.7%] to sGERD-OF interactions. Among women, 32.6% [95% CI = 27.0 – 39.9%] of the trend is attributable to OF, 13.6% [95% CI = 12.5 – 15.9%] to sGERD, and 47.4% [95% CI = 30.7 – 64.6%] to interactions. The predicted trends were compared with historical trends for obesity, smoking, and proton pump inhibitor use. Interestingly, predicted OF cohort trends correlated most highly with median body mass index (BMI) at age 50, (r = 0.988 for men; r = 0.998 for women).
Conclusions
sGERD and OF mechanistically increase premalignant cell promotion, which increases EAC risk exponentially with exposure duration.
Impact
Surveillance should target individuals with long-duration sGERD and OF exposures.
Keywords: symptomatic gastroesophageal reflux disease (sGERD), multiscale analysis, multistage clonal expansion model, premalignant promotion, esophageal adenocarcinoma (EAC)
Introduction
Esophageal adenocarcinoma (EAC) incidence rates have increased over 500% in the United States (US) since 1975, yet mechanistic drivers of this trend are not fully understood (1, 2). In particular, it is not clear how exposures may influence cellular processes during carcinogenesis to explain temporal trends for EAC, or the 5–6 fold increased EAC risk for US men compared with women (1–5). Established risk factors include age, symptomatic gastroesophageal reflux disease (sGERD), central-obesity, smoking, White race, male sex, and an inverse association with Helicobacter pylori infection (6–10). Progression to EAC occurs through development of Barrett’s esophagus (BE), dysplasia, and adenocarcinoma, with BE risk increasing with reflux severity (10–13). sGERD, defined as weekly or more frequent symptoms of reflux or heartburn, increases BE and EAC risk (10), although most individuals with sGERD do not have BE (14). Obesity and sGERD may be linked mechanistically (15–18), and with H. pylori, age, male gender, smoking, genetic factors, and gene-exposure interactions, influence BE development and EAC progression (19–26). These exposures and anthropometric factors likely account for most of the ~5-fold EAC incidence increase in the US and other Western countries from 1975–2009 (2, 3, 27–29).
Long-term sGERD trends are unknown, but three US population-based studies of residents of Olmsted County, Minnesota suggest that sGERD prevalence increased from about 13% to 20% in the early 1990s (30–32). Two UK studies of sGERD incidence show low incidence rates among children and higher rates among adults (33, 34). sGERD prevalence in Western countries ranges between 10–20% (35).
Long-term trends for other EAC risk factors are uncertain, especially for earlier birth cohorts. Cross-sectional data from the National Health and Nutrition Examination Surveys (NHANES) indicate US prevalence of overweight and obese individuals increased markedly from 1980–1999 with a leveling by 2010 (36–38). BMI increased for males born in the 1920s, and increased for both sexes after both world wars but decreased during the Great Depression (38). H. pylori seroprevalence decreased among older adults (> age 50) between 1980 and 1999 (39) with decreases by birth cohort (40).
BE is considered a requisite step for EAC development, with BE risk increasing linearly with earlier sGERD onset-age (41). A recent meta-analysis found an odds-ratio of 4.92, CI=(2.01–12.0) for at-least weekly sGERD in relation to long segment BE compared to no sGERD (42). Another meta-analysis also found an odds ratio of 4.92, CI= (3.92, 6.22) associating sGERD with EAC (10).
It is instructive to do a “back-of-the-envelope” calculation to estimate the potential impact of changing rates of sGERD on EAC incidence. If 20% of the population has sGERD (35) and the odds-ratio is ~5 for EAC given sGERD compared with no sGERD (10), then sGERD should increase EAC incidence by at most 80% (assuming no sGERD occurred in the past). This simple calculation suggests that sGERD should account for at most ~16% of the ~5-fold increase in EAC incidence. EAC trends must be largely driven by other factors – obesity, eradication of h. pylori, smoking, less frequent or non-symptomatic GERD, or perhaps unrecognized factors, e.g. some studies suggest proton pump inhibitors (PPI)s may reduce neutralization of damaging bile salts, increase gastrin production, and (also with antibiotics) alter the esophageal microbiome to increase EAC risk (43–48).
Further insight into EAC incidence trends in the US requires a better understanding of the mechanistic role of sGERD and its importance compared with other factors (OF). In this study, multiscale models are calibrated to EAC incidence data from the Surveillance, Epidemiology, and End Results (SEER) registries between 1975 and 2009 (27). Although multiscale models were fit previously to EAC incidence trends in the US (49, 50), this work represents the first systematic multiscale exploration of the mechanistic role of sGERD and OF as drivers of EAC incidence trends, while explicitly incorporating cohort and period trends for sGERD and OF that influence biological processes. The multiscale approach includes a model of sGERD prevalence that depends on age, birth cohort, and period. A sGERD onset-stratified population model is used to combine risks for individuals without sGERD and for individuals acquiring sGERD during different decades of life.
Materials and methods
Development of the multiscale model of EAC incidence and exploration of the mechanistic role of sGERD and OF proceeded in three phases. Phase 1 focused on identifying important biological mechanisms that are likely driving EAC trends. Phase 2 focused on understanding the mechanistic role of sGERD and OF in acting through the biological processes identified in Phase 1 to drive EAC incidence. Both phases of model development were informed by EAC incidence data from SEER, sGERD incidence data from the UK, and US sGERD prevalence data. Separate multiscale models of EAC incidence were built for all-race men and women. Phase 3 compared predicted trends for sGERD and OF with data on US trends for obesity, smoking, and PPI use.
EAC incidence and population data
EAC incidence and population data for all-race men and women by single years for ages 20–84 years and calendar years 1975–2009 were downloaded from nine SEER incidence databases. EAC incidence was defined using International Classification of Diseases for Oncology, third edition (ICD-O-3) histology codes 8140–8141, 8143–8145, 8190–8231, 8260–8263, 8310, 8401, 8480–8490, 8550–8551, 8570–8574, and 8576. US life tables for year 2000 were downloaded from the Centers for Disease Control website (51) to calculate age-adjusted rates for all-race males and females aged 20–84.
sGERD incidence and prevalence data
Data on sGERD incidence are from two cohort studies of the first diagnosis of weekly or more frequent GERD symptoms presenting in primary care in the UK. A study of children and adolescents (ages 1–17) identified 1700 incident sGERD cases diagnosed between years 2000–2005, with a comparison group of 5000 matched controls (34). A similar 1996 study identified 7451 incident sGERD patients aged 2–79 (mostly adults) with 10,000 controls (33). Estimated sGERD prevalence during years 1990–2000 are based on two US studies by Locke, et al. who found sGERD prevalence of ~20% (31, 32). These data were utilized to construct a sGERD prevalence model, described below.
Age-dependent sGERD prevalence model
Maximum likelihood estimation (MLE) and Markov chain Monte Carlo (MCMC) methods were used to model sGERD prevalence (in the 1990–2000 time frame) as a function of age by assuming non-prevalent individuals become prevalent at rates based on the UK data for age-specific sGERD incidence rates among children (34) and adults (33). The model includes an sGERD remission rate by which prevalent cases become non-prevalent, with calibration to an age-adjusted target of 20% sGERD prevalence around year 2000 based on the two US studies by Locke, et al. (31, 32). Finally, the resulting sGERD prevalence was re-fit using a three-parameter change-point exponential model representing the net rate of becoming prevalent during childhood, adulthood, and a change-point time. (See Supplementary Materials (SM) for details). This age-dependent sGERD prevalence model was used in all subsequent carcinogenesis models. During Phase 2 modeling, linear or sigmoidal cohort and period trends were estimated for sGERD prevalence while constraining the age-adjusted prevalence at approximately 20% in year 2000 in agreement with Locke, et al. (31, 32).
Multiscale EAC incidence models
MLE and MCMC methods were used to develop and compare models that represent the natural history of EAC while fitting to SEER EAC incidence data. All models include an age-dependent sGERD onset process, rates for transition to BE with or without sGERD, and a subsequent multistage carcinogenesis process that assumes any stem cells maintaining the BE tissue may acquire two initiating mutations to become premalignant, clonal expansion of premalignant cells, malignant transformations, and clonal expansions of malignant cells that may lead to cancer detection. (See Supplementary Figure S1).
The Phase 1 model family was designed to identify biological mechanisms that may potentially drive the observed EAC incidence trends. In these models, linear or sigmoidal trends for cohort and/or period were applied to one or more biological processes. Thus all individuals of a given age, period, birth cohort, and sex share the same set of biological rates, but these rates may change with birth cohort and calendar year.
The Phase 2 model family extended the Phase 1 models by stratifying the population according to sGERD duration, and then evaluating the mechanistic role of sGERD and OF acting on important biological mechanisms identified in Phase 1. In Phase 2, linear or sigmoidal trends for cohort and/or period were applied to sGERD and OF, which influence biological rates. Individuals of a given age, period, birth cohort, and sex were stratified by decade of sGERD onset, with individuals in each stratum modeled using baseline biological rates before acquisition of sGERD and different rates after sGERD onset.
Phase 1: Identifying biological processes that are likely driving EAC incidence trends
The Phase 1 model family introduced linear (two parameter) or sigmoidal (three parameter) trends by cohort and/or period that were applied to one or more of five biological processes represented by the model: 1) the transition from normal to BE tissue (νBE), 2) the (geometric mean) rate of two rate-limiting mutations transforming BE stem cells to premalignant cells (μ01), 3) clonal expansion of premalignant cells (γp and αp), 4) malignant transformation (μ2), and 5) clonal growth of malignant tissue (γm and αm). Beginning with linear trends, MLE and MCMC methods were used to systematically compare ten models with a single linear trend on either cohort or period applied to each of the five biological mechanisms. This was repeated for ten models using sigmoidal cohort or period trends while adjusting for the number of parameters. Comparisons continued with models of increasing complexity combining cohort and period trends acting on different biological processes, stopping at models with at most six trend parameters. These results were compared with a (non-nested) model with external (multiplicative) cohort and period adjustments similar to age-period-cohort (APC) models (49).
Phase 2: Estimating the influence of sGERD and OF acting on key biological processes
Models identified in Phase 1 that provid the best fits to EAC incidence in SEER were extended to include sGERD prevalence and OF modulated independently by cohort and period trends. The population was stratified by decade of sGERD onset. Using weights derived from the sGERD prevalence model, EAC hazards for each year of age and calendar year were summed over strata representing individuals with different decades of sGERD onset-age, including individuals without sGERD. Both MLE and MCMC methods were used for model selection and to infer mechanistic roles of sGERD and OF as modifiers of BE initiation, premalignant-, and malignant-promotion while estimating cohort and period effects. All models were initially evaluated using MLE methods to make model comparisons. Credibility intervals (CI)s for the best fitting models were estimated through extensive MCMC runs, including 36–40 independent chains with each chain running for approximately 75,000 cycles.
MLE’s and MCMC samples were used to estimate contributions to the observed EAC incidence trends from sGERD and OF and their interactions by cohort and period, and according to biological mechanisms mediating the actions of sGERD and OF. The respective contributions were calculated by switching on and off different model components using the stored (MLE and MCMC) parameter sets. Backgrounds were calculated by freezing period and cohort trends after year 1975 and after the 1900 birth cohort, respectively.
Phase 3: Comparing predicted OF and sGERD trends with US trends for obesity, smoking, and PPI utilization
We compared our predicted model trends for sGERD and OF with US trends for obesity, smoking, and prescription PPI use. Obesity trends data consist of median body mass index (BMI) of white males and females at age 50 by birth cohort using results from Komlos, et al. (38).
Smoking trends by birth cohort 1890–2000 were calculated by simulating 222,000 smoking histories each for men and women using the Smoking History Generator (SHG, version 6.3.2) from the Cancer Intervention and Surveillance Modeling Network (CISNET) (52, 53).
PPI trends data are from a PPI Drug Use Review and Duration Analysis by the US Food and Drug Administration (54), based on prescription claims for ~60 million de-identified patients (approximately 9% of the commercially insured US population) for years 2002–2009. PPIs were introduced in the US in 1989 (55).
Results
Refinement of an EAC incidence model
A family of nested multiscale models for EAC incidence was fit to SEER incidence data using maximum likelihood estimation. Models were compared and selected based on likelihood ratio tests.
The Phase 1 analysis identified a highly significant sigmoidal birth cohort trend affecting premalignant cell promotion as the most important mechanistic driver of the observed EAC incidence trends in SEER between 1975 and 2009. Significant additional improvements were found in models combining this cohort trend on premalignant cell promotion with either a sigmoidal cohort or period trend on BE initiation. See Supplementary Table S1 for likelihood comparison of selected models.
Phase 2 models compared different combinations of cohort and period trends on sGERD and OF that modify premalignant promotion and BE initiation, the two mechanisms found significant in Phase 1. Phase 2 models (stratified by sGERD onset-age decade) allowed rates for BE initiation and/or promotion to change at the sGERD onset-age and to follow the age-period-cohort profile of the sGERD function. Consistent with Phase 1 modeling, sGERD and OF were both found to significantly promote premalignant cells. Phase 2 models were extended to evaluate if sGERD and OF also promote malignant cells. The final EAC incidence model was selected as the best Phase 2 model using likelihood-based comparisons that account for the number of model parameters (see Supplementary Figure S1).
The final model includes 18 estimated parameters, including five background biological rates (BE initiation, initial mutation rates, premalignant promotion, malignant transformation, and malignant promotion), three response parameters for sGERD (increasing the rates for BE initiation, premalignant promotion, and malignant promotion), and ten parameters for cohort and period effects for sGERD and OF. All cohort and period effects were modeled using sigmoidal curves representing cohort and period trends, as these provided the best likelihoods. This initially required 12 parameters (three for each sigmoid curve). However, likelihood-ratio testing indicated that the estimated sGERD and OF trends share statistically indistinguishable inflection points for both cohort and period trends, thus reducing the count of trend parameters to ten. MLEs, MCMC medians, and 95% MCMC credibility intervals (CI)s for final model parameters are shown in Supplementary Table S1.
EAC incidence trends between 1975 and 2009
Between years 1975 and 2009, age-adjusted incidence (age-standardized to year 2000) increased among men from 1.12 to 8.11 (per 100,000 person years) representing an increase of 626% (95% CI = 558%–696%). Among women, age-adjusted EAC incidence increased from 0.28 to 1.21, an increase of 329% (95% CI =286%, 389%). (See Table 1, top).
Table 1.
Factors contributing to age-adjusted EAC incidence trends between 1975 and 2009
EAC incidence trends between 1975 and 2009 and attributable estimates of the direct effects and interactions due to sGERD, OF, cohort, period, and biological mechanism on these trends are shown for men and women. Cohort effects on OF promotion appear as the dominate drivers of the observed increase in EAC risk among both men and women. All estimates show MLEs, MCMC medians, and 95% MCMC credibility intervals (CI)s.
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| MLE | MCMC median | Lower 95% CI | Upper 95% CI | MLE | MCMC median | Lower 95% CI | Upper 95% CI | |
| Age adjusted incidence rate for 1975 (per 100,000 PY) | 1.12 | 1.14 | 1.01 | 1.28 | 0.28 | 0.30 | 0.24 | 0.36 |
| Age adjusted incidence rate for 2009 (per 100,000 PY) | 8.11 | 8.05 | 7.81 | 8.30 | 1.21 | 1.19 | 1.12 | 1.27 |
| Incidence increase between 1975 and 2009 | 626.31% | 610.23% | 517.19% | 708.61% | 329.24% | 300.96% | 232.42% | 403.65% |
| Direct effect of OF trends (affecting promotion only) | 487.43% | 470.94% | 373.57% | 569.48% | 107.24% | 112.02% | 67.59% | 166.28% |
| Direct effect of sGERD trends (affecting BE and promotion) | 83.73% | 86.83% | 58.85% | 101.83% | 45.58% | 47.38% | 36.73% | 63.09% |
| Interaction of sGERD with OF | 55.14% | 54.18% | 16.43% | 100.35% | 176.42% | 144.22% | 92.94% | 229.13% |
| Total increase from sGERD (direct + interaction with OF) | 138.87% | 137.84% | 105.72% | 190.59% | 222.00% | 191.80% | 139.66% | 280.37% |
| Direct effect of sGERD on BE | 6.77% | 6.37% | 0.00% | 17.49% | 1.41% | 1.90% | 0.47% | 3.63% |
| Direct effect sGERD promotion | 107.55% | 108.07% | 80.62% | 132.38% | 189.31% | 149.55% | 108.64% | 235.89% |
| Interaction of sGERD trends on BE initiation and promotion | 24.55% | 25.10% | 0.00% | 75.64% | 31.29% | 35.92% | 10.73% | 70.79% |
| Increase from direct effect of cohort on sGERD and OF | 465.36% | 455.25% | 366.42% | 554.50% | 202.95% | 184.05% | 139.40% | 256.87% |
| Direct cohort trends of OF (on promotion) | 440.55% | 425.11% | 335.56% | 515.59% | 106.68% | 111.54% | 67.24% | 165.70% |
| Direct cohort trends on sGERD and sGERD – OF interaction | 24.81% | 30.65% | 18.31% | 48.83% | 96.27% | 75.18% | 42.07% | 120.69% |
| Increase from direct effect of period on sGERD and OF | 52.79% | 52.43% | 28.64% | 71.19% | 45.00% | 46.97% | 36.44% | 61.85% |
| Direct period trends on sGERD | 40.65% | 41.16% | 15.48% | 60.38% | 44.80% | 46.76% | 36.24% | 61.63% |
| Direct period trends on OF and sGERD-OF interactions | 12.14% | 11.06% | 9.98% | 13.29% | 0.20% | 0.21% | 0.13% | 0.30% |
| Increase due to interaction between cohort and period | 108.16% | 100.99% | 79.00% | 131.73% | 81.29% | 68.93% | 43.62% | 117.78% |
| Interaction between cohort and period trends on sGERD | 69.38% | 63.29% | 40.68% | 92.75% | 80.71% | 68.54% | 43.14% | 117.29% |
| Interaction between cohort and period trends on OF and OF - sGERD interactions | 38.78% | 37.78% | 30.18% | 46.51% | 0.59% | 0.42% | 0.24% | 0.65% |
Changing patterns of EAC risk by age and calendar year – effects of sGERD and OF
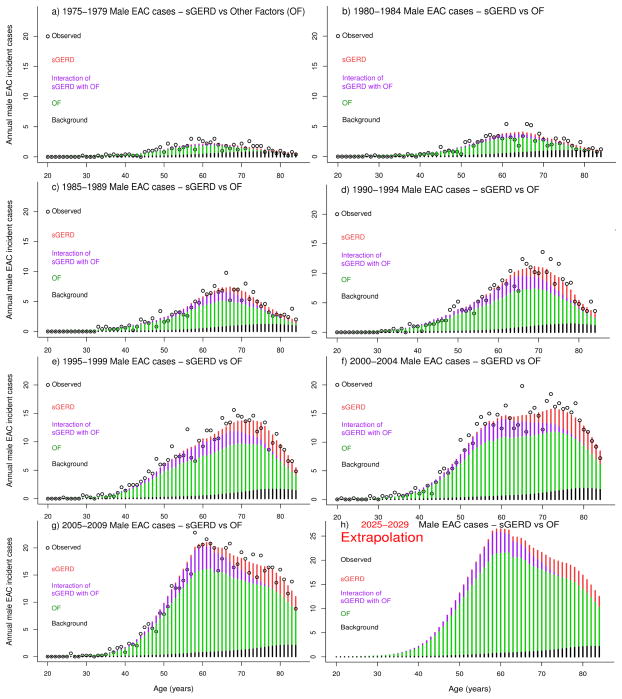
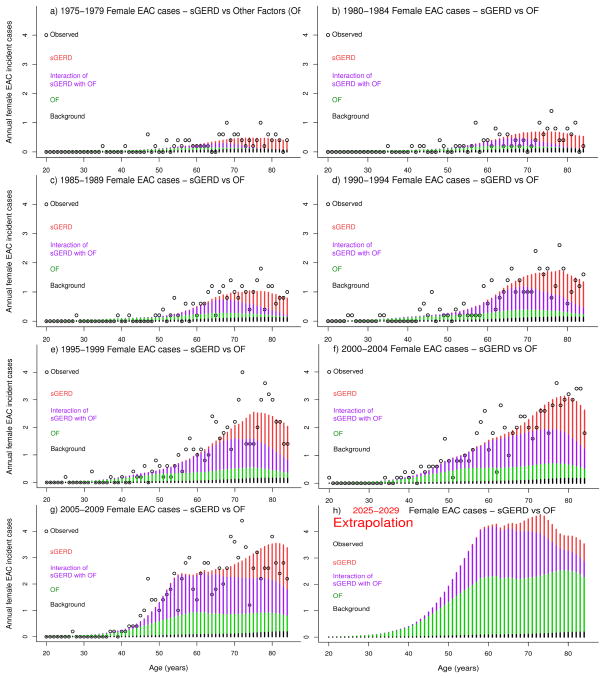
Observed EAC incident cases in SEER were compared with model predictions for incident cases attributable to changing patterns of sGERD, OF, sGERD-OF interactions, and background representing no cohort or period trends for sGERD or OF. Observed versus expected annual EAC cases are shown by year of age and by five-year age groups in Figure 1 for men and Figure 2 for women. Observed data are shown as black circles and model estimates by stacked bar graphs, including direct effects of sGERD (red), direct effects from OF (green), sGERD-OF interactions (violet), and background (black). Direct effects of OF make the largest contribution to the increase in EAC risk from 1975–2009, while sGERD and sGERD-OF interactions contribute less. sGERD-attributable EAC cases are generally delayed due to age-dependent sGERD prevalence patterns. EAC cases attributable to sGERD-OF interactions are due to an acceleration of premalignant promotion by OF followed by sGERD-associated promotion that decreases the time to EAC incidence. The decrease in number of EAC cases at older ages is due to normal population aging and death.
Figure 1.
Annual observed (black circles) and expected EAC incident cases (stacked bars) among men due to direct effects of sGERD (red), direct effects of OF (violet), sGERD-OF interactions (green), and Background (black), shown by five-year periods ranging from 1975–1979 in panel a), to 2005–2009 in panel f). Panel h) shows results of model extrapolation of the expected cases in years 2025–2029.
Figure 2.
Annual observed (black circles) and expected EAC incident cases (stacked bars) among women due to direct effects of sGERD (red), direct effects of OF (violet), sGERD-OF interactions (green), and Background (black), shown by five-year periods ranging from 1975–1979 in panel a), to 2005–2009 in panel f). Panel h) shows results of model extrapolation of the expected cases in years 2025–2029.
Extrapolation of age-dependent EAC risk to years 2025–2029
The parametric approach to modeling trends described here allows extrapolation of expected EAC cases into the future. An extrapolation to years 2025–2029 is shown in Figure panel 1h for men and 2h for women. The expected rates for women remain below those of men.
Estimated sGERD and OF trends driving EAC incidence
Estimated contributions of sGERD and OF trends to age-adjusted increases in EAC incidence from 1975–2009 are shown for men and women in Table 1. Among men, direct effects of OF account for a 487% increase in age-adjusted incidence, direct effects of sGERD for an 84% increase, and sGERD-OF interactions for a 55% increase, combining for a total 626% increase in age-adjusted incidence among men. Estimates for women suggest that direct OF effects account for a 107% increase in incidence, direct effects of sGERD for a 46% increase, and sGERD-OF interactions for a 176% increase, combining for a total 329% increase in age-adjusted incidence among women. Proportionately, the impact of sGERD on EAC incidence trends is somewhat larger for women than men. The estimated total impact of sGERD, including sGERD-OF interactions, is represented by an increase in EAC incidence from 1975–2009 of 139% (out of 626%) for men, and 222% (out of a 329%) for women. (See Table 1 for additional details, MLEs, and MCMC medians and 95% CIs for all estimates).
Birth cohort and period trends contribute significantly to the effects of sGERD and OF in driving EAC incidence trends, with birth cohort trends most important. Among men the 626% increase in age-adjusted incidence from 1975–2009 includes 465% directly due to cohort, 53% directly due to period, and 108% due to cohort-period interactions. The 329% age-adjusted incidence increase among women includes 203% increase due to birth cohort, 45% due to period, and 81% due to cohort-period interactions. Table 1 includes additional details.
Model predictions for sGERD prevalence, BE prevalence, prevalence of BE given sGERD, and the relative risk of developing BE given sGERD are shown in Table 2, including comparisons between sexes by six age groups and by early period (1975–1984) versus late period (2000–2009). Depending on age group, estimated sGERD prevalence increased by a factors of about 2–5 for men and 2–3 for women. These increases in sGERD prevalence do not translate into similar increases in BE prevalence. BE prevalence for both sexes approximately triples between the earliest (age 20–34) and the latest (age 75–84) groups, but increased generally less than 10% between early and late periods. Across age groups and periods, estimated BE prevalence among men is approximately twice that among women. Across groups, the estimated prevalence of BE given sGERD is approximately 50% higher than the prevalence of BE in the general population. Also, the estimated relative risk of developing BE given sGERD (at any age) is slightly over 3 for men, and somewhat over 4 for women.
Table 2.
Model Predictions for sGERD and BE
Model predictions for sGERD and BE prevalence, the prevalence of BE given sGERD, and the relative risk (RR) for BE given sGERD (versus no sGERD) are shown for males and females by five-year age group between 20 and 84, and for early years (1975–1984) versus late years (2000–2009) during the 1975–2009 study. Estimates are shown as MCMC medians, including 95% MCMC credibility intervals (CI)s.
| Males 1975–1984 | Males 2000–2009 | Females 1975–1984 | Females 2000–2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% |
Upper 95% CI |
|
|
|
||||||||||||
| Percent of population with sGERD | ||||||||||||
| Age 20–34 | 1.28% | 1.22% | 1.29% | 3.73% | 3.73% | 3.74% | 1.44% | 1.03% | 2.26% | 5.84% | 3.43% | 9.27% |
| Age 35–44 | 3.98% | 3.79% | 4.01% | 11.10% | 11.10% | 11.11% | 5.18% | 4.22% | 5.88% | 22.61% | 11.11% | 33.33% |
| Age 45–54 | 6.32% | 6.03% | 6.37% | 17.05% | 17.05% | 17.06% | 8.69% | 7.09% | 9.82% | 28.31% | 16.34% | 41.05% |
| Age 55–64 | 8.30% | 7.93% | 8.37% | 22.56% | 22.55% | 22.57% | 11.89% | 9.74% | 13.42% | 23.21% | 21.81% | 26.63% |
| Age 65–74 | 7.13% | 6.95% | 7.22% | 27.92% | 27.91% | 27.94% | 15.02% | 12.34% | 16.91% | 27.59% | 27.29% | 28.41% |
| Age 75–84 | 6.98% | 6.77% | 7.15% | 32.72% | 32.71% | 32.74% | 18.00% | 14.84% | 20.22% | 32.58% | 32.24% | 33.51% |
| BE prevalence | ||||||||||||
| Age 20–34 | 0.85% | 0.84% | 0.86% | 0.87% | 0.86% | 0.88% | 0.40% | 0.25% | 0.74% | 0.42% | 0.26% | 0.76% |
| Age 35–44 | 1.25% | 1.24% | 1.27% | 1.29% | 1.29% | 1.32% | 0.60% | 0.39% | 1.10% | 0.70% | 0.42% | 1.24% |
| Age 45–54 | 1.59% | 1.59% | 1.61% | 1.67% | 1.66% | 1.70% | 0.80% | 0.54% | 1.47% | 1.00% | 0.61% | 1.72% |
| Age 55–64 | 1.93% | 1.92% | 1.96% | 2.08% | 2.06% | 2.13% | 1.02% | 0.71% | 1.89% | 1.21% | 0.81% | 2.17% |
| Age 65–74 | 2.24% | 2.23% | 2.27% | 2.55% | 2.52% | 2.62% | 1.27% | 0.90% | 2.38% | 1.56% | 1.03% | 2.88% |
| Age 75–84 | 2.56% | 2.55% | 2.59% | 3.03% | 2.98% | 3.12% | 1.55% | 1.09% | 2.92% | 1.99% | 1.26% | 3.66% |
| Prevalence of BE given sGERD | ||||||||||||
| Age 20–34 | 1.43% | 1.38% | 1.52% | 1.42% | 1.38% | 1.51% | 0.94% | 0.53% | 1.75% | 0.89% | 0.53% | 1.64% |
| Age 35–44 | 1.77% | 1.73% | 1.86% | 1.79% | 1.74% | 1.88% | 1.06% | 0.65% | 1.95% | 1.09% | 0.67% | 2.01% |
| Age 45–54 | 2.35% | 2.27% | 2.49% | 2.35% | 2.27% | 2.48% | 1.69% | 0.92% | 3.12% | 1.70% | 0.91% | 3.15% |
| Age 55–64 | 2.94% | 2.84% | 3.14% | 2.96% | 2.85% | 3.15% | 2.31% | 1.17% | 4.34% | 2.32% | 1.17% | 4.35% |
| Age 65–74 | 3.56% | 3.43% | 3.81% | 3.62% | 3.48% | 3.87% | 2.95% | 1.42% | 5.60% | 3.01% | 1.44% | 5.70% |
| Age 75–84 | 4.26% | 4.04% | 4.54% | 4.27% | 4.09% | 4.58% | 3.59% | 1.68% | 6.83% | 3.69% | 1.71% | 7.02% |
| RR for BE given sGERD (versus no sGERD) | ||||||||||||
| All ages | 3.27 | 2.98 | 3.78 | 3.29 | 3.03 | 3.74 | 7.97 | 2.71 | 13.96 | 7.97 | 2.71 | 13.96 |
|
|
||||||||||||
sGERD duration increases BE risk ~ linearly, but EAC risk ~ exponentially
In Table 3, BE prevalence is compared for different sGERD durations (no sGERD, >0–10 yrs., 10–20 yrs., 20–40 yrs., and >40 yrs.) during early (1975–1984) and late (2000–2009) periods for both sexes. Across age groups, BE prevalence increases approximately 2.5 fold among men and over 3 fold for women for long-duration sGERD (over 40 years duration) compared to no sGERD. Controlling for sGERD duration, BE prevalence increases gradually with age.
Table 3.
Prevalence of BE given sGERD duration
Model predictions BE prevalence given sGERD duration (including No sGERD, >0–10 years, >10–20 years, >20–40 years, and >40 years) are shown for males and females for three age groups (55–64, 65–74, and 75–84), and for early years (1975–1984) versus late years (2000–2009) during the 1975–2009 study. Estimates are shown as MCMC medians, including 95% MCMC credibility intervals (CI)s.
| Males 1975–1984 | Males 2000–2009 | Females 1975–1984 | Females 2000–2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
|
|
|
||||||||||||
| sGERD duration - age 55–64 | ||||||||||||
| No sGERD | 1.91% | 1.90% | 1.95% | 1.90% | 1.89% | 1.94% | 0.90% | 0.57% | 1.68% | 0.89% | 0.57% | 1.67% |
| >0 – 10 years | 2.19% | 2.18% | 2.22% | 2.17% | 2.15% | 2.22% | 1.30% | 0.88% | 2.39% | 1.28% | 0.86% | 2.36% |
| >10–20 years | 2.99% | 2.89% | 3.18% | 2.95% | 2.86% | 3.14% | 2.49% | 1.24% | 4.71% | 2.44% | 1.21% | 4.58% |
| >20–40 years | 3.79% | 3.60% | 4.15% | 3.76% | 3.58% | 4.09% | 3.57% | 1.52% | 6.87% | 3.51% | 1.49% | 6.76% |
| >40 years | 5.34% | 4.96% | 6.02% | 5.31% | 4.96% | 5.93% | 5.93% | 2.14% | 11.56% | 5.88% | 2.11% | 11.49% |
| sGERD duration - age 65–74 | ||||||||||||
| No sGERD | 2.20% | 2.19% | 2.24% | 2.20% | 2.18% | 2.24% | 1.03% | 0.66% | 1.93% | 1.03% | 0.66% | 1.93% |
| >0 – 10 years | 2.47% | 2.46% | 2.49% | 2.47% | 2.45% | 2.52% | 1.43% | 0.97% | 2.62% | 1.44% | 0.98% | 2.63% |
| >10–20 years | 3.25% | 3.15% | 3.42% | 3.25% | 3.15% | 3.43% | 2.62% | 1.36% | 4.86% | 2.63% | 1.36% | 4.88% |
| >20–40 years | 4.30% | 4.09% | 4.68% | 4.29% | 4.08% | 4.66% | 4.14% | 1.76% | 7.96% | 4.15% | 1.77% | 7.98% |
| >40 years | 5.95% | 5.55% | 6.66% | 5.89% | 5.51% | 6.55% | 6.46% | 2.38% | 12.55% | 6.46% | 2.38% | 12.53% |
| sGERD duration - age 75–84 | ||||||||||||
| No sGERD | 2.50% | 2.48% | 2.54% | 2.49% | 2.47% | 2.54% | 1.17% | 0.75% | 2.19% | 1.17% | 0.75% | 2.19% |
| >0 – 10 years | 2.77% | 2.77% | 2.79% | 2.75% | 2.74% | 2.81% | 1.56% | 1.06% | 2.84% | 1.58% | 1.07% | 2.87% |
| >10–20 years | 3.58% | 3.46% | 3.73% | 3.52% | 3.43% | 3.69% | 2.74% | 1.48% | 5.03% | 2.76% | 1.49% | 5.07% |
| >20–40 years | 4.70% | 4.42% | 5.03% | 4.58% | 4.37% | 4.94% | 4.26% | 1.87% | 8.13% | 4.29% | 1.88% | 8.18% |
| >40 years | 6.13% | 5.66% | 6.71% | 5.93% | 5.57% | 6.54% | 5.87% | 2.32% | 11.36% | 5.88% | 2.32% | 11.38% |
EAC incidence is predicted to increase almost exponentially with sGERD duration as shown in Tables 4 and 5, in contrast to the gradual, essentially linear increase in risk for BE with sGERD duration, discussed above. These tables show relative and absolute EAC risk, respectively, by age group, sGERD duration, early versus late periods, and sex, using the same categories used in Table 3. In Table 4, the increase in estimated relative risk for EAC with sGERD duration is higher among women (who have lower baseline risk) than men. Similarly, relative risk increases with sGERD duration are higher in the earlier period (1975–1984) than later due to lower baseline risks in the earlier period. Table 5 shows absolute risks for EAC (per 100,000) with risk that increases nearly exponentially with sGERD duration. Absolute risks increase from early to late periods, and by age. Estimated risks for women with short or no sGERD duration are much lower than for men, but the relative difference decreases with increasing sGERD duration.
Table 4.
Relative Risk for EAC given sGERD duration
Model predictions of the relative risk (RR) for EAC given sGERD duration (including No sGERD, >0–10 years, >10–20 years, >20–40 years, and >40 years) are shown for males and females for three age groups (55–64, 65–74, and 75–84), and for early years (1975–1984) versus late years (2000–2009) during the 1975–2009 study. Estimates are shown as MCMC medians, including 95% MCMC credibility intervals (CI)s.
| Males 1975–1984 | Males 2000–2009 | Females 1975–1984 | Females 2000–2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
|
|
|
||||||||||||
| sGERD duration - age 55–64 | ||||||||||||
| No sGERD | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| >0 – 10 years | 1.10 | 1.09 | 1.11 | 1.08 | 1.07 | 1.09 | 1.03 | 1.02 | 1.03 | 1.06 | 1.03 | 1.11 |
| >10–20 years | 2.46 | 2.43 | 2.49 | 2.45 | 2.41 | 2.52 | 3.30 | 2.41 | 4.85 | 5.34 | 3.55 | 8.08 |
| >20–40 years | 9.37 | 8.92 | 9.87 | 3.96 | 3.87 | 4.08 | 23.38 | 14.92 | 43.66 | 15.41 | 9.76 | 27.02 |
| >40 years | 18.87 | 17.57 | 20.49 | 6.60 | 6.19 | 7.13 | 114.09 | 60.74 | 214.70 | 47.49 | 24.34 | 81.36 |
| sGERD duration - age 65–74 | ||||||||||||
| No sGERD | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| >0 – 10 years | 1.10 | 1.09 | 1.11 | 1.07 | 1.06 | 1.07 | 1.03 | 1.02 | 1.04 | 1.03 | 1.02 | 1.06 |
| >10–20 years | 1.73 | 1.72 | 1.74 | 2.09 | 2.06 | 2.14 | 2.10 | 1.58 | 2.96 | 4.50 | 2.96 | 6.50 |
| >20–40 years | 9.38 | 9.02 | 9.74 | 2.72 | 2.68 | 2.79 | 31.50 | 20.05 | 49.22 | 14.10 | 8.84 | 25.24 |
| >40 years | 16.00 | 15.00 | 17.16 | 4.38 | 4.13 | 4.79 | 102.41 | 55.12 | 196.09 | 38.20 | 20.16 | 64.76 |
| sGERD duration - age 75–84 | ||||||||||||
| No sGERD | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| >0 – 10 years | 1.12 | 1.11 | 1.13 | 1.07 | 1.06 | 1.08 | 1.03 | 1.03 | 1.04 | 1.03 | 1.02 | 1.03 |
| >10–20 years | 1.47 | 1.44 | 1.49 | 1.83 | 1.82 | 1.87 | 1.37 | 1.16 | 1.85 | 3.36 | 2.31 | 4.74 |
| >20–40 years | 9.81 | 9.50 | 10.20 | 2.43 | 2.40 | 2.49 | 27.13 | 17.95 | 49.16 | 13.64 | 8.21 | 24.62 |
| >40 years | 14.47 | 13.67 | 14.88 | 2.83 | 2.69 | 3.03 | 54.58 | 31.20 | 83.52 | 18.26 | 10.40 | 28.27 |
Table 5.
Absolute annual risk (per 100,000 individuals) for EAC given sGERD duration
Model predictions for the absolute risk (per 100,000 individuals) for EAC given sGERD duration (including No sGERD, >0–10 years, >10–20 years, >20–40 years, and >40 years) are shown for males and females for three age groups (55–64, 65–74, and 75–84), and for early years (1975–1984) versus late years (2000–2009) during the 1975–2009 study. Estimates are shown as MCMC medians, including 95% MCMC credibility intervals (CI)s.
| Males 1975–1984 | Males 2000–2009 | Females 1975–1984 | Females 2000–2009 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
MCMC median |
Lower 95% CI |
Upper 95% CI |
|
|
|
||||||||||||
| sGERD duration - age 55–64 | ||||||||||||
| No sGERD | 2.26 | 2.24 | 2.30 | 9.06 | 8.97 | 9.16 | 0.15 | 0.11 | 0.19 | 0.54 | 0.39 | 0.73 |
| >0 – 10 years | 2.69 | 2.64 | 2.76 | 10.42 | 10.24 | 10.58 | 0.16 | 0.11 | 0.21 | 0.60 | 0.43 | 0.79 |
| >10–20 years | 5.80 | 5.68 | 5.84 | 22.36 | 22.22 | 22.88 | 0.48 | 0.34 | 0.70 | 2.83 | 2.24 | 4.04 |
| >20–40 years | 21.96 | 21.31 | 23.08 | 35.95 | 35.61 | 36.94 | 3.58 | 2.49 | 5.16 | 8.39 | 6.57 | 10.88 |
| >40 years | 44.40 | 42.03 | 47.67 | 60.27 | 56.48 | 64.64 | 16.48 | 9.20 | 26.07 | 24.88 | 12.98 | 42.08 |
| sGERD duration - age 65–74 | ||||||||||||
| No sGERD | 3.20 | 3.17 | 3.26 | 16.25 | 16.07 | 16.45 | 0.22 | 0.15 | 0.31 | 0.88 | 0.61 | 1.25 |
| >0 – 10 years | 3.73 | 3.69 | 3.82 | 17.91 | 17.60 | 18.22 | 0.23 | 0.15 | 0.33 | 0.94 | 0.65 | 1.32 |
| >10–20 years | 5.84 | 5.76 | 5.94 | 34.05 | 33.75 | 34.79 | 0.46 | 0.27 | 0.80 | 4.00 | 3.11 | 5.18 |
| >20–40 years | 31.49 | 30.86 | 33.05 | 44.26 | 44.08 | 45.08 | 6.92 | 5.38 | 8.27 | 12.52 | 10.74 | 15.72 |
| >40 years | 54.12 | 51.05 | 57.65 | 71.55 | 67.32 | 77.36 | 22.57 | 12.80 | 33.64 | 33.65 | 17.58 | 52.94 |
| sGERD duration - age 75–84 | ||||||||||||
| No sGERD | 3.49 | 3.44 | 3.57 | 21.08 | 20.86 | 21.33 | 0.27 | 0.17 | 0.44 | 1.16 | 0.79 | 1.78 |
| >0 – 10 years | 3.90 | 3.82 | 3.96 | 22.82 | 22.52 | 23.22 | 0.29 | 0.18 | 0.46 | 1.22 | 0.83 | 1.87 |
| >10–20 years | 5.10 | 5.03 | 5.22 | 38.77 | 38.24 | 39.41 | 0.38 | 0.21 | 0.78 | 4.06 | 2.77 | 5.42 |
| >20–40 years | 33.79 | 33.14 | 36.15 | 51.16 | 50.99 | 52.28 | 8.06 | 5.18 | 10.38 | 16.11 | 13.98 | 20.11 |
| >40 years | 49.96 | 48.26 | 51.19 | 59.72 | 57.12 | 63.42 | 14.65 | 12.21 | 17.41 | 21.00 | 15.10 | 27.47 |
Supplementary Figure S2 in SM shows age-adjusted EAC incidence rates for men and women for calendar years 1975–2009, including observed age-adjusted rates (black circles) compared with age-adjusted model predictions (stacked bar graphs) showing contributions from sGERD versus OF, cohort versus period for OF and sGERD, and contributions from sGERD-associated promotion versus sGERD-associated development of BE. OF cohort effects play a dominant role while sGERD plays a smaller part in driving EAC incidence trends. Mechanistically, both sGERD and OF act primarily through premalignant promotion as drivers of EAC trends.
Correlation of OF and sGERD trends with US trends for obesity, smoking, and PPIs
In Phase 3 modeling, the Pearson product-moment correlation coefficient (r) was used to compare the likelihood-based model predictions for cohort and period trends on sGERD and OF with data for US trends of obesity, smoking, and PPI utilization. There is a very high correlation of predicted OF cohort trends with median BMI at age 50 by birth cohort for (r = 0.988) for men and (r = 0.998) for women.
A sensitivity analysis of the maximal potential impact of obesity on EAC incidence trends was made by assuming that obesity is responsible for all of the OF cohort trends and their interactions. Under this assumption, BMI accounts for, at most, 84.7% (95% CI = 79.9%, 89.6%) of the EAC incidence trend for men, and 86.1% (95% CI = 80.5%, 88.4%) of the incidence trend for women.
The correlation of smoking with OF or sGERD trends was poor, (r = −0.31) for men and (r = 0.23) for women. However, prescription PPI usage trends correlated highly with predicted sGERD period trends (r = 0.879) for men and (r = 0.992) for women.
A sensitivity analysis of the maximal potential impact of PPI use on EAC incidence was made assuming that PPIs are responsible for sGERD period trends and interactions. Under this assumption, PPIs could account for, at most, 18.2% (95% CI = 11.8%, 26.4%) of the incidence trend for men, and 38.1% (95% CI = 27.7%, 48.5%) for women.
Discussion
Although sGERD contributes significantly to EAC risk, it accounts for a small fraction of the increase in SEER incidence observed between 1975 and 2009. Other factors, modeled as OF, appear as the dominant driver of these increases. They interact with sGERD (at the tissue level) to increase risk beyond the additive contributions of sGERD and OF. As shown in Table 1, direct effects of sGERD are estimated to contribute approximately 13–14% of the observed increase in EAC incidence for men and women, which is consistent with the ‘back-of-the-envelope’ estimates of the maximum expected effects of sGERD on EAC trends. Mechanistically, both OF and sGERD appear to act primarily through premalignant cell promotion in driving EAC trends.
The importance of promotion as a mechanistic driver of EAC trends
Premalignant cell promotion may occur through esophageal inflammation and wounding from sGERD and OF that induces cytokine signaling and cell proliferation (56), leading to accelerated EAC development. Importantly, the effects of promotion are non-additive – the risk from two (independent) promoters may exceed the sum of effects from each promoter acting alone. Another important consequence of promotion is that risk increases almost exponentially with duration of promotion, since a promoter increases the exponential growth rate of premalignant clones throughout the duration of exposure. The effects of increasing risk with duration of exposure are seen in Tables 4 and 5, which show nearly exponential increases in relative and absolute EAC risks, respectively, with sGERD duration. This contrasts with roughly linear increases in risk for BE with sGERD duration seen in Table 3.
Why is the impact of sGERD dominated by promotion, and not BE initiation?
sGERD promotion leads to a nearly exponential increase in EAC risk with sGERD duration that generally exceeds the nearly linear increase in risk from BE due to sGERD. The latter translates into an approximately linear increase in EAC risk. Risk for EAC is highest among individuals developing BE at an early age. Since sGERD prevalence is low at young ages, individuals who develop EAC may not have had sGERD at BE onset, although risk from early onset of sGERD (even that occurring after BE development) increases exponentially with duration due to its promoting effect.
Age-dependent attributable risk patterns for sGERD and OF – direct effects and interactions
Figures 1 and 2 show estimates of attributable EAC risks by age for effects of cohort and period acting on sGERD and OF, and their interactions, along with background rates representing expected EAC incidence in the absence of trends. These contributions are calculated as differences between the integrated EAC model hazards when different combinations of factors are turned on or off. Direct effects of sGERD and OF represent independent actions of these exposures acting on the background rate. The OF increase promotion above the background, and if sGERD occurs, it further shortens the time to cancer compared with OF alone. That is why the interaction risk rises earlier than the direct sGERD risk. The attributable interaction represents a separation of the biological interaction into expected numbers of cancers due to different combinations of background, sGERD, and OF.
What is its potential impact of obesity on EAC trends?
The predicted OF cohort trends correlate very highly with US trends for median BMI at age 50, (r=0.988) for men, and (r=0.998) for women.
Does PPI usage potentially impact EAC trends?
sGERD period trends are highly correlated with recent prescription PPI usage in the US with predicted (r=0.879) for men, and (r=0.992) for women, possibly consistent with a study suggesting elevated risk for long-duration PPI usage (46). These results should be interpreted cautiously - correlation does not mean causation. Also, the estimated sGERD period trend begins to rise in the early 1980s for men, prior to the US introduction of PPIs in 1989. The match is better for women (See Supplemental Figure S1b,c).
Effects of less-frequent reflux and non-symptomatic GERD
The sensitivity of the model to estimate the effects of symptomatic reflux of frequency less than weekly or asymptotic reflux was tested by refitting the model under the assumption that reflux occurred with three times the frequency assumed for at-least weekly sGERD, and that this reflux acts on premalignant promotion, as found for sGERD. Under these assumptions, the reflux-associated attributable risk (direct plus interaction) increased by 25.8% (95% CI=13.0%, 53.6%) across males and females.
Similarities and differences between men and women in EAC risk
Although EAC incidence increased for both sexes between 1975 and 2009, risk for men increased over six-fold whereas risk increased slightly over three-fold for women. Premalignant cell promotion emerges as the dominant biological mechanism driving the incidence increase for both sexes, with estimated trends almost entirely due to birth cohort effects. OF appears as the primary driver of the increasing trends, especially among men, while both sGERD and OF contribute more evenly to the historical increase in incidence for women. In absolute terms, the increased risk in women associated with long duration sGERD and its interactions is about one-third to one-half that of men, consistent with a similar biological effect for both sexes given that rates for BE in women are about half those of men while sGERD rates are similar between sexes.
Limitations of this study
The analysis utilized SEER incidence data to simultaneously estimate cohort and period effects for sGERD and OF along with biological parameters in the EAC incidence model, and thus may be less precise than if detailed historical exposure data were available. The available data did not allow calculation of separate contributions of different risk factors comprising OF. Estimates of OF reflect period and birth cohort trends for the composite effects of obesity, H. pylori, non-symptomatic or less intense esophageal reflux, smoking, and other exposures. Thus the estimated effects of sGERD do not capture the full impact of esophageal reflux in general – instead less-intense and non-symptomatic reflux are modeled as contributions to OF. As discussed above, we attempted to address this issue by fitting models to SEER data while assuming rates of reflux three times that of sGERD. Extrapolations of future EAC risk are based on continuation of estimated sGERD and OF trends, and do not account for changes in population screening or gradual improvements in surveillance and treatment of BE patients over time. Finally, correlations do not imply causation, and thus in Phase 3 modeling, the high correlations between OF cohort trends and US median BMI trends, and between PPI usage and sGERD period trends should be interpreted with caution. Further studies are needed to establish a causal link between these exposures and the trends we have identified in this study
Conclusions
This analysis suggests that premalignant promotion is the most important biological mechanism driving EAC incidence trends, accounting for 95.0% [95% CI = 88.4 – 100.0%] of the increase among men from 1975–2009, and 90.1% [95% CI = 84.5 – 97.3%] among women. Individuals with early onset of both BE and sGERD are at highest risk. For extended duration of sGERD (greater than 40 years) the absolute sGERD associated EAC risk for women approaches one-third to one-half that of men, depending on age and calendar year, whereas the risk is 10–20 fold lower for women than men for individuals who never acquire sGERD.
Importantly, the dominant driver of promotion is OF. The high correlation of OF cohort trends with US median BMI trends for age 50 males and females is striking. If OF trends are driven by BMI, a sensitivity analysis suggests that BMI trends may account for a large proportion of the increase in US EAC incidence since 1975 (57).
Premalignant cell promotion is an important driver of carcinogenesis which causes incidence to increase exponentially with sGERD and OF exposure duration. Thus prevention and screening should focus on long-duration exposures, including early-onset sGERD (58–60).
Supplementary Material
Acknowledgments
This research was supported by the National Cancer Institute (NCI) under Grant Nos. UO1 CA152926 and UO1 CA182940 (W.D. Hazelton, J.M. Inadomi, C. Hur, and E.G. Luebeck), and by a Graduate Research Fellowship from the National Science Foundation, DGE-0718124 : KC, (K. Curtius).
We kindly acknowledge Drs. Komlos and Brabec for providing access to data on US obesity trends (38).
Footnotes
Conflict of interest: None.
References
- 1.Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. British journal of cancer. 2009;101:855–9. doi: 10.1038/sj.bjc.6605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149–58. doi: 10.1002/cncr.27834. Epub 2013/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histological types of oesophageal carcinomas in selected countries 1973–1995 (vol 99, pg 860, 2002) International Journal of Cancer. 2002;101:599. doi: 10.1002/Ijc.10687. [DOI] [PubMed] [Google Scholar]
- 4.Rutegard M, Nordenstedt H, Lu Y, Lagergren J, Lagergren P. Sex-specific exposure prevalence of established risk factors for oesophageal adenocarcinoma. British journal of cancer. 2010;103:735–40. doi: 10.1038/sj.bjc.6605804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubo A, Cook MB, Shaheen NJ, Vaughan TL, Whiteman DC, Murray L, et al. Sex-specific associations between body mass index, waist circumference and the risk of Barrett’s oesophagus: a pooled analysis from the international BEACON consortium. Gut. 2013;62:1684–91. doi: 10.1136/gutjnl-2012-303753. Epub 2013/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC, Bernstein L, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. International journal of epidemiology. 2012;41:1706–18. doi: 10.1093/ije/dys176. Epub 2012/11/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut. 2012;61:1261–8. doi: 10.1136/gutjnl-2011-300551. Epub 2011/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson LA, Murphy SJ, Johnston BT, Watson RG, Ferguson HR, Bamford KB, et al. Relationship between Helicobacter pylori infection and gastric atrophy and the stages of the oesophageal inflammation, metaplasia, adenocarcinoma sequence: results from the FINBAR case-control study. Gut. 2008;57:734–9. doi: 10.1136/gut.2007.132662. Epub 2007/11/21. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen NJ. What is behind the remarkable increase in esophageal adenocarcinoma? The American journal of gastroenterology. 2014;109:345–7. doi: 10.1038/ajg.2014.35. Epub 2014/03/07. [DOI] [PubMed] [Google Scholar]
- 10.Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther. 2010;32:1222–7. doi: 10.1111/j.1365-2036.2010.04471.x. Epub 2010/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falk GW, Jacobson BC, Riddell RH, Rubenstein JH, El-Zimaity H, Drewes AM, et al. Barrett’s esophagus: prevalence-incidence and etiology-origins. Ann Ny Acad Sci. 2011;1232:1–17. doi: 10.1111/j.1749-6632.2011.06042.x. [DOI] [PubMed] [Google Scholar]
- 12.Xian W, Ho KY, Crum CP, McKeon F. Cellular Origin of Barrett’s Esophagus: Controversy and Therapeutic Implications. Gastroenterology. 2012;142:1424–30. doi: 10.1053/j.gastro.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: Baseline histology and flow cytometry identify low- and high-risk patient subsets. American Journal of Gastroenterology. 2000;95:1669–76. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan X, Snyder N. Prevalence of Barrett’s esophagus in patients with or without GERD symptoms: role of race, age, and gender. Digestive diseases and sciences. 2009;54:572–7. doi: 10.1007/s10620-008-0395-7. Epub 2008/07/26. [DOI] [PubMed] [Google Scholar]
- 15.El-Serag HB, Tran T, Richardson P, Ergun G. Anthropometric correlates of intragastric pressure. Scandinavian journal of gastroenterology. 2006;41:887–91. doi: 10.1080/00365520500535402. [DOI] [PubMed] [Google Scholar]
- 16.Anand G, Katz PO. Gastroesophageal Reflux Disease and Obesity. Gastroenterology clinics of North America. 2010;39:39. doi: 10.1016/j.gtc.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Emerenziani S, Rescio MP, Guarino MPL, Cicala M. Gastro-esophageal reflux disease and obesity, where is the link? World J Gastroentero. 2013;19:6536–9. doi: 10.3748/wjg.v19.i39.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boeckxstaens GE. Review article: the pathophysiology of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;26:149–60. doi: 10.1111/j.1365-2036.2007.03372.x. Epub 2007/06/27. [DOI] [PubMed] [Google Scholar]
- 19.Edelstein ZR, Bronner MP, Rosen SN, Vaughan TL. Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. The American journal of gastroenterology. 2009;104:834–42. doi: 10.1038/ajg.2009.137. Epub 2009/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tselepis C, Perry I, Jankowski J. Barrett’s esophagus: disregulation of cell cycling and intercellular adhesion in the metaplasia-dysplasia-carcinoma sequence. Digestion. 2000;61:1–5. doi: 10.1159/000007729. Epub 2000/02/15. 7729. [DOI] [PubMed] [Google Scholar]
- 21.Reid BJ, Prevo LJ, Galipeau PC, Sanchez CA, Longton G, Levine DS, et al. Predictors of progression in Barrett’s esophagus II: baseline 17p (p53) loss of heterozygosity identifies a patient subset at increased risk for neoplastic progression. The American journal of gastroenterology. 2001;96:2839–48. doi: 10.1111/j.1572-0241.2001.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maley CC, Galipeau PC, Li X, Sanchez CA, Paulson TG, Reid BJ. Selectively Advantageous Mutations and Hitchhikers in Neoplasms: p16 Lesions Are Selected in Barrett’s Esophagus. Cancer research. 2004;64:3414–27. doi: 10.1158/0008-5472.CAN-03-3249. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez H, Opalinska J, Zhou L, Sohal D, Fazzari MJ, Yu Y, et al. Widespread hypomethylation occurs early and synergizes with gene amplification during esophageal carcinogenesis. PLoS genetics. 2011;7:e1001356. doi: 10.1371/journal.pgen.1001356. Epub 2011/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu IC, Zhao Y, Zhai R, Liu CY, Chen F, Ter-Minassian M, et al. Interactions between genetic polymorphisms in the apoptotic pathway and environmental factors on esophageal adenocarcinoma risk. Carcinogenesis. 2011;32:502–6. doi: 10.1093/carcin/bgq287. Epub 2011/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhai RH, Chen F, Liu G, Su L, Kulke MH, Asomaning K, et al. Interactions Among Genetic Variants in Apoptosis Pathway Genes, Reflux Symptoms, Body Mass Index, and Smoking Indicate Two Distinct Etiologic Patterns of Esophageal Adenocarcinoma. Journal of Clinical Oncology. 2010;28:2445–51. doi: 10.1200/Jco.2009.26.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhai RH, Liu G, Asomaning K, Su L, Kulke MH, Heist RS, et al. Genetic polymorphisms of VEGF, interactions with cigarette smoking exposure and esophageal adenocarcinoma risk. Carcinogenesis. 2008;29:2330–4. doi: 10.1093/carcin/bgn210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SEER. Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch; ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2011 Sub (1973–2010) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties. released April 2013, based on the November 2012 submission. [Google Scholar]
- 28.Levine DM, Ek WE, Zhang R, Liu X, Onstad L, Sather C, et al. A genome-wide association study identifies new susceptibility loci for esophageal adenocarcinoma and Barrett’s esophagus. Nature genetics. 2013;45:1487–93. doi: 10.1038/ng.2796. Epub 2013/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ek WE, Levine DM, D’Amato M, Pedersen NL, Magnusson PK, Bresso F, et al. Germline genetic contributions to risk for esophageal adenocarcinoma, Barrett’s esophagus, and gastroesophageal reflux. Journal of the National Cancer Institute. 2013;105:1711–8. doi: 10.1093/jnci/djt303. Epub 2013/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ. Dyspepsia and Dyspepsia Subgroups - a Population-Based Study. Gastroenterology. 1992;102:1259–68. [PubMed] [Google Scholar]
- 31.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–56. doi: 10.1016/s0016-5085(97)70025-8. Epub 1997/05/01. [DOI] [PubMed] [Google Scholar]
- 32.Locke GR, 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Risk factors associated with symptoms of gastroesophageal reflux. The American journal of medicine. 1999;106:642–9. doi: 10.1016/s0002-9343(99)00121-7. Epub 1999/06/23. [DOI] [PubMed] [Google Scholar]
- 33.Ruigomez A, Rodriguez LAG, Wallander MA, Johansson S, Graffner H, Dent J. Natural history of gastro-oesophageal reflux disease diagnosed in general practice. Aliment Pharm Ther. 2004;20:751–60. doi: 10.1111/j.1365-2036.2004.02169.x. [DOI] [PubMed] [Google Scholar]
- 34.Ruigomez A, Wallander MA, Lundborg P, Johansson S, Rodriguez LAG. Gastroesophageal reflux disease in children and adolescents in primary care. Scandinavian journal of gastroenterology. 2010;45:139–46. doi: 10.3109/00365520903428606. [DOI] [PubMed] [Google Scholar]
- 35.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–7. doi: 10.1136/gut.2004.051821. Epub 2005/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. Epub 1998/03/03. [DOI] [PubMed] [Google Scholar]
- 37.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : the journal of the American Medical Association. 2012;307:491–7. doi: 10.1001/jama.2012.39. Epub 2012/01/19. [DOI] [PubMed] [Google Scholar]
- 38.Komlos J, Brabec M. The trend of BMI values of US adults by deciles, birth cohorts 1882–1986 stratified by gender and ethnicity. Economics and human biology. 2011;9:234–50. doi: 10.1016/j.ehb.2011.03.005. Epub 2011/05/13. [DOI] [PubMed] [Google Scholar]
- 39.Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. American journal of epidemiology. 2012;175:54–9. doi: 10.1093/aje/kwr288. Epub 2011/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banatvala N, Mayo K, Megraud F, Jennings R, Deeks JJ, Feldman RA. The cohort effect and Helicobacter pylori. The Journal of infectious diseases. 1993;168:219–21. doi: 10.1093/infdis/168.1.219. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 41.Thrift AP, Kramer JR, Qureshi Z, Richardson PA, El-Serag HB. Age at onset of GERD symptoms predicts risk of Barrett’s esophagus. The American journal of gastroenterology. 2013;108:915–22. doi: 10.1038/ajg.2013.72. Epub 2013/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett’s esophagus. The American journal of gastroenterology. 2010;105:1729, 30–7. doi: 10.1038/ajg.2010.194. quiz 38. Epub 2010/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonde P, Sui G, Dhara S, Wang J, Broor A, Kim IF, et al. Cytogenetic characterization and gene expression profiling in the rat reflux-induced esophageal tumor model. The Journal of thoracic and cardiovascular surgery. 2007;133:763–9. doi: 10.1016/j.jtcvs.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 44.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, et al. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett’s metaplasia. Gastroenterology. 2010;138:1810–22. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Triadafilopoulos G. Proton pump inhibitors for Barrett’s oesophagus. Gut. 2000;46:144–6. doi: 10.1136/Gut.46.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hvid-Jensen F, Pedersen L, Funch-Jensen P, Drewes AM. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett’s oesophagus: a nationwide study of 9883 patients. Aliment Pharm Ther. 2014;39:984–91. doi: 10.1111/Apt.12693. [DOI] [PubMed] [Google Scholar]
- 47.Sital RR, Kusters JG, De Rooij FW, Kuipers EJ, Siersema PD. Bile acids and Barrett’s oesophagus: a sine qua non or coincidence? Scandinavian journal of gastroenterology Supplement. 2006;243:11–7. doi: 10.1080/00365520600664219. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Chaudhary N, Baghdadi J, Pei Z. Microbiome in reflux disorders and esophageal adenocarcinoma. Cancer journal. 2014;20:207–10. doi: 10.1097/PPO.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luebeck EG, Curtius K, Jeon J, Hazelton WD. Impact of tumor progression on cancer incidence curves. Cancer research. 2013;73:1086–96. doi: 10.1158/0008-5472.CAN-12-2198. Epub 2012/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong CY, Kroep S, Curtius K, Hazelton WD, Jeon J, Meza R, et al. Exploring the recent trend in esophageal adenocarcinoma incidence and mortality using comparative simulation modeling. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:997–1006. doi: 10.1158/1055-9965.EPI-13-1233. Epub 2014/04/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arias E. United States life tables, 2000. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2002;51:1–38. Epub 2003/02/14. [PubMed] [Google Scholar]
- 52.Holford TR, Levy DT, McKay LA, Clarke L, Racine B, Meza R, et al. Patterns of birth cohort-specific smoking histories, 1965–2009. Am J Prev Med. 2014;46:e31–7. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson C, Burns DM, Dodd KW, Feuer EJ. Chapter 2: Birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk Analysis. 2012;32(Suppl 1):S14–24. doi: 10.1111/j.1539-6924.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proton Pump Inhibitors BPCA Drug Use Review and Duration of Use Analysis, OSE RCM #: 2010–306. 10903 New Hampshire Avenue, Silver Spring, MD 20993: Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Surveillance and Epidemiology; Apr 30, 2010. pp. 1–28. [Google Scholar]
- 55.Rotman SR, Bishop TF. Proton Pump Inhibitor Use in the US Ambulatory Setting, 2002–2009. PloS one. 2013;8 doi: 10.1371/journal.pone.0056060. ARTN e56060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholson A, Jankowski J. Acid reflux and oesophageal cancer. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2011;185:65–82. doi: 10.1007/978-3-642-03503-6_4. Epub 2011/08/09. [DOI] [PubMed] [Google Scholar]
- 57.Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 58.Rubenstein JH, Morgenstern H, Appelman H, Scheiman J, Schoenfeld P, McMahon LF, Jr, et al. Prediction of Barrett’s Esophagus Among Men. The American journal of gastroenterology. 2013 doi: 10.1038/ajg.2012.446. Epub 2013/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lubin JH, Cook MB, Pandeya N, Vaughan TL, Abnet CC, Giffen C, et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett’s Esophagus and Esophageal Adenocarcinoma Consortium. Cancer epidemiology. 2012;36:306–16. doi: 10.1016/j.canep.2012.03.001. Epub 2012/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reid BJ, Li X, Galipeau PC, Vaughan TL. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nature reviews Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. Epub 2010/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.